Abstract
The bivalent anti-T-cell immunotoxin A-dmDT390-bisFv(G4S) was developed for treatment of T-cell leukemia and autoimmune diseases and for tolerance induction for transplantation. This immunotoxin was produced extracellularly in toxin-sensitive Pichia pastoris JW102 (Mut+) under control of the AOX1 promoter. There were two major barriers to efficient immunotoxin production, the toxicity of the immunotoxin for P. pastoris and the limited capacity of P. pastoris to secrete the immunotoxin. The immunotoxin toxicity resulted in a decrease in the methanol consumption rate, cessation of cell growth, and low immunotoxin productivity after the first 22 h of methanol induction. Continuous cell growth and continuous immunotoxin secretion after the first 22 h of methanol induction were obtained by adding glycerol to the methanol feed by using a 4:1 methanol-glycerol mixed feed as an energy source and by continuously adding a yeast extract solution during methanol induction. The secretory capacity was increased from 22.5 to 37 mg/liter by lowering the induction temperature. A low temperature reduced the methanol consumption rate and protease activity in the supernatant but not cell growth. The effects of adding glycerol and yeast extract to the methanol feed were synergistic. Adding yeast extract primarily enhanced methanol utilization and cell growth, while adding glycerol primarily enhanced immunotoxin production. The synergy was further enhanced by decreasing the induction temperature from 23 to 15°C, which resulted in a robust process with a yield of 37 mg/liter, which was sevenfold greater than the yield previously reported for a toxin-resistant CHO cell expression system. This methodology should be applicable to other toxin-related recombinant proteins in toxin-sensitive P. pastoris.
The bivalent anti-T-cell immunotoxin A-dmDT390-bisFv(G4S) was developed for treatment of T-cell leukemia and autoimmune diseases and tolerance induction for transplantation. This immunotoxin selectively kills human T cells and is the most efficacious of a variety of anti-T-cell immunotoxins that have been developed (20). The bivalent immunotoxin contains the first 390 amino acid residues of diphtheria toxin (DT) and two tandem sFv molecules that are responsible for binding the immunotoxin to the CD3ɛγ subunit of the T-cell receptor complex on human T cells. The first 390 amino acid residues of DT (DT390) contain the catalytic domain or A chain of DT that inhibits protein synthesis by ADP ribosylation of EF-2 and the translocation domain that translocates the catalytic domain to the cytosol by interactions with cytosolic Hsp90 and thioredoxin reductase (17).
The inhibition of protein synthesis by the catalytic domain makes it difficult to produce the toxin-related proteins in most eukaryotic cells (e.g., wild-type CHO cells, insect cells, and yeasts). The use of toxin-resistant eukaryotic cells can overcome the immunotoxin toxicity. However, selection and characterization of toxin-resistant eukaryotic cells are tedious, labor-intensive, and time-consuming. Furthermore, the bivalent immunotoxin production in an EF-2 mutant CHO cell expression system was limited to 5 mg/liter and could not be increased by selection for multiple gene insertions (Neville, unpublished data). Due to this limitation, except for three reports from our laboratory (12, 20, 25), all recombinant immunotoxin production for therapeutic uses has been limited to Escherichia coli production, which has required denaturation and refolding from inclusion bodies (6). However, refolding of the multidomain structure of the bivalent immunotoxin from E. coli was inefficient, and complete bioactivity was not recovered (25). Therefore, our attempt to develop a robust Pichia pastoris production system for the bivalent immunotoxin was driven by the inadequacy of the existing productions systems.
The expression of bivalent immunotoxin in shake flask cultures of P. pastoris without any genetic modification for toxin resistance has been described previously (25). Although P. pastoris EF-2 was sensitive to the toxic effects of the toxin moiety, P. pastoris was relatively resistant to the immunotoxin when it was expressed under the AOX1 promoter and directed into the secretory pathway in the presence of rich medium. For example, immunotoxin levels as high as 10 mg/liter were obtained after 4 days in shake flask cultures in rich medium. However, examples of toxicity of the immunotoxin for P. pastoris were noted. There was a 55% loss of viable cells over a 24-h period of immunotoxin secretion in methanol-containing rich medium (25). On minimal methanol medium, there was a marked reduction in the growth rate of the immunotoxin-producing strain (JW102) compared to the growth rate of wild-type strain X-33 (13). The introduction of a second gene for immunotoxin production into P. pastoris failed to increase production but resulted in an increase in immunotoxin degradation products, suggesting that there is a limitation in the P. pastoris secretory machinery with respect to bivalent immunotoxin (13).
Our objective in this study was to establish a method for optimized, scalable bioreactor production of a toxic protein, the bivalent immunotoxin, in toxin-sensitive P. pastoris. To accomplish this goal, we hypothesized that varying the composition of the rich medium (18) and the fermentation carbon source (27) would attenuate the toxicity of the immunotoxin for host cells. We further hypothesized that a reduction in the temperature (9, 10) during induction would have an enhancing effect on immunotoxin secretion. In this study we found that a toxin-sensitive P. pastoris strain (JW102 [Mut+]) can secrete the bivalent immunotoxin at a level of 37 mg/liter under optimized fermentation conditions. This methodology should broaden the means for production of other toxin-related recombinant proteins in toxin-sensitive P. pastoris.
MATERIALS AND METHODS
Yeast strains.
We used genetically engineered P. pastoris strain JW102 (Mut+) (previously designated pJHW #2, but the designation was changed to avoid confusion with a plasmid), which was generated for production of the bivalent immunotoxin from the host strain GS115 (Invitrogen, Carlsbad, Calif.) (25). Expression of the integrated immunotoxin gene in the P. pastoris genome was controlled by the AOX1 (alcohol oxidase 1) promoter during methanol induction. Secretion of the gene product was directed by the alpha-prepro leader sequence. To compare the growth profiles and fermentation parameters in a fermentor, wild-type strain X-33 (Invitrogen) and the toxin-resistant strain mutEF2JC307-8 (2), which produces immunotoxin (13), were used as control strains. The mutEF2JC307-8 (2) strain had the same expression cassette as JW102 for immunotoxin secretion. Host strain GS115 was not used as the control strain, because it was a histidine auxotroph.
Initial complex fermentation medium and YSG broth.
Yeast extract, Bacto Peptone, Soytone Peptone, and Casamino Acids were all obtained from Difco (Detroit, Mich.). The initial complex fermentation medium contained (per liter) 20 g of yeast extract, 20 g of Soytone Peptone, 40 g of glycerol, 13.4 g of yeast nitrogen base with ammonium sulfate but without amino acids (Difco), and 4.3 ml of the PTM1 salt solution, as well as 0.02% (vol/vol) antifoam 289 (Sigma Chemical Co., St. Louis, Mo.). The PTM1 salt solution (Invitrogen) contained 24.0 mM (6 g/liter) cupric sulfate (CuSO4 · 5H2O), 0.534 mM (80 mg/liter) sodium iodide (NaI), 17.8 mM (338.6 mg/liter) manganese sulfate (MnSO4 · 5H2O), 0.827 mM (200 mg/liter) sodium molybdate (NaMoO4 · 2H2O), 0.323 mM (20 mg/liter) boric acid (H3BO3), 2.1 mM (500 mg/liter) cobalt chloride (CoCl2 · 6H2O), 147.0 mM (20 g/liter) zinc chloride (ZnCl2), 234.0 mM (65.1 g/liter) ferrous sulfate (FeSO4 · 7H2O), 1.64 mM (400 mg/liter) biotin, and 188.0 mM (18.4 g/liter) sulfuric acid (H2SO4).
YSG broth contained 1% (wt/vol) yeast extract, 2% (wt/vol) Soytone Peptone, and 1% (wt/vol) glycerol.
Strain maintenance and preparation of seed cultures.
Strain JW102 expressing the bivalent immunotoxin was maintained as a frozen stock at −80°C in 25% (vol/vol) glycerol. One milliliter of the frozen stock was inoculated into 50 ml of YSG broth and then cultivated for 2 days at 28°C at 250 rpm (orbit diameter, 1.9 cm). Thirty milliliters from a 50-ml culture was used as the first seed culture for inoculating 600 ml of YSG broth in two 1-liter flasks. After cultivation for 1 day at 28°C at 250 rpm (orbit diameter, 1.9 cm), the cultures were used as the second seed culture for inoculation of 10 liters of initial complex fermentation medium in a fermentor.
Fermentation.
We used a BioFlo 4500 fermentor (New Brunswick Scientific Company, Edison, N.J.) with a methanol sensor and controller (Raven Biotechnology Company, Vancouver, Canada) that maintained the methanol concentration at 0.15% (vol/vol) during induction. This fermentor was linked to a computer running AFS-BioCommand Windows-based software (New Brunswick Scientific Company), which allowed us to control all parameters by programmed processes.
The glycerol batch phase was completed within 18 h of inoculation, and complete consumption of glycerol in the culture was detected by monitoring the dissolved oxygen spike. Then there was a glycerol-fed batch phase, during which 75% (vol/vol) glycerol was fed by ramping up the feeding rate from 0.1 to 3.0 g/min for 7 h. A 75% (vol/vol) glycerol solution containing 18 ml of the PTM1 salt solution per liter was used to obtain the desired cell density for 7 h before methanol induction. Induction was performed with a continuous feed containing methanol or methanol-glycerol (4:1, vol/vol) with or without 10 mM phenylmethylsulfonyl fluoride (PMSF). The feeding rate for methanol or 4:1 methanol-glycerol was automatically controlled so that it was maintained at the set point (0.15% [vol/vol] methanol in the culture) by the methanol sensor and controller. The methanol consumption rate was measured by weighing a methanol solution or a methanol-glycerol mixed solution every 1 min with a computer-interfaced balance (PG5002S; Mettler Toledo, Greiβensee, Switzerland). PMSF was added at a final concentration of 1 mM just prior to induction when PMSF was added during methanol induction. A Casamino Acids or yeast extract solution (10%, wt/vol) was added continuously at a rate of 10 ml/h during methanol induction.
All parameters were automatically managed by running processes programmed in the AFS-BioCommand software. The dissolved oxygen level in the fermentor was maintained at >40% for the entire fermentation by adding O2 as needed. The pH in the fermentor was kept at 3.5 during the growth phase and at 7.0 during the methanol induction phase by adding 29% (vol/vol) NH4OH or 40% (vol/vol) H3PO4. The pH was ramped up from 3.5 to 7.0 for 2 h before the initiation of methanol induction. The pH shift procedure reduced the secretion of contaminant proteins (75- and 35-kDa bands) into the supernatant (Woo, unpublished data). The temperature was set at 28°C for growth and at 15 to 25°C during methanol induction. The induction temperature was ramped down from 28°C to 25 to 15°C during the first 4 h of methanol induction.
After the culture was harvested, a supernatant was prepared by centrifugation (2,800 × g at 4°C for 30 min). EDTA was added to a final concentration of 5 mM to prevent protein degradation during storage at 4°C.
Measurement of wet cell density for monitoring cell growth.
One milliliter of a culture sample was placed in a tared 1.5-ml microcentrifuge tube and centrifuged at 20,800 × g at 25°C for 2 min. The supernatant was removed with a pipette, and the residual liquid in the tube was blotted with filter paper. After the tube containing the cell pellet was weighed, the wet cell density was calculated.
Measurement of protease activity in the supernatant.
Unnicked CRM9 (with one point mutation in the recognition domain of DT [7]) was used as the substrate for measurement of serine protease activity in the supernatant at a final concentration of 225 μg/ml. The supernatant was incubated at 28°C with shaking at 250 rpm (orbit diameter, 1.9 cm) for 20 h before it was applied to a 4 to 20% Tris-glycine precast sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel in the presence of a reducing agent (100 mM dithiothreitol). CRM9 contains a well-exposed furin/Kex-2 cleavage site between the A fragment (22 kDa) and the B fragment (40 kDa) spanned by a disulfide bond. Protease activity in the medium was detected by loss of unnicked CRM9 under reducing conditions, and the band intensity of the unnicked CRM9 was quantified by densitometry on Coomassie blue-stained gels.
Western blotting.
Proteins were fractionated on 4 to 20% Tris-glycine precast SDS-PAGE gels (Invitrogen) run under nonreducing or reducing conditions and were transferred to nitrocellulose membranes by electroblotting. Nonspecific binding was blocked with 5% nonfat skim milk in Tris-buffered saline buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing 0.1% Tween 20. Goat polyclonal antibody directed against DT (19) diluted 1:2,000 was used as the primary antibody, and alkaline phosphatase-conjugated rabbit anti-goat immunoglobulin G (Roche Applied Science, Indianapolis, Ind.) diluted 1:5,000 was used as the secondary antibody. The immunotoxin was visualized with the one-step nitroblue tetrazolium—5-bromo-4-chloro-3-indolylphosphate substrate (Pierce Chemical Company, Rockford, Ill.). Alternatively, rabbit polyclonal antibody directed against (G4S)3 linker was used as the primary antibody for detecting intact immunotoxin and degraded products since the bivalent immunotoxin contained three (G4S)3 linkers. This antibody was raised against a synthetic peptide whose amino acid sequence was GGGGSGGGGSGGGGS.
Purification.
We used a previously developed scalable three-step procedure for purification of the bivalent immunotoxin (26) in which we utilized borate anion-exchange chromatography to eliminate contaminating host glycoproteins. Purification was performed with 1 liter of centrifuged supernatant. The specific T-cell cytotoxicity and purity of the purified immunotoxin were confirmed by a 20-h protein synthesis assay and by Superdex gel filtration high-performance liquid chromatography, respectively, as described previously (25).
Measurement of cell viability.
To measure cell viability, we used a modification of Ormerod's method (14), in which fluorescein diacetate (FDA) and propidium iodide (PI) were used as vital dyes. FDA taken up by P. pastoris was converted to fluorescein. If a cell had an intact plasma membrane, fluorescein was retained and PI was excluded. In brief, 500 μl of a suspension of P. pastoris cells (106 cells/ml in phosphate-buffered saline) was mixed with 50 μl of an FDA solution (10 μg/ml) and 50 μl of a PI solution (100 μg/ml). After incubation at room temperature for 10 min, cell viability was measured by flow cytometry by counting 10,000 events within the gates. The viable cell gate included green fluorescence and excluded red fluorescence. Confidence limits were calculated by using the binomial distribution (4).
Quantification of the concentration of the bivalent immunotoxin.
For quantification of the concentration of the purified bivalent immunotoxin, we used Superdex gel filtration. A Superdex 200 10/300 GL prepacked column (1.0 by 30 cm; Amersham Biosciences Corp., Piscataway, N.J.) connected to a high-performance liquid chromatography system (GBC Scientific Equipment, Arlington Heights, Ill.) was electrophoresed with 90 mM Na2SO4-10 mM NaH2PO4 · H2O-1 mM EDTA (pH 8.0) at a rate of 0.5 ml/min. Purified immunotoxin whose concentration was known (based on UV absorbance) (25) was used as a standard.
Quantification of the concentration of the bivalent immunotoxin in supernatants or liquid samples was performed by comparing the intensities of Coomassie blue-stained precast 4 to 20% SDS gels with the intensities of immunotoxin standards whose concentrations were known.
RESULTS
Immunotoxin expression and methanol utilization.
Wild-type strain X-33 did not produce immunotoxin (Fig. 1A) and served as a control for monitoring methanol consumption and cell growth. This strain had a maximum methanol consumption rate of 1.95 ml/min at 25°C. This consumption rate was maintained at >70% of the maximum rate for the entire methanol induction phase (Fig. 1A). The wet cell density increased continuously during the 44-h methanol induction. The DT-resistant immunotoxin-producing EF-2 mutant strain mutEF2JC307-8 (2) was used as another control for comparing methanol consumption and cell growth when the immunotoxin was secreted. This EF-2 mutant strain had profiles for methanol consumption and wet cell growth similar to those of wild-type strain X-33 during induction (Fig. 1A). The maximum methanol consumption rate and the wet cell gain during 44 h of methanol induction were 2.2 ml/min and 9.17%, respectively. However, use of the EF-2 mutant did not improve immunotoxin secretion under the fermentation conditions used for the JW102 strain producing immunotoxin. For strain JW102, the maximum methanol consumption rate was 1.30 ml/min at 25°C. After peaking at 7 to 8 h following the initiation of methanol induction, the consumption rate decreased to 15% of the maximum rate after 44 h of methanol induction. Within the first 22 h of methanol induction, the methanol consumption rate dropped to <50% of the maximum methanol consumption rate (Fig. 1B). This low level of methanol consumption resulted in a smaller increase in the wet cell density for JW102 (2.0%) than for X-33 (10.5%) (Fig. 1A and B). There was little or no increase in the wet cell density after the first 22 h of methanol induction, and the secreted level of immunotoxin decreased from 15 to 10 mg/liter. Immunotoxin breakdown products were not detectable on the SDS gels used to monitor product stability (data not shown).
FIG. 1.
Comparison of profiles of cell growth, methanol consumption, and immunotoxin secretion during methanol induction. (A) Strain X-33 and the immunotoxin-producing toxin-resistant EF-2 mutant mutEF2JC307-8(2). These two strains had similar profiles for the methanol consumption rate and for wet cell density gain during methanol induction. The data are for X-33. For the toxin-resistant mutant, the maximum methanol consumption rate and wet cell density gain after 44 h of methanol induction were 2.2 ml/min and 9.17%, respectively. (B to F) Strain JW102. The constant conditions for panels A to F included a glycerol batch phase, followed by a glycerol-fed batch phase prior to induction. For induction, either pure methanol alone or a 4:1 methanol-glycerol was used. PMSF at a concentration of 10 mM in methanol was infused continuously during induction. Casamino Acids was added when yeast extract was not added. The induction conditions were as follows: 4:1 methanol-glycerol feeding and no yeast extract feeding (A); methanol feeding and no yeast extract feeding (B); methanol feeding and yeast extract feeding (C); 4:1 methanol-glycerol feeding and yeast extract feeding (D); 4:1 methanol-glycerol feeding and no yeast extract feeding (E); and 4:1 methanol-glycerol feeding and yeast extract feeding (F). The induction temperature was 23 to 25°C (A to E) or 15°C (F) (note that the right axis in panel F is compressed twofold compared to the right axes in the other panels). Dotted line, methanol consumption rate; solid line, wet cell density; dashed line, level of secreted immunotoxin. Because of the large amount of work involved in 10-liter bioreactor fermentations, it was not practical to replicate the results in panels A to E. The optimized method (F) was performed three times, and the points are averages; the standard error of the mean is indicated when it is greater than 10%. The actual data points for wet cell density and the level of secreted immunotoxin are indicated (▪ and •, respectively). The actual data points for the methanol consumption rate are not shown because the methanol consumption rate was measured every minute.
Yeast extract feeding, methanol consumption, and immunotoxin production.
The decreased methanol consumption and cell growth rate associated with immunotoxin production may be due to the toxicity of the immunotoxin for P. pastoris. If yeast extract was added continuously to the bioreactor with methanol as the sole carbon source (Fig. 1C), then the peak methanol consumption was less than that with the wild-type strain and the EF-2 mutant strain (Fig. 1A), but the decrease after 10 h was eliminated and cell growth increased throughout the induction period. This growth response was coupled with a loss of immunotoxin in the medium after 8 h, suggesting that there was protease activity. Immunotoxin fragments were present 4 h after induction, and no intact immunotoxin was detected by 19 h after induction (Fig. 2A). If the medium collected at various times was incubated with purified immunotoxin, the amount of immunotoxin fragments formed depended on the age of the medium (Fig. 2B). For example, at 49 h postinduction the intact immunotoxin band was greatly reduced, and the 36.5-kDa band representing degraded fragments was greatly increased relative to samples from earlier times.
FIG. 2.
Protein degradation and immunotoxin production. (A) Time course for immunotoxin levels during methanol induction in cultures with methanol and yeast extract feeding (see Fig. 1C). The immunotoxin (IT) band is indicated by an arrow. (B) Analysis of residual immunotoxin by SDS-PAGE after incubation (28°C, 20 h, shaking at 250 rpm) of purified immunotoxin (250 μg/ml) with equal volumes of the supernatants collected at the times indicated following methanol induction. Mixtures of equal volumes of purified immunotoxin and phosphate-buffered saline or supernatant from zero time were used as the controls (CON). Ten-microliter portions of the prepared samples were loaded for SDS-PAGE and fractionated on 4 to 20% SDS-Tris-glycine gels under nonreducing conditions. The gels were stained with Coomassie blue dye. Mark12 marker (Invitrogen) was used as the protein marker (lane M).
Addition of glycerol to the methanol feed with yeast extract feeding.
The protease activity observed when methanol was the sole carbon source could have been a result of leaking from dead or injured cells. When we substituted methanol-glycerol (4:1, vol/vol) (Fig. 1D) for the pure methanol (Fig. 1C), the level of immunotoxin in the medium rose to 20 mg/liter at 44 h. Only minimal levels of degradation products were detected in SDS gels of proteins in this medium (Fig. 3, 23°C panel). The methanol-glycerol mixed feed without yeast extract could not sustain the methanol consumption or the continual increase in cell mass, and the final immunotoxin concentration was 15 mg/liter (Fig. 1E).
FIG. 3.
Effect of temperature on immunotoxin production. Samples taken at different induction times (44, 50, and 67 h) from runs at different induction temperatures (15 to 23°C) were fractionated on 4 to 20% SDS-Tris-glycine gels under nonreducing conditions. Continuous feeding of yeast extract and methanol-glycerol was used for all runs. The gels were stained with Coomassie blue dye. IT, bivalent immunotoxin; IT-dp, degraded products of the bivalent immunotoxin. The degradation products were identified by Western blotting by using anti-DT antibody and anti-(G4S)3 linker antibody. The anti-(G4S)3 linker antibody could detect the bivalent immunotoxin and degraded products, because the immunotoxin contained three (G4S)3 linkers. The arrows indicate bands not related to the bivalent immunotoxin. Mark12 marker (Invitrogen) was used as the protein marker (lane M).
Low temperature and secretion of bivalent immunotoxin.
Low temperature can improve the yield of heterologous protein expression in P. pastoris by enhancing protein folding within the endoplasmic reticulum and/or by reducing the medium protease activity (9). At 15°C methanol consumption at 44 h was reduced by 25%; however, cell growth was maintained. Immunotoxin production increased by 50% at 44 h (30 ± 0 mg/liter; n = 3) and by almost 100% at 67 h (37 ± 2.9 mg/liter; n = 3). Most of the increase in immunotoxin secretion occurred at temperatures between 20 and 15°C (data not shown).
The highest expression level was observed at 17.5°C (data not shown), but the final yield obtained by the three-step purification procedure for the immunotoxin was greatest at 15°C and the average values were 13.8 ± 1 mg/liter (n = 3) and 16.0 ± 1 mg/liter (n = 3) at 44 and 67 h, respectively. The purified immunotoxin produced at 15°C was fully functional, as confirmed by measuring specific T-cell cytotoxicity with a protein synthesis assay that yielded 50% inhibitory concentration values for three individual production runs of 1.2 × 10−13 ± 0.1 × 10−13 M, compared to 2 × 10−13 M for the average of three runs for a shake flask culture.
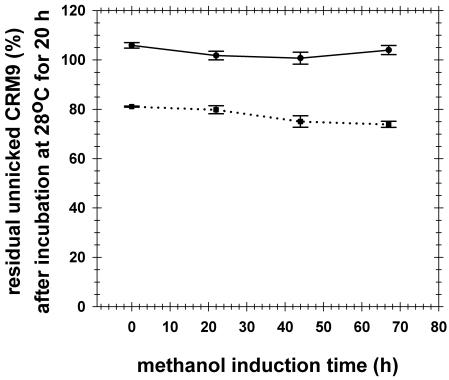
The levels for degraded immunotoxin bands on SDS gels from bioreactor supernatants (all of which were continuously fed with 10 mM PMSF) ranged from modest at 23°C to undetectable at 15°C (Fig. 3). When we used a sensitive assay for serine Kex-2-like proteases and a mutant DT (CRM9) substrate, protease activity was undetectable at 67 h when the temperature was 15°C, although activity was detected at 67 h when PMSF was not infused (Fig. 4). At 15°C the gel patterns and immunotoxin yields were identical whether PMSF was infused or not (data not shown).
FIG. 4.
Analysis of protease activity in supernatants in the absence and presence of PMSF during methanol induction at 15°C. Supernatants were removed after 0, 22, 44, and 67 h of methanol induction from fermentations with continuous feeding of yeast extract and methanol-glycerol. The supernatants were incubated with unnicked CRM9 as the substrate. After incubation, 10 μl of each sample was fractionated on a 4 to 20% SDS-Tris-glycine gel under reducing conditions. After staining and drying, the gel was digitized and analyzed to determined the band intensity of unnicked CRM9 by using NIH Image software. Solid line, PMSF added during methanol induction; dotted line, no PMSF. Each data point is the average for three fermentations; the error bars indicate the standard errors of the means.
A cell viability analysis of 15°C bioreactor samples that received the methanol-glycerol mixed feed plus yeast extract medium and were assayed by flow cytometery revealed low levels of dead cells, as follows: for the glycerol fed-batch phase, 0.7% ± 0.22% (confidence limit, 99%); and for the glycerol-methanol mixed feed, 1.2% ± 0.58% (confidence limit, 99%) at 22 h, 1.7% ± 0.61% (confidence limit, 99%) at 44 h, and 1.1% ± 0.51% (confidence limit, 99%) at 67 h (the dead cell fraction was determined from one fermentation run). Viable cells showing intracellular esterase activity were present in over 96% of the cells at all times during methanol induction.
DISCUSSION
We increased the secretion of bivalent immunotoxin fourfold in a toxin-sensitive P. pastoris bioreactor culture compared to the secretion in a shake flask culture by optimizing the fermentation conditions. We (i) changed the energy source by adding glycerol to the methanol feed (by using a 4:1 methanol-glycerol mixed feed), (ii) added yeast extract continuously, and (iii) lowered the temperature during methanol induction to 15°C. Adding glycerol decreased immunotoxin proteolysis and enhanced immunotoxin production, while added yeast extract primarily enhanced methanol utilization and cell growth. Glycerol feeding and yeast extract feeding acted synergistically to increase immunotoxin production, and this synergy was enhanced at 15°C.
The reduction in methanol utilization that was corrected by yeast extract feeding (Fig. 1C) was apparently secondary to inhibition of protein synthesis by the immunotoxin following ADP ribosylation of EF-2. This was shown by the fact that a P. pastoris strain producing immunotoxin and engineered for toxin resistance in the EF-2 gene (13) consumed methanol at the wild-type strain rate (Fig. 1). In the toxin-sensitive strain inhibition of protein synthesis could occur if the immunotoxin gained access to the cytosol compartment where EF-2 resides. Two distinct mechanisms could produce this effect. One possible mechanism is posttranslational translocation, in which the entire immunotoxin is translated before it enters the Sec61 translocon (16). This should provide a brief opportunity for ADP ribosylation of EF-2. Posttranslational translocation is common when the signal peptide is alpha mating factor, as it is in this case (24). Another possibility is the well-documented proton-mediated catalytic domain translocation across an internal membrane compartment (2). This could occur from the mildly acidic Golgi compartment or the more acidic vacuole. Whichever immunotoxin translocation mechanism is dominant, yeast extract feeding appears to interfere either with this step or with the subsequent ADP ribosylation of EF-2 either directly or by attenuating the catalytic activity of the translocated toxin A chain.
Inhibition of protein synthesis by immunotoxin could decrease methanol utilization due to a loss of alcohol oxidase (AOX) and/or catalase activities since these enzymes usually are rate limiting in methylotrophic yeasts (5). AOX can be inactivated by accumulation of H2O2, and catalase can be inactivated by accumulation of formaldehyde (23). Both of these compounds require an adequate energy source, NAD+, reducing equivalents, and O2 for further metabolism (8). In mammalian cells inhibition of protein synthesis following contact with DT is associated with apoptosis and DNA fragmentation (21) and with a marked decline in ATP levels (Neville, unpublished data). Yeast cells also can undergo apoptosis (3). Therefore, there are multiple potential pathways by which a decrease in protein synthesis can decrease methanol metabolism.
Although continuous addition of yeast extract largely corrected the reduction in methanol metabolism, immunotoxin production was low and was associated with extensive proteolysis (Fig. 1C and 2). This extensive proteolysis was reversed by providing supplemental carbon in the form of a mixed 4:1 methanol-glycerol feed (Fig. 1D and Fig. 3, 23°C panel), which increased the immunotoxin concentration to 20 mg/liter. It has been reported that there is an optimal maximal specific growth rate during P. pastoris methanol fed-batch culture, which, when exceeded, depresses heterologous protein production (27). Adding methanol at the optimal rate and adding glycerol at a rate that is 20% of the rate for maximal growth in the presence of glycerol increased heterologous protein production by 50% (27). This increase could have resulted from increased metabolism of formaldehyde and H2O2 and higher activity of catalase and AOX. In the case of secreted proteins, these metabolic changes also might reduce the amount of excreted proteases and reduce the number of dead or injured cells leaking proteolytic enzymes.
Increased immunotoxin secretion in response to changes in medium composition also could result from changes in the level of the unfolded protein response (UPR) within the endoplasmic reticulum. If a large fraction of the immunotoxin unfolds in the endoplasmic reticulum, the presence of a robust UPR could enhance secretion of the immunotoxin (22). In Saccharomyces cerevisiae the basal level of HAC1 splicing that generates the UPR in rich medium was 25% with nonfermentable carbon sources (ethanol and acetate), compared to 1 to 3% with glucose (18). Therefore, culture medium composition can strongly affect the UPR induction status.
Lowering the induction temperature from 23 to 25°C to 15°C further increased the immunotoxin level to 30 mg/liter at 44 h and to 37 mg/liter at 67 h (Fig. 1F). A low induction temperature was associated with a low and constant level of dead cells during induction (<2.0%) and reduced protease activity toward immunotoxin within the bioreactor even though small amounts of protease activity could be detected by a sensitive assay (Fig. 3 and 4). These results are consistent with the results of a study in which a temperature-limited (12°C) fed-batch technique was used (9). For the temperature-limited fed-batch technique, the level of dead cells was reduced from 9 to <1% at 44 h compared to the level in a methanol-limited fed-batch reactor at 30°C. This reduction in the level of dead cells was associated with a marked reduction in the amount of degraded product (lipase) and a twofold increase in the amount of intact product at late times. These changes were attributed to avoidance of oxygen deprivation at high cell densities. AOX activity increased more than twofold at 67 h with the temperature-limited fed-batch technique.
Lowering the induction temperature might also result in increased immunotoxin secretion due to balancing of the immunotoxin input and output through the secretory pathway by reducing the overall protein synthesis rate. In the expression and secretion of heterologous proteins, each protein appears to have an optimal secretion level. Expression greater than the optimal level (overexpression) can reduce secreted protein yields (1, 11, 13, 15). The bivalent immunotoxin also might require a longer processing time for correct folding because of the multidomain structure of the protein, which has low activity after in vitro refolding following expression in E. coli (25). Therefore, we hypothesized that low temperature might allow more time for the immunotoxin to fold correctly by reducing other cellular activities. The methanol consumption rate was reduced by only 25% when the temperature was changed from 23 to 15°C, and the cell growth rate was unchanged at 44 h. Further studies (e.g., pulse-chase experiments) are needed to determine which mechanisms are the most important mechanisms for enhancing immunotoxin production at low temperatures.
Most recombinant immunotoxins are currently produced intracellularly in E. coli and therefore require refolding from solubilized inclusion bodies. While this works well for smaller immunotoxins, multidomain immunotoxins having high-affinity bivalent binding moieties, such as the immunotoxin described here, are difficult to refold and may never achieve full bioactivity. P. pastoris is an attractive alternative expression system that has the full complement of eukaryotic protein folding machinery. Although P. pastoris was relatively resistant to the toxic effects of DT, there were toxic effects that limited high-cell-density bioreactor production under the AOX1 promoter. This study demonstrated that there is a synergy between carbon source supplementation with glycerol and continuous yeast extract feeding that attenuates the toxic effects of the immunotoxin and increases production, especially at 15°C. The yield of this robust process is 37 mg/liter, which is sevenfold greater than the yield previously reported for the toxin-resistant CHO cell expression system (25). This methodology should be useful for production of other recombinant immunotoxins and other toxic proteins in toxin-sensitive P. pastoris.
Acknowledgments
The Department of Health and Human Services (DHHS) holds several patents relating to composition and methods of use of bivalent anti-T-cell immunotoxin. Novartis International AG (Switzerland) has currently licensed these patents. J. H. Woo, Y. Y. Liu, and D. M. Neville as inventors receive royalty payments from the Department of Health and Human Services.
REFERENCES
- 1.Bannister, S. J., and K. D. Wittrup. 2000. Glutathione excretion in response to heterologous protein secretion in Saccharomyces cerevisiae. Biotechnol. Bioeng. 68:389-395. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, M. J., and D. Eisenberg. 1994. Refined structure of monomeric diphtheria toxin at 2.3A resolution. Protein Sci. 3:1464-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, S. R., D. D. Dunigan, and M. B. Dickman. 2003. Bcl-2 family members inhibit oxidative stress-induced programmed cell death in Saccharomyces cerevisiae. Free Radic. Biol. Med. 34:1315-1325. [DOI] [PubMed] [Google Scholar]
- 4.Conover, W. J. 1999. Some tests based on the binomial distribution, p. 123-178. In B. Wiley (ed.), Practical nonparametric statistics, 3rd ed. John Wiley and Sons, Inc., New York, N.Y.
- 5.Couderc, R., and J. Baratti. 1980. Oxidation of methanol by the yeast, Pichia pastoris. Purification and properties of the alcohol oxidase. Agric. Biol. Chem. 44:2279-2289. [Google Scholar]
- 6.Frankel, A. E., D. M. Neville, T. A. Bugge, R. J. Kreitman, and S. H. Leppla. 2003. Immunotoxin therapy of hematologic malignancies. Semin. Oncol. 30:545-557. [DOI] [PubMed] [Google Scholar]
- 7.Hu, V. W., and R. K. Holmes. 1987. Single mutation in the A domain of diphtheria toxin results in a protein with altered membrane insertion behavior. Biochim. Biophys. Acta 902:24-30. [DOI] [PubMed] [Google Scholar]
- 8.Jahic, M., J. C. Rotticci-Mulder, M. Martinelle, K. Hult, and S. O. Enfors. 2002. Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess Biosyst. Eng. 24:385-393. [Google Scholar]
- 9.Jahic, M., F. Wallberg, M. Bollok, P. Garcia, and S. O. Enfors. 2003. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb. Cell Fact. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Z., F. Xiong, Q. Lin, M. d'Anjou, A. J. Daugulis, D. S. Yang, and C. L. Hew. 2001. Low-temperature increases the yield of biologically active herring antifreeze protein in Pichia pastoris. Protein Expr. Purif. 21:438-445. [DOI] [PubMed] [Google Scholar]
- 11.Liebman, J. M., D. LaSala, W. Wang, and P. M. Steed. 1999. When less is more: enhanced baculovirus production of recombinant proteins at very low multiplicities of infection. BioTechniques 26:36-38, 40, 42. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Y. Y., I. Gordienko, A. Mathias, S. Ma, J. Thompson, J. H. Woo, and D. M. Neville, Jr. 2000. Expression of an anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin in a mutant CHO cell line. Protein Expr. Purif. 19:304-311. [DOI] [PubMed] [Google Scholar]
- 13.Liu, Y. Y., J. H. Woo, and D. M. Neville. 2003. Targeted introduction of a diphtheria toxin resistant mutation into the chromosomal EF-2 locus of Pichia pastoris and expression of immunotoxin in the EF-2 mutants. Protein Expr. Purif. 30:262-274. [DOI] [PubMed] [Google Scholar]
- 14.Ormerod, M. G. 2000. Further applications to cell biology, p. 249-258. In M. G. Ormerod (ed.), Flow cytometry, a practical approach, 3rd ed. Oxford University Press, New York, N.Y.
- 15.Pendse, G. J., S. Karkare, and J. E. Bailey. 1992. Effect of cloned gene dosage on cell growth and hepatitis B surface antigen synthesis and secretion in recombinant CHO cells. Biotechnol. Bioeng. 40:119-129. [DOI] [PubMed] [Google Scholar]
- 16.Pilon, M., K. Romisch, D. Quach, and R. Schekman. 1998. Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol. Biol. Cell 9:3455-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratts, R., H. Zeng, E. A. Berg, C. Blue, M. E. McComb, C. E. Costello, J. C. VanderSpek, and J. R. Murphy. 2003. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 160:1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroder, M., J. S. Chang, and R. J. Kaufman. 2000. The unfolded protein response represses nitrogen-starvation induced developmental differentiation in yeast. Genes Dev. 14:2962-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson, J., H. Hu, J. Scharff, and D. M. Neville. 1995. An anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin avoids inhibition by pre-existing antibodies in human blood. J. Biol. Chem. 270:28037-28041. [DOI] [PubMed] [Google Scholar]
- 20.Thompson, J., S. Stavrou, M. Weetall, J. M. Hexham, M. E. Digan, Z. Wang, J. H. Woo, Y. Yu, A. Mathias, Y. Y. Liu, S. Ma, I. Gordienko, P. Lake, and D. M. Neville, Jr. 2001. Improved binding of a bivalent single-chain immunotoxin results in increased efficacy for in vivo T-cell depletion. Protein Eng. 14:1035-1041. [DOI] [PubMed] [Google Scholar]
- 21.Thorburn, J., A. E. Frankel, and A. Thorburn. 2003. Apoptosis by leukemia cell-targeted diphtheria toxin occurs via receptor-independent activation of Fas-associated death domain protein. Clin. Cancer Res. 9:861-865. [PubMed] [Google Scholar]
- 22.Valkonen, M., M. Penttila, and M. Saloheimo. 2003. Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veenhuis, M., J. P. Van Dijken, and W. Harder. 1983. The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts. Adv. Microb. Physiol. 24:1-82. [DOI] [PubMed] [Google Scholar]
- 24.Willer, M., A. J. Jermy, G. J. Steel, H. J. Garside, S. Carter, and C. J. Stirling. 2003. An in vitro assay using overexpressed yeast SRP demonstrates that cotranslational translocation is dependent upon the J-domain of Sec63p. Biochemistry 42:7171-7177. [DOI] [PubMed] [Google Scholar]
- 25.Woo, J. H., Y. Y. Liu, A. Mathias, S. Stavrou, Z. Wang, J. Thompson, and D. M. Neville. 2002. Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr. Purif. 25:270-282. [DOI] [PubMed] [Google Scholar]
- 26.Woo, J. H., and D. M. Neville. 2003. Separation of bivalent anti-T cell immunotoxin from P. pastoris glycoproteins by borate anion exchange. BioTechniques 35:392-398. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, W., K. J. Hywood Potter, B. A. Plantz, V. L. Schlegel, L. A. Smith, and M. M. Meagher. 2003. Pichia pastoris fermentation with mixed-feeds of glycerol and methanol: growth kinetics and production improvement. J. Ind. Microbiol. Biotechnol. 30:210-215. [DOI] [PubMed] [Google Scholar]