Abstract
Context
Relatively few data are available about symptoms among cancer patients.
Objectives
To describe the prevalence and severity of symptoms among a large, representative cohort of newly diagnosed cancer patients.
Methods
We collected survey data about symptoms (pain, fatigue, depression, nausea/vomiting, cough, dyspnea, diarrhea) from 5422 patients with incident lung and colorectal cancer from the diverse, nationally representative Cancer Care Outcomes Research and Surveillance (CanCORs) Consortium cohort. We described the prevalence of any symptoms and moderate/severe symptoms approximately four to six months following diagnosis. We used logistic regression to identify patient and clinical characteristics associated with symptoms, and calculated adjusted proportions of patients with symptoms.
Results
In total, 5067 (93.5%) patients reported at least one symptom in the four weeks before their survey, with 51% reporting at least one moderate/severe symptom. Lung cancer patients reported more symptoms than colorectal cancer patients. Patients who received treatment or had more comorbidities were more likely to report symptoms. For example, after adjustment, patients who received chemotherapy during the six weeks before the survey were more likely than others to report at least one symptom (97.3% vs. 90.8%, P<0.001), and at least one moderate/severe symptom (56.8% vs. 46.2%, P<0.001). After adjustment, early vs. late stage patients did not differ in reports of at least one symptom (93.6% vs. 93.4%, P=0.853) and differed only slightly in reports of at least one moderate/severe symptom (53.3% vs. 49.6%, P=0.009).
Conclusion
Most recently diagnosed lung and colorectal cancer patients have cancer-related symptoms regardless of stage, and more than half have at least one moderate/severe symptom.
Keywords: Cancer, symptoms, prevalence, colorectal neoplasms, lung neoplasms
Introduction
Quality of life is increasingly recognized as an important outcome for cancer patients both in research and in clinical practice (1–6), and quality of life is integrally related to the symptoms that patients experience. In classic medical training, symptoms are important because they provide subjective information that leads to the diagnosis of medical problems and treatment of disease (7). Often, treatment of disease is aimed at cure or prolonged survival, and resolution of symptoms is a valued byproduct of the treatment. However, in a patient-centered approach to disease, symptoms are the patient’s experience of a disease and, therefore, become more central in the overall treatment plan for the patient. This is especially important in cancer patients where symptoms are often caused by both the disease itself and the side effects and toxicities of treatment.
In 2002, a National Institutes of Health State of the Science Report on management of cancer symptoms, including pain, fatigue and depression, identified the need for research on the occurrence, assessment, and treatment of cancer symptoms occurring alone and together (8). Although several studies have assessed symptoms in a cancer population, most are relatively small and geographically limited (9–18). A systematic review of studies assessing symptoms in patients with incurable cancer not undergoing active treatment found that patients with advanced cancer described many symptoms, most notably pain and fatigue (3). A recent Canadian study linked routinely collected Edmonton Symptom Assessment System (ESAS) data with administrative data to describe one of the first estimates of symptom prevalence in a population-based cohort of cancer patients (19). Although this study filled important gaps in our knowledge by providing estimates of the prevalence of symptoms in a heterogeneous cohort of cancer patients attending an oncology clinic for treatment or survivorship care, it did not include data on stage of disease, timing of assessment relative to treatment, and other factors that may influence the burden of symptoms across the continuum of cancer care. Similarly, a 2013 study by Cleeland et al. assessed symptoms for patients presenting for an ambulatory clinic visit during any point in their disease trajectory using the M. D. Anderson Symptom Inventory (MDSI) (20).
Using data from the diverse, nationally representative Cancer Care Outcomes Research and Surveillance (CanCORs) study, we assessed the prevalence and severity of self-reported symptoms, including pain, fatigue, depression, nausea/vomiting, cough, dyspnea, and diarrhea, among patients approximately four to six months after diagnosis with lung or colorectal cancer. This multisite incident cohort provides the opportunity to analyze a large number of patients at approximately the same point in time of their disease trajectory. Using these data, we present prevalence data adjusted for patient characteristics, including stage of disease and types of treatments received.
Methods
Study Population
Data for this study were collected as part of the CanCORS study, a demographically representative national study of the care and outcomes experienced by approximately 10,000 patients diagnosed with lung or colorectal cancer between 2003 and 2005 (21–23). The CanCORS study enrolled patients from five geographic areas, including Alabama, Iowa, Los Angeles County, eight counties in northern California, and 22 counties in central and eastern North Carolina, five integrated health care delivery systems from the Cancer Research Network, and 15 Veteran Administration hospitals. Information about study design and procedures has been published previously (21, 23). The human subjects committees at all participating institutions approved the study.
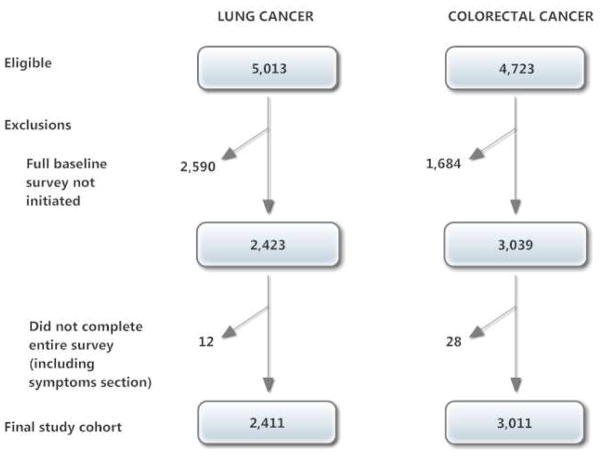
Patients aged 21 and older diagnosed with lung or colorectal cancer were identified within weeks of their diagnosis through rapid case ascertainment. Collaborative stage (24) at diagnosis was obtained from medical records (available for 87% of patients) or cancer registries if medical records were unavailable. Patients were considered late stage if they had stage IV colorectal cancer or stage IIIb or IV lung cancer. Patients (or their surrogates if they were deceased or too ill to participate) were interviewed by telephone approximately three to six months after diagnosis, after informed consent. Survey instruments were translated into Spanish and Chinese and administered by bilingual interviewers for patients who preferred these languages. The American Association for Public Opinion Research survey response rate was 51.0% (25) and the cooperation rate was 59.9%. We restricted this analysis to the 5422 patients who completed the full baseline interview themselves, as the brief and surrogate versions of the survey did not include questions about the patient’s symptoms (Fig. 1). Data collection procedures for the study of symptom prevalence soon after diagnosis of lung and colorectal cancer were established in advance of data collection, included in sections 8 and 9 of the patient survey, and approved by CanCORS investigators (21,26).
Fig. 1.
Flowchart for study cohort derived from CanCORS sampled patients.
Survey Instrument
Interviewers queried patients about the prevalence of symptoms including pain, fatigue, depressive symptoms, nausea/vomiting, cough, dyspnea, and diarrhea during the four weeks prior to the survey (survey instrument available at http://www.cancors.org/public).
Data on demographics (age, gender, race), language spoken at home, education, insurance status, wealth, marital status, comorbidity count (adapted from the self-administered Charlson Index and the comorbidity questions from the Prostate Cancer Outcomes Study [PCOS]) and treatment (receipt of chemotherapy, radiation, or surgery within the past six weeks) also were collected with the survey instrument (26,27). Wealth was assessed by asking the question, “If you lost all of your current sources of income and had to live off of your savings, how long could you continue to live at your current address and standard of living?”
Symptom Prevalence
Overall symptom prevalence was estimated using the purposefully low threshold of any report of a symptom during the four weeks before the patient survey. Those who screened positive for a symptom were queried further about symptom severity using validated scales: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) questions for nausea/vomiting, dyspnea, cough, and diarrhea (28–30); SF-36® vitality scale for fatigue (31); the Center for Epidemiologic Studies Depression Scale (CESD-8) for depressive symptoms (32, 33); and the Brief Pain Inventory (BPI) for pain (34). Dyspnea and cough questions were only asked of lung cancer patients and diarrhea questions were only asked of colorectal cancer patients (26).
Pain was considered present if the patient described either “pain beyond every day kinds of pain (minor headaches, sprains, and toothaches)” or “use of treatments or medications for pain.” Depressive symptoms were considered present if patients replied that they were experiencing any of the symptoms included in the CESD-8 (32). Fatigue was considered present if patients reported “a lot of energy” only some of the time (middle option) or less frequently using a five-point Likert scale. Patients were considered to have had nausea/vomiting, dyspnea, cough, or diarrhea in the past four weeks if they reported having at least “a little bit.”
Moderate to Severe Symptoms
The following criteria were used to classify symptoms as moderate to severe. For symptoms calculated from the SF-36 or EORTC items, we converted scores to a 0–100 range scale:
A score of 5 or greater (out of 10) on the BPI for pain (34, 35).
A score of 6 or greater on the CESD-8 for depressive symptoms (32, 33).
A score of less than 40 on a 0 to 100 range scale derived from the SF-36 vitality scale (31) for fatigue to indicate a value that is one standard deviation or more below published norms (36).
A score of greater than 66 on a 0 to 100 range scale for nausea and vomiting (from EORTC QLQ-C30) (28).
A score of greater than 66 on a 0 to 100 range scale for cough (from EORTC QLQ-LC13) (29).
A score of greater than 66 on a 0 to 100 range scale for dyspnea (from EORTC QLQ-LC13) (29).
A score of greater than 66 on a 0 to 100 range scale for moderate to severe diarrhea based on seven questions from the EORTC QLQ-CR29 including stool frequency during day and night, stool urgency, stool incontinence and other bothersome symptoms such as bloody stool, painful and difficult bowel movements (30).
Statistical Analysis
Patient Factors Associated With Symptom Prevalence and Severity
We conducted bivariate analyses to study the relationship between patient characteristics (age, race/language, gender, wealth, insurance status, cancer type, stage, comorbitity, marital status), treatment status, and symptom prevalence. We also tested whether timing of survey administration relative to diagnosis was associated with symptom prevalence. We used multivariable logistic regression to study patient factors associated with the patient reporting any symptom or any moderate to severe symptom in two separate models. Item non-response was less than 1% for variables used in our study with the exception of wealth, which had a 16% non-response rate. We used multiple imputations to account for missing data for the independent variables in the multivariable model (37). We calculated proportions of patients with symptoms for patient subgroups defined by each covariate, adjusted for all other covariates, by direct standardization under the regression model (38). Specifically, using the regression coefficients, we computed the predicted probability for each subject with and without the characteristics of interest, holding all other characteristics at their observed value. We report the average of these predicted probabilities. Data management and descriptive analyses were performed using SAS software, v. 9.3 (SAS Institute, Inc., Cary, NC), and modeling using the multiply-imputed data was conducted in Stata 12 (StataCorp LP, College Station, TX).
Results
Study Population
The 5422 patients who completed the symptom survey comprised 51% of the entire CanCORS cohort (Fig. 1) and were similar to the overall demographically representative cohort except that they were less likely to have lung cancer and more likely to have earlier stage cancer (23).
Prevalence of Any Symptoms and Moderate to Severe Symptoms
Overall, 5067 (93.5%) patients in our analytic cohort reported at least one symptom (pain, fatigue, depression, nausea/vomiting, cough, dyspnea, diarrhea) of any severity in the four weeks prior to the survey, and for 50.7% of our analytic cohort, at least one of these symptoms was moderate or severe. The prevalence of symptoms of any severity for patients with lung cancer ranged from 32.9% for nausea/vomiting in early-stage patients to 84.1% for cough in late-stage patients. The prevalence of symptoms among colorectal cancer patients ranged from 32.4% with nausea/vomiting in early-stage patients to 79% for depressive symptoms in late-stage patients (Table 1).
Table 1.
Symptoms in Early and Late Stage Lung and Colorectal Cancer (N=5422)
| Symptom | Presence of Symptom N (%) | |||
|---|---|---|---|---|
| Lung Cancer (N=2411) | Colorectal Cancer (N=3011) | |||
| Early Stage (N=1295) | Late Stage (N=1116) | Early Stage (N=2426) | Late Stage (N=585) | |
| Any Symptom | 1272 (98.2%)a | 1100 (98.6%) b | 2151 (88.7%) a, c | 544 (93.0%)b, c |
| Pain | 756 (58.4%) a | 650 (58.2%) b | 939 (38.7%)a, c | 269 (46.0%)b, c |
| Fatigue | 964 (74.4%) a, c | 885 (79.3%)b, c | 1519 (62.6%) a, c | 402 (68.7%)b, c |
| Depressive symptoms | 1030 (79.5%)a, c | 935 (83.8%) c | 1717 (70.8%) a, c | 462 (79.0%) c |
| Nausea/vomiting | 426 (32.9%) c | 481 (43.1%) c | 786 (32.4%) c | 276 (47.2%) c |
| Cough | 1055 (81.5%) | 939 (84.1%) | N/A | N/A |
| Dyspnea | 1052 (81.2%) | 890 (79.8%) | N/A | N/A |
| Diarrhea | N/A | N/A | 1015 (41.8%) | 272 (46.5%) |
| Total | 6555 | 5880 | 8127 | 2225 |
P<0.01 Comparison between early stage lung and early stage colorectal.
P<0.01 Comparison between late stage lung and late stage colorectal.
P<0.01 Comparison between early stage and late stage within cancer type.
The prevalence of symptoms reported to be moderate to severe varied with cancer type and by stage (Table 2). Moderate to severe cough was frequently reported among lung cancer patients with both early-stage (39.6%) and late-stage (44.5%) disease. Moderate to severe pain was present in 18.4% of lung cancer and 12.4% of colorectal cancer patients. Moderate to severe fatigue was more prevalent in lung cancer than colorectal cancer patients in early stage (36.9% v. 24.7%) and late stage (43.4% v. 28.6%). Moderate to severe depressive symptoms occurred in 15% of patients. Moderate to severe nausea was infrequent (6% overall) (Table 2).
Table 2.
Moderate to Severe Symptoms in Early and Late Stage Lung and Colorectal Cancer (N=5422)
| Symptom | Symptom is Moderate/Severe N (%) | |||
|---|---|---|---|---|
| Lung Cancer (N=2411) | Colorectal Cancer (N=3011) | |||
| Early Stage (N=1295) | Late Stage (N=1116) | Early Stage (N=2426) | Late Stage (N=585) | |
| Any Symptom | 851 (65.7%) a, b | 820 (73.5%) a, c | 840 (34.6%) a, b | 240 (41.0%) a, c |
| Pain | 220 (17.0%) b | 223 (20.0%) | 278 (11.5%) a, b | 94 (16.1%) a |
| Fatigue | 478 (36.9%) a, b | 484 (43.4%) a, c | 598 (24.7%) b | 167 (28.6%) c |
| Depressive symptoms | 227 (17.5%) b | 209 (18.7%) | 306 (12.6%) b | 82 (14.0%) |
| Nausea/vomiting | 79 (6.1%) a | 102 (9.1%) a | 104 (4.3%) a | 40 (6.8%) a |
| Cough | 513 (39.6%) | 497 (44.5%) | N/A | N/A |
| Dyspnea | 289 (22.3%) | 273 (24.5%) | N/A | N/A |
| Diarrhea | N/A | N/A | 29 (1.2%) | 11 (1.9%) |
| Total | 2657 | 2608 | 2155 | 634 |
P<0.01 Comparison between early stage and late stage within cancer type.
P<0.01 Comparison between early stage lung and early stage colorectal.
P<0.01 Comparison between late stage lung and late stage colorectal.
Patient Factors Associated with Symptom Prevalence
In bivariate analyses, symptoms were significantly more likely to be reported for female, younger, unmarried, less educated, less wealthy, uninsured patients; patients with more comorbidity; and for those who recently received treatment or those with late stage or lung cancer (Table 3). Moderate to severe symptoms also were more likely to be reported in these patient subgroups as well as among Hispanic or Latino patients. There was no significant difference between the timing of the survey in relation to diagnosis and having any symptom or any moderate to severe symptom (data not shown).
Table 3.
Unadjusted Prevalence and Severity for at Least One Symptom by Patient Characteristics
| N=5422 | Patient Characteristics | Any Symptom | Any Moderate to Severe Symptom | ||
|---|---|---|---|---|---|
| N (%) | % | P-value | % | P-value | |
| Gender | |||||
| Male | 2888 (53.3%) | 92.6% | 47.5% | ||
| Female | 2534 (46.7%) | 94.5% | 0.004 | 54.4% | <0.001 |
| Age (yrs) | |||||
| 21–59 | 1840 (33.9%) | 95.7% | 55.0% | ||
| 60–69 | 1584 (29.2%) | 93.1% | 51.6% | ||
| 70–79 | 1424 (26.3%) | 91.4% | 47.8% | ||
| 80+ | 574 (10.6%) | 92.3% | <0.001 | 42.0% | <0.001 |
| Race | |||||
| White | 3870 (69.7%) | 93.3% | 50.9% | ||
| Hispanic or Latino | 371 (6.8%) | 93.5% | 55.0% | ||
| African American | 726 (13.4%) | 94.8% | 51.7% | ||
| Other | 545 (10.1%) | 93.0% | 0.48 | 45.3% | 0.02 |
| Education | |||||
| Less than high school | 919 (16.9%) | 94.5% | 56.7% | ||
| High school/some college | 3153 (58.2%) | 94.2% | 52.9% | ||
| College degree or more | 1343 (24.8%) | 91.0% | <0.001 | 41.7% | <0.001 |
| (Missing) | 7 (0.1%) | ||||
| Insurance status | |||||
| VA | 634 (11.7%) | 94.2% | 60.7% | ||
| Private | 1910 (35.2%) | 94.2% | 48.7% | ||
| Supplemental | 1996 (36.8%) | 92.1% | 46.7% | ||
| Public | 622 (11.5%) | 93.1% | 56.6% | ||
| None | 214 (3.9%) | 97.7% | 0.007 | 57.0% | <0.001 |
| (Missing) | 46 (0.8%) | ||||
| Wealth (how much money saved to live at current cost of living) | |||||
| < 1 month | 1034 (19.1%) | 96.4% | 63.7% | ||
| 1–2 months | 568 (10.5%) | 96.3% | 62.3% | ||
| 3–6 months | 574 (10.6%) | 95.1% | 51.6% | ||
| 7–12 months | 359 (6.6%) | 95.5% | 49.0% | ||
| More than one year | 2019 (37.2%) | 90.7% | <0.001 | 42.1% | <0.001 |
| (Missing) | 868 (16.0%) | ||||
| Marital Status | |||||
| Not married | 2259 (41.7%) | 94.7% | 56.2% | ||
| Married | 3160 (58.3%) | 92.6% | 0.001 | 46.8% | <0.001 |
| (Missing) | 3 (0.1%) | ||||
| Cancer Type | |||||
| Lung | 2411 (44.5%) | 98.4% | 69.3% | ||
| Colorectal | 3011 (55.5%) | 89.5% | <0.001 | 35.9% | <0.001 |
| Stage | |||||
| Early | 3721 (68.6%) | 92.0% | 45.4% | ||
| Late | 1701 (31.4%) | 96.7% | <0.001 | 62.3% | <0.001 |
| Surgery in last 6 weeks | |||||
| Not received | 5204 (96.0%) | 93.4% | 50.4% | ||
| Received | 218 (4.0%) | 95.0% | 0.36 | 59.6% | 0.007 |
| Radiation in last 6 weeks | |||||
| Not received | 4808 (88.7%) | 92.8% | 47.9% | ||
| Received | 614 (11.3%) | 98.7% | <0.001 | 73.3% | <0.001 |
| Chemotherapy in last 6 weeks | |||||
| Not received | 3085 (56.9%) | 90.4% | 45.1% | ||
| Received | 2337 (43.1%) | 97.4% | <0.001 | 58.2% | <0.001 |
| Comorbidity | |||||
| 0–1 | 3286 (60.6%) | 91.7% | 42.9% | ||
| 2+ | 2125 (39.2%) | 96.2% | <0.001 | 62.8% | <0.001 |
| (Missing) | 11 (0.2%) | ||||
In multivariable analyses controlling for patient characteristics, female patients and those who had less wealth, had lung vs. colorectal cancer, patients with two or more comorbidities and patients recently treated with chemotherapy or surgery were more likely than others to report any symptoms (Table 4). These same factors as well as younger age, less education, Hispanic or Latino ethnicity, non-private insurance, being unmarried, early vs. late stage cancer, and reporting recent radiation were associated with reporting moderate or severe symptoms. Many of these differences, although statistically significant, reflected relatively small absolute differences in adjusted proportions, whereas others had greater absolute differences (Table 4). After adjustment, there was no difference in report of at least one symptom among early vs. late stage patients (93.6% vs. 93.4%, P=0.85), and only a small difference in report of at least one moderate to severe symptom (53.3% vs. 49.6%, P=0.01). However, controlling for other variables, patients who received chemotherapy during the six weeks before the survey were more likely to report at least one symptom (97.3% vs. 90.8%, P<0.001), and at least one moderate/severe symptom (56.8% vs. 46.2%, P<0.001). Lung cancer patients were much more likely than colorectal cancer patients to report moderate to severe symptoms (66.3% vs. 38.5%, P<0.001), as were patients with two or more vs. 0–1 comorbidities (60.4% vs. 44.5%, P<0.001).
Table 4.
Adjusted Rates of Symptoms in Patients
| N=5422 | Any Symptom | Any Moderate to Severe Symptom | ||
|---|---|---|---|---|
| Adjusted Proportions for Patients with Any Symptoms | P-value | Adjusted Proportions for Patients with Any Moderate to Severe Symptoms | P-value | |
| Gender | ||||
| Male (reference) | 92.7% | 47.2% | ||
| Female | 94.3% | 0.02 | 54.7% | <0.001 |
| Age (yrs) | ||||
| 21–59 | 95.3% | 0.31 | 57.7% | <0.001 |
| 60–69 | 92.5% | 0.17 | 49.3% | 0.04 |
| 70–79 | 91.7% | 0.03 | 45.7% | 0.61 |
| 80+ (reference) | 94.2% | 44.6% | ||
| Race | ||||
| White (reference) | 93.4% | 51.1% | ||
| Hispanic or Latino | 93.4% | 0.97 | 56.2% | 0.04 |
| African American | 93.9% | 0.61 | 48.5% | 0.17 |
| Other | 93.3% | 0.93 | 47.5% | 0.09 |
| Education | ||||
| College degree or more (reference) | 92.6% | 48.2% | ||
| High school/some college | 93.8% | 0.12 | 51.4% | 0.04 |
| Less than high school | 93.9% | 0.23 | 52.3% | 0.05 |
| Insurance status | ||||
| Private (reference) | 93.4% | 48.3% | ||
| VA | 93.4% | 0.95 | 58.3% | <0.001 |
| Supplemental | 93.6% | 0.90 | 50.3% | 0.31 |
| Public | 92.4% | 0.41 | 53.0% | 0.05 |
| None | 96.4% | 0.16 | 48.4% | 0.99 |
| Wealth (how much money saved to live at current cost of living) | ||||
| More than one year (reference) | 91.9% | 46.2% | ||
| 7–12 months | 95.4% | 0.03 | 50.5% | 0.10 |
| 3–6 months | 94.4% | 0.06 | 51.0% | 0.04 |
| 1–2 months | 95.1% | 0.03 | 56.2% | <0.001 |
| < 1 month | 95.3% | 0.001 | 56.9% | <0.001 |
| Marital Status | ||||
| Not married (reference) | 94.0% | 53.2% | ||
| Married | 93.1% | 0.23 | 49.0% | 0.001 |
| Cancer Type | ||||
| Colorectal (reference) | 90.2% | 38.5% | ||
| Lung | 98.2% | <0.001 | 66.3% | <0.001 |
| Stage | ||||
| Early (reference) | 93.4% | 49.6% | ||
| Late | 93.6% | 0.85 | 53.3% | 0.01 |
| Surgery in last 6 weeks | ||||
| Not received (reference) | 93.3% | 50.0% | ||
| Received | 96.2% | 0.05 | 65.6% | <0.001 |
| Radiation in last 6 weeks | ||||
| Not received (reference) | 93.3% | 49.5% | ||
| Received | 96.2% | 0.08 | 61.0% | <0.001 |
| Chemotherapy in last 6 weeks | ||||
| Not received (reference) | 90.8% | 46.2% | ||
| Received | 97.3% | <0.001 | 56.8% | <0.001 |
| Comorbidity | ||||
| 0–1 | 92.0% | 44.5% | ||
| 2+ | 95.9% | <0.001 | 60.4% | <0.001 |
Discussion
This is the first, large scale study to report estimates of the prevalence of symptoms in a nationally representative incident cohort of cancer patients. Strikingly, we found that almost all patients – 93.5% – reported experiencing symptoms in the past four weeks, with more than half experiencing moderate to severe symptoms. Cancer symptoms are known to be prevalent among patients with advanced cancers who are dying, yet we found that symptoms were consistently prevalent in the six months following diagnosis across all stages of cancer in association with most treatments, and even among untreated patients
Although we found many patient factors statistically associated with the presence of symptoms or moderate to severe symptoms, the absolute difference in adjusted proportions varied. Some factors, such as recent receipt of cancer-directed treatment, having two or more comorbidities, younger age, less wealth and lung vs. colorectal cancer were associated with a greater than 10 percentage point difference in adjusted prevalence of symptoms, suggesting that these factors are more clinically significant compared with other factors such as stage, education and marital status.
Although receipt of recent treatment was one of the most influential patient factors associated with moderate to severe symptoms, more than 40% of patients not receiving treatment reported at least one such symptom. The high rates of symptom burden in all patients emphasize the pervasive need for symptom management and palliation throughout the trajectory of disease.
In the setting of limited time during routine follow-up visits for most cancer patients, the development of tools to systematically screen for symptoms and protocols to enable assessment outside the clinic visit warrant further evaluation. The ESAS has been incorporated into routine care in Ontario and findings to date are positive and suggest that routine screening with rapid cycle change quality improvement processes may improve symptom management for cancer patients. However, there has been some resistance by physicians to incorporating such models into care, because of concerns about efficiency and time constraints (39–41). Other innovative methods of symptom monitoring that may be more suitable for physician work flow include nurse-assisted and automated telephone symptom management tools, both of which have been shown to reduce symptom severity for patients (42–44) and may be cost saving (45).
Patient-reported data are increasingly being used to evaluate and improve the quality of supportive care for patients with cancer (46). Prospectively monitoring symptoms and other patient reported outcomes in clinical care and research is likely to lead to a better understanding of the patient experience of disease and help guide interventions for improvement that will have an important impact on quality of life for patients (47,48).
Consistent with a recent Canadian study (19), we found fatigue was prevalent in three-quarters of our cohort, pain was present in about half of the patients, and nausea and vomiting was present in less than half of the patients. Depressive symptoms as well as cough and dyspnea among lung cancer patients also were highly prevalent. Unadjusted prevalence rates for moderate to severe fatigue, pain and depressive symptoms also were similar to those rates found in Cleeland et al.’s study (20).
Controlling for other variables, in our cohort of newly diagnosed patients, we found that recent treatment with chemotherapy was associated with a higher adjusted prevalence for moderate to severe symptoms (56.8% vs. 46.2%, P<0.001). It is also interesting to note that a common side effect of chemotherapy, nausea, was infrequently moderate to severe (6%), which may reflect highly effective prophylactic pharmacological interventions (49). Further work should investigate if a higher prevalence of symptoms among patients receiving tumor-directed therapy could be addressed with available prophylactic and therapeutic regimens. This reflects a target area for quality improvement.
The strengths of this study include identifying symptom burden at a defined time in the patient’s disease course with validated tools for symptom assessment among a large nationally representative cohort of cancer patients. In order to increase the reliability of patient self-reports on their symptom experiences and to limit recall bias, we only asked about symptoms in the four weeks prior to the survey (50,51). Therefore, this is only a snapshot of the prevalence of symptoms in a cohort of cancer patients for a period of one month, suggesting that over the entire trajectory of a cancer patient’s experience, symptom prevalence is higher. Also, because this was part of a larger survey with many research objectives, there was limited opportunity to include questions related to symptom prevalence in the survey design. Therefore, not all symptoms are addressed here; for example, rates of dyspnea were studied in lung but not in colorectal cancer patients, and other symptoms such as constipation were not assessed. In addition, these estimates likely underestimate the overall prevalence of symptoms in the population, as patients who were too ill to participate or who died shortly after diagnosis did not provide data on their experiences.
Understanding the prevalence and severity of symptoms that patients face across the continuum of cancer and which patients are at higher risk for these symptoms is a critical first step to identifying the needs of this population. Prevalence data can inform efforts to implement strategies for management of distressing symptoms and inform efforts to transform the health delivery system to deliver high quality patient-centered care. We found that symptoms were nearly universal in this population-based cohort of patients with newly diagnosed lung and colorectal cancer, suggesting that efforts to better manage symptoms should be of high priority.
Acknowledgments
This work was supported by the American Cancer Society grant number (119663-RSG-10–176–01-PCSM). The Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center at the Dana-Farber Cancer Institute (U01 CA093344) and the Primary Data Collection and Research Centers at the Harvard Medical School and Northern California Cancer Center (U01 CA093324); Dana-Farber Cancer Institute and Cancer Research Network (U01 CA093332); RAND and University of California, Los Angeles (U01 CA093348); University of Alabama at Birmingham (U01 CA093329); University of Iowa (U01 CA093339); University of North Carolina (U01 CA093326); and by a Department of Veteran Affairs grant to the Durham VA Medical Center (U01 CDA093344 (MOU) and HARQ 03-438MO-03). Dr. Keating’s effort also was funded by the National Institutes of Health (NIH) (R011CA164021- 01A1). Dr. Walling also was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number (UL1TR000123).
Footnotes
Some of the preliminary data from this study were presented at the American Academy of Hospice and Palliative Medicine Annual Meeting in March 2012.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the ACS, NCI or the NIH.
Drs. Walling, Kahn, Tisnado, Keating, Dy, Arora, and Mack and Mr. Pantoja have no conflicts of interest to disclose. Dr. Malin is currently employed at Wellpoint, Inc. and has stock ownership in same.
The authors dedicate this paper to Dr. Jane C. Weeks, who passed away prior to submission of this paper; her contributions were substantial and very much appreciated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arora NK. Importance of patient-centered care in enhancing well-being from a cancer survivor’s perspective. Qual Life Res. 2009;18:1–4. doi: 10.1007/s11136-008-9415-5. [DOI] [PubMed] [Google Scholar]
- 2.Oliver A, Greenberg CC. Measuring outcomes in oncology treatment: the importance of patient centered outcomes. Surg Clin N Am. 2009;89:17–25. doi: 10.1016/j.suc.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality. Evidence report/Technology assessment. 61. Rockville, MD: U.S. Department of Health and Human Services; 2002. Management of cancer symptoms: pain, depression, and fatigue. [Google Scholar]
- 5.Liao Y, Liao W, Shun S, et al. Symptoms, psychological distress, and supportive care needs in lung cancer patients. Support Car Cancer. 2011;19:1743–1751. doi: 10.1007/s00520-010-1014-7. [DOI] [PubMed] [Google Scholar]
- 6.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–179. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 7.Weed LL. The problem oriented record as a basic tool in medical education, patient care and clinical research. Ann Clin Res. 1971;3:131–134. [PubMed] [Google Scholar]
- 8.Patrick DI, Ferkerick SI, Frame PS, et al. National Institutes of Health State of the Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J, Malin JK, Tao ML, et al. Symptoms after breast cancer treatment: are they influenced by patient characteristics? Breast Cancer Res Treat. 2008;108:153–165. doi: 10.1007/s10549-007-9599-3. [DOI] [PubMed] [Google Scholar]
- 10.Chang VT, Hwang SS, Feuman M, et al. Symptom and quality of life survey of medical oncology patients at a Veterans affairs medical center: a role for symptom assessment in cancer. Cancer. 2000;88:1175–1183. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9:372–382. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 12.Coyle N, Adelhardt, Foley KM, et al. Character of terminal illness in the advanced cancer patient: pain and other symptoms during the last four weeks of life. J Pain Symptom Manage. 1990;5:83–93. doi: 10.1016/s0885-3924(05)80021-1. [DOI] [PubMed] [Google Scholar]
- 13.Hoekstra J, Vernooj-Dassen MJ, de Vos R, Bindels PJ. The added value of assessing the ‘most troublesome’ symptom among patients with cancer in the palliative phase. Patient Educ Couns. 2007;65:223–229. doi: 10.1016/j.pec.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi A, Morita T, Miyaghita M, et al. Symptom prevalence and longitudinal follow-up in cancer patients receiving chemotherapy. J Pain Symptom Manage. 2009;37:823–830. doi: 10.1016/j.jpainsymman.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Lidstone V, Bullers E, Seed PT, et al. Symptoms and concerns amongst cancer outpatients: identifying the need for specialist palliative care. Palliat Med. 2003;17:588–595. doi: 10.1191/0269216303pm814oa. [DOI] [PubMed] [Google Scholar]
- 16.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics, and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 17.Tranmer JE, Heyland D, Dudgeon D, et al. Measuring the symptom experience of seriously ill cancer and noncancer hospitalized patients near the end of life with the Memorial Symptom Assessment Scale. J Pain Symptom Manage. 2003;25:420–429. doi: 10.1016/s0885-3924(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 18.Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO guidelines: a longitudinal follow-up study of symptom prevalence, severity, and etiology. Pain. 2001;93:247–257. doi: 10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 19.Barbera L, Seow H, Howell D, et al. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119:4333–4340. doi: 10.1002/cncr.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. [Last accessed May 20, 2014];Cancer Care Outcomes Research and Surveillance Consortium. Available from http://healthservices.cancer.gov/cancors/
- 23.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the Cancer Care Outcomes Research and Surveillance (CanCORS) consortium relative to Surveillance, Epidemiology and End Results (SEER) program. Med Care. 2012;50:277–362. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Joint Committee on Cancer. AJCC cancer staging atlas. Chicago, IL: Spingerlink; 2006. [Google Scholar]
- 25.American Association for Public Opinion Research. [Last accessed May 20, 2014];Standard definitions. Available from http://www.aapor.org/Standard_Definitions2.htm.
- 26.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 27.Tisnado DM, Adams JL, Liu H, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44:132–140. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 29.European Organization for Research and Treatment of Cancer. [Last accessed May 20, 2014];EORTC QLQ-LC13. Available from http://groups.eortc.be/qol/sites/default/files/img/specimen_lc13_english.pdf.
- 30.European Organization for Research and Treatment of Cancer. [Last accessed May 20, 2014];EORTC QLQ-CR29. Available from http://groups.eortc.be/qol/sites/default/files/img/specimen_cr29_english.pdf.
- 31.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 32.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53:1117–1125. [Google Scholar]
- 33.Keating NL, Landrum M, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28:4364–4370. doi: 10.1200/JCO.2009.26.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 35.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 36.Ware J. SF-36 survey update. [Last accessed May 20, 2014];Table 1: Summary of information about SF-36 scales and physical and mental health component summary measures. Available from http://www.sf-36.org/tools/sf36.shtml.
- 37.He Y, Zaslavsky AM, Harrington DP, et al. Imputation in a multiformat and multiwave survey of cancer care. Proceedings in Health Policy Statistics; American Statistical Association; 2007. pp. 1541–1549. [Google Scholar]
- 38.Little RJA. Direct standardization: a tool for teaching linear models for unbalanced data. Am Stat. 1982;36:38–43. [Google Scholar]
- 39.Bainbridge D, Seow H, Sussman J, et al. Multidisciplinary health care professionals perceptions of the use and utility of a symptom assessment system for oncology patients. J Oncol Pract. 2011;7:19–23. doi: 10.1200/JOP.2010.000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert JE, Howell D, King S. Quality improvement in cancer symptom assessment and control: the Provincial Palliative Care Integration Project (PPCIP) J Pain Symptom Manage. 2012;43:663–678. doi: 10.1016/j.jpainsymman.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Dudgeon D, King S, Howell D, et al. Cancer Care Ontario’s experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21:357–364. doi: 10.1002/pon.1918. [DOI] [PubMed] [Google Scholar]
- 42.Sikorskii A, Given CW, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Head BA, Keeney C, Studts J, et al. Feasibility and acceptance of a telehealth intervention to promote symptom management during treatment for head and neck cancer. J Support Oncol. 2011;9:e1–e11. doi: 10.1016/j.suponc.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroenke K, Theobald D, Wu J, et al. Effect of a telecare management on pain and depression in patients with cancer. JAMA. 2010;304:163–171. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Given CW, Bradley C, You M, et al. Costs of novel symptom mangement interventions and their impact on hospitalizations. J Pain Symptom Manage. 2010;39:663–672. doi: 10.1016/j.jpainsymman.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dy SM, Walling AM, Mack JW, et al. Evaluating the quality of supportive oncology using patient-reported data. J Oncol Pract. 2014 Mar 11; doi: 10.1200/JOP.2013.001237. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hay JL, Atkinson TM, Reeve BB, et al. Cognitive interviewing of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Qual Life Res. 2014;23:257–269. doi: 10.1007/s11136-013-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 49.Schwartberg LS, Grunberg SM, Kris MG. Recent advances and updated guidelines in the management of chemotherapy-induced nausea and vomiting. Clin Adv Hematol Oncol. 2011;9:1–14. [PubMed] [Google Scholar]
- 50.Sudman S, Bradburn NM. Response effects in surveys. Chicago, IL: Aldine; 1974. [Google Scholar]
- 51.Andersen RM, Kasper J, Frankel MR, et al. Applications to improve health surveys. San Francisco, CA: Jossey-Bass; 1979. Total survey error. [Google Scholar]