Abstract
Objectives:
To identify guidelines on the clinical use of CBCT in dental and maxillofacial radiology, in particular selection criteria, to consider how they were produced, to appraise their quality objectively and to compare their recommendations.
Methods:
A literature search using MEDLINE (Ovid®) was undertaken prospectively from 1 January 2000 to identify published material classifiable as “guidelines” pertaining to the use of CBCT in dentistry. This was supplemented by searches on websites, an internet search engine, hand searching of theses and by information from personal contacts. Quality assessment of publications was performed using the AGREE II instrument. Publications were examined for areas of agreement and disagreement.
Results:
26 publications were identified, 11 of which were specifically written to give guidelines on the clinical use of CBCT and contained sections on selection criteria. The remainder were a heterogeneous mixture of publications that included guidelines relating to CBCT. Two had used a formal evidence-based approach for guideline development and two used consensus methods. The quality of publications was frequently low as assessed using AGREE II, with many lacking evidence of adequate methodology. There was broad agreement between publications on clinical use, apart from treatment planning, in implant dentistry.
Conclusions:
Reporting of guideline development is often poorly presented. Guideline development panels should aim to perform and report their work using the AGREE II instrument as a template to raise standards and avoid the risk of suspicions of bias.
Keywords: cone-beam computerized tomography; radiography, dental; patient selection practice guideline; evidence-based dentistry
Introduction
The arrival of any new medical intervention, diagnostic or therapeutic, brings new challenges to clinicians. Will its introduction be worthwhile in terms of financial cost? Will it give benefits to the patients in terms of quality of life? Will not using it put clinicians at a professional disadvantage? The introduction of CBCT for dental and maxillofacial radiology has posed many questions such as these. As described by Fryback and Thornbury,1 a new radiological technique should be efficacious at all levels, from technical accuracy efficacy to societal efficacy, yet the introduction and growth of CBCT has moved faster than the acquisition of the evidence. CBCT has been available in dental and maxillofacial radiology for well over a decade. Numerous models of equipment are in existence,2 and there is evidence of widespread use in some countries.3,4
Clinical guidelines are a means of providing a framework for the use of a new technology or technique. Guidelines are systematically developed statements designed to assist the clinician and patient in making decisions about appropriate healthcare for certain specific clinical circumstances.5 There are three fundamental approaches to guideline development. The first is to rely on the opinion of an expert panel's considered judgment. The second is to employ some kind of consensus method and the third is to use “evidence-based” guideline development methodology. Each has advantages and disadvantages, but the aim with any guideline must be to limit the influence of individual opinion and bias. Evidence-based methods are promoted as having the best chance of achieving this by using defined and objective methods based upon systematic review of the literature, with quality assessment of evidence and the grading of recommendations.6
In radiology, guidelines can provide assistance in choosing the appropriate imaging pathway and are often called “referral criteria”, “selection criteria” or “appropriateness criteria”. These are descriptions of clinical conditions derived from patient signs, symptoms or history that identify patients who are likely to benefit from a particular radiographic technique.7 In medical imaging, the availability of such guidelines is well established.8,9 There may be a requirement for selection criteria to be available to clinicians. For example, in the European Union, the directive relating to medical uses of ionizing radiation requires that the “Holder” (employer) responsible for an establishment using X-rays on patients provides referral criteria for clinicians.10 While this may not be the case elsewhere, there remains the ethical need for justification of medical exposures, for which referral criteria provide a framework of good practice.
In the context of CBCT in dentistry, where higher radiation doses are usually seen than in conventional dental radiography,11,12 it is particularly important to adhere to the radiation protection principle of justification. Guidelines, in the form of selection criteria, can provide the clinician with a helpful framework within which to work. The aim of this review was to identify guidelines on the clinical use of CBCT in dental and maxillofacial radiology, in particular selection criteria, to consider how they were produced, to appraise their quality objectively and to compare and contrast their recommendations.
Methods and materials
The reporting of this review follows, wherever possible, the format recommended in the preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.13
A literature search was undertaken to identify published material classifiable as “guidelines” pertaining to the use of CBCT in dentistry.
Eligibility criteria
To be included, the identified guidelines had to meet three criteria:
make recommendations on the clinical use (relating to justification and selection criteria) of CBCT in any dentally related speciality
be aimed at the individual practitioner (any health professional working within dentistry) and/or patient level
be published in 2000 or after.
No a priori language restrictions were set, as it was anticipated that many non-English publications might be amenable to translation by the authors or by colleagues if needed.
Information sources and search strategy
It was anticipated that guideline documents would not necessarily be identifiable by a simple search of the scientific literature, so several strategies were used. The primary method of sourcing guideline publications, a MEDLINE (Ovid®) search, was performed prospectively from 1 January 2000, with a final search date of 18 June 2014. The terms used for the MEDLINE (Ovid) search are shown in Table 1.
Table 1.
Guideline-related search terms used for MEDLINE (Ovid®)
| 1. guideline*.mp. or exp guideline/ |
| 2. position statement.mp. |
| 3. position paper.mp. |
| 4. clinical recommendation*.mp. |
| 5. or/1–4 |
| 6. cone beam computed tomography.mp. |
| 7. volumetric radiography.mp. |
| 8. volumetric tomography.mp. |
| 9. digital volumetric tomography.mp. |
| 10. digital volume tomography.mp. |
| 11. Cone-beam.mp. or exp Cone-Beam Computed Tomography/ |
| 12. (volume ct or volumetric ct).mp. |
| 13. (volume computed tomography or volumetric computed tomography).mp. |
| 14. CBCT.mp. |
| 15. or/6–14 |
| 16. (dental or dentistry).mp. |
| 17. exp dentistry/ |
| 18. or/16–17 |
| 19. 15 and 18 |
The search was performed prospectively from 1 January 2000. An initial, focused search was undertaken using these terms, along with a second, broader search excluding lines 6–15, run on the same date.
In addition, the US National Guideline Clearinghouse (www.guideline.gov) and the Royal College of Surgeons of England (https://www.rcseng.ac.uk/fds/publications-clinical-guidelines/clinical_guidelines) websites were searched on the same date, and an ad hoc search of Google using a variety of relevant search terms was undertaken in the expectation of identifying grey literature (e.g. governmental agency reports, specialist society documents). Requests for information on guidelines were made on two occasions over the preceding 2 years on the ORADLIST Oral Radiology online discussion group (http://lists.ucla.edu/cgi-bin/mailman/listinfo/oradlist) hosted by the University of California (Los Angeles, CA) requesting information about guidelines or position papers on CBCT. The reference lists of two PhD theses from the University of Manchester, UK, were also hand-searched for relevant guideline publications.
Where guidelines had been updated or published more than once, the most recent version was used for the assessment, taking into consideration any methods published in previous publications.
Selection of publications
The search results were managed in Endnote X4® (Adept Scientific Ltd, Letchworth, UK). An initial screen of the results was undertaken by a single assessor (A-MG) to remove any documents that were clearly not relevant. A second, more focused screening was undertaken by a second assessor (KH) to determine whether the identified documents truly met the inclusion criteria.
Data collection process
Each identified guideline document that met the inclusion criteria was assessed for quality by two, independent, assessors from a team of five (the four listed authors plus one other). The fifth assessor was used on two occasions where the allocation of publications would have resulted in assessment of a guideline by someone involved in their development. The AGREE Collaboration has defined quality of guidelines as “the confidence that the potential biases of guideline development have been addressed adequately and that the recommendations are both internally and externally valid, and are feasible for practice”.14 The AGREE II instrument assesses the methodological rigour and transparency with which a guideline has been developed, and was used for this review.14 To make this assessment, the AGREE II instrument requires the appraiser to make a judgment on each of 23 items in 6 domains (Table 2) allocating a quality score between 1 and 7. A score of one was given when there is no information that is relevant to the AGREE II item or if the concept is very poorly reported. A score of seven was given if the quality of reporting was exceptional and where the full criteria and considerations articulated in the AGREE II user's manual were met. Domain scores were calculated by summing up all the scores of the individual items in a domain and by scaling the total as a percentage of the maximum possible score for that domain. AGREE II gives no threshold of adequacy for domain scores but advises that such decisions should be made by the user and guided by the context in which the instrument is being used.
Table 2.
Domains and key items assessed using the AGREE II instrument14
| Domain | Key item |
|---|---|
| Scope and purpose | The overall objective(s) of the guideline is (are) specifically described The health question(s) covered by the guideline is (are) specifically described The population (patients, public etc.) to whom the guideline is meant to apply is specifically described |
| Stakeholder involvement | The guideline development group includes individuals from all relevant professional groups The views and preferences of the target population (patients, public etc.) have been sought The target users of the guideline are clearly defined |
| Rigour of development | Systematic methods were used to search for evidence The criteria for selecting the evidence are clearly described The strengths and limitations of the body of evidence are clearly described The health benefits, side effects and risks have been considered in formulating the recommendations There is an explicit link between the recommendations and the supporting evidence The guideline has been externally reviewed by experts prior to its publication A procedure for updating the guideline is provided |
| Clarity of presentation | The recommendations are specific and unambiguous The different options for management of the condition or health issue are clearly presented Key recommendations are easily identifiable |
| Applicability | The guideline describes facilitators and barriers to its application The guideline provides advice and/or tools on how the recommendations can be put into practice The potential resource implications of applying the recommendations have been considered The guideline presents monitoring and/or auditing criteria |
| Editorial independence | The views of the funding body have not influenced the content of the guideline Competing interests of guideline development group members have been recorded and addressed |
Each included publication was classified into one of three categories according to the method used in developing guideline statements:
expert based
consensus based with a clearly defined methodology
formal evidence based with a clearly defined methodology for assessing evidence and grading of recommendations.
Guidelines in publications described as being achieved by “consensus” were only classified as such where there was demonstrable evidence of a methodology having been used (e.g. voting process, Delphi methods).
Guideline statements relating to aspects of justification and selection criteria for CBCT were extracted and classified according to clinical use (e.g. implant dentistry, orthodontics, trauma). Agreement and disagreement between guidelines were noted.
Results
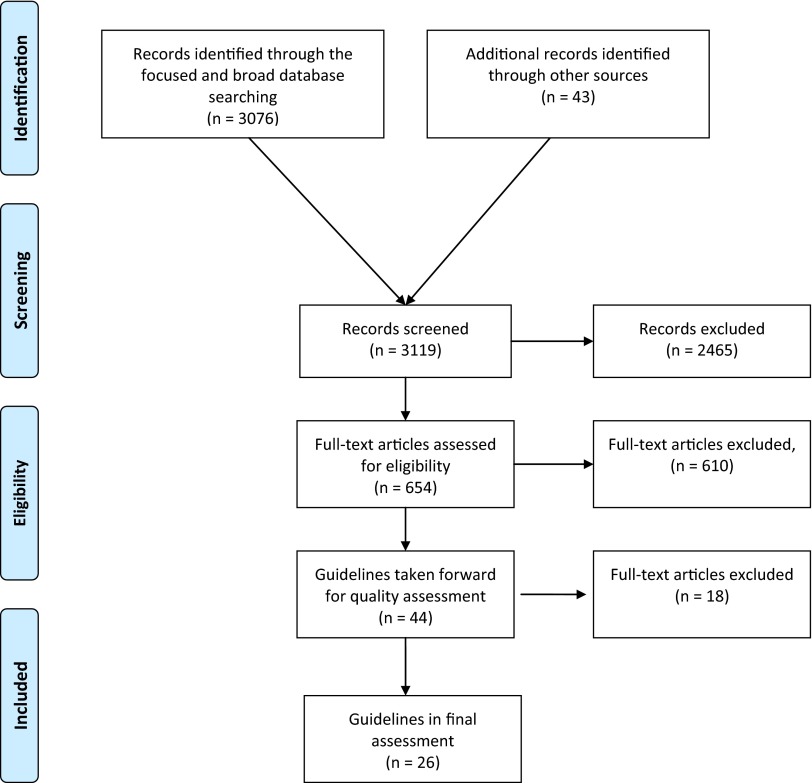
Figure 1 shows the flow of articles identified through our searches. Following full text screening and removal of duplicates, 44 publications remained for quality assessment. However, during the course of quality assessment, some publications were excluded because they were clearly updated or revised versions of other guidelines. In addition, a pragmatic decision was taken to exclude some publications in which the content regarding clinical use (aspects of justification and selection criteria) was extremely limited and which only made explicit referral to other publications included in the review. The final number of publications subjected to quality assessment was 26. Table 3 shows these 26 publications, their date of publication, their mode of development and the clinical areas at which guidelines were aimed.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow chart13 depicting the number of records identified, included and excluded at the different stages in the review.
Table 3.
The 26 publications identified in the review, with their year of publication, their method of development and the clinical areas at which guidelines were aimed
| Publication | Year of publication | Country or region of origin | Method of guideline development | Focus of CBCT clinical use |
|---|---|---|---|---|
|
Guideline publications related specifically to CBCT | ||||
| Haute Autorité de Santé15 | 2009 | France | Expert opinion | Comprehensive |
| Horner et al16 | 2009 | Europe | Consensus | Comprehensive |
| American Association of Endodontists; American Academy of Oral and Maxillofacial Radiology3 | 2011 | USA | Expert opinion | Endodontics |
| Hoge Gezondheidsraad17 | 2011 | Belgium | Expert opinion | Comprehensive |
| Noffke et al18 | 2011 | South Africa | Expert opinion | Comprehensive |
| American Dental Association Council on Scientific Affairs19 | 2012 | USA | Expert opinion | Comprehensive |
| Benavides et al20 | 2012 | International | Expert opinion | Implant dentistry |
| European Commission11 | 2012 | Europe | Evidence-based methods | Comprehensive |
| American Academy of Oral and Maxillofacial Radiology21 | 2013 | USA | Consensus | Orthodontics |
| Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften22 | 2013 | Germany | Evidence-based methods | Comprehensive |
| European Society of Endodontology23 | 2014 | Europe | Expert opinion | Endodontics |
|
Publications including guideline statements on the use of CBCT | ||||
| Handelsman24 | 2006 | USA | Expert opinion | Implant dentistry |
| Isaacson et al25 | 2008 | UK | Expert opinion | Orthodontics |
| Academy of Osseointegration26 | 2010 | USA | Expert opinion | Implant dentistry |
| Drago and Carpentieri27 | 2011 | USA | Expert opinion | Implant dentistry |
| Diangelis et al28 | 2012 | International | Expert opinion | Dental trauma |
| Evans et al29 | 2012 | UK | Expert opinion | Endodontics |
| Harris et al30 | 2012 | Europe | Expert opinion | Implant dentistry |
| Husain et al31 | 2012 | UK | Expert opinion | Orthodontics |
| Tyndall et al32 | 2012 | USA | Expert opinion | Implant dentistry |
| Walter et al33 | 2012 | Switzerland | Expert opinion | Periodontology |
| American Association of Endodontists34 | 2013 | USA | Expert opinion | Dental trauma |
| Cooper and Pin-Harry35 | 2013 | USA | Expert opinion | Implant dentistry |
| Counihan et al36 | 2013 | UK | Expert opinion | Orthodontics |
| Faculty of General Dental Practice (UK)37 | 2013 | UK | Expert opinion | Comprehensive |
| Ngiam et al38 | 2013 | Australia | Expert opinion | Sleep apnoea |
11 of the included publications (Table 3) were specifically focused on the use of CBCT, whereas the remaining 15 contained recommendations on the use of CBCT. Two of these could have been classified in the former category, as although both related to imaging in implant dentistry, they were both primarily concerned with CBCT.30,32 Most of the guideline publications were from the USA, UK or European institutions/organizations. Eight publications were judged to be “comprehensive”, in that they either gave general guidance on clinical use and aspects of justification or provided multiple guidelines relating to different dental uses.11,15–20,22,37 Only two documents showed clear reporting of an evidence-based methodology for guideline development.11,22 The FGDP (UK)37 used grading of evidence for guideline statements, but there was much inconsistency between sections of the document and it was clear that many guidelines were based on expert opinion only, so this was judged to be an expert-based publication. Two publications could reasonably be classified as consensus guidelines.16,21 Other publications may have used the term “consensus” in their titles or within the text but gave no evidence of how consensus was achieved; in these cases, it was assumed that “consensus” was being used synonymously with “agreement” rather than indicating the use of a defined methodology. All other publications were classified as expert opinion, although there was great variation in the number and nature of the “experts” involved, ranging from a single author publication24 to extensive and multidisciplinary panels.15,37
The results of the quality assessment using the AGREE II instrument are shown in Table 4. Looking at the six domains considered by the AGREE II tool, some patterns could be seen. There were generally good quality scores for Domain 1 (scope and purpose) because authors usually communicated the focus of the guideline(s) and the intended context adequately. Scores for Domain 2 (stakeholder involvement) were relatively low, but variable, usually reflecting the absence of any patient or public involvement in guideline development, but also the multidisciplinary nature of the team involved. Domain 3 (rigour of development) also scored variably, with only four publications exceeding 50% for this key aspect.11,15,22,37 This disappointing result usually reflected incomplete or absent detail of methodology, but all of the key items in this domain were often absent. Clarity of presentation (Domain 4) typically produced a positive (>50%) score. The few exceptions that scored low were usually owing to recommendations being positioned within the text of the document and hard to identify, rather than being highlighted, or were ambiguous in their wording. The ratings for Domain 5 (applicability) were generally very poor, demonstrating an almost uniform failure to consider the implications of guideline implementation. Only one publication was scored positively (58%) in this domain;37 this was owing to the inclusion of a section on tools for clinical audit in practice.
Table 4.
Quality scores of each domain defined by the AGREE II instrument for the included guideline publications, calculated according to the method of Brouwers et al14
| Publication | AGREE II domains |
|||||
|---|---|---|---|---|---|---|
| Scope and purpose | Stakeholder involvement | Rigour of development | Clarity of presentation | Applicability | Editorial independence | |
|
Guidelines related specifically to CBCT | ||||||
| Haute Autorité de Santé15 | 86 | 42 | 65 | 78 | 25 | 58 |
| Horner et al16 | 72 | 53 | 43 | 78 | 13 | 25 |
| American Association of Endodontists; American Academy of Oral and Maxillofacial Radiology3 | 36 | 19 | 7 | 41 | 0 | 0 |
| Hoge Gezondheidsraad17 | 92 | 42 | 28 | 72 | 6 | 17 |
| Noffke et al18 | – | – | – | – | – | – |
| American Dental Association Council on Scientific Affairs19 | 53 | 50 | 13 | 61 | 2 | 33 |
| Benavides et al20 | 81 | 44 | 23 | 72 | 21 | 17 |
| European Commission11 | 92 | 78 | 94 | 97 | 38 | 63 |
| American Academy of Oral and Maxillofacial Radiology21 | 72 | 36 | 17 | 72 | 4 | 0 |
| Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften22 | 92 | 58 | 75 | 86 | 19 | 58 |
| European Society of Endodontology23 | 64 | 44 | 26 | 58 | 8 | 63 |
|
Guidelines including statements on use of CBCT | ||||||
| Handelsman24 | 39 | 6 | 0 | 25 | 0 | 0 |
| Isaacson et al25 | 89 | 53 | 32 | 81 | 2 | 13 |
| Academy of Osseointegration26 | 42 | 22 | 3 | 28 | 2 | 0 |
| Drago and Carpentieri27 | 58 | 3 | 0 | 19 | 0 | 0 |
| Diangelis et al28 | 83 | 47 | 19 | 83 | 0 | 0 |
| Evans et al29 | 81 | 36 | 13 | 50 | 0 | 13 |
| Harris et al30 | 80 | 64 | 20 | 67 | 6 | 33 |
| Husain et al31 | 69 | 33 | 5 | 39 | 0 | 0 |
| Tyndall et al32 | 91 | 47 | 19 | 69 | 2 | 13 |
| Walter et al33 | 64 | 19 | 38 | 53 | 27 | 46 |
| American Association of Endodontists34 | 81 | 25 | 2 | 69 | 0 | 0 |
| Cooper and Pin-Harry35 | 42 | 0 | 0 | 17 | 0 | 0 |
| Counihan et al36 | 50 | 14 | 0 | 6 | 4 | 0 |
| Faculty of General Dental Practice (UK)37 | 92 | 61 | 61 | 92 | 56 | 38 |
| Ngiam et al38 | 78 | 28 | 10 | 56 | 6 | 29 |
Scores are the mean of two assessors and are expressed as a percentage of the maximum achievable score for that domain.
There was reasonable agreement on the fundamental principle of justification and individual selection of patients for CBCT examinations. On several occasions, it was recommended that CBCT should be reserved as a supplementary imaging technique where conventional radiography failed to answer the question for which imaging was required. The main guideline documents dealing with endodontic uses of CBCT follow this approach.3,11,23,37 The US-based guideline3 statement that “CBCT should only be used when the question for which imaging is required cannot be answered adequately by lower dose conventional dental radiography or alternate imaging modalities” concurs almost exactly with the wording of European-based guidelines.11,15,23,37 This conservative view is reinforced in the recent European Society of Endodontology position statement, which states that “…a CBCT scan should only be considered if the additional information from reconstructed three-dimensional images will potentially aid [in] formulating a diagnosis and/or enhance the management of a tooth with an endodontic problem(s)”.23
However, there was one example of conflicting guidelines: the use of CBCT in implant dentistry planning, where three publications have recommended, in the context of CBCT, that cross-sectional imaging should be used in planning all dental implant placements.18,27,32 Other guidelines maintain that a selective approach is appropriate,11,15,17,20,22,30,37 while a further group of publications gave equivocal statements.24,26,35
Discussion
One challenge in conducting this review was in identifying guidelines. Unlike research studies, which will generally be published in journals, guidelines may be found in a wide variety of locations, such as the websites of specialist societies and colleges, and access may be restricted to members. Thus, we used a variety of search strategies to perform this review, but it is likely that relevant publications were missed. Language was also a limitation, with most non-English documents being identified by personal contacts, the Google search or from the reference lists in the European guidelines.11 Despite these limitations, the guidelines identified are probably a reasonable reflection of the range of material available to clinicians. It would be of value if developers of clinical guideline documents were required to submit them to national or international repositories, such as the National Guideline Clearing House in the USA. Recently, the Cochrane Collaboration Oral Health Group has established an International Oral Health Care Guideline Depository (http://ohg.cochrane.org/international-oral-health-guideline-repository) to help identify priority review topics that could inform guideline development, to identify areas of duplication/overlap, where evidence tables could be shared between guideline development groups, and to increase stakeholder involvement in guideline development by widening dissemination. These are admirable aspirations, bearing in mind the limitations of guidelines observed in this review and the duplication of efforts on areas such as the use of CBCT in implant dentistry and endodontics.
Most of the publications were classified as expert opinion based, although some had clearly involved substantial effort of many people to develop. This is disappointing when clinical guideline development methods, such as those described by Scottish Intercollegiate Guidelines Network (SIGN),39 National Institute for Health and Care Excellence (NICE)40 and the American College of Radiology9 are well established. This probably reflects the considerable time commitment involved in undertaking such activities. Looking at the publications listed in Table 3, publications ranged from substantial multinational efforts through to single or double author articles. It could be argued that some or all of the latter should have been excluded. However, these items were self-described as offering “guidelines” to clinicians and were often written in the authoritative and engaging manner of experienced clinicians. Readers of these articles may accept such well-illustrated and easily understood publications as being preferable to other, more “academic” guidelines, so a decision was made to include them in the review.
We restricted the evidence gathering to clinical applications of CBCT, i.e. aspects of justification and selection criteria. Consequently, some important publications on CBCT, from national and provincial radiation safety authorities, were not included where they dealt principally with aspects of administrative requirements and radiation safety.41–45 In terms of guidelines on clinical use (selection criteria), the guideline documents identified were heterogeneous. Some were comprehensive and lengthy, including numerous detailed selection criteria for particular clinical situations. Others limited their recommendations to basic principles and did not attempt detailed guideline development. Some publications were guidelines on clinical procedures (Table 3), in which imaging was only a small part, and detailed selection criteria for CBCT were, perhaps understandably, lacking.
There are enormous challenges in developing selection criteria for CBCT in dentistry. The evidence base is still very limited for some clinical uses. While some studies of diagnostic accuracy are achievable where a valid laboratory model can be used (e.g. dental fracture diagnosis); for other applications such as periapical inflammatory pathosis, it is impossible to achieve a study design entirely free of risk of bias or applicability problems. There are few studies at the higher levels of hierarchy of diagnostic efficacy in accordance with Fryback and Thornbury1, to the authors' knowledge at the time of conducting this review, with only one randomized controlled trial having been published on the impact of CBCT on patient outcomes.46 The many CBCT machines on the market have different image quality and the diagnostic capability of any machine will vary depending upon mode of operation. Thus, it might be argued that we will never be able to develop “definitive” guidelines with high grading of supporting evidence for CBCT. This may well be true, but it does not validate a passive approach from researchers and guideline developers. Instead, efforts should remain focused on producing the best achievable guidelines with a transparent approach to acknowledging where evidence is lacking.
We used the AGREE II appraisal tool14 to conduct this review in an attempt to assess quality. The AGREE Collaboration defined quality of guidelines as “the confidence that the potential biases of guideline development have been addressed adequately and that the recommendations are both internally and externally valid, and are feasible for practice”.47 The results of our appraisal suggest that many guidelines on clinical use of CBCT fall well short of the ideal. Such a finding is not unique to CBCT or to radiology in general. It is important to recognize that the AGREE II quality scores (Table 3) should not be interpreted as a “league table” or a condemnation of poorly scoring publications. Some guideline documents might be valid in their recommendations; agreement between poorly and highly scoring guidelines for many aspects of clinical use suggest that this is the case. Rather, the quality scores should be seen for what they are: an indicator of the clarity of reporting. Without such clarity, there is a risk that guidelines could inappropriately be accused of bias or errors. The AGREE II instrument should be seen as a template for those developing and presenting guidelines, in a manner analogous to the use of the Standards for Reporting of Diagnostic Accuracy statement for the reporting of diagnostic accuracy studies (http://www.stard-statement.org/). Furthermore, there was not perfect agreement between assessors of each publication using the AGREE II tool. While there was no evidence of contrary judgments on specific items, the scores allocated might not agree. In retrospect, the use of three, or ideally four, assessors might have been desirable.
Patient and public involvement is widely seen as an essential part of guideline development and implementation48,49 and is often straightforward when guidelines are being developed for particular clinical conditions, such as cancer or chronic diseases, because well-established and highly motivated patient organizations and pressure groups usually exist. By contrast, it is particularly challenging to identify a means of patient and public involvement when developing guidelines on diagnostic tests, as highlighted in the guideline document from Germany.22 Nonetheless, patient and public involvement is not impossible to achieve, and strategies have been developed.50 It is reasonable to expect that development of any clinical guideline would involve representation from all professional groups that it may affect. Some publications in this review demonstrated a multidisciplinary authorship, notably the European Commission11 document, but others were restricted to one professional group, such as the radiologists. This inevitably weakens any guideline and can increase the risk of accusations of bias. The AGREE II instrument specifically highlights the need for involvement of at least one methodology expert within the development group (e.g. systematic review expert, epidemiologist, statistician, library scientist etc.).14
It is important to recognize that some publications may have performed a comprehensive and systematic literature review and have linked their recommendations to the strength of the evidence yet simply failed to report this. Statements were seen in the preamble to some reviewed publications such as “a systematic literature review was performed”. The absence of detail about the search strategy and method of critical appraisal was insufficient and might add to suspicion of bias. Few publications recorded that external review had been performed. Of course, in cases published in journals, it would be expected that peer review would have been performed prior to acceptance, but review by independent assessors prior to journal submission is a desirable feature in guideline development and is essential where a guideline is published outside the normal journal framework, e.g. on a website.
The practicalities of implementation of clinical guidelines, both facilitators and barriers, should be considered when presenting them to the target user groups. Failure to do this may partially explain why guidelines may be ignored. The final domain (editorial independence) was scored very poorly for most publications, owing to the typical absence of acknowledgment of the interests of the funding body and records of potential or real conflicts of interest within the authors/guideline development group. These are straightforward items to include in publications, and there should be no difficulty in scoring high here.
The conflict observed between guidelines on the use of CBCT in implant dentistry planning was notable. It is not the purpose of this review to argue either case, and the subject has recently been considered in a systematic review by Bornstein et al.51 Nonetheless, it is important to remember that the publications reviewed by us were presumably based on the same evidence, yet came to different conclusions. Disagreement between guidelines may be an influential factor in health professionals ignoring them. This emphasizes the need for a transparent and robust methodology in guideline development.
Whether to use CBCT or not in the “real” world is influenced by numerous factors.52 There is evidence that there is high variation in prescription of dental radiographs nationally and internationally, which is not explained by levels of dental health or wealth in society. Dentists are inevitably influenced by teachers, both as undergraduates and during continuing education. Financial pressures may favour the use of certain clinical techniques, such as CBCT, if they can increase profits; it has been demonstrated in the UK that removal of payments for radiography and other clinical activities leads to a reduction in their use.53 It is therefore of interest that, in the context of pre-surgical use of CBCT as an aid in third molar surgery, recent evidence suggests that using CBCT substantially increased costs compared with using panoramic radiography but without any change in the resources used for surgery, post-surgical treatment or patient complication management.54 An influential factor is that some dentists' training on the appropriate use of CBCT may be limited to that received from manufacturers and suppliers who may be selective in their communication of research evidence for their product. Clinical use of CBCT is as open to such influences as any other dental procedure. Thus, it is important to “fight the good fight” and promote practice based on the evidence. Furthermore, it is in the interests of those of us who are involved in guideline development to follow best practice, as indicated by AGREE, to limit the risks of bias and potential criticism.
Conclusions
Reporting of guidelines on clinical use of CBCT is often poorly presented. Prospectively, guideline development panels should aim to perform and report their work using the AGREE II instrument as a means of raising standards and avoiding the risk of suspicions of bias. In particular, bearing in mind the limitations and deficiencies in publications reviewed here, guideline developers should be sure to assemble a multidisciplinary team of stakeholders to formulate guidelines. They should perform systematic review and critical appraisal of the evidence, recognize the limitations of the available evidence and clearly link their recommendations to it. Guidelines should be externally reviewed prior to publication, and clear implementation strategies and tools for monitoring and clinical audit should be available.
Conflict of interest
KH was involved in the development of four publications included in the review, while A-MG was involved in the development of one publication.
Acknowledgments
Acknowledgments
The authors thank Dr Tanya Walsh for assisting with the quality appraisal of the reviewed publications.
References
- 1.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991; 11: 88–94. [DOI] [PubMed] [Google Scholar]
- 2.Nemtoi A, Czink C, Haba D, Gahleitner A. Cone beam CT: a current overview of devices. Dentomaxillofac Radiol 2013; 42: 20120443. doi: 10.1259/dmfr.20120443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Association of Endodontists American Academy of Oral and Maxillofacial Radiology. Use of cone-beam computed tomography in endodontics Joint Position Statement of the American Association of Endodontists and the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111: 234–7. doi: 10.1016/j.tripleo.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 4.Han S, Lee B, Shin G, Choi J, Kim J, Park C, et al. Dose area product measurement for diagnostic reference levels and analysis of patient dose in dental radiography. Radiat Prot Dosimetry 2012; 150: 523–31. doi: 10.1093/rpd/ncr439 [DOI] [PubMed] [Google Scholar]
- 5.Field MJ, Lohr KN. Guidelines for clinical practice: from development to use. Washington, DC: National Academy Press; 1992. [PubMed] [Google Scholar]
- 6.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet 2003; 362: 1225–30. [DOI] [PubMed] [Google Scholar]
- 7.European Commission. Radiation protection 136. European guidelines on radiation protection in dental radiology: the safe use of radiographs in dental practice. Luxembourg: Office for Official Publications of the European Communities; 2004. Available from: http://ec.europa.eu/energy/nuclear/radioprotection/publication/doc/136_en.pdf [Google Scholar]
- 8.European Commission. Radiation protection 118. Referral guidelines for imaging. Luxembourg: Office for Official Publications of the European Communities; 2001. Available from: http://ec.europa.eu/energy/nuclear/radioprotection/publication/doc/118_en.pdf [Google Scholar]
- 9.ACR.org. Reston, VA: American College of Radiology. [Updated May 2014; cited 29 June 2014.] Available from: http://www.acr.org/Quality-Safety/Appropriateness-Criteria [Google Scholar]
- 10.European Commission. Council directive 97/43/Euratom of 30 June 1997 on health protection of individuals against the dangers of ionising radiation in relation to medical exposure. Luxembourg: European Commission; 1997. [DOI] [PubMed] [Google Scholar]
- 11.European Commission. Radiation protection 172. Evidence based guidelines on cone beam CT for dental and maxillofacial radiology. Luxembourg: Office for Official Publications of the European Communities; 2012. Available from: http://ec.europa.eu/energy/nuclear/radiation_protection/doc/publication/172.pdf [Google Scholar]
- 12.Rottke D, Patzelt S, Poxleitner P, Schulze D. Effective dose span of ten different cone beam CT devices. Dentomaxillofac Radiol 2013; 42: 20120417. doi: 10.1259/dmfr.20120417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–41. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. ; on behalf of the AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in healthcare. CMAJ 2010; 182: E839–42. doi: 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haute Autorité de Santé. Tomographie Volumique a Faisceau Conique de la Face (Cone Beam Computerized Tomography). Rapport d’évaluation Technologique. Service évaluation des actes professionnels. Saint-Denis La Plaine: Haute Autorité de Santé; 2009. Available from: http://www.has-sante.fr/portail/upload/docs/application/pdf/2009-12/rapport_cone-beam_version_finale_2009-12-28_17-27-28_610.pdf [Google Scholar]
- 16.Horner K, Islam M, Flygare L, Tsiklakis K, Whaites E. Basic principles for use of dental cone beam computed tomography: consensus guidelines of the European Academy of Dental and Maxillofacial Radiology. Dentomaxillofac Radiol 2009; 38: 187–95. doi: 10.1259/dmfr/74941012 [DOI] [PubMed] [Google Scholar]
- 17.Advies van de Hoge Gezondheidsraad nr. 8705. Dentale Cone Beam Computed Tomography. Brussel: Hoge Gezondheidsraad; 2011. Available from: www.health.belgium.be/internet2Prd/groups/public/@public/@shc/documents/ie2divers/19068321_en.pdf [Google Scholar]
- 18.Noffke CE, Farman AG, Nel S, Nzima N. Guidelines for the safe use of dental and maxillofacial CBCT: a review with recommendations for South Africa. SADJ 2011; 66: 262, 264–6. [PubMed] [Google Scholar]
- 19.American Dental Association Council on Scientific Affairs. The use of cone-beam computed tomography in dentistry: an advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2012; 143: 899–902. [DOI] [PubMed] [Google Scholar]
- 20.Benavides E, Rios HF, Ganz SD, An CH, Resnik R, Reardon GT, et al. Use of cone beam computed tomography in implant dentistry: the International Congress of Oral Implantologists consensus report. Implant Dent 2012; 21: 78–86. doi: 10.1097/ID.0b013e31824885b5 [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Oral and Maxillofacial Radiology. Clinical recommendations regarding use of cone beam computed tomography in orthodontics. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: 238–57. doi: 10.1016/j.oooo.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 22.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). s2k-Leitlinie Dentale digitale Volumentomographie Version Nr. 9 vom 5 August 2013. AWMF-Register-Nummer:083–05. Düsseldorf: AWMF; 2013. Available from: http://www.awmf.org/uploads/tx_szleitlinien/083-005l_S2k_Dentale_Volumentomographie_2013-10.pdf [Google Scholar]
- 23.European Society of Endodontology; Patel S, Durack C, Abella F, Roig M, Shemesh H, Lambrechts P, et al. European Society of Endodontology position statement: the use of CBCT in endodontics. Int Endod J 2014; 47: 502–4. doi: 10.1111/iej.12267 [DOI] [PubMed] [Google Scholar]
- 24.Handelsman M. Surgical guidelines for dental implant placement. Br Dent J 2006; 201: 139–52. [DOI] [PubMed] [Google Scholar]
- 25.Isaacson KG, Thom AR, Horner K, Whaites E. Guidelines for the use of radiographs in clinical orthodontics. 3rd edn. London, UK: British Orthodontic Society; 2008. [Google Scholar]
- 26.Academy of Osseointegration. 2010 guidelines of the Academy of Osseointegration for the provision of dental implants and associated patient care. Int J Oral Maxillofac Implants 2010; 25: 620–7. [PubMed] [Google Scholar]
- 27.Drago C, Carpentieri J. Treatment of maxillary jaws with dental implants: guidelines for treatment. J Prosthodont 2011; 20: 336–47. doi: 10.1111/j.1532-849X.2011.00717.x [DOI] [PubMed] [Google Scholar]
- 28.Diangelis AJ, Andreasen JO, Ebeleseder KA, Kenny DJ, Trope M, Sigurdsson A, et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 1. Fractures and luxations of permanent teeth. Dent Traumatol 2012; 28: 2–12. doi: 10.1111/j.1600-9657.2011.01103.x [DOI] [PubMed] [Google Scholar]
- 29.Evans G, Bishop K, Renton T. Guidelines for Surgical Endodontics version 2. 2012. Available from: http://www.rcseng.ac.uk/fds/publications-clinical-guidelines/clinical_guidelines/documents/surgical_endodontics_2012.pdf [DOI] [PubMed]
- 30.Harris D, Horner K, Grondahl K, Jacobs R, Helmrot E, Benic GI, et al. E.A.O. guidelines for the use of diagnostic imaging in implant dentistry 2011. A consensus workshop organized by the European Association for Osseointegration at the Medical University of Warsaw. Clin Oral Implants Res 2012; 23: 1243–53. doi: 10.1111/j.1600-0501.2012.02441.x [DOI] [PubMed] [Google Scholar]
- 31.Husain J, Burden D, McSherry P, Morris D, Allen M; Clinical Standards Committee of the Faculty of Dental Surgery RCoSoE. National clinical guidelines for management of the palatally ectopic maxillary canine. Br Dent J 2012; 213: 171–6. doi: 10.1038/sj.bdj.2012.726 [DOI] [PubMed] [Google Scholar]
- 32.Tyndall DA, Price JB, Tetradis S, Ganz SD, Hildebolt C, Scarfe WC; American Academy of Oral and Maxillofacial Radiology. Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 817–26. doi: 10.1016/j.oooo.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Walter C, Weiger R, Zitzmann NU. Periodontal surgery in furcation-involved maxillary molars revisited–an introduction of guidelines for comprehensive treatment. Clin Oral Investig 2011; 15: 9–20. doi: 10.1007/s00784-010-0431-9 [DOI] [PubMed] [Google Scholar]
- 34.American Association of Endodontists. Recommended guidelines of the AAE for the treatment of traumatic dental injuries (revised 2013). Available from: http://www.aae.org/guidelines/
- 35.Cooper LF, Pin-Harry OC. “Rules of six”—diagnostic and therapeutic guidelines for single-tooth implant success. Compend Contin Educ Dent 2013; 34: 94–8, 100–1. [PubMed] [Google Scholar]
- 36.Counihan K, Al-Awadhi EA, Butler J. Guidelines for the assessment of the impacted maxillary canine. Dent Update 2013; 40: 770–2, 775–7. [DOI] [PubMed] [Google Scholar]
- 37.Faculty of General Dental Practice (UK). In: Horner K, Eaton KA, eds. Selection criteria for dental radiography. 3rd edn. London, UK: Faculty of General Dental Practice (UK) Royal College of Surgeons of Surgeons of England; 2013. [Google Scholar]
- 38.Ngiam J, Balasubramaniam R, Darendeliler MA, Cheng AT, Waters K, Sullivan CE. Clinical guidelines for oral appliance therapy in the treatment of snoring and obstructive sleep apnoea. Aust Dent J 2013; 58: 408–19. doi: 10.1111/adj.12111 [DOI] [PubMed] [Google Scholar]
- 39.NICE.org.uk. London, UK: National Institute for Health and Care Excellence. [Cited 29 June 2014.] Available from: http://www.nice.org.uk/Guidance [Google Scholar]
- 40.SIGN.ac.uk. Edinburgh, UK: Scottish Intercollegiate Guidelines Network. Available from: http://www.sign.ac.uk/ [Google Scholar]
- 41.Sundhedsstyrelsen. Statens Institut for Strålebeskyttelse. Krav til 3D dental. Herlev: Statens Institut for Strålebeskyttelse; 2009. [Google Scholar]
- 42.Health Protection Agency. Guidance on the safe use of dental cone beam CT (computed tomography) equipment. HPA-CRCE-010. Chilton, UK: Health Protection Agency; 2010. [Google Scholar]
- 43.Statens strålevern. Stråleverninfo 8:2010. Krav for bruk av Cone Beam CT ved odontologiske virksomheter. Østerås: Statens strålevern; 2010. [Google Scholar]
- 44.Einarsson G. Geislavarnir vegna notkunar sérhæfðra tölvusneiðmyndatækja við tannlækningar (CBCT). Reykjavik: Geislavnir Rikisins (Icelandic Radiation Protection Authority). Available from: http://www.gr.is/media/leidbeiningar/Rit_GR1101_GeislavarnirvegnanotkunarCBCT.pdf [Google Scholar]
- 45.British Columbia Centre for Disease Control, Environmental Health Services. Guidelines on radiation protection & quality assurance applicable to dental cone beam computed tomography (CBCT). 2014. Available from: www.bccdc.ca/healthenv/Radiation/RadiationInMedicine/default.htm#Dental [Google Scholar]
- 46.Guerrero ME, Botetano R, Beltran J, Horner K, Jacobs R. Can preoperative imaging help to predict postoperative outcome after wisdom tooth removal? A randomized controlled trial using panoramic radiography versus cone-beam CT. Clin Oral Investig 2014; 18: 335–42. doi: 10.1007/s00784-013-0971-x [DOI] [PubMed] [Google Scholar]
- 47.AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care 2003; 12: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boivin A, Currie K, Fervers B, Gracia J, James M, Marshall C, et al. Patient and public involvement in clinical guidelines: international experiences and future perspectives. Qual Saf Health Care 2010; 19: e22. doi: 10.1136/qshc.2009.034835 [DOI] [PubMed] [Google Scholar]
- 49.Légaré F, Boivin A, van der Weijden T, Pakenham C, Burgers J, Légaré J, et al. Patient and public involvement in clinical practice guidelines: a knowledge synthesis of existing programs. Med Decis Making 2011; 31: E45–74. doi: 10.1177/0272989X11424401 [DOI] [PubMed] [Google Scholar]
- 50.Díaz Del Campo P, Gracia J, Blasco JA, Andradas E. A strategy for patient involvement in clinical practice guidelines: methodological approaches. BMJ Qual Saf 2011; 20: 779–84. doi: 10.1136/bmjqs.2010.049031 [DOI] [PubMed] [Google Scholar]
- 51.Bornstein MM, Scarfe WC, Vaughn VM, Jacobs R. Cone beam computed tomography in implant dentistry: a systematic review focusing on guidelines, indications, and radiation dose risks. Int J Oral Maxillofac Implants 2014; 29: 55–77. doi: 10.11607/jomi.2014suppl.g1.4 [DOI] [PubMed] [Google Scholar]
- 52.Hollender L. Decision making in radiographic imaging. J Dent Educ 1992; 56: 834–43. [PubMed] [Google Scholar]
- 53.Tickle M, McDonald R, Franklin J, Aggarwal VR, Milsom K, Reeves D. Paying for the wrong kind of performance? Financial incentives and behaviour changes in National Health Service dentistry 1992–2009. Community Dent Oral Epidemiol 2011; 39: 465–73. doi: 10.1111/j.1600-0528.2011.00622.x [DOI] [PubMed] [Google Scholar]
- 54.Petersen LB, Olsen KR, Christensen J, Wenzel A. Image and surgery-related costs comparing cone beam computed tomography and panoramic imaging before removal of impacted mandibular third molars. Dentomaxillofac Radiol 2014; 43: 20140001. doi: 10.1259/dmfr.20140001 [DOI] [PMC free article] [PubMed] [Google Scholar]