Abstract
Many components of the CHIEF (Convergence of Hormones, Inflammation, and Energy Related Factors) pathway could influence survival given their involvement in cell growth, apoptosis, angiogenesis, and tumor invasion stimulation. We used ARTP (Adaptive Rank Truncation Product) to test if genes in the pathway were associated with colorectal cancer-specific mortality. Colon cancer (n = 1555) and rectal cancer (n = 754) cases were followed over five years. Age, center, stage at diagnosis, and tumor molecular phenotype were considered when calculating ARTP p values. A polygenic risk score was used to summarize the magnitude of risk associated with this pathway. The JAK/STAT/SOC was significant for colon cancer survival (PARTP = 0.035). Fifteen genes (DUSP2, INFGR1, IL6, IRF2, JAK2, MAP3K10, MMP1, NFkB1A, NOS2A, PIK3CA, SEPX1, SMAD3, TLR2, TYK2, and VDR) were associated with colon cancer mortality (PARTP <0.05); JAK2 (PARTP = 0.0086), PIK3CA (PARTP = 0.0098), and SMAD3 (PARTP = 0.0059) had the strongest associations. Over 40 SNPs were significantly associated with survival within the 15 significant genes (PARTP<0.05). SMAD3 had the strongest association with survival (HRGG 2.46 95% CI 1.44,4.21 PTtrnd = 0.0002). Seven genes (IL2RA, IL8RA, IL8RB, IRF2, RAF1, RUNX3, and SEPX1) were significantly associated with rectal cancer (PARTP<0.05). The HR for colorectal cancer-specific mortality among colon cancer cases in the upper at-risk alleles group was 11.81 (95% CI 7.07, 19. 74) and was 10.99 (95% CI 5.30, 22.78) for rectal cancer. These results suggest that several genes in the CHIEF pathway are important for colorectal cancer survival; the risk associated with the pathway merits validation in other studies.
Introduction
The CHIEF (Convergence of Hormones, Inflammation, and Energy Related Factors) pathway integrates elements central to the etiology of colorectal cancer (CRC) [1]. The pathway was developed based on our knowledge of the epidemiology of CRC and genes that may influence cancer risk through major components of the pathway, including hormones, inflammation, and energy-related factors [1]. Many genes in the pathway could influence tumor progression and prognosis given their involvement cell growth, apoptosis, promotion of inflammation and angiogenesis, immune response, and stimulation of tumor invasion and metastasis [2]. The main trunk of the pathway contains a serine/threonine protein kinase 11 (STK11 or LKB1), mammalian target of rapamycin (MTOR), and the tumor suppressor PTEN (phosphatase tensin homolog deleted on chromosome 10). STK11 responds to changes in cellular energy balance (ATP levels) [3], [4] and governs whole body insulin sensitivity [5], [6]. NFκB is an important nuclear transcription factor that regulates cytokines and is critical for the regulation of tumorigenesis, cell proliferation, apoptosis, response to oxidative stress, and inflammation while vascular endothelial growth factor (VEGF) plays an important role in regulation of cell growth signaling and is a major mediator of tumor angiogenesis [7] [8].
Cytokines such as interleukins, TGFβ-signaling pathway, interferons, and tumor necrosis factor (TNF), are key elements of the inflammatory process in the CHIEF pathway. The TGF-β-signaling pathway is involved in all aspects of tumorigenesis, including stimulation of tumor invasion and metastasis [2]. Signal transduction and activation of transcription (STAT) and mitogen-activated kinases (MAPK) genes are involved in both inflammation and metabolic signaling associated with hormones and energy-related factors. MAPKs serve as an integration point for multiple biological signals and are involved in a variety of cellular processes such as proliferation. Angiogenesis and inflammation are hallmark features of tumorigenesis [9] as well as key elements in the CHIEF pathway, thus it is reasonable to hypothesis that pathway influences survival.
In this paper, we summarize the significance of this pathway as it relates to survival after being diagnosed with colon or rectal cancer using Adaptive Rank Truncation Product (ARTP), building on our previous work that evaluated the pathway with colon and rectal cancer risk where we documented overall risk as well as risk specific to tumor molecular phenotype [10]. This statistical program utilizes a permutation method that allows us to summarize across genes within sub-pathways of the overall pathway to estimate the association with survival of the pathway, genes, and SNPs within the pathway. To further estimate the magnitude of the association of this pathway on survival, we utilize a polygenic risk score that is based on the permutated ARTP findings.
Methods
Two study populations are included in these analyses. The first study, a population-based case-control study of colon cancer, included cases (n = 1,555 with complete genotype data) identified between October 1, 1991 and September 30, 1994 living in the Twin Cities Metropolitan Area or a seven-county area of Utah or enrolled in the Kaiser Permanente Medical Care Program of Northern California (KPMCP) [11]. The second study, with identical data collection methods, included cases with cancer of the rectosigmoid junction or rectum (n = 754 cases with complete genotype data) who were identified between May 1997 and May 2001 in Utah and at the KPMCP [12]. Eligible cases were between 30 and 79 years of age at the time of diagnosis, living in the study geographic area, English speaking, mentally competent to complete the interview, and with no previous history of CRC, and no previous diagnosis of familial adenomatous polyposis, ulcerative colitis, or Crohn's disease. Cases who did not meet these criteria were ineligible as were individuals who were not black, white, Hispanic, or Asian (for the rectal cancer study). All study participants provided written informed consent on Institutional Review Board approved consent forms prior to completing the study questionnaire; the consent form and study protocol was approved by the Institutional Review Board on Human Subjects at the University of Utah, Kaiser Permanente Medical Research Program, and the University of Minnesota.
Tumor Registry Data
Tumor registry data were obtained to determine disease stage at diagnosis and months of survival after diagnosis. Disease stage was categorized using the sixth edition of the American Joint Committee on Cancer (AJCC) staging criteria. One pathologist in Utah did all disease staging. Local tumor registries provided information on patient follow-up including vital status, cause of death, and contributing cause of death. Follow-up was obtained for all study participants and was terminated for the Colon Cancer Study in 2000 and for the Rectal Cancer Study in 2007. At that time all study participants had over five years of follow-up.
Tumor Marker Data
Tumors were defined by specific molecular alterations: any TP53 mutation; any KRAS mutation; MSI+; and CpG Island Methylator Phenotype (CIMP). CIMP status was based on the classic panel and defined as positive if at least two of five markers were methylated [13]. Microsatellite instability (MSI) was based on BAT26, TGFβRII, and a panel of 10 tetranucleotide repeats that has been shown to correlate highly with the Bethesda Panel [14]; our study was done prior to the Bethesda Panel development. These data are included in analysis since we have shown that tumor molecular phenotype influences survival and is associated with SNPs in this pathway [10], [15]
TagSNP Selection and Genotyping
TagSNPs were selected using the following parameters: r2 = 0.8 defined LD blocks using a Caucasian LD map, minor allele frequency (MAF)>0.1, range = −1500 bps from the initiation codon to +1500 bps from the termination codon, and 1 SNP/LD bin. All markers were genotyped using a custom multiplexed bead array assay format based on GoldenGate chemistry (Illumina, San Diego, California). A genotyping call rate of 99.85% was attained. Blinded internal replicates represented 4.4% of the sample set. The duplicate concordance rate was 100.00%. S1 Table list all genes included in the sub-pathway while S2 Table list number of SNPs assessed for each gene and the PARTP value for each gene on the platform. We analyzed data from 155 genes which included 10 genes that were previously assessed in our lab (VDR, ESR1, ESR2, AR, IGF1, IGF1R, IGFBR3, IRS1, IRS2, and PPARG) along with 145 genes from the Illumina platform. The initial platform included 1536 SNPs, of these, 1381 were successfully analyzed by Illumina. We included in our analysis only those SNPs were>95% of the population had results, leaving 1246 SNPs for analysis No imputation was done.
Statistical Methods
The goal of the analysis was to evaluate the overall associations between genes and pathways as they relate to colon and rectal cancer survival. To do this, we used ARTP, a statistical program that utilizes a highly efficient permutation algorithm to determine significance at the gene, sub-pathway, and pathway level for survival after diagnosis with colon or rectal cancer [16]. Vital status and survival months were permuted 10,000 times within R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Since our focus was on colorectal cancer-specific mortality, people who died from other causes or who were lost to follow-up were censored at the date of death or last contact. Months of survival were calculated from date of diagnosis until end of follow-up or date of last contact. Cox Proportional Hazards models were adjusted for age, race/ethnicity, sex, AJCC stage, and tumor molecular phenotype. Tumors were defined by specific molecular alterations: any TP53 mutation; any KRAS mutation; MSI+; and CIMP high. As the proportion of MSI+ tumors in the rectal cases was <3% [17], we did not include these tumor markers as an adjustment variable for rectal cancer. Associations with SNPs within ARTP were assessed assuming an additive model unless a preliminary check of the hazard ratios indicated a dominant or recessive mode of inheritance. For SNPs with gene p values <0.05 that were associated with colon or rectal cancer based on ARTP results, we report Hazard Ratios (HR) and 95% confidence intervals (CIs) assessed from Cox Proportional Hazard models in SAS to show the magnitude of the association between these SNPs and hazard of dying after diagnosis with colon or rectal cancer; we also report p values for likelihood ratio test (LRT). We include those genes which contributed to the ARTP permutated gene p value for reference since they could possibly indicate greater significance and are of interest for replication elsewhere. We did not further adjust SNP associations for multiple comparisons since our analytic approach is top down: looking at the overall pathway (where number of genes are adjusted), genes (where number of SNPs are adjusted), and SNPs that contribute to significant permutated PARTP values. Genes were assigned to only one sub-pathway prior to the hierarchical analyses. However, we realize many genes could function in other sub-pathways to which they were not assigned for analysis.
To summarize the risk associated with the CHIEF pathway, we calculated polygenic summary scores. To conservatively estimate risk, we included in the risk models SNPs from genes where the gene ARTP p values were 0.10 or less and the SNP p values within those genes were 0.10 or less. Our analysis includes SNPs with p<0.10 only from those genes where the PARTP was <0.10. Thus, we include SNPs that were not statistically significant and we omit SNPs that were statistically significant in genes where the PARTP was>0.10. Since genes are associated with multiple sub-pathways, we did not restrict to genes where the sub-pathway was significant. If SNPs within the same gene had r2 values of 0.80 or greater only one SNP was included in the model. Risk was modeled using at-risk alleles, using all genotypes with the low-risk genotype or referent group as zero. For the co-dominant or additive model a score of zero, one, or two was assigned relative to the number of at-risk alleles, while scores of zero or two were assigned for the dominant and recessive models in order to capture the risk associated with the various genotypes. Polygenic scores were then used to summarize risk across the genes and SNPs to better capture the risk associated with the pathway.
Results
The majority of study participants were over 60 years of age, were non-Hispanic white, and male (Table 1). Most cases were diagnosed with an AJCC Stage 1 or 2 tumor. At the end of follow-up roughly 35% of study participants had died. The overall pathway was not statistically significantly associated with survival for either colon or rectal cancer (Table 2). However, the JAK/STAT/SOC was significant for colon cancer survival (PARTP = 0.035) and the interleukin pathway was of borderline significance for rectal cancer (PARTP = 0.06).
Table 1. Description of study population.
| Colon | Rectal | ||
| n (%) | n (%) | ||
| Age | 30–39 | 23 (1.48) | 19 (2.52) |
| 40–49 | 102 (6.57) | 96 (12.73) | |
| 50–59 | 289 (18.61) | 196 (25.99) | |
| 60–69 | 537 (34.58) | 250 (33.16) | |
| 70–79 | 602 (38.76) | 193 (25.60) | |
| Center | Utah | 249 (16.03) | 274 (36.34) |
| KPMCP | 742 (47.78) | 480 (63.66) | |
| Minnesota | 562 (36.19) | ||
| Race/Ethnicity | NHW | 1426 (91.82) | 625 (82.89) |
| Hispanic | 59 (3.80) | 61 (8.09) | |
| Black | 68 (4.38) | 29 (3.85) | |
| Asian | 39 (5.17) | ||
| Sex | Male | 868 (55.89) | 451 (59.81) |
| Female | 685 (44.11) | 303 (40.19) | |
| Tumor Molecular Phenotypes | CIMP+ | 272 (26.96) | 59 (11.11) |
| KRAS2 Mutation | 348 (31.93) | 173 (29.37) | |
| TP53 Mutation | 515 (45.90) | 277 (49.64) | |
| MSI Unstable | 185 (15.76) | 14 (2.39) | |
| AJCC Stage | 1 | 468 (30.14) | 381 (50.53) |
| 2 | 404 (26.01) | 124 (16.45) | |
| 3 | 374 (24.08) | 175 (23.21) | |
| 4 | 128 (8.24) | 57 (7.56) | |
| Unknown | 179 (11.53) | 17 (2.25) | |
| Vital Status | Dead | 520 (33.48) | 259 (34.35) |
| Alive1 | 1033 (66.52) | 495 (65.65) | |
| Cause of Death | Colorectal Cancer | 309 (59.42) | 171 (66.02) |
| Other Cancer | 58 (11.15) | 14 (5.41) | |
| Non-Cancer | 90 (17.31) | 37 (14.29) | |
| Unspecified/Unknown | 63 (12.12) | 37 (14.29) | |
| Percent Five-Year Survival2 | 65.71% | 73.09% | |
| Median Survival Time (months)3 | 62 | 74 |
Includes cases lost to follow-up within five years of diagnosis.
Excludes cases lost to follow-up within five years of diagnosis.
Time from diagnosis to death or last follow-up.
Table 2. Overall pathway PARTP 1.
| Colon | Rectal | |||
| Sub-Pathway | Sub-Pathway | Pathway | Sub-Pathway | Pathway |
| PARTP | PARTP | PARTP | PARTP | |
| Angiogenesis | 0.2426 | 0.2479 | 0.8865 | 0.6248 |
| Hormone/Insulin/Growth | 0.4030 | 0.7416 | ||
| Interferons | 0.0770 | 0.1720 | ||
| Interleukins | 0.4662 | 0.0609 | ||
| Jak/Stat/Socs | 0.0353 | 0.5152 | ||
| Pathway Core | 0.2036 | 0.7114 | ||
| MAP Kinase (MAPK) | 0.3160 | 0.3529 | ||
| Selenoproteins | 0.1834 | 0.3659 | ||
| Telomere | 0.5166 | 0.9729 | ||
| TGFβ | 0.1503 | 0.4647 | ||
| Toll-Like Receptors (TLR) | 0.1109 | 0.9874 | ||
| Tumor Necrosis Factor (TNF) | 0.8566 | 0.1712 | ||
Adjusted for age, study center, race/ethnicity, sex, AJCC stage, and tumor markers: CIMP, KRAS, TP53; MSI for colon only. ARTP p values based on 10,000 permutations.
However several genes within the sub-pathways were significant for colon (Table 3) and rectal (Table 4) cancer mortality. Fifteen genes (DUSP2, INFGR1, IL6, IRF2, JAK2, MAP3K10, MMP1, NFkB1A, NOS2A, PIK3CA, SEPX1, SMAD3, TLR2, TYK2, and VDR) were significantly associated with colon cancer mortality at the <0.05 level; an additional 15 genes had gene PARTP values between 0.05 and 0.10 (see S3 Table). The genes that were most significantly associated with survival were JAK2 (PARTP = 0.0086), PIK3CA (PARTP = 0.0098), and SMAD3 (PARTP = 0.0059). Over 40 SNPs were significantly associated with survival within the 15 significant genes (PARTP<0.05). Of these SNPs, SMAD3 had the strongest association with survival (HRGG 2.46 95% CI 1.44,4.21 PLRT = 0.0002). Ten SNPs in five genes had P values less than 0.005, including IL6 rs1800796 (HRGG 0.55 95% CI 0.36, 0.84), IRF2 rs12504466 (HRTT 1.51 95% CI 1.14,1.99), rs793814 (HRTT/AA 0.57 95% CI 0.39,0.83), and rs3775582 (HRAA/AT 0.67 95% CI 0.50,0.89), JAK2 rs7043371 (HRAT/TT 0.63 95% CI 0.47,0.84) and rs10815160 (HRTT 1.62 95% CI 1.07,2.47), SEPX1 rs732510 (HRAA/AG 1.47 95% CI 1.13,1.90), and SMAD3 rs893473 (HRCC 1.45 95% CI 1.14,1.83) rs1866317 (HRCC 1.47 95% CI 1.14,1.90), and rs12708492 (HRCC 1.52 95% CI 1.16,2.00).
Table 3. Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with colon cancer (gene PARTP≤0.05; SNP Ptrend≤0.10).
| Gene | PARTP | SNP | Genotype | HR (95%CI)1 | Ptrend |
| DUSP2 | 0.0225 | rs1724120 | AA vs. GG/GA | 0.72 (0.54, 0.96) | 0.0199 |
| IFNGR1 | 0.0121 | rs3799488 | TC/CC vs. TT | 1.30 (0.98, 1.72) | 0.0772 |
| rs9376267 | CT/TT vs. CC | 1.37 (1.09, 1.73) | 0.0079 | ||
| rs1327474 | GG vs. AA/AG | 0.69 (0.50, 0.94) | 0.0158 | ||
| IL6 | 0.0417 | rs1800796 | GC/CC vs. GG | 0.55 (0.36, 0.84) | 0.0032 |
| IRF2 | 0.0207 | rs6856910 | CC vs. TT | 1.42 (0.99, 2.04) | 0.0835 |
| rs793777 | GG vs. CC | 0.67 (0.46, 0.98) | 0.0426 | ||
| rs2797507 | CA/AA vs. CC | 0.77 (0.61, 0.98) | 0.0380 | ||
| rs12504466 | TC/CC vs. TT | 1.51 (1.14, 1.99) | 0.0027 | ||
| rs793814 | AA vs. TT/TA | 0.57 (0.39, 0.83) | 0.0018 | ||
| rs7655800 | AG/GG vs. AA | 1.33 (1.04, 1.70) | 0.0234 | ||
| rs9684244 | CC vs. GG | 0.56 (0.37, 0.84) | 0.0124 | ||
| rs13139310 | AA vs. GG | 0.35 (0.16, 0.74) | 0.0220 | ||
| rs11723606 | TT vs. CC | 0.45 (0.24, 0.86) | 0.0341 | ||
| rs13116389 | GT/TT vs. GG | 1.38 (1.09, 1.75) | 0.0073 | ||
| rs793801 | AA vs. GG/GA | 1.39 (1.01, 1.91) | 0.0506 | ||
| rs3775582 | GA/AA vs. GG | 0.67 (0.50, 0.89) | 0.0038 | ||
| rs1044873 | CT/TT vs. CC | 1.32 (1.04, 1.68) | 0.0231 | ||
| JAK2 | 0.0086 | rs1887429 | GT/TT vs. GG | 1.34 (1.07, 1.69) | 0.0113 |
| rs7043371 | TT vs. AA/AT | 0.63 (0.47, 0.84) | 0.0010 | ||
| rs10974947 | AA vs. GG | 1.34 (0.86, 2.10) | 0.0319 | ||
| rs3780379 | GA/AA vs. GG | 1.32 (1.04, 1.67) | 0.0221 | ||
| rs10815160 | GG vs. TT | 1.62 (1.07, 2.47) | 0.0017 | ||
| MAP3K10 | 0.0306 | rs1129156 | TT vs. CC | 1.49 (0.89, 2.52) | 0.0073 |
| MMP1 | 0.0289 | rs470215 | CC vs. TT | 1.45 (0.99, 2.12) | 0.0278 |
| NFKBIA | 0.0252 | rs696 | AA vs. GG | 1.41 (1.00, 1.99) | 0.0696 |
| rs2233409 | TT vs. CC | 0.62 (0.37, 1.03) | 0.0562 | ||
| rs3138053 | GG vs. AA | 0.56 (0.35, 0.90) | 0.0177 | ||
| NOS2A | 0.0421 | rs7406657 | CC vs. GG | 0.59 (0.32, 1.09) | 0.0061 |
| rs9906835 | GG vs. AA | 0.62 (0.43, 0.89) | 0.0105 | ||
| rs2297516 | CC vs. AA | 0.59 (0.40, 0.86) | 0.0095 | ||
| PIK3CA | 0.0098 | rs2699905 | GA/AA vs. GG | 0.73 (0.58, 0.93) | 0.0101 |
| rs7640662 | CG/GG vs. CC | 0.71 (0.54, 0.94) | 0.0154 | ||
| rs2677760 | CC vs. TT/TC | 1.43 (1.11, 1.83) | 0.0067 | ||
| rs1607237 | CC vs. TT/TC | 1.45 (1.10, 1.92) | 0.0104 | ||
| SEPX1 | 0.0217 | rs732510 | GG vs. AA/AG | 1.47 (1.13, 1.90) | 0.0049 |
| SMAD3 | 0.0059 | rs1498506 | CC vs. AA | 0.69 (0.48, 0.99) | 0.0837 |
| rs9972423 | AA vs. TT | 0.82 (0.56, 1.19) | 0.0950 | ||
| rs2118611 | GG vs. AA | 1.89 (1.19, 2.99) | 0.0188 | ||
| rs11071933 | GG vs. CC | 1.60 (1.15, 2.24) | 0.0111 | ||
| rs7163381 | AA vs. GG | 1.67 (1.09, 2.58) | 0.0113 | ||
| rs4776892 | TT vs. AA | 1.64 (0.93, 2.91) | 0.0292 | ||
| rs2414937 | CC vs. GG | 2.46 (1.44, 4.21) | 0.0002 | ||
| rs745103 | CC vs. TT | 1.50 (1.08, 2.08) | 0.0186 | ||
| rs893473 | CT/TT vs. CC | 1.45 (1.14, 1.83) | 0.0024 | ||
| rs1866317 | CG/GG vs. CC | 1.47 (1.14, 1.90) | 0.0040 | ||
| rs4601989 | TT vs. CC | 0.48 (0.24, 0.93) | 0.0719 | ||
| rs11639295 | TT vs. CC/CT | 0.54 (0.33, 0.89) | 0.0083 | ||
| rs12708492 | CT/TT vs. CC | 1.52 (1.16, 2.00) | 0.0019 | ||
| TLR2 | 0.0302 | rs5743704 | CA/AA vs. CC | 1.80 (1.20, 2.68) | 0.0077 |
| rs5743708 | GA/AA vs. GG | 1.77 (1.15, 2.72) | 0.0160 | ||
| TYK2 | 0.0178 | rs12720356 | TG/GG vs. TT | 1.30 (0.96, 1.76) | 0.0933 |
| rs280521 | GA/AA vs. GG | 0.69 (0.52, 0.92) | 0.0078 | ||
| rs280523 | GA/AA vs. GG | 0.59 (0.38, 0.91) | 0.0105 | ||
| VDR | 0.0499 | VDR_Bsm1 | BB vs. bb | 1.50 (1.06, 2.12) | 0.0453 |
| VDR_Fok1 | ff vs. FF | 1.47 (1.01, 2.15) | 0.0709 | ||
| VDR_Poly | SS vs. LL | 1.47 (1.03, 2.10) | 0.0483 |
Hazard Ratio (HR) and 95% Confidence Intervals (CI) adjusted for age, study center, race/ethnicity, sex, AJCC stage, and tumor molecular phenotype: MSI, CIMP, KRAS, and TP53. PARTP based on 10,000 permutations.
Table 4. Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with rectal cancer (gene PARTP≤0.05; SNP Ptrend≤0.10).
| Gene | PARTP | SNP | Genotype | HR (95%CI)1 | Ptrend |
| IL2RA | 0.0216 | rs2386841 | AA vs. CC | 3.10 (1.50, 6.41) | 0.0298 |
| rs7072398 | GA/AA vs. GG | 0.62 (0.45, 0.85) | 0.0035 | ||
| rs11256456 | CC vs. TT | 1.90 (0.97, 3.70) | 0.0049 | ||
| rs11256457 | CG/GG vs. CC | 0.70 (0.51, 0.96) | 0.0282 | ||
| rs6602398 | GT/TT vs. GG | 0.76 (0.56, 1.04) | 0.0861 | ||
| rs11256497 | AA vs. GG | 0.59 (0.34, 1.01) | 0.0588 | ||
| rs791587 | AA vs. GG | 0.57 (0.36, 0.90) | 0.0129 | ||
| rs10905669 | TT vs. CC | 1.73 (0.93, 3.21) | 0.0054 | ||
| rs2476491 | AA vs. TT | 0.56 (0.29, 1.09) | 0.0210 | ||
| rs2256774 | AG/GG vs. AA | 0.68 (0.50, 0.93) | 0.0153 | ||
| rs706779 | GG vs. AA | 0.64 (0.41, 1.01) | 0.0388 | ||
| rs706778 | GA/AA vs. GG | 1.58 (1.10, 2.26) | 0.0103 | ||
| rs3118470 | TC/CC vs. TT | 1.45 (1.06, 2.00) | 0.0201 | ||
| IL8RA | 0.0189 | rs1008563 | CT/TT vs. CC | 0.71 (0.52, 0.98) | 0.0368 |
| rs1008562 | GG vs. CC | 1.60 (1.04, 2.46) | 0.0278 | ||
| rs16858808 | CT/TT vs. CC | 0.51 (0.23, 1.11) | 0.0637 | ||
| rs16858811 | TG/GG vs. TT | 0.52 (0.25, 1.08) | 0.0571 | ||
| IL8RB | 0.0306 | rs4674258 | CT/TT vs. CC | 0.72 (0.52, 0.99) | 0.0436 |
| rs1126579 | TT vs. CC | 1.60 (1.05, 2.46) | 0.0235 | ||
| IRF2 | 0.0091 | rs809909 | TA/AA vs. TT | 0.76 (0.56, 1.05) | 0.0986 |
| rs10009261 | TT vs. CC | 1.52 (0.93, 2.49) | 0.0730 | ||
| rs1425551 | CC vs. AA/AC | 1.51 (1.03, 2.20) | 0.0396 | ||
| rs807684 | GG vs. AA/AG | 0.31 (0.14, 0.67) | 0.0005 | ||
| rs3756094 | AA vs. GG/GA | 0.37 (0.20, 0.67) | 0.0003 | ||
| RAF1 | 0.0158 | rs3729931 | TT vs. CC | 0.65 (0.39, 1.09) | 0.0690 |
| rs9809501 | TG/GG vs. TT | 0.62 (0.40, 0.95) | 0.0229 | ||
| rs11923427 | CG/GG vs. CC | 0.58 (0.40, 0.85) | 0.0039 | ||
| rs11711419 | AT/TT vs. AA | 0.71 (0.50, 1.00) | 0.0452 | ||
| rs4684871 | GG vs. AA | 0.56 (0.33, 0.96) | 0.0260 | ||
| rs904453 | AA vs. CC | 1.73 (1.12, 2.68) | 0.0132 | ||
| RUNX3 | 0.0244 | rs7517302 | CC vs. TT | 1.77 (1.15, 2.71) | 0.0098 |
| rs2135756 | GG vs. AA/AG | 0.54 (0.35, 0.82) | 0.0022 | ||
| SEPX1 | 0.0311 | rs13331553 | TC/CC vs. TT | 1.45 (1.06, 1.98) | 0.0202 |
| rs732510 | GG vs. AA/AG | 1.47 (1.04, 2.07) | 0.0335 |
Hazard Ratio (HR) and 95% Confidence Intervals (CI) adjusted for age, study center, race/ethnicity, sex, AJCC stage, and tumor molecular phenotype: CIMP, KRAS, and TP53. PARTP based on 10,000 permutations.
Fewer genes were associated with survival after diagnosis with rectal cancer than for colon cancer (Table 4). Seven genes (IL2RA, IL8RA, IL8RB, IRF2, RAF1, RUNX3, and SEPX1) had PARTP values <0.05, while nine genes (BMP1, BMPR1A, ESR2, IL1A, IL3, PRKAG2, SOCS1, STK11, and TSC2) had PARTP values between 0.05 and 0.10 (S4 Table). SEPX1 rs732510 was associated with both colon and rectal mortality with similar magnitudes of association. Several SNPs in the genes with PARTP<0.05 also had linear trend P values of <0.005, including IR2RA rs7072398 (HRGG 0.62 95% CI 0.45,0.86), IRF2 rs807684 (HRAA/AG 0.31 95% CI 0.14, 0.67 PLRT 0.0005) and rs3756094 (HRGG/GA 0.37 95% CI 0.20,0.67 PLRT 0.0003), RAF1 rs11923427 (HRCC 0.58 95% CI 0.40,0.65), and RUNX3 rs2135756 (HRAA/AG 0.54 95% CI 0.35,0.82).
The polygenic risk score (Fig. 1) showed increased risk with increasing number of at risk alleles. The overall HR for colorectal cancer mortality among colon cancer cases in the highest risk group (upper sixth of the at-risk allele distribution) was 11.81 (95% CI 7.07, 19. 74) and was 10.99 (95% CI 5.30,22.78) among rectal cancer cases.
Figure 1. Polygenic summary score associated with CHIEF pathway for colorectal cancer survival.
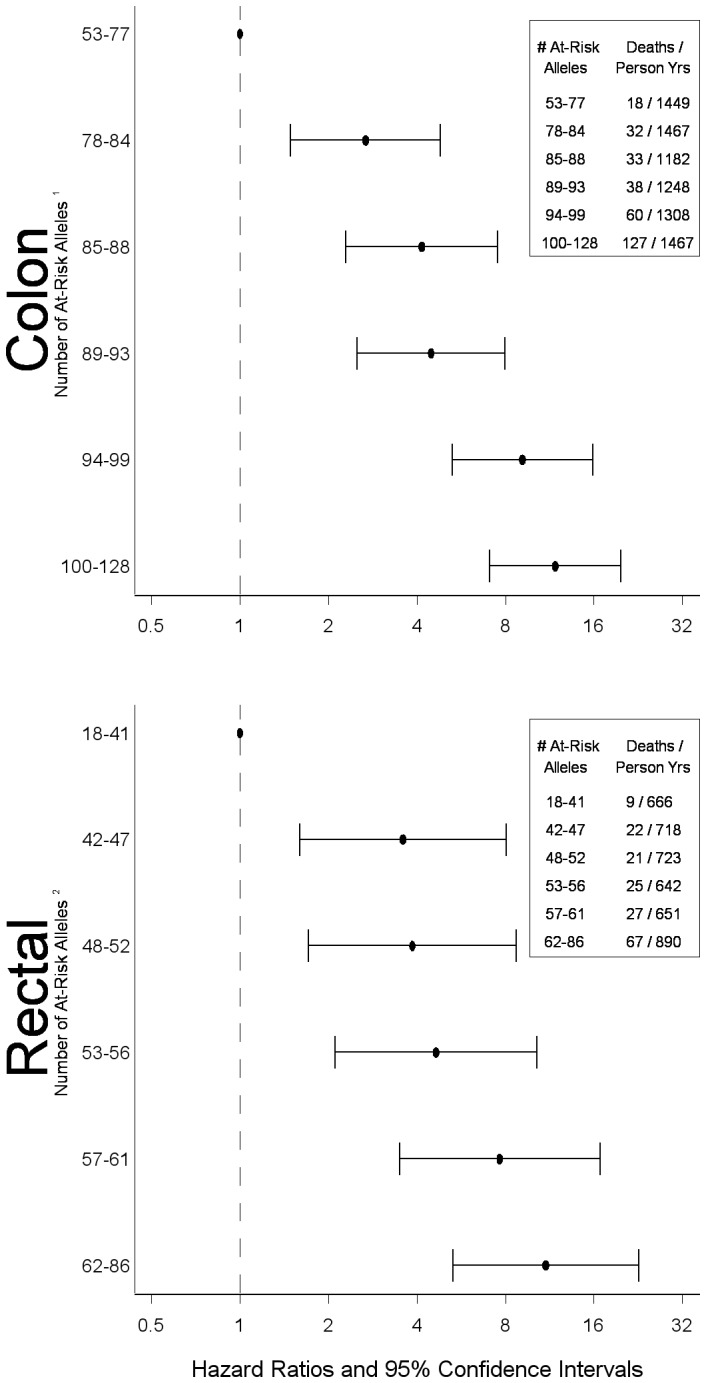
1SNPs included in score: BMP2 rs1979855, rs3178250, BMPR1A rs7895217, rs10887668, BMPR1B rs10049681, rs4699673, rs12508087, rs9307147, rs4490463, rs2120834, DUSP2 rs1724120, EIF4EBP3 rs250425, IFNGR1 rs3799488, rs9376267, rs1327474, IGF1, IKBKB rs5029748, rs10958713, IL1B rs1143627, rs1143623, IL6 rs1800796, IRF2 rs6856910, rs793777, rs2797507, rs12504466, rs793814, rs7655800, rs9684244, rs13139310, rs13116389, rs793801, rs3775582, IRF8 rs305083, rs305080, rs11649318, rs13338943, rs10514611, rs1044873, JAK2 rs1887429, rs7043371, rs10974947, rs3780379, rs10815160, JUNB rs2229510, MAP3K10 rs1129156, MMP1 rs470215, MMP3 rs3025066, NFKBIA rs696, rs2233409, rs3138053, NOS2A rs7406657, rs2297516, PIK3CA rs2699905, rs7640662, rs2677760, rs1607237, RPS6KA2 rs2049956, rs1894660, rs6918886, rs932356, rs9459715, rs1883361, rs4710090, rs661325, rs2345067, rs2072638, rs1309150, rs7745781, SEP15 rs9433110, SEPX1 rs732510, SMAD3 rs1498506, rs9972423, rs2118611, rs11071933, rs7163381, rs4776892, rs2414937, rs745103, rs893473, rs1866317, rs4601989, rs11639295, rs12708492, SOCS1 rs4780355, STAT3 rs1053005, rs2293152, rs8069645, STAT5A rs12601982, TLR2 rs5743704, rs5743708, TYK2 rs12720356, rs280521, rs280523, VDR_Fok1, VDR_Poly. 2SNPs included in score: BMP1 rs12114940, rs3924229, rs3857979, BMPR1A rs7088641, rs2168730, rs7895217, rs4934275, ESR2_Rsa, IL1A rs3783546, IL2RA rs2386841, rs7072398, rs11256456, rs11256457, rs6602398, rs11256497, rs791587, rs10905669, rs2476491, rs2256774, rs706779, rs706778, rs3118470, IL3 rs181781, IL8RA rs1008563, rs1008562, rs16858811, IL8RB rs1126579, IRF2 rs809909, rs10009261, rs1425551, rs807684, rs3756094, PRKAG2 rs1541538, rs2536082, rs6947064, rs7805747, rs1860743, rs10278273, rs7801616, rs7784818, rs3934597, RAF1 rs3729931, rs9809501, rs11923427, rs4684871, rs904453, RUNX3 rs7517302, rs2135756, SEPX1 rs13331553, rs732510, SOCS1 rs193779, STK11 rs8111699, rs741765, TSC2 rs2074968.
Discussion
Several genes were associated with survival after diagnosis with colorectal cancer, although the overall pathway was not statistically significant and only the JAK/STAT/SOCs sub-pathway had a PARTP<0.05. Fifteen genes were associated with colon cancer survival (PARTP<0.05) and seven genes were associated with rectal cancer survival. It should be noted this represents 9.6% of genes analyzed for colon cancer and approximately 5% of genes analyzed for rectal cancer and could be chance findings; thus these findings need replications. We observed that the hazard of dying after being diagnosed with either colon or rectal cancer increased with increasing number of at-risk alleles. The lack of statistical significance observed for the overall pathway could reflect sub-pathway groupings that did not optimize the data. Further evaluation at the gene and SNP level suggested that many components of the pathway contributed to survival, although a large segment of the pathway did not.
The JAK/STAT-signaling pathway was the only sub-pathway that was statistically significant using ARTP. This pathway plays a critical role in immune response and regulation of inflammation given its essential affiliation with cytokine signaling. STAT3 specifically has been shown to promote uncontrolled cell growth and survival through dysregulation of gene expression involved in apoptosis, cell-cycle regulation, and angiogenesis. [18] JAK1, JAK2, and STAT3 have been associated with colorectal cancer progression [19]. In our analysis, STAT3 and STAT5 were of marginal significance with colon cancer survival, while JAK2 and TYK2 were statistically significant. Within these genes, several SNPs were significantly associated with survival.
Several genes in the backbone of the CHIEF pathway were associated with survival, including PIK3CA for colon cancer and PRKAG2, STK11, and TSC2 for rectal cancer. Phosphoinositide 3-kinase (PI3K gene official name PIK3CA) is an early event in cells responding to growth factors, cytokines, and insulin [20]. PI3K induces the activation of Akt1 (alias PDK). The PI3K/Akt pathway is recognized as an important regulator of cell proliferation and survival and is thought to be involved in mediating the effects of MTOR [21]. It has been shown that inflammation-related factors can activate MTOR can promote tumor angiogenesis by phosphorylating TSC1 (also known as hamartin) and thereby inactivating the TSC1-TSC2 complex [22], [23]. TSC2, also known as tuberin, specifically has been shown to be involved in insulin signaling, tumor suppressor functions, and regulation of cell growth. A study by Lee and colleagues showed that STK11, PRKAA1, and TSC1 polymorphisms were associated with disease-free survival after diagnosis with colorectal cancer; they did not see an association with TSC2 [24]. Other studies have shown that STK11 is associated with tumor metastasis and more aggressive tumors [25], [26].
Increased tumor vascularization and inflammation have been associated with advanced tumor stage and poor prognosis [27]. Thus, we hypothesized that genes associated with angiogenesis would influence survival. We observed that NOS2A, MMP1, and VDR were associated with survival after colon cancer diagnosis and no major angiogenesis genes on our platform were associated with rectal cancer. Inducible nitric oxide synthase (NOS2) is induced by inflammatory cytokines and hypoxia and produces large amounts of nitric oxide. Nitric oxide can affect cancer through many ways, it can increase apoptosis and inhibit carcinogenesis or promote carcinogenesis through increasing angiogenesis [28]. MMPs are involved in normal physiological processes required for development and morphogenesis; a loss of control of MMPs can result in pathological processes including inflammation, angiogenesis, and cellular proliferation that are central to diseases such as cancer. MMPs, and MMP1 specifically, have been studied using indicators of metastatic potential by evaluating tumor stage at time of diagnosis, tumor grade and histology and been shown to be associated with greater metastatic potential [29]. VDR expression has been associated with better survival for colon and breast cancer [30]–[32]. Previously, we reported that FLT1 SNPs were significantly associated with the hazard of dying of colorectal cancer after diagnosis with colon cancer and KDR SNPs were associated significantly with colorectal deaths after diagnosis with rectal cancer [33].
The TGF-β-signaling pathway has been shown to be one of the strongest pathways associated with colon cancer risk in our data. Others have shown that improved disease-free survival after diagnosis with CRC was associated with increased TGF-β expression [34]. Forsti and colleagues looked at nine polymorphisms in the TGF-β-signaling pathway and CRC among 308 cases of colorectal cancer [35] and observed that TGFβRA IVS7G+24A minor allele was associated with better survival. Several others studies have focused on SMAD2, SMAD4, and SMAD7 and found associations with prognosis after CRC diagnosis [36], [37]. We only observed marginally significant associations with BMP2 (PARTP = 0.083), BMPR1A (PARTP = 0.053), BMPR1B (PARTP = 0.069) for colon cancer survival. RUNX3 was significantly associated with rectal cancer survival, while BMP1 (PARTP = 0.099) and BMPR1A (PARTP = 0.085) were marginally significant.
Two MAPKs genes were associated with survival in our data; these genes mediate intracellular signaling and are involved in diverse cellular processes that include cell proliferation and differentiation and apoptosis and implicated in progression [38]. The three major categories of MAPK are the stress-activated protein kinase c-Jun NH-2 terminal kinase (JNK or SAPK1), stress-activated protein kinase 2 (p38 or SAPK2), and the extracellular signal-regulated protein kinases (ERK1/2) [38], [39]. JNK, which includes MAP3K10 that was associated with survival in our data, is generally associated with apoptosis induction [40]. DUSPs attenuate the effect of MAPK [41].
SEPX1 was associated with survival for both colon and rectal cancer while SEP15 was marginally associated (PARTP = 0.068) with colon cancer survival. We previously reported that three SNPs in this pathway were associated with rectal cancer survival, SEPN1 rs718391 (HR 1.67, 95% CI 1.11,2.51) and SEPX1 rs13331553 (HR 1.46 95%CI 1.07,2.00) and SEPX1 rs732510 (HR 1.68 95% CI 1.09,2.60) after adjustment for multiple comparisons using FDR. However, taking the gene approach as we did with ARTP, SEPX1 remained significant for both colon and rectal cancer.
Several cytokines, including interleukins and interferons, and other mediators of inflammation were associated with both colon (INFGR1, IL6, IRF2, NFκB1A, TLR2) and rectal cancer survival (IL1A and IL3), as was suppressor of cytokine signaling (SOCS1). Functions of cytokine-related pathways include apoptosis and cell proliferation. INFG has been shown to regulate the expression of apoptosis-related genes and has been hypothesized to regulate cell sensitivity to apoptosis [42]. TLRs can promote inflammation, cell survival and tumor progression [43]. Studies analyzing associations between risk or survival and SNPs in interleukin genes such as IL1B, IL1RA, IL10 have reported conflicting results; some SNPs being associated with increased risk or survival while others associated with a lower risk or survival for colorectal cancer [44]–[46].
To estimate the magnitude of risk associated with carrying multiple high-risk alleles, we created a polygenic risk score. Our results suggest that the genetic variant load is important for survival after diagnosis since we observed substantial increased risk of dying with increasing numbers of variant genotypes. While one could hypothesize that a single insult to the pathway could influence risk and that additional insults would have minimal effect on risk, our data suggest otherwise. Inflammatory pathways are somewhat redundant, composed of multiple cytokines with overlapping functions; this supports that multiple insults to the pathways would result in increased risk. Our data support the hypothesis that increases in risk and hazard of dying is linear and that as genetic variant load of high-risk genotypes increases, so does the risk of developing cancer and dying after being diagnosed with cancer. However, caution is in order given the data used to identify at-risk alleles, was then used in the polygenic risk score. While we did not just take significant SNPs in creating the risk score, but used our permutated data to identify at-risk alleles, these results still warrant caution, especially in terms of the magnitude of the associations detected. Furthermore, to help place the risk observed in these data to other risk factors for survival, it should be noted that disease stage remains the strongest predictor of survival, with those being diagnosed at AJCC Stage 4 having over a 12-fold increased risk of dying than those diagnosed at a local disease stage.
The pathway approach we used was novel in that it summarized the statistical significance of the pathway and genes rather than focus on individual SNPs. ARTP allowed us to combine single SNP p values using the rank truncated product statistic and assess significance via permutations at multiple levels, including the gene, sub-pathway, and overall pathway level. While we selected genes that we believed were most important to the pathway, there are many other genes and SNPs involved in this pathway that could be important and contribute to colorectal cancer-specific mortality. We also are limited in our ability to assess interaction between genes and with lifestyle factors that could influence risk, since ARTP at this time does not allow for assessment of interactions. Unfortunately, we do not have a separate population to validate these findings and therefore encourage others with similar data to replicate these findings. Likewise, we did not attempt a test and training set, given the impact of that method on study power; lack of replication thus could be from lack of power. Other limitations to our assessment is lack of treatment and other related medical conditions that could impact survival. While we can argue that it is unlikely that these genes and SNPs are associated with treatment, we do not have the ability to test that. However, treatment is highly correlated with AJCC stage, and we have adjusted for stage in our analysis.
It is noteworthy that our findings for colon and rectal cancer are for the most part different. There are several potential explanations for these findings. First, disease pathways could be different for the two cancer sites, and thus genes and sub-pathways that are important could also differ. Another explanation for these differences, could stem from a smaller sample size for rectal than colon cancer. This could explain the lack of replication in rectal cancer from colon cancer findings, however it would explain differences observed in rectal cancer that are not replicated in colon cancer. While the underlying cause of these differences is not clear, it has been observed that risk factors differ between colon and rectal cancer [11], [47]–[54].
In conclusion, there is support that genes within the CHIEF pathway are associated with colorectal cancer-specific mortality, although the overall pathway did not influence risk. Replication of these findings, along with more detailed assessment of the specific genes may help identify key variants that could importantly contribute to prognosis.
Supporting Information
List of genes, aliases, and chromosomal location.
(DOCX)
Table. List of sub-pathways and genes included in each sub-pathway for ARTP analysis.
(DOCX)
Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with colon cancer (0.05> gene PARTP≤0.10; SNP Ptrend≤0.10).
(DOCX)
Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with rectal cancer (0.05> gene PARTP≤0.10; SNP Ptrend≤0.10).
(DOCX)
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute. We would like to acknowledge the contributions of Dr. Bette Caan, Judy Morse and Donna Schaffer and the Kaiser Permanente Medical Research Program, and Sandra Edwards, Roger Wolff, Erica Wolff, Michael Hoffman and Jennifer Herrick at the University of Utah, and Dr. Kristin Anderson and Dr. John Potter for data management and collection at the University of Minnesota.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Ethical restrictions apply to the patient-level dataset underlying the analyses presented, because of nature of consent forms signed, personnel information contained in the database, and the IRB approval. These restrictions prevent the data from being made fully available in a public repository. Interested researchers are kindly asked to contact the corresponding author for additional information.
Funding Statement
Funding was provided by the National Institute of Health (CA48998), which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Slattery ML, Fitzpatrick FA (2009) Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev Res (Phila Pa) 2:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon KJ, Blobe GC (2008) Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 1782:197–228. [DOI] [PubMed] [Google Scholar]
- 3. Carling D (2004) Ampk. Curr Biol 14:R220. [DOI] [PubMed] [Google Scholar]
- 4. Hardie DG (2003) Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144:5179–5183. [DOI] [PubMed] [Google Scholar]
- 5. Carling D (2004) The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29:18–24. [DOI] [PubMed] [Google Scholar]
- 6. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, et al. (2003) Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans 31:216–219. [DOI] [PubMed] [Google Scholar]
- 7. Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE (2000) Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 12:1232–1235. [DOI] [PubMed] [Google Scholar]
- 8. Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, et al. (2010) VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. The Journal of experimental medicine 207:2855–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ono M (2008) Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 99:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slattery ML, Wolff RK, Lundgreen A (2014) A Pathway Approach to Evaluating the Association between the CHIEF Pathway and Risk of Colorectal Cancer. Carcinogenesis. [DOI] [PMC free article] [PubMed]
- 11. Slattery ML, Potter J, Caan B, Edwards S, Coates A, et al. (1997) Energy balance and colon cancer–beyond physical activity. Cancer Res 57:75–80. [PubMed] [Google Scholar]
- 12. Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, et al. (2003) Physical activity and colorectal cancer. Am J Epidemiol 158:214–224. [DOI] [PubMed] [Google Scholar]
- 13. Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, et al. (2005) Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology 129:837–845. [DOI] [PubMed] [Google Scholar]
- 14. Slattery ML, Curtin K, Anderson K, Ma KN, Ballard L, et al. (2000) Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst 92:1831–1836. [DOI] [PubMed] [Google Scholar]
- 15. Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, et al. (2001) Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 10:917–923. [PubMed] [Google Scholar]
- 16. Yu K, Li Q, Bergen AW, Pfeiffer RM, Rosenberg PS, et al. (2009) Pathway analysis by adaptive combination of P-values. Genetic epidemiology 33:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slattery ML, Curtin K, Wolff RK, Boucher KM, Sweeney C, et al. (2009) A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum 52:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsieh FC, Cheng G, Lin J (2005) Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun 335:292–299. [DOI] [PubMed] [Google Scholar]
- 19. Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, et al. (2008) Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 10:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alessi DR, Downes CP (1998) The role of PI 3-kinase in insulin action. Biochim Biophys Acta 1436:151–164. [DOI] [PubMed] [Google Scholar]
- 21. Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501. [DOI] [PubMed] [Google Scholar]
- 22. Lee DF, Hung MC (2007) All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle 6:3011–3014. [DOI] [PubMed] [Google Scholar]
- 23. Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, et al. (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130:440–455. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Kang BW, Chae YS, Kim HJ, Park SY, et al. (2014) Genetic Variations in STK11, PRKAA1, and TSC1 Associated with Prognosis for Patients with Colorectal Cancer. Ann Surg Oncol. [DOI] [PubMed]
- 25. Guervos MA, Marcos CA, Hermsen M, Nuno AS, Suarez C, et al. (2007) Deletions of N33, STK11 and TP53 are involved in the development of lymph node metastasis in larynx and pharynx carcinomas. Cell Oncol 29:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakanishi C, Yamaguchi T, Iijima T, Saji S, Toi M, et al. (2004) Germline mutation of the LKB1/STK11 gene with loss of the normal allele in an aggressive breast cancer of Peutz-Jeghers syndrome. Oncology 67:476–479. [DOI] [PubMed] [Google Scholar]
- 27. Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 28. Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, et al. (1998) The multifaceted roles of nitric oxide in cancer. Carcinogenesis 19:711–721. [DOI] [PubMed] [Google Scholar]
- 29. Przybylowska K, Kluczna A, Zadrozny M, Krawczyk T, Kulig A, et al. (2006) Polymorphisms of the promoter regions of matrix metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast Cancer Research and Treatment 95:65–72. [DOI] [PubMed] [Google Scholar]
- 30.Zgaga L, Theodoratou E, Farrington SM, Din FV, Ooi LY, et al. (2014) Plasma Vitamin D Concentration Influences Survival Outcome After a Diagnosis of Colorectal Cancer. J Clin Oncol. [DOI] [PubMed]
- 31. Fedirko V, Riboli E, Tjonneland A, Ferrari P, Olsen A, et al. (2012) Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European ppulations. Cancer Epidemiol Biomarkers Prev 21:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ditsch N, Toth B, Mayr D, Lenhard M, Gallwas J, et al. (2012) The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J Histochem Cytochem 60:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slattery ML, Lundgreen A, Wolff RK (2013) VEGFA, FLT1, KDR and colorectal cancer: Assessment of disease risk, tumor molecular phenotype, and survival. Mol Carcinog. [DOI] [PubMed]
- 34. Tsamandas AC, Kardamakis D, Ravazoula P, Zolota V, Salakou S, et al. (2004) The potential role of TGFbeta1, TGFbeta2 and TGFbeta3 protein expression in colorectal carcinomas. Correlation with classic histopathologic factors and patient survival. Strahlenther Onkol 180:201–208. [DOI] [PubMed] [Google Scholar]
- 35. Forsti A, Li X, Wagner K, Tavelin B, Enquist K, et al. (2010) Polymorphisms in the transforming growth factor beta 1 pathway in relation to colorectal cancer progression. Genes Chromosomes Cancer 49:270–281. [DOI] [PubMed] [Google Scholar]
- 36. Isaksson-Mettavainio M, Palmqvist R, Forssell J, Stenling R, Oberg A (2006) SMAD4/DPC4 expression and prognosis in human colorectal cancer. Anticancer Res 26:507–510. [PubMed] [Google Scholar]
- 37. Xie W, Rimm DL, Lin Y, Shih WJ, Reiss M (2003) Loss of Smad signaling in human colorectal cancer is associated with advanced disease and poor prognosis. Cancer J 9:302–312. [DOI] [PubMed] [Google Scholar]
- 38. Park J, Park E, Han SW, Im SA, Kim TY, et al. (2012) Down-regulation of P-cadherin with PF-03732010 inhibits cell migration and tumor growth in gastric cancer. Investigational new drugs 30:1404–1412. [DOI] [PubMed] [Google Scholar]
- 39. Scharf PJ, Witney J, Daly R, Lyons BA (2004) Solution structure of the human Grb14-SH2 domain and comparison with the structures of the human Grb7-SH2/erbB2 peptide complex and human Grb10-SH2 domain. Protein science: a publication of the Protein Society 13:2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puri MC, Bernstein A (2003) Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America 100:12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, et al. (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. Journal of the National Cancer Institute 97:975–980. [DOI] [PubMed] [Google Scholar]
- 42. Tekautz TM, Zhu K, Grenet J, Kaushal D, Kidd VJ, et al. (2006) Evaluation of IFN-gamma effects on apoptosis and gene expression in neuroblastoma–preclinical studies. Biochim Biophys Acta 1763:1000–1010. [DOI] [PubMed] [Google Scholar]
- 43. Yang H, Zhou H, Feng P, Zhou X, Wen H, et al. (2010) Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J Exp Clin Cancer Res 29:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, et al. (2006) Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev 15:1126–1131. [DOI] [PubMed] [Google Scholar]
- 45.Miteva LD, Stanilov NS, Deliysky TS, Stanilova SA (2014) Significance of -1082A/G polymorphism of IL10 gene for progression of colorectal cancer and IL-10 expression. Tumour Biol. [DOI] [PubMed]
- 46. Ting WC, Chen LM, Huang LC, Hour MJ, Lan YH, et al. (2013) Impact of interleukin-10 gene polymorphisms on survival in patients with colorectal cancer. J Korean Med Sci 28:1302–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Habermann N, Ulrich CM, Lundgreen A, Makar KW, Poole EM, et al. (2013) PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer. Genes & nutrition 8:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murtaugh MA, Sweeney C, Ma KN, Potter JD, Caan BJ, et al. (2006) Vitamin d receptor gene polymorphisms, dietary promotion of insulin resistance, and colon and rectal cancer. Nutr Cancer 55:35–43. [DOI] [PubMed] [Google Scholar]
- 49. Slattery ML, Caan BJ, Benson J, Murtaugh M (2003) Energy balance and rectal cancer: an evaluation of energy intake, energy expenditure, and body mass index. Nutr Cancer 46:166–171. [DOI] [PubMed] [Google Scholar]
- 50. Slattery ML, Curtin K, Wolff R, Ma KN, Sweeney C, et al. (2006) PPARgamma and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States). Cancer Causes Control 17:239–249. [DOI] [PubMed] [Google Scholar]
- 51. Slattery ML, Lundgreen A, Welbourn B, Corcoran C, Wolff RK (2012) Genetic variation in selenoprotein genes, lifestyle, and risk of colon and rectal cancer. PLoS One 7:e37312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slattery ML, Lundgreen A, Welbourn B, Wolff RK, Corcoran C (2012) Oxidative balance and colon and rectal cancer: interaction of lifestyle factors and genes. Mutation Research 734:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Slattery ML, Murtaugh M, Caan B, Ma KN, Neuhausen S, et al. (2005) Energy balance, insulin-related genes and risk of colon and rectal cancer. Int J Cancer 115:148–154. [DOI] [PubMed] [Google Scholar]
- 54. Caan BJ, Coates AO, Slattery ML, Potter JD, Quesenberry CP Jr, et al. (1998) Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord 22:178–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes, aliases, and chromosomal location.
(DOCX)
Table. List of sub-pathways and genes included in each sub-pathway for ARTP analysis.
(DOCX)
Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with colon cancer (0.05> gene PARTP≤0.10; SNP Ptrend≤0.10).
(DOCX)
Genes and related SNPs associated with colorectal cancer-specific mortality among patients diagnosed with rectal cancer (0.05> gene PARTP≤0.10; SNP Ptrend≤0.10).
(DOCX)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Ethical restrictions apply to the patient-level dataset underlying the analyses presented, because of nature of consent forms signed, personnel information contained in the database, and the IRB approval. These restrictions prevent the data from being made fully available in a public repository. Interested researchers are kindly asked to contact the corresponding author for additional information.


