Abstract
Background
The incidence of stroke in adulthood increases with advancing age, but there is little understanding of how post-stroke treatment should be tailored by age.
Objective
The goal of this study was to determine if age and task-specificity of rehabilitative training affect behavioral improvement and motor cortical organization after stroke.
Methods
Young and aged mice were trained to proficiency on the Pasta Matrix Reaching Task prior to lesion induction in primary motor cortex with endothelin-1. After a short recovery period, mice received 9 weeks of rehabilitative training on either the previously learned task: Pasta Matrix Reaching, a different reaching task: Tray Reaching, or no training. To determine the extent of relearning, mice were tested once weekly on the Pasta Matrix Reaching Task. Mice then underwent intracortical microstimulation mapping to resolve the remaining forelimb movement representations in peri-lesion motor cortex.
Results
Although aged mice had significantly larger lesions compared to young mice, Pasta Matrix Reaching served as effective rehabilitative training for both age groups. Young animals also showed improvement after Tray Reaching. Behavioral improvement in young mice was associated with an expansion of the rostral forelimb area (“premotor” cortex), but we failed to see reorganization in the aged brain, despite similar behavioral improvements.
Conclusions
Our results indicate that reorganization of motor cortex may be limited by either aging or greater tissue damage, but the capacity to improve motor function via task-specific rehabilitative training continues to be well maintained in aged animals.
Keywords: aging, intracortical microstimulation, motor map, pasta matrix, reaching
Introduction
Stroke affects nearly 800,000 Americans each year. The majority of strokes during adulthood occur after the age of 60 and stroke risk continues to increase with advancing age[1]. Aged animal models of stroke are important for elucidating mechanisms of effective rehabilitative therapies. Following experimental induction of stroke, aged animals show long-lasting performance deficits on sensorimotor tasks[2]. Rehabilitative tasks can be effective in promoting improvements in forelimb function in older rats and monkeys[3-5], but these improvements are somewhat limited. While young animals’ wrist and digit movement patterns during grasping normalize over time, older animals’ do not[4, 5], evidence that the aged brain does not recover from stroke as well as the young brain.
In young adult squirrel monkeys, motor cortical infarcts cause a reduction in the areal extent of the functional map and a loss of skilled forelimb use[6]. Rehabilitative training induces a beneficial reorganization of the peri-lesion motor map concurrent with behavioral improvement[7]. However, it is unknown what effect stroke has on the aged motor cortical representations of the forelimb, which we have previously shown have already lost some complexity and learning-related plasticity in the intact brain[8]. The goal of the current study was to determine how age and task-specificity of rehabilitative training affects motor cortical organization and the ability to regain a previously learned motor skill following focal ischemic motor cortical lesions in a clinically relevant animal model.
Methods
Subjects
A total of 25 3-7 month old and 24 16-20 month old male C57BL/6 mice were used. All mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 1 month of age, except for a subset of aged mice obtained as retired breeders at 9 months of age (n=17). Animals were housed in groups of 3-4 except for retired breeders, who were housed singly to prevent aggressive behavior. All mice received standard cage supplementation[9]. Differences in housing had no effect on behavioral recovery after stroke (main effect of group: F(1,22)=1.45, p=0.24; group by day interaction: F(10,220)=1.05, p=0.41) or cortical representation size (t-values=-0.22-1.71, p-values=0.10-0.89).
To ensure proper levels of motivation during the reaching tasks, mice were maintained on scheduled feeding, receiving a daily allowance of 2.5-3g of rodent chow immediately following each training session. Seven young and 10 aged mice were omitted from the study (and the above animal numbers) due to post-operative mortality. Animal use was in accordance with a protocol approved by the University of Texas at Austin Animal Care and Use Committee.
Experimental overview
Mice received daily training on the Pasta Matrix Reaching Task (PMRT) for 8 weeks preoperatively to ensure task mastery[8]. Following task acquisition, ischemic lesions of the forelimb motor cortex were induced with endothelin-1 (ET-1), a vasoconstricting peptide[9]. Post-operative reaching success on the PMRT was assessed 4 days after surgery. Young and aged mice were randomly divided into three treatment conditions: 1) No Rehabilitation (No-Rehab; n=8 young, n=8 aged), 2) Tray Reaching rehabilitation (Tray-Rehab; n=8 young, n=8 aged), or 3) Pasta Matrix Rehabilitation (PM-Rehab; n=9 young, n=8 aged). All mice were tested once weekly (probe trials) on the PMRT, following a 5 day period of rehabilitation or control procedures. Rehabilitation and probe trials were continued in this fashion for 9 weeks total. Following the final probe trial, mice underwent a terminal intracortical microstimulation (ICMS) procedure to resolve remaining forelimb movement representations in the caudal forelimb area (CFA) of the primary motor cortex and the rostral forelimb area (RFA), which is putatively homologous to premotor cortex[10]. Experimenters were blinded to the training conditions of animals during ICMS procedures.
Pre-operative training
The PMRT requires mice to reach for and break small pieces of vertically oriented, uncooked capellini pasta pieces arranged in a matrix distal and lateral to the reaching chamber aperture. In order to successfully retrieve a piece, the mouse must break the pasta by grasping and pulling forward. Detailed methods were described previously[9].
Mice were trained to reach only with the preferred limb by filling only the half of the matrix contralateral to that limb (Fig. 1A). Daily training sessions consisted of up to 100 reach attempts or 15 minutes, whichever occurred first. The number of pasta pieces successfully broken was recorded. Pre-operative training was conducted 6 days per week, with 1 day off, for a total of 10 weeks. Mice received no pre-operative training on the Tray Reaching Task (Fig. 1B).
Figure 1.
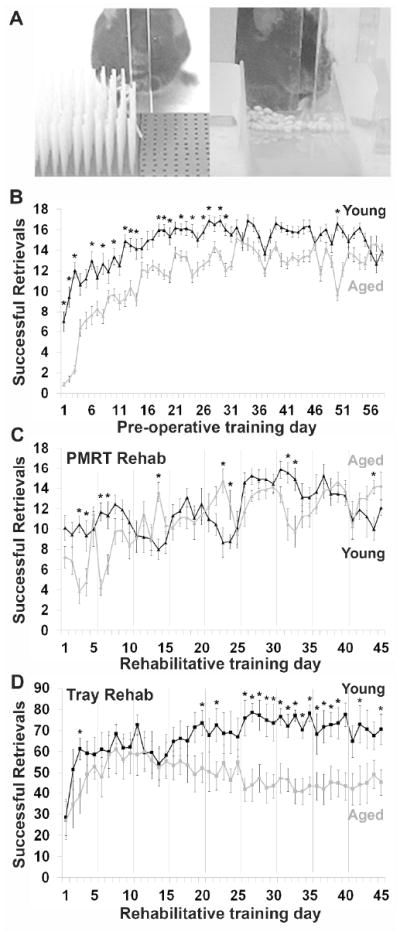
Aged mice are able to learn and perform skilled motor tasks. (A) Examples of mice performing the two rehabilitative training tasks: the PMRT (left) and the Tray Reaching Task (right). (B) Results of post-operative PMRT training trials in young and aged mice. Successful retrievals indicate the number of pasta pieces broken and eaten per training session. (C) Results of PMRT rehabilitative training trials in young and aged mice. (D) Results of Tray Reaching rehabilitative training trials in young and aged mice. Successful retrievals indicate the number of millet seeds grasped and eaten per training session. Gray vertical lines indicate 2 day breaks in rehabilitative training to allow for probe trials on the PMRT and days off of training. *p<0.05, Young vs. Aged. Data are means±S.E.
Endothelin-1 lesions
ET-1 induced lesion surgeries were performed as previously described[9]. Under anesthesia (100mg/kg ketamine and 10mg/kg xylazine, i.p.), a small burr hole was drilled through the skull over the forelimb representation of primary motor cortex, at 1.5mm lateral to midline and +0.3mm anterior to Bregma[11]. The dura was punctured and a calibrated pipette with a tip diameter of ~50μm was lowered into the cortex to a depth of 800μm. For young mice, 4μl of ET-1 (American Peptide; 320pmol in saline) was infused into the cortex. Aged mice received a smaller volume of ET-1 (3μl) based on a pilot study that indicated that the larger volume (and dose) increased mortality rates. The burrhole was then filled with gelfoam and covered with UV curing dental cement prior to suturing. Each animal was allowed to fully awaken in a heated chamber, and received buprenorphine (3ml/kg, s.c.) before it was returned to the home cage. Post-lesion mortality rates were not significantly different between young and aged mice (21% vs. 29%, respectively; χ2(1, N=66)=0.49, p=0.52).
Rehabilitative training
Rehabilitative training began 5 days after lesion induction surgery, and was then conducted 5 days per week, followed by 1 probe trial day and 1 day off, for a total of 9 weeks. Probe trials of the PMRT were used to access deficits in, and relearning of, this previously acquired task. Post-operative PMRT sessions, including probe trials, were conducted exactly as pre-operative sessions. Control procedures for mice in the No-Rehab condition consisted of eating pieces of pasta too small to handle from the floor of a reaching chamber for a maximum of 15 minutes per day.
Mice in the Tray-Rehab condition performed the Tray Reaching Task, in which they were allowed to reach for 100 millet seeds placed in an inclined glass tray (2.5•2.5•7.5cm, with a 6mm tall front lip; Fig. 1A). Rehabilitation was restricted to the contralesional limb by aligning the edge of the tray with the lateral edge of the reaching slit, on the contralateral side of the reaching chamber. The close proximity of the reaching slit to the chamber wall (1.8cm) forced a reaching angle in which mice were only able to successfully obtain seeds with the contralateral paw.
Intracortical microstimulation
Mice underwent a terminal ICMS procedure 1-2 days following the final PMRT probe session (9 weeks post-infarct). Motor cortical representation areas were defined by the movements generated at the lowest stimulation thresholds, the approach traditionally used to characterize motor cortical maps in primates and rats[6, 7, 12] and which we’ve previously refined for mice[8, 11]. Aged mice were more sensitive to the effects of anesthesia, and received a smaller anesthetic dose for the procedure (young mice: 150mg/kg ketamine and 10mg/kg xylazine; aged mice: 100mg/kg ketamine and 4mg/kg xylazine, i.p.)
ICMS was conducted as previously described[8, 11]. A large craniotomy was made over the motor cortex. Intracortical penetrations with a glass microelectrode (15μm tip) were made at 790-800μm depths in 250μm increments, bordering in all forelimb responsive sites with non-forelimb or non-responsive sites. Penetrations were made throughout the typical extent of the mouse forelimb area[11] to ensure that all forelimb responsive sites were detected.
At each site, a 40ms train of 13 200μs monophasic cathodal pulses was delivered at 350Hz from an electrically isolated, constant current stimulator (BAK Electronics) at a rate of 1Hz. Stimulation was increased until a visible movement was evoked on the contralateral side of the body, up to a maximum of 100μA. We utilized a higher maximal current than our previous studies in intact mice (60μA)[8, 11] to compensate for potential dampening of cortical responses near the infarct[13]. The movement evoked at the lowest current (stimulation threshold) was recorded for each site. If no movement was seen at or below 100μA, the site was considered non-responsive.
Histology and infarct analysis
Immediately following the ICMS procedure, mice were euthanized with an overdose of sodium pentobarbital (175mg/kg, i.p.) and perfused intracardially with 0.1M phosphate buffer and 4% paraformaldehyde. Brains were post-fixed in paraformaldehyde and sliced on a vibratome into 50μm thick sections. Every sixth section was mounted onto gelatin-coated slides and Nissl stained with toluidine blue.
Coronal sections were viewed at a magnification of 50x. The cortical boundaries of 9 coronal sections per animal from approximately 2.0mm anterior to 1.2mm posterior to Bregma were traced using Neurolucida software. Cavalieri’s method was used to calculate total remaining cortical volume as the product of summed section areas and distance between sections[14]. Lesion volume was indirectly estimated as the difference between the volumes of the damaged and intact cortices. Remaining peri-infarct forelimb movement representation areas were estimated by comparing total map areas to previously published baseline cortical area values[8].
Statistics
SPSS software was used for all statistical analyses. A priori power analysis indicated that a sample size of 6 mice per group was necessary to detect significant effects in skilled reaching behavior and ICMS-evoked maps at 80% power with an α level of 0.05. Two-tailed t-tests were used to compare cortical volumes and interhemispheric volume differences between young and aged mice. A repeated-measures analyses of variance (ANOVAs was used to compared pre-operative learning curves of young and aged mice on the PMRT. Repeated-measures ANOVAs were conducted on post-operative behavioral data to determine 1) if either type of rehabilitative training improved reaching success on probe trials, relative to controls (PM-Rehab vs. No-Rehab and Tray-Rehab vs. No-Rehab), and 2) if one task was more effective in promoting improvement over the other (PM-Rehab vs. Tray-Rehab), using separate analyses for young and aged animals and bonferroni-corrected post-hoc comparisons. Young and aged performance on initial postoperative and post-rehabilitative training probe trials was compared with two-tailed t-tests. To directly assess the effect of age on behavioral improvement within each treatment condition, we used repeated-measures ANOVAs and bonferroni-corrected post-hoc tests to compare the change from initial post-operative performance of young and aged mice.
Two-tailed t-tests were conducted to determine whether 1) the remaining representation areas or ratio of proximal to distal forelimb representations were affected by either type of rehabilitative training (PM-Rehab vs. No-Rehab and Tray-Rehab vs. No-Rehab) and 2) if the two tasks resulted in differing cortical organization (PM-Rehab vs. Tray-Rehab). Data from the CFA and RFA, as well as young and old mice, were analyzed separately.
Results
Pre-operative learning on the PMRT
Pre-operative training was conducted 6 days per week for a total of 10 weeks, in order to ensure that both young and aged mice reached proficiency on the task prior to lesion induction (Fig. 1B). Young mice initially performed better on the task, and started at a higher success level on the first day of training (t(47)=5.99, p<0.001). A significant main effect of age (F(1,47)=20.61, p<0.001) and a day•age interaction (F(57,2679)=8.45, p<0.001) suggest that the pattern of learning differed between young and aged mice. This was especially evident in the first month of training. However, over the course of pre-operative training, aged mice were able to catch up to young mice. By the last three days of training, which were averaged to compute a baseline score for comparison to post-operative rehabilitative training, there was no significant difference between the age groups (t(47)=0.46, p=0.44). These results agree with previously published work from our lab, showing that aged mice are able to learn a new skilled reaching task to a similar extent as young mice, albeit more slowly[15].
Infarct analysis
ET-1 infusion resulted in significantly more tissue damage in aged mice compared to young mice (Fig. 2), despite a smaller ET-1 dose in aged mice (3μl vs. 4μl in young mice). There was no difference in the absolute volume of the contralesional hemisphere between young and aged mice (t(47)=0.29, p=0.77). However, the ipsilesional hemisphere was significantly smaller (t(47)=-3.92, p<0.001), and the interhemispheric volume differences were greater (t(47)=-2.87, p=0.006) for aged mice than for young mice (Fig. 2A). There was no significant difference in infarct size between the training conditions in young (F(2,24)=0.69, p=0.51) or aged animals (F(2,23)=0.74, p=0.49). Representative coronal sections from young and aged animals are shown in Fig. 2B.
Figure 2.
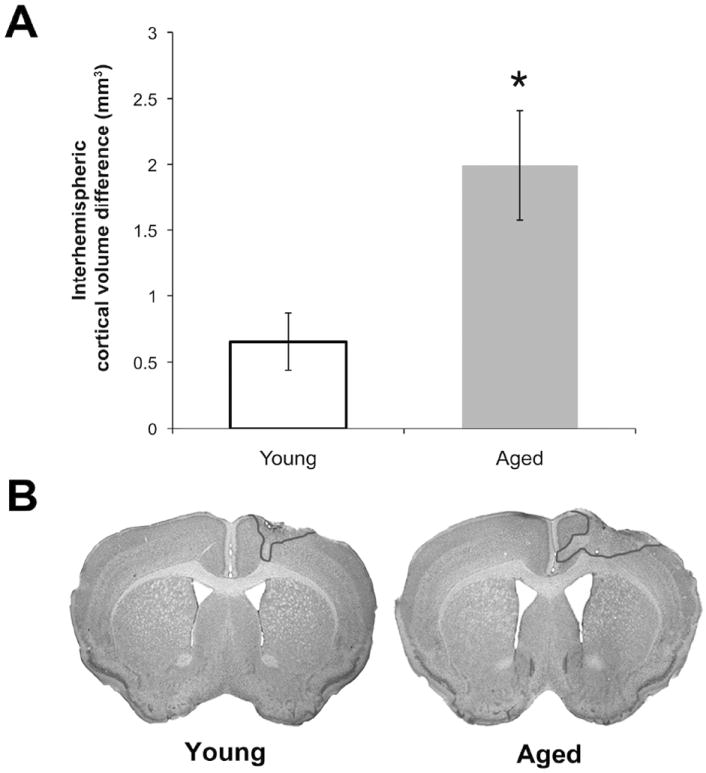
Endothelin-1 induced infarcts were larger in the aged mouse brain. (A) Interhemispheric volume differences were used to estimate infarct size. (B) Representative nissl-stained coronal sections from young (left) and aged (right) animals. The infarct is outlined in the right hemisphere in both cases. *p<0.05, Young vs. Aged. Data are means±S.E.
Rehabilitative training
Young and aged mice were equally as successful during rehabilitative training sessions on the PMRT, in which they “relearned” the previously acquired reaching task (Fig. 1C). There was a significant effect of rehabilitative training day (F(44,660)=9.14, p<0.001), but no significant effect of age (F(1,15)=0.58, p=0.46). A significant day•age interaction (F(44,660)=4.57, p<0.001) suggested that the pattern of relearning differed between young and aged mice. Although aged mice were initially more impaired on the PMRT after stroke, they quickly relearned the task and performed at a similar success level to young mice.
When mice received rehabilitative training on the untrained reaching task, Tray Reaching, we found a significant effect of rehabilitative training day (F(44,616)=3.65, p<0.001) and a significant day•age interaction (F(44,616)=3.28, p<0.001). There was a strong trend towards an effect of age, but this did not reach statistical significance (F(1,14)=4.53, p=0.052). The two groups had similar performance for the first month after stroke (Fig. 1D). Only after this time did young mice begin to surpass aged mice in successful performance on this task. Thus, while aged mice were able to successfully learn this new task, they were ultimately not as skilled on this task as young mice.
PMRT probe trials: Young mice
Initially, the three groups of young mice had similar post-operative deficits (Fig. 3A). Rehabilitative training on either reaching task began to improve success levels in the first training week. The greatest post-operative performance levels on the PMRT were achieved in mice that received rehabilitative training on this task. When the results of post-operative weekly probe trials were compared, there were significant overall improvements in reaching performance as a result of training on both the PMRT (F(1,15)=4.13, p=0.04, PM- vs. No-Rehab) and Tray Reaching Task (F(1,14)=4.70, p=0.048, Tray- vs. No-Rehab) compared with no-training controls. Neither analysis indicated significant day•group interactions (p’s=0.16 and 0.31, respectively). There was no significant group effect (F(1,15)=1.01, p=0.33, PM- vs. Tray-Rehab) or day•group interaction (F(1,150)=1.40, p=0.21) when the two types of training were directly compared. These results indicate that both reaching tasks were effective as rehabilitative training in young mice.
Figure 3.
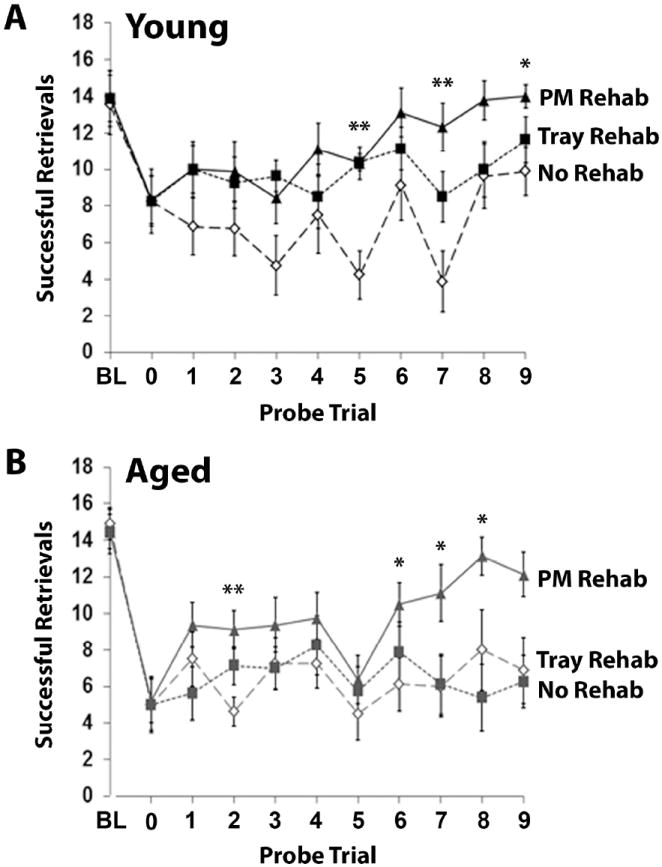
Relearning a complex motor skill after ischemia is dependent on the type of rehabilitative training provided. (A) Results of PMRT probe trials throughout the rehabilitative training period in young (A) and aged (B) mice. Successful retrievals indicate the number of pasta pieces broken and eaten per training session. BL=baseline. Data are means±S.E.
PMRT probe trials: Aged mice
Initially, all groups of aged mice had similar post-operative deficits (Fig. 3B). Like young mice, training on the PMRT in aged mice improved reaching success compared no training. While PMRT training significantly improved overall performance compared with no-training controls (F(1,14)=4.63, p=0.049, PM- vs. No-Rehab), Tray Reaching did not (F(1,14)<0.001, p=0.99, Tray- vs. No-Rehab). When the two types of training were compared, there was a significant group effect (F(1,14)=5.27, p=0.04, PM- vs. Tray-Rehab). None of the analyses revealed significant group•day interactions (p-values>0.05). These results indicate that only the PMRT was effective as rehabilitative training in aged mice.
Comparison of young and aged mice
Pre-operative performance on the PMRT was similar in young and aged mice (t(47)=-0.78, p=0.44). Aged mice, which had larger lesions than young mice, were more impaired in the first post-operative probe session (t(47)=2.85, p=0.007) and at the end of the 9 weeks of rehabilitative training compared to young mice (t(47)=2.91, p=0.005). To assess the effect of age on behavioral improvement in each rehabilitative training group, we directly compared young and aged mice for their change from baseline performance (Fig. 4). This revealed that there were no effects of age on reaching performance in the No-Rehab (Fig. 4A; F(1,14)=2.14, p=0.17) and PM-Rehab conditions (Fig. 4C; F(1,15)=2.12, p=0.17). However, there was a significant age difference in the effectiveness of Tray-Rehab (Fig. 4B; F(1,14)=7.64, p=0.02). There were no day•age interactions within any of the three rehabilitative training condition (p-values=0.16, 0.71, and 0.29, respectively). These results indicate that even though aged mice were initially more impaired and had more cortical damage than young mice, rehabilitative training on the Pasta Matrix Task was similarly effective across ages in promoting behavioral improvement. However, rehabilitative training on the Tray Reaching Task failed to induce sufficient behavioral improvement in aged mice, despite being relatively effective in younger mice.
Figure 4.
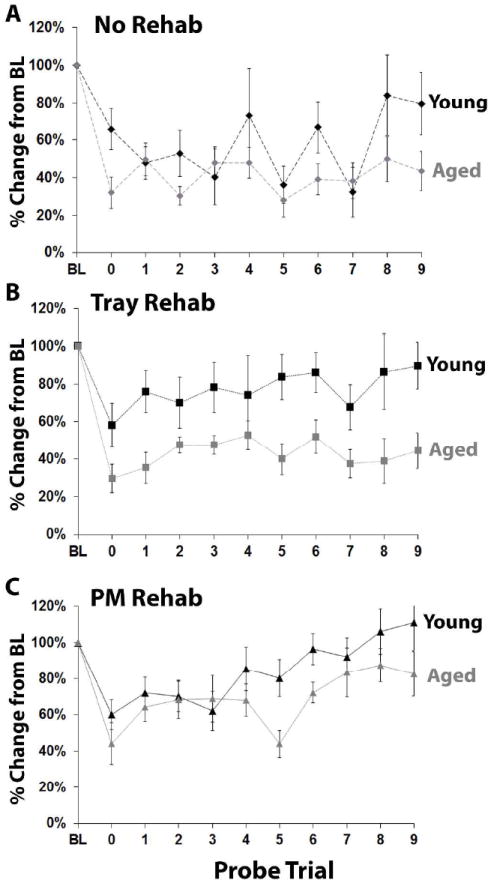
Rehabilitative training on the Pasta Matrix Task, but not the Tray Reaching Task, was similarly effective across ages in promoting behavioral improvement. (A-C) Results of PMRT probe trials comparing young and aged animals that received (A) no rehabilitation, (B) Tray Reaching rehabilitation, or (C) PMRT rehabilitation. There was a significant effect of age only in the Tray Rehab condition.
Intracortical microstimulation
All infarcts were within the boundaries of the CFA in the motor cortex. Notably, although aged mice had larger infarcts than young mice, both age groups retained a large amount of cortical map area (Table 1). As previously found[11], only a subset of mice had a discernible RFA, located just anterior to the CFA. The number of aged animals with an evident RFA (n=10 out of 24) was not different from that of young animals (n=12 out of 25; χ2(49)=0.20, p=0.90). However, all animals, including those without a discernable RFA (i.e. RFA area = 0 mm2), were included in the analyses of ICMS data.
Table 1.
Estimated remaining forelimb motor cortical representation areas (mm2) relative to intact brains
| Typical intact cortical area (mm2) | Post-infarct cortical area (mm2) | Estimated remaining cortical area (%) | |
|---|---|---|---|
| Young CFA | 1.49±0.08(n=35) | 0.87±0.09(n=25) | 58±6 |
| Aged CFA | 1.48±0.12(n=28) | 0.88±0.10(n=24) | 60±7 |
| Young RFA | 0.16±0.02(n=25) | 0.22±0.05(n=12) | 140±32 |
| Aged RFA | 0.24±0.03(n=23) | 0.17±0.04(n=10) | 70±15 |
All data are means±S.E. Typical intact cortical area values are from a previously published data set[8]. Remaining map percentages were estimated by dividing individual post-infarct areas by the intact mean.
Neither rehabilitative training strategy had a significant effect on the overall size of the remaining peri-infarct CFA in young mice (Fig. 5A; t(14)=1.40, p=0.18, PM- vs. No-Rehab; t(9)=-0.06, p=0.95, Tray- vs. No-Rehab). However, young mice that received rehabilitative training on the PMRT had a significantly larger RFA compared to mice that received no training (Fig. 5B; t(9)=-2.34, p=0.04). Tray reaching seemed to have an intermediate effect on RFA size, as this group was not significantly different from either the PM-Rehab (t(11)=-1.85, p=0.09) or No-Rehab (t(10)=0.67, p=0.52) groups. There were no group differences in the areas of digit, wrist, elbow or shoulder representations within either the CFA (t-statistics=-1.71-1.09, p-values=0.11-0.88) or RFA (t-statistics=-1.75-1.00, p-values=0.11-0.88), nor in the sum or ration of proximal and distal movement representations (CFA: t-statistics=-1.13–1.56, p-values= 0.13–0.71; RFA: t-statistics=-2.10–1.44, p-values=0.07–0.88).
Figure 5.
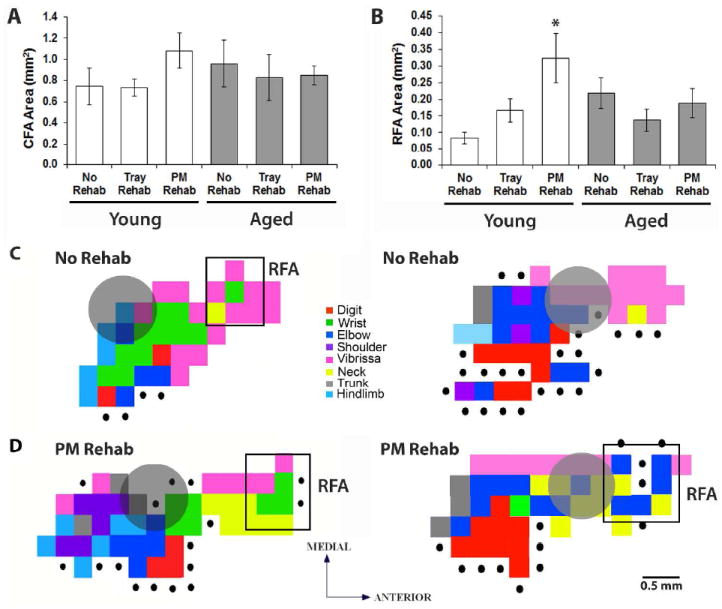
Rehabilitative training on the PMRT resulted in a larger RFA in young mice. (A) CFA areas across age and training conditions. (B) RFA areas across age and training conditions. (C) Representative ICMS-evoked forelimb representations from young mice that received no rehabilitative training (C) and rehabilitative training on the PMRT (D). Grey circles indicate the infarct location. *p<0.05 PM- vs. No-Rehab. Data are means±S.E.
Despite remarkable behavioral improvements following rehabilitative training, we failed to see reorganization of aged forelimb motor representations (Fig. 5A,B). There were no rehabilitative training effects on the overall area of the CFA (t(9)=-0.48, p=0.65, PM- vs. No-Rehab; t(13)=-0.42, p=0.68, Tray- vs. No-Rehab) or RFA (t(8)=-1.43, p=0.19, PM- vs. No-Rehab; t(11)=0.41, p=0.69, Tray- vs. No-Rehab). There were no group differences in digit, wrist, elbow or shoulder representations within either the CFA (t-statistics=-1.03-2.05, p-values=0.07-0.98) or RFA (t-statistics=-1.44-1.45, p-values=0.18-1.00) nor in the sum or ratio of proximal and distal representations (CFA: t-statistics=-0.81-1.65, p-values=0.13-0.85; RFA: t-statistics=-1.43-1.43, p-values=0.19-1.00). The direct comparison of young and aged motor maps is complicated by baseline differences in the size of RFA (Table 1; Tennant et al., 2012). Thus, even though there was a significant enlargement of RFA in young mice following rehabilitative training on the PMRT compared to young No Rehab controls, there was no difference in CFA or RFA size between young and aged mice when comparing within treatment groups (t-values=0.43-1.12, p-values=0.27-0.67). Our conclusion that reorganization is age-dependent is based on the evidence that rehabilitation resulted in map reorganization in young, but not aged, mice compared to age-matched controls.
Discussion
Following focal ischemic lesions, aged mice exhibit deficits similar to those seen in human stroke survivors, including impairments in coordinated and dexterous use of the contralesional upper extremity. Task-specific rehabilitation has been shown to improve motor function (i.e. dexterity, range of motion) of the affected arm and hand[16-19] and prevent compensation with the less-impaired side of the body[20, 21].Our results suggest that task-specific rehabilitative training may be an especially effective way to induce behavioral improvement of the upper extremities in older stroke survivors and drive beneficial plasticity in premotor areas of the younger brain.
Relearning skilled movements through rehabilitative training results in the maintenance and reorganization of surviving motor representations in young rats and primates[7, 22, 23]. In squirrel monkeys, lesions of the MI hand area result in enlargement of the hand representation within the ventral premotor cortex (PMv)[24]. Following lesions of both the MI and PMv, there is an enlargement of the hand representation in the supplementary area (SMA)[25]. Recent evidence implicates that similar plasticity is occurring in areas of the rodent brain that are analogous to premotor or supplementary motor areas of the primate brain. Gharbawie et al.[26] showed that rats with complete lesions of the motor area, inclusive of CFA and RFA, were more impaired on a skilled reaching task than animals that only had damage to the CFA, leaving RFA intact[26]. Zeiler et al.[27] found a reduction in inhibitory markers in the medial agranular cortex (AGm) following lesions of the CFA and post-operative retraining on a skilled reaching task. The deficits were reinstated by damaging the AGm after relearning had occurred, suggesting that other areas of the mouse brain, such as the RFA, also play a role in stroke recovery. Our finding that ICMS-evoked motor representations in the RFA expanded and reorganized in young mice following successful rehabilitative training further supports the idea that plasticity in non-primary motor areas is important for post-stroke behavioral improvement with rehabilitative training, at least in the young brain.
Although aged animals regained reaching ability following rehabilitative training on the PMRT, we failed to find any changes in ICMS-evoked motor maps. This could be explained by the larger lesions seen in aged brains resulting in greater damage to the areas that would normally compensate for lost functions. Interestingly, although aged mice had larger infarcts than young mice, they retained a similar amount of CFA representation area compared to typical intact representations areas[11]. This either suggests that much of the CFA was spared after ischemia or that the forelimb movement representations extended into peri-infarct tissue. If this second point is true, then cortical plasticity may not be as constrained in the aged brain as the lack of plasticity in RFA would imply. Alternatively, because reorganization of movement representations occurs late in the process of skill learning[28], it is feasible that plasticity in the aged RFA has not yet happened, but could occur following a longer duration of rehabilitative training. We have previously reported that, in the absence of brain damage, there is a dissociation between behavioral performance and cortical reorganization in aged animals[8]. It is not unheard of for a cortical area to undergo reorganization after brain injury and rehabilitative training, but not after motor skill learning in the absence of brain damage. In rats, reorganization of the RFA, which was not seen due to motor learning alone[12], could be resolved using long-duration ICMS after ischemic lesions of the CFA[29]. However, this does not seem to be the case in the aged brain. It is likely that motor learning or relearning in the aged brain is simply not reflected in the reorganization of movement representations as detected using the ICMS methods of this study. This hardly rules out the possibility of an age-related recruitment of other brain areas for acquisition or relearning of motor skills. In the human motor system, the aged brain is able to compensate for the slowed processing capabilities of primary motor areas by recruiting additional areas of the brain, including contralateral motor areas and ipsilateral sensory and cognitive areas, during performance of a motor task[30]. This same process could be occurring in the aged brain. Future studies will investigate learning- and stroke-induced plasticity occurring in other cortical and subcortical areas (such as the somatosensory cortex and thalamus) that are strongly interconnected to the motor cortex. Thus, although we did not see clear evidence of plasticity of the aged premotor cortex in the current study, it does not rule out the possibility that the aged brain retains plasticity in response to ischemia and rehabilitative training.
Overall, the results of this study show that while the aged brain may suffer more damage in response to ischemia, aged animals are able to regain a remarkable amount of motor function if provided with task-specific rehabilitative training. In contrast to young animals, in which rehabilitative training on a novel task, the Tray Reaching Task, produced some behavioral improvement, aged mice benefitted less from post-operative training on the Tray Reaching Task. This suggests that the ability to master new tasks after stroke or to generalize between motor tasks is lost or impaired during aging. Thus, in order for rehabilitative therapy to be effective in older stroke survivors, clinicians must take care to target therapy so that it is specific to the task being relearned.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NS056839 to TAJ) and the National Institute on Aging (F31AG034032 to KAT).
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soleman S, Yip P, Leasure JL, Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Experimental neurology. 2010 Mar;222(1):13–24. doi: 10.1016/j.expneurol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabilitation and neural repair. 2008 May-Jun;22(3):250–61. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alaverdashvili M, Whishaw IQ. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010 Apr 28;167(1):21–30. doi: 10.1016/j.neuroscience.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, Rosene DL. Recovery from ischemia in the middle-aged brain: a nonhuman primate model. Neurobiology of aging. 2012 Mar;33(3):619 e9–e24. doi: 10.1016/j.neurobiolaging.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of neurophysiology. 1996 May;75(5):2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 7.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996 Jun;272(5269):1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 8.Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, et al. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012 Sep 23; doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. Journal of neuroscience methods. 2009 Jun 30;181(1):18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10(3):269–89. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- 11.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011 Apr;21(4):865–76. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998 Dec;80(6):3321–5. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 13.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010 Dec;27(12):2221–32. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. Journal of anatomy. 1989 Dec;167:167–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, et al. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012 Oct;98(3):291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, et al. Critical decline in fine motor hand movements in human aging. Neurology. 1999 Oct;53(7):1458–61. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- 17.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000 Oct;31(10):2390–5. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 18.Williams BK, Galea MP, Winter AT. What is the functional outcome for the upper limb after stroke? Aust J Physiother. 2001;47(1):19–27. doi: 10.1016/s0004-9514(14)60295-6. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006 Nov;296(17):2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 20.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006 Apr;37(4):1045–9. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 21.Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009 Jul-Aug;16(4):237–53. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003 Dec;25(8):789–93. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 23.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003 Nov;28(11):1757–69. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 24.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003 Jun;89(6):3205–14. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 25.Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008 Sep;100(3):1498–512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharbawie OA, Karl JM, Whishaw IQ. Recovery of skilled reaching following motor cortex stroke: do residual corticofugal fibers mediate compensatory recovery? Eur J Neurosci. 2007 Dec;26(11):3309–27. doi: 10.1111/j.1460-9568.2007.05874.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, et al. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013 Feb;44(2):483–9. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004 Jan;24(3):628–33. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci U S A. 2006 Jul;103(30):11370–5. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008 Jan;28(1):91–9. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


