Abstract
In the bone marrow (BM), hematopoietic progenitors (HP) reside in specific anatomical niches near osteoblasts (Ob), macrophages (MΦ) and other cells forming the BM microenvironment. A connection between immunosurveillance and traffic of HP has been demonstrated but the regulatory signals that instruct immune regulation on HP circulation are unknown. We discovered that the BM microenvironment deficiency of p62, an autophagy regulator and signal organizer, results in loss of autophagic repression of macrophage contact-dependent activation of Ob NF-κB signaling. Consequently, Ob p62-deficient mice lose bone, Ob Ccl4 expression and HP chemotaxis towards Cxcl12 resulting in egress of short-term hematopoietic stem cells and myeloid progenitors. Finally, Ccl4 expression and myeloid progenitor egress are reversed by the deficiency of the p62 PB1 binding partner Nbr1. A functional ‘MΦ-Ob niche’ is required for myeloid progenitor/short-term stem cell retention, in which Ob p62 is required to maintain NF-κB signaling repression, osteogenesis and BM progenitor retention.
INTRODUCTION
Steady-state blood formation during most adulthood depends on long-lived hematopoietic progenitors (HP) (Sun, 2014). Constitutive egress of bone marrow (BM)-resident HP into the blood is a well-established phenomenon. Circulating HP can survey peripheral organs and foster the local production of tissue-resident innate immune cells under both steady-state conditions and in response to inflammatory signals (Baldridge et al., 2010; Essers et al., 2009; Massberg et al., 2007). Dysregulation of stromal components of the HP niches within the BM, such as changes in the levels of chemokines from osteoblasts (Ob) and other mesenchymal cells, has been associated with HP egress (Ding and Morrison, 2013; Greenbaum et al., 2013; Mendez-Ferrer et al., 2010; Omatsu et al., 2010; Petit et al., 2002; Sugiyama et al., 2006; Visnjic et al., 2004). Specifically, the deletion of the major hematopoietic stem cell and progenitor (HSC/P) traffic regulator Cxcl12 (Peled et al., 1999; Peled et al., 2000) from Cxcl12-abundant reticular cells and Ob, results in constitutive HP mobilization and a loss of B-lymphoid progenitors, while their HSC function is normal (Greenbaum et al., 2013). Physiological regulation of these mesenchymal components modulates HP trafficking, and is afforded by several mechanisms, including signals derived from BM-resident macrophages (MΦ) (Casanova-Acebes et al., 2013; Chow et al., 2011; Christopher et al., 2011; Winkler et al., 2010). Cellular cross-talk between MΦ and Ob in the HP niche may critically regulate the response of HP to cytokines and chemokines.
The transcription factor NF-κB has a key role in inflammation and immune responses (Ghosh and Karin, 2002; Silverman and Maniatis, 2001; Sun et al., 2013), and has been recently shown to play a role in the response of myeloid progenitors to stress hematopoiesis (Zhao et al., 2014). NF-κB can also control mesenchymal derived osteogenesis, and mice with a loss of function of NF-κB signaling show osteopetrosis (Iotsova et al., 1997). IκB kinase (IKK)-dependent NF-κB activation is essential for the bone remodeling function of osteoclasts (Ruocco et al., 2005) and the restoration of NF-κB in IKK-deficient mice prevents Ob differentiation (Chang et al., 2009). However, the mechanisms and regulatory pathways that control NF-κB activation in the BM Ob niche, and the putative effect of NF-κB signaling on HP activity in the BM remain unknown.
p62 (also called Sqstm-1) is a master regulator of ubiquitinated protein turnover via autophagy and the ubiquitin-proteasome system (Moscat and Diaz-Meco, 2009). p62 also has a central role in osteoclastogenesis. It controls the receptor activator of NF-κB (RANK) signaling by interacting with TRAF6 and activating NF-κB through atypical protein kinase C (aPKC)-mediated activation of IKK in osteoclasts (Duran et al., 2008; Duran et al., 2004). The loss of p62 signaling is implicated in osteolytic lesions in multiple myeloma and adipogenesis (Hiruma et al., 2009; Rodriguez et al., 2006), while gain-of-function mutations of p62 are associated with aberrant and excessive bone turnover in Paget disease (Rodriguez et al., 2006). p62 has also been implicated in the selective autophagy of components of the NF-κB signaling pathway (Chang et al., 2013), however, the specific cellular and molecular roles of p62 in Ob, and its role in the Ob control of HP activity, have not yet been elucidated. In this manuscript, we reveal that upstream BM-MΦ signaling and cell-to-cell interaction is required for Ob differentiation and the expression of the chemokine Ccl4 (MΦ inflammatory protein-1β, Mip-1β). While the cell autonomous deficiency of p62 does not translate into significant HP activity defects, the deficiency of p62 in the non-hematopoietic compartment of BM results in osteopenia due to defective Ob differentiation and HP egress. Mechanistically, the p62 within Ob attenuates NF-κB signaling through the downregulation of phospho-focal adhesion kinase (p-FAK), NF-κB and p-IκBα, thus impairing NF-κB activation, MΦ-dependent Ob differentiation and Ccl4 production.
RESULTS
Deficiency of p62 induces non-cell-autonomous HP egress in vivo
p62−/− mice exhibit egress of myeloid HP (Fig. 1A) and short-term (ST) repopulating stem cells (Fig. 1B–C) to the peripheral blood (PB), but not long- or medium-term repopulating hematopoietic stem cells (Fig. S1A–C), common lymphoid progenitors Fig. S1D), or B-cell lineage populations (Fig. S1E). This egress of myeloid progenitors was also observed in the spleen (Fig. 1D). However, p62-deficiency was not associated with expansion of the BM content of cells (data not shown), repopulating HSC (Fig. S1F–H), myeloid or common lymphoid progenitors, or B-cell lineage populations (Fig. S1I–K), or changes in the hematopoietic regenerative response to 5-fluorouracil administration (Fig. S1L–M).
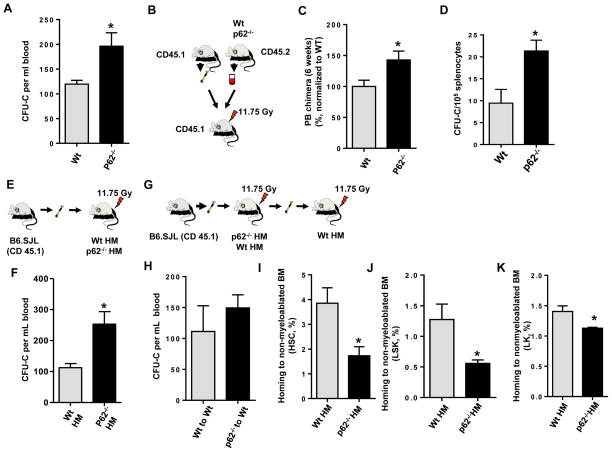
Figure 1. p62 regulates HP trafficking in a non-cell autonomous manner.
(A) CFU-C content in the PB (PB) of Wt or p62−/− mice (n=8–10 mice per group). Values represent average of three independent experiments. (B) Experimental set up. PB mononuclear cells (PBNC) from CD45.2+ Wt or p62−/− mice were mixed with CD45.1+ B6.SJLPtprca Pep3b/BoyJWt bone marrow (BM) cells and competitively transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice. (C) CD45.2+ chimera in the PB of recipient mice (5–8 mice per group) after 6 weeks of competitive transplantation. (D) Frequency of hematopoietic progenitors in spleen of Wt or p62−/− mice (n=3 mice per group). (E) Experimental set up. BM cells from CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice were non-competitively transplanted into lethally irradiated CD45.2+ Wt or p62−/− mice to generate chimeric Wt HM or HM p62−/− mice.(F) Absolute numbers of CFU-C present in the PB of Wt HM or HM p62−/− mice (n=4–7 mice per group). (G) Experimental set up.BM cells from CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice were non-competitively transplanted into lethally irradiated CD45.2+ Wt or p62−/− mice to generate chimeric Wt HM or HM p62−/− mice. BM cells isolated from primary Wt HM or HM p62−/− mice were transplanted into lethally irradiated CD45.2+ to generate secondary Wt recipients. (H) Absolute numbers of CFU-C present in the PB of secondary Wt recipient mice (n=4–6 mice per group). Values represent mean ± SEM. p=ns. (I–J) Homing (%) of Wt ST-HSC (I), LSK (J) and LK (K) BM cells to non-myeloablated BM from Wt or p62−/− mice. A minimum of 5 mice were analyzed per group. Values represent mean ± SEM. * p<0.05.
To determine whether the myeloid progenitor egress is hematopoietic intrinsic or if it depends on the hematopoietic microenvironment (HM), we generated full chimeric animals of wild-type hematopoiesis (H-Wt) and p62-deficient hematopoiesis (H-p62−/−) by BM transplantation into CD45.1+ animals (Fig. S2A), or through reverse transplantation of Wt HSC into lethally irradiated CD45.2+ Wt (Wt HM) or p62−/− animals (p62−/− HM) (Fig. 1E). We observed that the effect of p62 on HP traffic is non-cell-autonomous since H-p62−/− HP did not recapitulate the increased egress of HP (Fig. S2B). Conversely, mice lacking p62 in the HM (p62−/− HM) did phenocopy the HP egress of primary mice (Fig. 1F), and increased HP egress of p62−/− HM mice was rescued by secondary transplantation into Wt recipients (Fig. 1G–H). Altogether these data indicate that the effect of p62-deficiency on HP egress is non-cell-autonomous.
It has been reported that over-expression of AAAGUGC seed-containing micro-RNA promotes cell expansion, replating capacity, and signaling in hematopoietic cells, by interfering with p62-regulated pathways in myeloid cell lines, and that these changes may reflect the effect of p62-deficiency on HSC and myeloid progenitor mobilization (Meenhuis et al., 2011). To identify whether p62 regulates in vivo hematopoietic cell proliferation, we analyzed the cell cycle status of primary Wt and p62-deficient as well as Wt hematopoietic cells engrafted in full chimeric WtHM and p62−/− HM mice. We found no significant differences in the cell cycle status of BM long-term (LT)-HSC (Fig. S2C), ST-HSC (Fig. S2D) and Lin−/c-kit+/Sca-1− (LK) (Fig. S2E) cells from primary mice or in Wt HM or p62−/− HM mice (Figs. S2F–H). Our data using models of primary loss-of-function of p62 did not support the existence of a cell-autonomous or microenvironment-dependent role of p62 on HSC/P cell cycle regulation.
To determine whether a defect in the homing of circulating HP is responsible for HP egress in p62-deficient HM, we measured the ability of Wt HSC and HP to home into non-myeloablated Wt HM or p62−/− HM. As compared to Wt HM recipients, there was a 50–60% reduction in the homing of immunophenotypically defined BM HSC (Lin−/c-kit+/Sca-1+/CD34−/CD135− cells) to p62−/− HM recipient mice (Fig. 1I), and the progenitor containing population of Lin−/c-kit+/Sca-1+ (LSK) BM cells (Fig. 1J), as well as LK BM cells (Fig. 1K),. This homing defect suggested a significant impairment in the ability of the p62−/− HM to lodge HSC/P upon transplantation into an unmanipulated host. Nevertheless, this difference in HSC/P homing ability disappeared when the recipient mice were myeloablated following lethal irradiation (Figs. S2I–K), suggesting the existence of an accessory contribution from a radiosensitive cell population which can be regenerated by Wt HSC/P transplantation.
Ob deficiency of p62 is responsible for osteogenic defects and HP egress
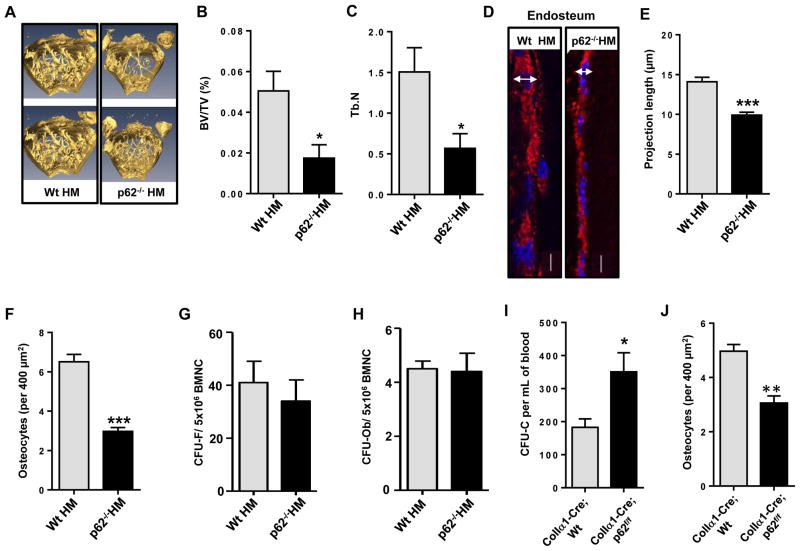
The mammalian BM microenvironment consists of multiple cell types, including mesenchymal progenitors, CXCL12-expressing adventitial reticular cells, Ob lineage cells, endothelial cells, pericytes, fibroblasts and adipocytes, among others. Functional studies support a role for Ob-lineage cells to maintain HP in the BM in vivo. Long-term HSC are connected to Ob cells (Zhang et al., 2003). Ob in the BM contribute to HP expansion through activation of Notch signaling (Calvi et al., 2003), whereas targeted ablation of Ob in vivo results in a loss of HP from the BM (Visnjic et al., 2004). To understand whether osteogenesis was impaired in p62−/− HM mice, and to delineate the role of p62 in the non-hematopoietic HP niche, we examined the bone architecture of p62−/− HM chimeric mice. Bone histomorphometric analysis using micro-computed tomography (micro-CT, Fig. 2A) revealed significantly decreased femoral trabecular bone volume (Fig. 2B) and trabecular number (Fig. 2C) in p62−/− HM mice as compared to Wt HM mice. In p62-deficient femoral sections, histological analysis of endosteal ColIα1-expressing Ob demonstrated a flatter appearance (Fig. 2D–E) and a decreased number of cortical osteocytes (Fig. 2F). However, the frequency (and content) of mesenchymal progenitors (colony-forming unit fibroblasts, CFU-F, Fig. 2G) and osteoprogenitors (CFU-Ob, Fig. 2H) did not change, suggesting that p62 controls Ob maturation and terminal differentiation, but not mesenchymal progenitor differentiation. Finally, to confirm whether the presence of p62 in Ob is responsible for HP mobilization, we enumerated the number of circulating HP in mice with a specific deletion of p62 in Ob. We crossed Colα1(I)-Cre mice with p62f/f mice to obtain Ob-specific Colα1(I)-Cre;WT and Colα1(I)-Cre;p62f/f mice. Interestingly, Colα1(I)-Cre;p62f/f mice phenocopied increased HP egress as observed in p62−/− and p62−/− HM mice (Fig. 2I–J), suggesting that the loss of p62 in Ob is responsible for non-cell-autonomous HP egress in vivo.
Figure 2. Ob p62-deficiency impairs Ob differentiation and osteogenesis in vivo resulting in HP egress.
(A) Representative micro-computer tomography (micro-CT) analyses of femoral trabecular bone of Wt HM or HM p62−/− mice after 16 weeks of transplantation. (B and C) Percent ratio of trabecular bone volume versus tissue volume (BV/TV) and trabecular number (Tb.N) of Wt HM or p62−/− HM mice (4–7 mice per group) at 16 weeks after transplantation. (D) Representative confocal microscopic images of collagen type1α1 (red) and nuclear counterstaining (DAPI, blue) in femur sections from Wt HM or p62−/− HM. (E) Measurement of Ob length projections of bone lining Obs in longitudinal femoral sections from Wt HM (Ob n=24) or p62−/− HM (Ob n>17 per group). Analysis was performed as measurement of the transversal diameter at the widest point of the Ob expressing Col1α1 (space between arrow ends). (F) Counts of osteocytes in femoral cortical bone from Wt HM (n=47 fields) or p62−/− HM (n=42 fields). Values represent mean ± SEM. ***p<0.001. (G and H) CFU-F and CFU-Ob from BMNC of chimeric Wt HM or HM p62−/− mice. (I) ColIa1-Cre;p62 p62f/f mice mice phenocopy the hematopoietic egress of primary p62−/− mice and p62−/− HM mice. Values represent mean ± SEM. *p<0.05. (J) Osteocyte counts in femoral cortical bone from Coll1α1-Cre; Wt or Coll1α1-Cre; p62f/f. N=33 fields were analyzed for each group. ***p<0.001.
p62 regulates NF-κB-dependent Ob differentiation in presence of MΦ
The homing defect which is associated with a deficiency of p62 in non-conditioned HM (Fig. 1H, S1L–M) is not found in mice with irradiated HM, this may suggest that radiosensitive hematopoietic cells are required as effectors to retain HP in the BM. Myeloid mononuclear accessory cells, in addition to Ob, have been proposed to form a myeloid signaling network responsible for HP homing and engraftment in the BM (Katsumoto et al., 2005). Specifically, MΦ have been known to mediate HP retention in the BM (Casanova-Acebes et al., 2013; Chow et al., 2011). It has been shown that depletion of BM MΦ, but not other lineage-related cells such as osteoclasts (Miyamoto et al., 2011), is adequate to suppress endosteal Ob, inhibit the expression of HP-supportive cytokines at the endosteum, and elicit HP mobilization into the PB (Winkler et al., 2010). The content of MΦ in contact with Ob in the trabecular and endosteal lining of BM from Wt HM and p62−/− HM mice was similar (Figs. S3A–B). Similar to untreated mice with global deficiency of p62 (Duran et al., 2004), the loss of p62 in osteoblasts did not modify significantly the bone osteoclast content (Figs. S3C–D). Together, these data suggested that the content of osteogenic MΦ or osteoclasts was not causative of the loss of BM retention observed in HM- or Ob- p62-deficient mice.
To identify whether MΦ are required for p62-mediated Ob activity, we isolated and expanded F4/80+/CD68+/CD115+/CD169− MΦ derived from the BM of ubiquitin C-EGFP mice (Fig. S3E) and cocultured with Ob in vitro at a 1:1 ratio for 24 hours in the absence of MΦ or osteogenic differentiation factors, and then analyzed their biochemical and expression effect on downstream signaling. Notably, the expression levels of Ob differentiation genes are significantly reduced in p62−/−Ob that have been cocultured with Wt MΦ (Fig. 3A), implying that MΦ induce specific signaling that interfere with Ob differentiation. Ob focal adhesion kinase (FAK) phosphorylation and activation has been shown to depend on MΦ β2-integrin binding which regulates Ob differentiation (Kim et al., 2007). By using phospho-flow cytometry analysis, we found that MΦ are capable of activating FAK in Wt or p62−/− Ob (Fig. S3F). The absence of p62 expression in Ob resulted in a modest reduction of MΦ-dependent Ob FAK activation, suggesting that the moderate decrease in FAK activation in p62−/− Ob may not be responsible for the dramatic loss of p62−/− Ob differentiation.
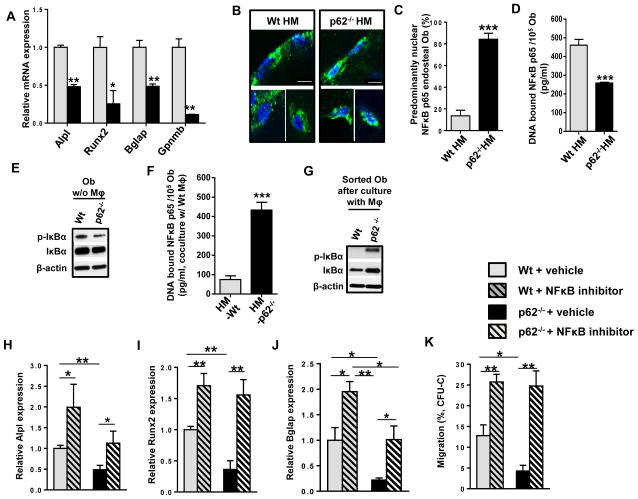
Figure 3. MΦs are in close proximity to endosteal Obs in vivo and p62 regulates NF-κB-dependent Ob differentiation through MΦ-mediated FAK signaling.
(A) Relative gene expressions of Alpl, Runx2, Bglap, and Gpnmb in Wt (grey bars) or p62-deficient (black bars) Ob after 24 hours of culture with Wt MΦ. (B) Representative images of femoral bones from chimeric Wt HM (n=4) or p62−/− BM (n=3) stained with anti-NF-κB p65 (green) and nuclear counterstaining (DAPI, blue). (C) Percent of Ob with predominant nuclear localization of NF-κB p65 from confocal microscopy images of a minimum of 25 Ob per group. (D and F) DNA-bound NF-κB p65 was measured by ELISA using isolated nuclear fractions of sorted Wt or p62-deficient Ob after culture alone (D) or with EGFP+ Wt MΦ for 24 hours (F). (E and G) Representative immunoblots of phosphorylated IκBα and IκBα expression in sorted Wt or p62-deficient Ob after culture alone (E), or with EGFP+ Wt MΦ for 24 hours (G). β-actin was used as a loading control. (H–K) Migration of HP towards Ob and Wt MΦ supplemented with 100 ng/mL Cxcl12, and either vehicle control or BAY 11-7085. (H). Relative gene expressions of Alpl (I), Runx2 (J), and Bglap (K) in Wt or p62-deficient sorted Ob after 24 hours of coculture with EGFP+ Wt MΦ in the presence of 1 μM BAY 11-7085 (hatched bars) or vehicle control (DMSO, solid bars). Values represent mean ± SEM. **p<0.05, **p<0.005.
MAPK and NF-κB transcriptional signatures have been associated with p62 activity. Erk or p38 activation has been shown to be required for Ob differentiation. The Erk or p38 pathway mediates the signal transduction from hormone and growth factors, such as fibroblast growth factor-2 (Xiao et al., 2002) and parathyroid hormone (Chen et al., 2004). They also stimulate Runx-2 phosphorylation and its transcriptional activity (Franceschi et al., 2009; Xiao et al., 2000). In addition, Erk or p38 signaling is required for p62 activity in other mesenchymal lineage BM cells such as adipocytes. It was reported that absence of Erk1 was sufficient to restore abrogated adipogenesis and energy homeostasis of p62-deficient animals in vivo (Lee et al., 2010). However, while MΦ signaling is associated with increased activation of Erk, but not p38, in FACS sorted Ob (Fig. S3G), the deficiency of p62 resulted in inhibition (Fig. S3G), rather than further upregulation as was reported in adipocytes (Lee et al., 2010; Rodriguez et al., 2006). Furthermore, HP egress was not rescued by deletion of Erk1 (Fig. S3H), strongly suggesting that Erk upregulation is not responsible for HP mobilization in p62-deficient Ob.
NF-κB signaling has also been shown to be crucial in Ob differentiation. Mice with disrupted NF-κB signaling show severe osteopetrosis (Iotsova et al., 1997). IκB kinase (IKK)-dependent NF-κB activation is essential for the bone remodeling function of osteoclasts (Ruocco et al., 2005), and gain-of-function of NF-κB activity prevents Ob differentiation as demonstrated in mice deficient in IKK (Chang et al., 2009). Since osteoclasts may be dispensable for HP mobilization in vivo (Miyamoto et al., 2011), we analyzed whether NF-κB-dependent signaling in Ob correlates with HP egress. Histological analysis of active NF-κB p65 in endosteal and trabecular Ob showed increased NF-κB nuclear localization in p62−/− Ob in vivo (Fig. 3B–C). Similar to previous reports in osteoclasts and tumor cells (Duran et al., 2008; Duran et al., 2004), Ob cultured from p62-deficient animals showed decreased levels of the p65 subunit of NF-κB bound to DNA (Fig. 3D), and IκBα, the phosphorylated inhibitor of NF-κB, (p-IκBα, Fig. 3E) was responsible for degradation. However, cocultures of MΦ with Ob resulted in increased NF-κB p65 activity, as demonstrated by translocation of NF-κB to the nucleus (Fig. 3F). In addition, p62−/− Ob isolated after coculture with MΦ showed increased phosphorylation and expression of IκBα (Fig. 3G). Taken together, these data demonstrate that MΦ reverses p62-dependent NF-κB signaling in Ob. To ascertain whether NF-κB is crucial for p62-dependent Ob differentiation, we evaluated the changes of bone specific gene expression after in vitro treatment with BAY 11-7085, an NF-κB inhibitor. The attenuation of NF-κB activity was validated by the reduced amount of DNA bound-NF-κB p65 concentration seen in both Ob and MΦ (Fig. S3I–J). Interestingly, addition of BAY 11-7085 to Ob-MΦ cocultures derepressed Alpl-, Runx2- and Bglap-expression in Wt and p62−/−sorted Ob (Fig. 3H–J), suggesting that p62-deficiency enhances the ability of IKK/NF-κB activity to inhibit Ob differentiation. Finally, Cxcl12-driven chemotaxis in the presence of WT MΦ-p62-deficient Ob cocultures was significantly reduced (Fig. 3K), indicating that Ob p62-deficiency results in a loss of sensitivity to Cxcl12 chemotaxis gradients. The inhibition of NF-κB in WT MΦ-Wt Ob cocultures results in an ~2-fold increase in chemotaxis of Wt HP, and eliminates the effect of p62-deficiency in Ob, suggesting that NF-κB activity is responsible for the effect of Ob p62 on directed migration towards Cxcl12 gradients (Fig 3K).
HM p62-deficiency impairs MΦ-dependent Ob expression of the chemokine Ccl4 and recapitulates part of the mobilization phenotype associated with BM MΦ activity
In order to determine whether the osteogenic deficiency of p62 in p62−/− HM mice could result in the alteration of the levels of chemokines responsible for HP egress, we performed an in vivo expression screen of a panel of chemokines or cytokines known to be relevant in HP traffic (Figs. S4A–N). We hypothesized that their expression and secretion in plasma and/or femoral extracellular fluid may depend on Ob activity, which could be regulated by NF-κB activity.
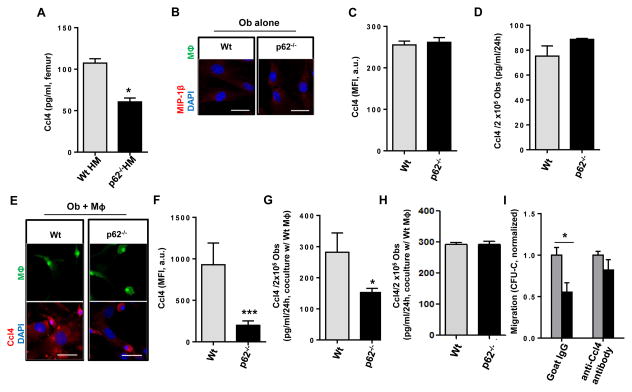
Interestingly, Ccl4 (MΦ inflammatory protein-1β, Mip-1β) was uniquely found to be downregulated in the femoral extracellular fluid of p62-deficient mice (Fig. 4A), and trended to be decreased in plasma as well (Fig. S4A). Ccl4 is a C-C motif chemokine that modulates BM HP chemotactic response to Cxcl12 (Basu and Broxmeyer, 2009). Cxcl12 levels in the plasma and bones of p62−/− HM mice were normal or upregulated (Fig. S5A–B), which was consistent with a null or minimal contribution of mature Ob to systemic or local Cxc12 levels, as previously reported (Ding and Morrison, 2013; Greenbaum et al., 2013). As expected, Ob from Wt mice did not show any significant expression or secretion of Ccl4, neither did the deficiency of p62 modify its expression or secretion (Fig. 4B–D). Interestingly, the expression and secretion of Wt Ob Ccl4 protein was significantly increased upon coculture with MΦ, whereas the deficiency of p62 in Ob resulted in diminished levels of Ccl4 expression and secretion, similar to the levels of unstimulated Ob (Fig. 4E–G, to be compared with Figs. 4B–D).
Figure 4. p62 regulates Ccl4-dependent HP retention.
(A) BM extracellular levels of Ccl4. Extracellular fluid from Wt HM or p62−/− HM femora (at 16 weeks after transplantation) were processed for ELISA. (B–D) In vitro analysis of Ccl4 in explanted Ob from Wt and p62−/− mice cultured for 24 hours. (B) Representative confocal microscopic images of Ccl4 (red) and nuclear counterstaining (DAPI, blue) in Wt or p62−/− Ob. Data are representative of two independent experiments with similar results. (C) Mean fluorescent intensity (MFI) of Ccl4 expression measured in (B). (D) Concentration of Ccl4 secreted from 105 Ob after 24 hours culture without contact with Wt MΦ. Values are derived from two independent experiments. (E–G) In vitro analysis of Ccl4 in explanted Ob from Wt and p62−/− mice cultured for 24 hours with EGFP+ Wt MΦ at a 1:1 ratio. (E) Representative confocal microscopic images of Ccl4 (red) and nuclear counterstaining (DAPI, blue). Data are representative of three independent experiments with similar results. (F) MFI of Ccl4 expression measured in (E). A minimum of 25 Ob were measured. (G) Concentration of Ccl4 secreted from 105 Ob after 24 hours culture in contact with EGFP-expressing Wt MΦ. Values are derived from three independent experiments. (H) Effect of MΦ-Ob contact on Ccl4 production. Ccl4 production was not upregulated in Ob (lower chamber) from Wt and p62-deficient animals cultured in non-contact (transwell) systems after 24 hours of culture with MΦ (upper chamber). (I) Chemotaxis of HP towards Ob and of Wt MΦ towards a Cxcl12 gradient, in the presence or absence of an anti Ccl4 antibody. For all panels, values represent mean ± SEM. *p<0.05. ***p<0.001.
Administration of clodronate liposomes has been shown to deplete MΦ and induce HP mobilization (Winker et al., 2010). We compared the effect of MΦ depletion by clodronate with the deficiency of Ob p62, in terms of their effect on HP mobilization. We found that clodronate did result in a depletion of CD11b+/F4/80+/CD68+/CD115 (c-fms)+ MΦ in vivo, did not affect other CD11b+/F4/80+ BM cell populations (Fig S5C). Exhaustion of CD11b+/F4/80+/CD68+/CD115+ induced an ~4-fold increase in the number of circulating HP while Ob p62-deficiency resulted in only ~2-fold increase (Fig. S5D). Interestingly, the effect of HM p62-deficiency on HP mobilization was lost in CD11b+/F4/80+/CD68+/CD115+ depleted animals (Fig S5D). These results indicate that CD11b+/F4/80+/CD68+/CD115+ MΦ signal through Ob p62, and that Ob p62 is, at least partly, responsible for the effect of CD11b+/F4/80+/CD68+/CD115+ cell depletion on myeloid progenitor retention in the BM.
We confirmed that direct cell-to-cell interaction between Ob and MΦ was necessary to modulate Ccl4 production, since culture of Ob and MΦ in different chambers of a non-contact transwell culture system failed to reproduce the difference in expression of Ccl4 (Fig. 4H). Moreover, addition of an anti-Ccl4 antibody to cocultures of p62-deficient Ob and Wt MΦ, restored the ability of Wt HP to respond to Cxcl12-driven chemotaxis (Fig 4I). Collectively, our data indicate that p62 prevents activation of IKK and NF-κB in Ob, and that p62 is required for osteogenesis, Ob differentiation, expression of the chemokine Ccl4, Cxcl12-directed chemotaxis and HP retention within the BM microenvironment.
p62 signaling attenuates IKK/NF-κB activity through its autophagic activity, facilitating Ob differentiation and Ccl4 expression
To mechanistically ascertain the role of p62 in Ob differentiation and Ob Ccl4 expression, p62−/− Ob were transduced with a retroviral EGFP-expressing bicistronic vector expressing full-length p62 or an empty vector (mock, Fig. S6A). Transduced cells were cocultured (or not) with Wt (unlabeled or ubiquitin C-EGFP transgenic) MΦ for 24 hours. This experimental setting was used to determine the effect of the restoration of p62 expression on NF-κB activity and signaling pathway. As a positive control of optimal NF-κB translocation, we used stimulation with TNF-α on Ob (Fig. S6B). We confirmed that MΦ signaling was necessary for p62-dependent NF-κB nuclear translocation, since exogenous expression of p62 did rescue and abrogate the nuclear localization of active NF-κB p65 in p62-deficient Ob in the presence of MΦ (Fig. 5A), but not in their absence (Fig. S6B). Reintroduction of p62 decreased the cellular levels of NF-κB p65 protein (Fig. 5B) due to both lisosomal degradation and a loss of nuclear translocation, as assessed by co-localization in lisosomes and diminished nuclear translocation (Fig. 5A- and 5C). Inhibition of NF-κB p65 activation by p62 was secondary to restored catalytic activity of IKK activity, since the absolute cytosolic levels of p-IκBα were found to inversely correlate with the expression of p62 (Fig. 5D). These changes were not associated with changes in the expression levels of the IKK catalytic subunits α, β, or γ (Fig. S6C), indicating that p62 controls IKK activity, but not its expression.
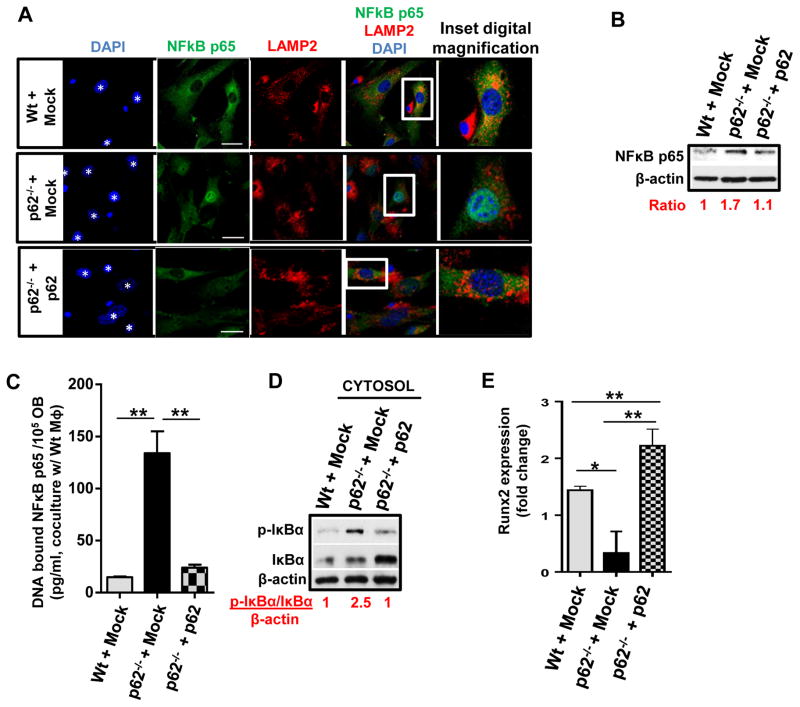
Figure 5. Ectopic expression of p62 abrogates nuclear translocation of NF-κB p65 andrescues differentiation and Ccl4 expression of p62-deficient Ob.
(A) Representative confocal microscopy images of NF-κB p65 (green), lisosomes (LAMP2, red) and nuclear counterstaining (DAPI, blue) in cocultures of FACS-sorted, mock vector transduced Wt (Wt + Mock), p62-deficient (p62−/− + Mock) or p62 transduced- p62-deficient (p62−/− + p62) Ob (DAPI, asterisks) cultured with unlabeled MΦs (DAPI, small nuclei with condensed chromatin) for 24 hours. (B) Representative immunoblot of total NFκB p65 in post-culture isolated Ob cell lysates from (A). β-actin was used as a loading control. (C) DNA-bound NF-κB p65 in lysates of the nuclear fraction of Ob cells isolated post-culture from (B). (D) Immunoblot of phosphorylated IκBα and total IκBα expression in the cytosolic fraction of cells from (B). (E) Change (fold) in Runx2 mRNA expression of transduced Ob after 24 hours of coculture with Wt MΦ. Values are derived from three independent experiments. For all panels, values represent mean ± SEM. *p<0.05; **p<0.01.
Similarly, exogenous expression of p62 in p62−/− Ob in the presence of MΦ rescued the expression of Ob Runx2 (Fig. 5E), confirming that p62 expression is associated with Ob differentiation. Overexpression of p62 also rescued the expression and production of Ccl4 by Ob (Fig. 6A–C) indicating that the concentration of Ccl4 is directly related to p62 expression, and that it is inversely related to the activity of NF-κB in Ob cultured with MΦ.
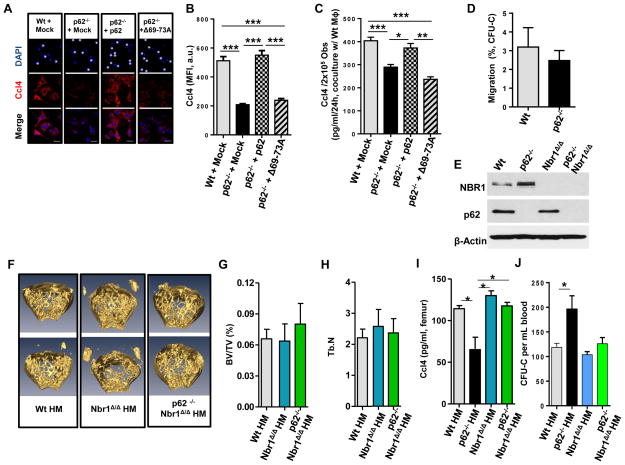
Figure 6. p62 oligomerization is required for Ccl4 expression and a double deficiency of p62 and Nbr1 rescues Ccl4 expression and HP mobilization in vivo.
(A) Representative confocal microscopy images of Ccl4 (red) and nuclear counterstaining (DAPI, blue) in mock vector transduced Wt (Wt + Mock), p62-deficient (p62−/− + Mock), p62 transduced- p62-deficient (p62−/− + p62) or deletion of 69-73A in p62, disrupting PB1-mediated p62 oligomerization, transduced-p62-deficient (p62−/− + Δ69-73A) Ob (DAPI, asterisks) after coculture for 24 hours with Wt MΦ (DAPI, small nuclei with condensed chromatin). (B) MFI of Ccl4 expression measured in (A). Values are derived from three independent experiments. (C) Secreted Ccl4 concentration from 2×105 sorted, retrovirally transduced Obs after 24 hours culture in contact with Wt MΦ. Values represent mean ± SEM. *p<0.05;**p<0.01; ***p<0.001. (D) Chemotaxis of CFU-C (%) towards Ob and Wt MΦ supplemented with Cxcl12 and bafilomycin A1 or vehicle (control). (E) Representative immunoblot confirming the protein expressions of Nbr1 and p62 in Ob from p62−/−, Nbr1Δ/Δ, or p62−/−Nbr1 Δ/Δ mice. (F) Representative micro-CT analyses of femoral trabecular bone of Wt HM, Nbr1Δ/Δ HM or p62−/− Nbr1Δ/Δ HM mice at 16 weeks post-transplantation. (G and H) Percent ratio of trabecular bone volume versus tissue volume (BV/TV) and trabecular number (Tb.N) of the Wt HM, Nbr1Δ/Δ HM or p62−/− Nbr1Δ/Δ HM mice analyzed in (F). (I) BM extracellular levels of Ccl4 from Wt, p62−/−, Nbr1Δ/Δ, or p62−/− Nbr1Δ/Δ mice (n=12 mice per group). (J) CFU-C contents in the PB of Wt HM, p62−/−, Nbr1Δ/ΔHM, or p62−/− Nbr1Δ/Δ HM mice (n=4–7 mice per group).
Protein degradation by p62-dependent autophagy mechanisms may be determined by the oligomerization-dependent autophagosome localization (Itakura and Mizushima, 2011). To identify the functional domain in p62 responsible for Ccl4 production, p62−/− Ob were transduced with a retroviral vector expressing the p62 Δ69-73 mutant which fails to oligomerize, a process required for autophagosome formation (Duran et al., 2011; Duran et al., 2008; Moscat et al., 2006). The chemoattraction of HP towards Cxcl12 which was added to cultures of Wt MΦ and Wt Ob was significantly inhibited by the autophagy inhibitor, bafilomycin A1, to the same level seen in cultures of Wt MΦ and p62−/− Ob (Fig. 6D, compare with Fig. 3K). This suggests that the effect of p62-deficiency is equivalent to that induced by bafilomycin A1 through autophagic inhibition. Ectopic expression of the p62 Δ69-73 mutant (Fig. S6D) which disrupts the p62 oligomerization required for autophagosome formation failed to rescue Ccl4 expression or production (Fig. 6A–C), suggesting that p62-dependent Ccl4 expression depends on oligomerization which is required for localization to the autophagosome formation site.
Together, these data indicate that p62 represses MΦ-dependent NF-κB signaling, and that it is necessary for Ob differentiation and Ccl4 production through its role in autophagy.
Nbr1 antagonizes p62 and its deficiency rescues BM MΦ-dependent Ob Ccl4 expression and HP retention
Phox bemp1-(PB1)-domain-containing proteins p62 and Nbr1 share domain architecture and play overlapping roles in cell signaling through protein-protein interaction. A C-terminus deletion of Nbr1 results in osteoblast differentiation (Whitehouse et al., 2010). To identify whether Nbr1 has overlapping or distinct roles in osteogenesis and HP retention, we crossed CMV-Cre; Nbr1f/f (Nbr1 Δ/Δ) mice (Yang et al., 2010), where Nbr1 is absent in germinal cells, with p62−/− mice (p62−/−; Nbr1Δ/Δ). We analyzed the effect of HM deficiency of full-length Nbr1 and of double Nbr1/p62 on osteogenesis and HP mobilization in chimeric animals. We identified that Ob isolated from p62−/− bones express increased levels of Nbr1 (Fig. 6E), suggesting a compensatory role. The deficiency of Nbr1 in vivo does not significantly impair osteogenesis, Ccl4 production, or levels of circulating HP (Fig. 6F–J). Surprisingly, the double deletion of Nbr1 in p62−/− HM mice (p62−/−; Nbr1Δ/Δ HM) ameliorates bone architecture, Ccl4 production, and HP egress, which suggests distinct roles for p62 HM and Nbr1 in hematopoiesis, and an antagonistic effect of Nbr1 on p62 activity..
DISCUSSION
The circulation of HSC/P in PB is crucial as part of a system of immunosurveillance of the peripheral organs and to foster the local production of tissue-resident innate immune cells (Massberg et al., 2007). Niche-regulation of HP trafficking in vivo is incompletely understood. Cellular and molecular specificity, as well as crosstalk, are important aspects in order to understand how cell signaling within complex networks generates such precise cellular responses to a myriad of different stimuli. A key to this process are intracellular protein scaffolds, which are multidomain proteins that assemble specific signaling complexes in different cellular locations so as to assure a spatially and temporarily controlled signal.
The role of Ob in HSC/P traffic remains controversial, and the genetic characterization of the molecular signatures within the Ob microenvironment which have been implicated in contributing to HSC/P trafficking in vivo, is quite incomplete. Previous reports have indicated that Ob activity controls HSC/P retention within the BM (Calvi et al., 2003; Raaijmakers et al., 2010; Visnjic et al., 2004; Zhang et al., 2003), and MΦ that are in contact with Ob (called osteomacs) (Winkler et al., 2010) have been recently implicated as intermediate cells in G-CSF mobilization, Ob depletion and in HSC/P egress from the BM (Katayama et al., 2006; Winkler et al., 2012). However, modulation of Ob numbers does not necessarily alter HSC numbers (Kiel et al., 2007; Zhu et al., 2007), suggesting that functional changes in Ob activity may be responsible for their effect on BM HSC/P retention. Cxcl12-dependent progenitor retention (Greenbaum A et al, 2013) and B-lymphopoiesis (Zhu et al., 2007; Greenbaum et al., 2013; Ding et al., 2013) are functional roles that have been assigned to Ob lineage populations of the BM. However, the specific role of Ob in HSC/P mobilization remains controversial (Kiel et al., 2007; Ding and Morrison, 2013).
The deficiency of p62 in the non-hematopoietic compartment of BM results in osteopenia as a result of loss of Ob differentiation, and induces egress of ST-HSC and myeloid progenitors. This effect is due to impaired Ob signaling since the deficiency of p62 in Ob, as identified by the expression of ColIα1 driven by its 2.3Kb promoter/enhancer, phenocopies the mobilization of HSC/P of mice with p62-deficient HM (Dacquin et al., 2002). However, the deficiency of p62 does not result in either significant mobilization of long- or medium-term repopulating HSC or changes in the BM content or mobilization of B lymphopoietic cells, suggesting that p62 regulates some, but not all, of the activities associated with Ob activity in the BM (Winkler et al., 2010; Chouo et al., 2011; Zhu et al., 2007; Greenbaum et al., 2013). This mobilization effect results in decreased homing of ST-HSC and LSK/LK progenitors in non-myeloablated recipients, but not in myeloablated recipients. The effect of myeloablation is restored by transplantation, since p62−/− HM mice display increased numbers of circulating HSC/P after transplantation. We hypothesized that a Wt radiosensitive cell population with the ability to be regenerated by 6 weeks post-transplantation may be responsible for this differential effect, and may contribute to significant changes in the content or activity of the osteoblastic niche. Several reports have provided information about the functional defects and apoptosis of irradiated MΦ in vivo in C57Bl/6 mice as early as within the first 24 hours after irradiation (Coates et al., 2008; reviewed in Mukherjee et al., 2014). While the mechanisms of these changes induced by myeloablation remain unclear and are likely to be indirect, we hypothesized that a radiosensitive cell which could be regenerated by as early as 6 weeks post-transplantation may be responsible of this differential effect, being tissue macrophages major candidates in relation to the BM Ob niche. Other radiosensitive populations such as osteoclasts, which have also been reported to control HSC/P traffic in the BM (Kollet et al., 2006), were analyzed in the BM of Wt HM and p62−/−HM mice and found not to be regenerated by 6 weeks post-transplantation (data not shown).
A putative effect of p62 on BM HSC/P proliferation (Meenhuis et al., Blood 2011) as a downstream target of microRNAs 17/20/93/106 was explored. Our data could not confirm changes in HSC/P proliferation, as assessed by flow cytometry analysis of BrdU uptake in vivo, in either primary mice or HM chimeric BM HSC/P. Analysis of p62−/− and p62−/− HM mice for as long as 6 months after birth or transplantation did not show any significant sign of hematopoietic failure (data not shown). While it is possible that long-term aging of p62−/− or p62−/− HM mice results in significant defects in BM HSC/P content, our data focuses on the effect of p62 expression on the ST-HSC and myeloid progenitor populations.
This report describes and analyzes a novel mechanism of MΦ-Ob-dependent hematopoietic progenitor retention in the BM, in which MΦ regulate Ob differentiation. We explored the existence of functional crosstalk between MΦ and Ob lineage cells that would regulate HSC/P trafficking, and identified a novel three-cell (MΦ, Ob and HSC/P) interplay of interactions in vivo which could be recapitulated and mechanistically analyzed in cellular models.. Our data provide evidence that a) cell contact between p62 in MΦ and Ob induces Ob NF-κB activity and differentiation; b) the maintenance of low levels of Ob NF-κB activity is crucial for Ob differentiation, Cxc12-directed chemotaxis, BM retention of myeloid progenitors, and the upregulation of NF-κB activity secondary to the loss of p62 results in egression of myeloid progenitors and ST-HSC to the PB; c) autophagy p62 is a negative regulator of NF-κB activity controlling the levels of p-FAK, p-IκBα, and NF-κB translocation; and d) Nbr1, a PB1 binding partner of p62, antagonizes p62 activity as a negative regulator, and the loss of Nbr1 rescues the deficient osteogenesis of p62-deficient animals. This signaling pathway is summarized in Fig. S6E.
Circulating HP can foster the local production of tissue-resident innate immune cells in response to inflammatory signals (Baldridge et al., 2010; Essers et al., 2009; Massberg et al., 2007). The transcription factor NF-κB has a key role in inflammation and immune responses and has been recently shown to play a role in myeloid progenitor response to stress hematopoiesis (Zhao et al., 2014). Inflammatory signals through NF-κB probably underlie the stress circulation of HSC/P. MΦ are well known mediators of intrinsic activation of NF-κB activity in response to infection or other inflammatory cues. Here, we demonstrate that MΦ signaling is required for Ob differentiation and Ob NF-κB activation, and is crucial for the expression and production of Ob Ccl4, a modulator of Cxcl12 activity which effects HP retention in the BM (Basu and Broxmeyer, 2009). Ob would act as signal amplifiers and coordinators of inflammatory signals initiated by infection responsive macrophages. Our data reveals for the first time Ccl4as a chemokine expressed and secreted by differentiated Ob in contact with BM MΦ, and confirms in vivo previous data on the role of Ccl4 as a modulator of Cxcl12-dependent HSC/P traffic in the BM (Basu and Broxmeyer, 2009).
Ob have high autophagy activity during differentiation (Liu et al., 2013) and NF-κB signaling prevents Ob differentiation (Chang et al., 2009). Oligomerization of p62 is required for autophagosome formation (Itakura and Izushima, 2011), where p62 aggregates ubiquitinated proteins (Bjorkoy et al., 2006) and has been shown to regulate the degradation of the RelA component of NF-κB in Ob through selective autophagy (Chang et al., 2013). We have identified that p62 attenuates MΦ-dependent NF-κB signaling in Ob through the downregulation of pFAK, NF-κB and p-IκBα, which results in impaired NF-κB activation and Ob differentiation in vivo (Fig. S6E). Pharmacological inhibition of NF-κB in MΦ/p62−/− Ob cocultures restored Ob differentiation and Cxcl12-driven chemotaxis, confirming the mechanistic role of NF-κB in the osteogenic defect of p62-deficient mice. Neutralization of Ccl4 and inhibition of autophagy also restored the deficient Cxcl12-directed chemotaxis seen in the same coocultures indicating that all are dependent on the same pathway and regulated by p62. Finally, restoration of p62 expression in the Ob represses NF-κB activity to levels similar to Wt, and induces Ob differentiation, Ccl4 expression, and progenitor retention in vivo. Our data also suggest that during Ob-MΦ crosstalk, p62 relies on p62-PB1 oligomerization for Ccl4 expression.
BM MΦ are essential to understand Ob activity, osteogenesis and HP traffic. The iinteraction between differentiating cell- and niche-derived signals have also been shown to play an important role in Drosophila HP maintenance (Mondal et al., 2011). Clodronate-induced deficiency of MΦ results in progenitor mobilization (Winkler et al., 2010). We have confirmed that the deficiency of CD11b+/F4/80+/CD68+/CD115+ BM MΦ does result in 4-fold increase in myeloid progenitor mobilization, which is ~2-fold higher than the mobilization observed in p62-deficient animals. Interestingly, the Ob p62-deficiency does not add or synergize with the depletion of MΦ, indicating that the effect of Ob p62-deficiency on myeloid progenitor retention depends on the existence of BM MΦ in vivo. Subpopulations of MΦ may be specifically controlling myeloid progenitor retention. BM CD169+ MΦ have been shown to promote the retention of HSC/P in the mesenchymal stem cell niche (Chow et al., 2011). Our experiments did not specifically address the role of Ob activity in relation to CD169+ MΦ, since the cultured primary MΦ did not express CD169. It is possible that CD169+ BM MΦ share the activities of BM CD169− MΦ in regards to the activation of Ob NF-κB activity and downstream effects, however, this point is unproven and will require further dissection in vivo of the specific MΦ populations responsible for Ob NF-κB activity.
In our system the interaction between Ob and MΦ has been exclusively contact-dependent as some of the phenotypes, such as upregulation of Ccl4 expression could not be reproduced in non-contact transwell systems. We provide evidence that p62 regulates osteogenetic signals in an Ob-MΦ coculture setup. This signal, probably mediated by integrins, relies, at least in part, on FAK or other redundant family proteins that are responsible for cell-to-cell anchoring. Integrins link the inside of a cell with its outside environment and in doing so regulate a wide variety of cell behaviors. Integrins play an important role in angiogenesis and cell migration, however their functions in bone formation are less clear. The majority of integrin signaling proceeds through FAK, an essential component of the focal adhesion complex. The loss of FAK does not perturb Ob differentiation in vitro or in vivo, owing to the compensatory increase in Pyk2 in Ob (Kim et al., 2007). FAK and Pyk2 are substrates of autophagy, and there is emerging evidence implicating autophagy as an important mediator of bone cell function in normal physiology (Hocking et al., 2012) and in pathology as documented by the role of p62 mutations in Paget disease of the bone (Rea et al., 2013).
Genetic truncation of Nbr1, a selective autophagic receptor for degradation of ubiquitinated substrates which can interact with p62 but not LC3, leads to increased Ob differentiation and activity in vivo (Whitehouse et al., 2010). As shown by our data, its deficiency restores in vivo BM Ccl4 production and HP mobilization, suggesting that Nbr1 plays an inhibitory role on the p62-dependent regulation of NF-κB activity in Ob.
Our data define for first time a signaling network between BM MΦ and neighboring Obs with activity on the HP niche. We propose the existence of a regulatory signal from BM resident “osteo-macrophages” where Ob NF-κB signaling is connected with the immunosurveillance functions of circulatory HP. We also propose the existence of a homeostatic regulatory role of selective autophagy regulated by p62 on NF-κB signaling pathway in Ob which is required for osteogenesis and BM progenitor retention. A connection between bone, innate immunity and progenitor traffic is proposed which has the ability to amplify or inhibit MΦ-dependent inflammatory signals. Our data support a role for p62 in the regulation of the intercellular signaling at the BM MΦ-Ob niche, and identifies the key molecular determinants of signaling regulating ST-HSC and myeloid progenitor trafficking.
EXPERIMENTAL PROCEDURES
Mice
All animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all protocols were approved by institutional care and use committees for animal research at the Cincinnati Children’s Hospital Research Foundation. p62−/−, p62−/−; Erk1−/−, and CMV-Cre; Nbr1Δ/Δ mice have been described previously (Lee et al., 2010; Rodriguez et al., 2006; Yang et al., 2010). p62−/− mice were crossed with Nbr1Δ/Δ mice to generate p62−/−; Nbr1Δ/Δ mice. Genetically modified Colα1(I)(2.3 Kb promoter/enhancer)–Cre mice (Dacquin et al., 2002) were crossed with p62f/fmice (Muller et al., 2013) for generation of osteolineage-specific deletion of p62. All primary mice were analyzed between 6 and 10 weeks of age. Chimeric (HM) mice were generated by non-competitive transplantation of Wt BM nucleated cells (BMNC) from B6.SJLPtprca Pepcb/BoyJ(CD45.1+) mice into lethally irradiated 6–8 week old CD45.2+ Wt or p62-deficient mice. All mice were maintained in C57Bl/6 background. C57Bl/6 (CD45.2+) and B6.SJLPtprca Pepcb/BoyJ(CD45.1+) mice were obtained commercially (Jackson Laboratory, Bar Harbor, ME; Harlan Laboratories, Frederick, MD). Littermate mice from the same breeding were used in all experiments. Ubiquitin C-EGFP mice have been described previously. These mice had been backcrossed >10 generations into C57Bl/6 mice.
Quantification of HP egress
PB total and differential counts were analyzed using a Hemavet 950 (DREW Scientific). PB was isolated by retro-orbital bleeding. Hematopoietic progenitors isolated from BM, spleen or PB were grown on methylcellulose medium supplemented with cytokine cocktails (Stem Cell Technologies) and colony forming progenitors (CFU-C) were scored on day 9.
HSC repopulation
Adult recipient mice were lethally irradiated with a Cs137 gamma irradiator as previously described (Sengupta et al., 2011). For competitive repopulation experiments CD45.2+ BMNC or PB (50–150 μL) were mixed with 500,000–3×106 CD45.1+ BMNC and were transplanted into lethally-irradiated B6.SJLPtprca Pepcb/BoyJ(CD45.1+) recipient mice. HSC engraftment was measured by chimera assessment using flow cytometry at 6 weeks, 10 weeks and 16 weeks post-transplantation. Chimera level was normalized to Wt levels as 100% to allow inter-experiment comparison.
Homing assays
For homing assay to nonmyeloablated BM, 20–25×106carboxyfluorescein diacetate, succinimidyl ester (CFSE, Invitrogen) labeled BM cells from Wt mice were prepared and used for transplantation though the tail vein injection. Wt or p62−/−animals were used as recipients and sacrificed after three hours post transplantation. The single cell suspensions of the BM were subjected to FACS analysis to measure homing of HSC (CFSE+lineage−Sca-1+cKit+CD135−), LSK, and LK cells. The percent of homing was calculated from the input and output cell numbers. To measure homing to myeloablated BM, 23×106 Wt BM cells were transplanted into lethally irradiated Wt or p62−/− recipient mice. Sixteen hours after transplant, the recipient mice were sacrificed and the BM cells were harvested and cultured in triplicate for CFU-C assay as well as FACS analysis. BM homing was calculated as previously reported (Boggs, 1984).
Rest of experimental procedures is described in the Supplemental Experimental Procedures section.
Supplementary Material
Figure S1. Loss of p62 does not affect the levels of circulating medium- and long-term HSC or induce cell-autonomous HSC/P loss of activity. Related to Figure 1. (A) Count of circulating LT-HSC (Lin−/c-kit+/Sca-1+/CD34−/CD135−) in PB from Wt and p62−/− mice from mice in Fig. 1A. (B–C) Normalized medium (10-week)- and long (16 weeks) term chimera of Wt and p62−/− mice from experiment described in Fig. 1B (p=N.S.). (D–E) Counts of common lymphoid progenitor (D) and B-lymphoid cell populations in peripheral blood (E) immunophenotypically defined as described in Supplementary Material and Methods and in Fig. 1A, of Wt (grey bars) and p62-deficient mice (black bars). (F) Schema of competitive transplantation to generate primary and secondary chimeric mice. BM cells from CD45.2+ Wt or p62−/− mice were mixed with CD45.1+ B6.SJLPtprca Pep3b/BoyJ wild type BM cells and competitively transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ primary and secondary recipient mice. CD45.2+ chimera was monitored at different time points. (G–H) Evolution of CD45.2+ chimera in PB of primary recipient mice (G, n=6–8 mice per group) and secondary recipient mice (H, n=5–8 mice per group). Data are presented as mean ± SD. (I–K) Femoral content of BM myeloid progenitors (CFU-C) (I), CLP (J) and B-cell populations in Wt and p62−/− mice. N≥5 mice per group. (L–M) Evolution of absolute neutrophil (L) and platelet (M) counts in the PB of Wt or p62−/− mice after 5-FU administration. N=6–8 mice per group. Data are presented as mean ± SD.
Figure S2. p62 deficiency does not impair the proliferation of HSC or myeloid progenitors. Related to Figure 1. (A) Schema of non-competitive transplantation to generate hematopoietic p62−/− mice (H-p62−/−) and H-Wt controls. BM cells from CD45.2+ Wt or p62−/− mice were transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ wild type mice. (B) Frequency of hematopoietic progenitors in PB of recipient mice (n=8 mice per group) after 6 weeks of transplantation. (C–E) Cell cycle analysis of LT-HSC (C), ST-HSC (D) and LK (E) BM cells in primary Wt and p62−/− mice. (F–H) Cell cycle analysis of LT-HSC (F), ST-HSC (G) and LK (H) BM cells in Wt HM and p62−/− HM mice generated as described in Fig. 1E. N≥4 mice per group. Values represent mean ± SD. p=N.S. (I–K) Homing of Wt BM ST-HSC (I) and LSK cells (J) and LK cells (K) into myeloablated BM of Wt or p62−/− mice. N=5–8 mice per group in a minimum of two independent experiments. Values represent mean ± SEM. p=N.S.
Figure S3. BM derived MΦ contact with Obs and signal through FAK and NF-kB but not Erk or p38 MAPK and p62-deficient osteoclasts are indispensable. Related to Figures 2 and 3. (A) MΦ can be found in close proximity to endosteal Obs in vivo. F4/80 (green), Collagen I (ColI, red) and DAPI counterstain (blue) in longitudinal femoral sections of Wt HM and p62−/− HM mice. (B) Fraction of high magnification fields where MΦ (F4/80+, green) and Ob (ColI+) were found in either trabecular or endosteal bone. A minimum of 12 high magnification (scale bar= 20μm) fields were analyzed per bone. A minimum of three femora from different chimeric mice were analyzed per group. (C) Representative examples of TRAP staining of longitudinal sections of femurs from Coll1α-Cre;Wt and Coll1α-Cre; p62f/f femora, from Fig. 2J. (D) TRAP positive osteoclasts per field. A minimum of 17 fields were analyzed. Scale bar = 100μm. (E) BMNC from ubiquitin C-EGFP mice were cultured for 7 days with 100ng/ml M-CSF then phenotypically characterized by flow cytometry. (F) Activation of Ob FAK measured by phospho-flow analysis. The pFAK/FAK levels of Wt or p62 deficient Ob alone (solid bars) were compared to the levels of pFAK in Ob cultured for 24 hours with Wt EGFP+ MΦ (mosaic bars). N=3 independent cultures per group done by duplicate. **p<0.005; *** p<0.001. (G) Representative immunoblots of phosphorylated ERK and phosphorylated p38 expression in Wt or p62 deficient Ob with or without 24 hours contact of wild type MΦ. β-actin was used as a loading control. (H) Frequency of hematopoietic progenitors in PB of Wt or p62−/−Erk1−/− mice (n=6 mice per group). Values represent mean ± SEM. *p<0.05. (I–J) NF-κB p65 in nucleus from flow cytometry sorted Wt or p62−/− Ob (I) or MΦ (J) after 24 hours co-culture with wild type MΦ. 1 μM BAY 11-7085 (hatched bars) treated cells were compared to vehicle control (DMSO, solid bars) treated cells as in Figs. 3H–J. Values represent mean ± SEM. *p<0.05, **p<0.005, *** p<0.001.
Figure S4. Quantification of relevant chemokines and cytokines in plasma and extracellular femoral fluid of Wt HM and p62−/− HM mice. Related to Figure 4. Plasma concentrations of Ccl4 (A), Il-1α (B), Il-1β (C), Tnf-α (D), Ifn-γ (E), Il-10 (F), and G-csf (G) from Wt HM or p62−/− HM mice were detected and measured by luminex assay. Rnkl (H), Osteoprotegerenin (I), Osteocalcein (J), Leptin (K), Ccl3 (L), RANTES (M), and Vegf (N) in BM from Wt HM or p62−/− HM mice were also measured by luminex assay similarly to Fig. 4A. Values represent mean ± SEM with a minimum of 3 mice per group.
Figure S5. Cxcl12 levels are not reduced in plasma or bone of HM p62−/− mice, however, in vivo depletion of MΦ induces hypermigration to the level of HM p62−/− mice. Related to Figure 4. Plasma (A) and femoral (B) levels of Cxcl12 were determined by Elisa in specimens from Fig. 4A. * p<0.05. N≥4 mice per group. (C) Representative FACS dot plots to confirm the depletion of subsets of MΦ in vivo after clodronate-liposome administration. (D) Circulating CFU-C content in peripheral blood before or after injection of clodronate containing liposomes. Vehicle (PBS) containing liposomes were administered as control. N≥8 mice per group. Values represent mean ± SEM. *p<0.05;**p<0.01.
Figure S6. Ectopic expression of p62 and p62 mutant in p62−/− Ob and relation to IKK/NF-κB and Ccl4 expression or activity. Related to Figures 5 and 6. (A) Representative immunoblot of p62 expression in mock vector transduced Wt (Wt + Mock) or p62 deficient Ob (p62−/− + Mock) or full length of p62 transduced-p62 deficient Ob (p62−/− + p62) analyzed in Figs. 5A–E. Retroviral transduced cells were sorted by flow cytometry as a population of EGFP+. β-actin was used as a loading control. (B) Representative confocal microscopic images of NF-κB p65 (red) and nuclear (DAPI, blue) in retroviral-transduced (EGFP) Wt or p62−/− Ob. TNFα (10 ng/mL) was added to culture media of Ob for positive control of NF-κB p65 nuclear translocation. Data are representative of two independent experiments with similar results. (C) Representative immunoblots of IKK catalytic subunits α, β or γ cytosolic expression in in mock vector transduced Wt (Wt + Mock) or p62 deficient Ob (p62−/− + Mock) or full length of p62 transduced- p62 deficient Ob (p62−/− + p62) after 24 hours contact of wild type MΦ. β-actin was used as a loading control. (D) Representative immunoblot of P62 expression in mock vector transduced p62 deficient Ob (Mock) or Δ69-72A mutant of p62 transduced-p62 deficient Ob (Δ69-72A) used in Figs. 6A–C. Retroviral transduced cells were sorted by flow cytometry as a population of EGFP+. β-actin was used as a loading control. (E) Proposed model of interplay between MΦ, Ob and HSC/P. Contacting MΦ induces NF-κB activation in Ob. Activation of FAK, IKK and NF-κB in Ob is induced by macrophages resulting in inhibition of Ob differentiation and Ccl4 expression. NF-κB signaling intermediate levels are controlled by p62-dependent mechanisms of autophagic degradation and in absence of p62, osteogenesis and Ccl4 production are decreased in vitro and in vivo, resulting in loss of BM HSC/P retention.
Acknowledgments
This study was supported by the Heimlich Institute of Cincinnati, the Department of Defense (10580355), the National Institutes of Health (NIH; R01-HL087159 and HL087159S1) and funds from Hoxworth Blood Center and Cincinnati Children’s Hospital Medical Center (to J.A.C.); R01-CA132847 and R01-CA170225 (to J.M.), R01-CA134530 (to M.T.D-M); and R01-AR041255, R01-AR055913 and R01-AR056678 (to R.C.). K.H.C is a National Blood Foundation research funding awardee. A.S. was an awardee of The International Lady Tata Memorial Trust, London, and A,S. acknowledges funding from CSIR, DST-SERB (SB/SO/HS-053/2013) and Ramalingaswami Fellowship (BT/RLF/RE-ENTRY/06/2010), Department of Biotechnology, Govt. of India. We thank Cincinnati Children’s Research Foundation Mouse, Flow Cytometry and Immunobiology Cores for services, supported by the Center of Excellence in Molecular Hematology (P30 DK090971). We also thank Yi Zheng and Hartmut Geiger for critical reading of the manuscript and Ms. Margaret O’Leary for editorial revision.
Footnotes
AUTHOR CONTRIBUTIONS
K.H.C., A.S., M.D.-M., J.M. and J.A.C. designed research. K.H.C. and A.S. performed majority of the experiments with the help from A.D., S.J.L, D.G-N, S.R.H. and A.M.W. R.C.N. and K.H.C. performed confocal image analysis. R.P. performed micro CT analysis of femurs. D.T.S. contributed with reagents and experimental design in NFκB activation experiments. M.W and R.C. contributed to bone histomorphometry analysis and bone experimental design and interpretation. B.J.A. performed bioinformatics analysis of Wt and p62-deficient Ob. K.H.C., A.S. and J.A.C. analyzed data; and K.H.C., A.S. and J.A.C. wrote the paper.
The authors declare not to have any relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Broxmeyer HE. CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells. J Immunol. 2009;183:7478–7488. doi: 10.4049/jimmunol.0900542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Johansen T. p62/p62: a missing link between protein aggregates and the autophagy machinery. Autophagy. 2006;2:138–139. doi: 10.4161/auto.2.2.2405. [DOI] [PubMed] [Google Scholar]
- Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16:277–286. doi: 10.1002/ajh.2830160309. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, NAG, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Su YC, Hu CW, Lei HY. TLR2-dependent selective autophagy regulates NF-kappaB lysosomal degradation in hepatoma-derived M2 macrophage differentiation. Cell Death Diff. 2013;20:515–523. doi: 10.1038/cdd.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D’Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279:29121–29129. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450–6. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Cell Tiss Org. 2009;189:144–152. doi: 10.1159/000151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma Y, Honjo T, Jelinek DF, Windle JJ, Shin J, Roodman GD, Kurihara N. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood. 2009;113:4894–4902. doi: 10.1182/blood-2008-08-173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J Bone Min Res. 2012;27:1439–1447. doi: 10.1002/jbmr.1668. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Itakura E, Mizushima N. p62 Targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J Cell Biol. 2011;192:17–27. doi: 10.1083/jcb.201009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Katsumoto TR, Duda J, Kim A, Wardak Z, Dranoff G, Clapp DW, Shannon K. Granulocyte/macrophage colony-stimulating factor and accessory cells modulate radioprotection by purified hematopoietic cells. J Exp Med. 2005;201:853–858. doi: 10.1084/jem.20041504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kim JB, Leucht P, Luppen CA, Park YJ, Beggs HE, Damsky CH, Helms JA. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone. 2007;41:39–51. doi: 10.1016/j.bone.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Pfluger PT, Kim JY, Nogueiras R, Duran A, Pages G, Pouyssegur J, Tschop MH, Diaz-Meco MT, Moscat J. A functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogenesis. EMBO Rep. 2010;11:226–232. doi: 10.1038/embor.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–8. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Min Res. 2013;28:2414–2430. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenhuis A, van Veelen PA, de Looper H, van Boxtel N, van den Berge IJ, Sun SM, Taskesen E, Stern P, de Ru AH, van Adrichem AJ, Demmers J, Jongen-Lavrencic M, Löwenberg B, Touw IP, Sharp PA, Erkeland SJ. MiR-17/20/93/106 promote hematopoietic cell expansion by targeting sequestosome 1-regulated pathways in mice. Blood. 2011;118:916–25. doi: 10.1182/blood-2011-02-336487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by PB1 domain interactions. Mol Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Coates PJ, Lorimore SA, Wright EG. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232:289–99. doi: 10.1002/path.4299. [DOI] [PubMed] [Google Scholar]
- Muller TD, Lee SJ, Jastroch M, Kabra D, Stemmer K, Aichler M, Abplanalp B, Ananthakrishnan G, Bhardwaj N, Collins S, et al. p62 links beta-adrenergic input to mitochondrial function and thermogenesis. J Clin Invest. 2013;123:469–478. doi: 10.1172/JCI64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Science. 1999;283:845–8. [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–96. [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Walsh JP, Layfield R, Ratajczak T, Xu J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocrine Rev. 2013;34:501–524. doi: 10.1210/er.2012-1034. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metabolism. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu LC, Cao Y, Schett G, Wagner EF, Karin M. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, Cancelas JA. Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci USA. 2011;108:9957–9962. doi: 10.1073/pnas.1103132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Gene Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–7. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Chang JH, Jin J. Regulation of nuclear factor-kappaB in autoimmunity. Trends Immunol. 2013;34:282–289. doi: 10.1016/j.it.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Whitehouse CA, Waters S, Marchbank K, Horner A, McGowan NW, Jovanovic JV, Xavier GM, Kashima TG, Cobourne MT, Richards GO, et al. Neighbor of Brca1 gene (Nbr1) functions as a negative regulator of postnatal osteoblastic bone formation and p38 MAPK activity. Proc Natl Acad Sci USA. 2010;107:12913–12918. doi: 10.1073/pnas.0913058107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, Nowlan B, Cisterne A, Bendall LJ, Sims NA, Levesque JP. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- Yang JQ, Liu H, Diaz-Meco MT, Moscat J. NBR1 is a new PB1 signalling adapter in Th2 differentiation and allergic airway inflammation in vivo. EMBO J. 2010;29:3421–3433. doi: 10.1038/emboj.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Ma C, O’Connell RM, Mehta A, Diloreto R, Heath JR, Baltimore D. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 2014;14:445–459. doi: 10.1016/j.stem.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JR, Garrett Y, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Loss of p62 does not affect the levels of circulating medium- and long-term HSC or induce cell-autonomous HSC/P loss of activity. Related to Figure 1. (A) Count of circulating LT-HSC (Lin−/c-kit+/Sca-1+/CD34−/CD135−) in PB from Wt and p62−/− mice from mice in Fig. 1A. (B–C) Normalized medium (10-week)- and long (16 weeks) term chimera of Wt and p62−/− mice from experiment described in Fig. 1B (p=N.S.). (D–E) Counts of common lymphoid progenitor (D) and B-lymphoid cell populations in peripheral blood (E) immunophenotypically defined as described in Supplementary Material and Methods and in Fig. 1A, of Wt (grey bars) and p62-deficient mice (black bars). (F) Schema of competitive transplantation to generate primary and secondary chimeric mice. BM cells from CD45.2+ Wt or p62−/− mice were mixed with CD45.1+ B6.SJLPtprca Pep3b/BoyJ wild type BM cells and competitively transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ primary and secondary recipient mice. CD45.2+ chimera was monitored at different time points. (G–H) Evolution of CD45.2+ chimera in PB of primary recipient mice (G, n=6–8 mice per group) and secondary recipient mice (H, n=5–8 mice per group). Data are presented as mean ± SD. (I–K) Femoral content of BM myeloid progenitors (CFU-C) (I), CLP (J) and B-cell populations in Wt and p62−/− mice. N≥5 mice per group. (L–M) Evolution of absolute neutrophil (L) and platelet (M) counts in the PB of Wt or p62−/− mice after 5-FU administration. N=6–8 mice per group. Data are presented as mean ± SD.
Figure S2. p62 deficiency does not impair the proliferation of HSC or myeloid progenitors. Related to Figure 1. (A) Schema of non-competitive transplantation to generate hematopoietic p62−/− mice (H-p62−/−) and H-Wt controls. BM cells from CD45.2+ Wt or p62−/− mice were transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ wild type mice. (B) Frequency of hematopoietic progenitors in PB of recipient mice (n=8 mice per group) after 6 weeks of transplantation. (C–E) Cell cycle analysis of LT-HSC (C), ST-HSC (D) and LK (E) BM cells in primary Wt and p62−/− mice. (F–H) Cell cycle analysis of LT-HSC (F), ST-HSC (G) and LK (H) BM cells in Wt HM and p62−/− HM mice generated as described in Fig. 1E. N≥4 mice per group. Values represent mean ± SD. p=N.S. (I–K) Homing of Wt BM ST-HSC (I) and LSK cells (J) and LK cells (K) into myeloablated BM of Wt or p62−/− mice. N=5–8 mice per group in a minimum of two independent experiments. Values represent mean ± SEM. p=N.S.
Figure S3. BM derived MΦ contact with Obs and signal through FAK and NF-kB but not Erk or p38 MAPK and p62-deficient osteoclasts are indispensable. Related to Figures 2 and 3. (A) MΦ can be found in close proximity to endosteal Obs in vivo. F4/80 (green), Collagen I (ColI, red) and DAPI counterstain (blue) in longitudinal femoral sections of Wt HM and p62−/− HM mice. (B) Fraction of high magnification fields where MΦ (F4/80+, green) and Ob (ColI+) were found in either trabecular or endosteal bone. A minimum of 12 high magnification (scale bar= 20μm) fields were analyzed per bone. A minimum of three femora from different chimeric mice were analyzed per group. (C) Representative examples of TRAP staining of longitudinal sections of femurs from Coll1α-Cre;Wt and Coll1α-Cre; p62f/f femora, from Fig. 2J. (D) TRAP positive osteoclasts per field. A minimum of 17 fields were analyzed. Scale bar = 100μm. (E) BMNC from ubiquitin C-EGFP mice were cultured for 7 days with 100ng/ml M-CSF then phenotypically characterized by flow cytometry. (F) Activation of Ob FAK measured by phospho-flow analysis. The pFAK/FAK levels of Wt or p62 deficient Ob alone (solid bars) were compared to the levels of pFAK in Ob cultured for 24 hours with Wt EGFP+ MΦ (mosaic bars). N=3 independent cultures per group done by duplicate. **p<0.005; *** p<0.001. (G) Representative immunoblots of phosphorylated ERK and phosphorylated p38 expression in Wt or p62 deficient Ob with or without 24 hours contact of wild type MΦ. β-actin was used as a loading control. (H) Frequency of hematopoietic progenitors in PB of Wt or p62−/−Erk1−/− mice (n=6 mice per group). Values represent mean ± SEM. *p<0.05. (I–J) NF-κB p65 in nucleus from flow cytometry sorted Wt or p62−/− Ob (I) or MΦ (J) after 24 hours co-culture with wild type MΦ. 1 μM BAY 11-7085 (hatched bars) treated cells were compared to vehicle control (DMSO, solid bars) treated cells as in Figs. 3H–J. Values represent mean ± SEM. *p<0.05, **p<0.005, *** p<0.001.
Figure S4. Quantification of relevant chemokines and cytokines in plasma and extracellular femoral fluid of Wt HM and p62−/− HM mice. Related to Figure 4. Plasma concentrations of Ccl4 (A), Il-1α (B), Il-1β (C), Tnf-α (D), Ifn-γ (E), Il-10 (F), and G-csf (G) from Wt HM or p62−/− HM mice were detected and measured by luminex assay. Rnkl (H), Osteoprotegerenin (I), Osteocalcein (J), Leptin (K), Ccl3 (L), RANTES (M), and Vegf (N) in BM from Wt HM or p62−/− HM mice were also measured by luminex assay similarly to Fig. 4A. Values represent mean ± SEM with a minimum of 3 mice per group.
Figure S5. Cxcl12 levels are not reduced in plasma or bone of HM p62−/− mice, however, in vivo depletion of MΦ induces hypermigration to the level of HM p62−/− mice. Related to Figure 4. Plasma (A) and femoral (B) levels of Cxcl12 were determined by Elisa in specimens from Fig. 4A. * p<0.05. N≥4 mice per group. (C) Representative FACS dot plots to confirm the depletion of subsets of MΦ in vivo after clodronate-liposome administration. (D) Circulating CFU-C content in peripheral blood before or after injection of clodronate containing liposomes. Vehicle (PBS) containing liposomes were administered as control. N≥8 mice per group. Values represent mean ± SEM. *p<0.05;**p<0.01.
Figure S6. Ectopic expression of p62 and p62 mutant in p62−/− Ob and relation to IKK/NF-κB and Ccl4 expression or activity. Related to Figures 5 and 6. (A) Representative immunoblot of p62 expression in mock vector transduced Wt (Wt + Mock) or p62 deficient Ob (p62−/− + Mock) or full length of p62 transduced-p62 deficient Ob (p62−/− + p62) analyzed in Figs. 5A–E. Retroviral transduced cells were sorted by flow cytometry as a population of EGFP+. β-actin was used as a loading control. (B) Representative confocal microscopic images of NF-κB p65 (red) and nuclear (DAPI, blue) in retroviral-transduced (EGFP) Wt or p62−/− Ob. TNFα (10 ng/mL) was added to culture media of Ob for positive control of NF-κB p65 nuclear translocation. Data are representative of two independent experiments with similar results. (C) Representative immunoblots of IKK catalytic subunits α, β or γ cytosolic expression in in mock vector transduced Wt (Wt + Mock) or p62 deficient Ob (p62−/− + Mock) or full length of p62 transduced- p62 deficient Ob (p62−/− + p62) after 24 hours contact of wild type MΦ. β-actin was used as a loading control. (D) Representative immunoblot of P62 expression in mock vector transduced p62 deficient Ob (Mock) or Δ69-72A mutant of p62 transduced-p62 deficient Ob (Δ69-72A) used in Figs. 6A–C. Retroviral transduced cells were sorted by flow cytometry as a population of EGFP+. β-actin was used as a loading control. (E) Proposed model of interplay between MΦ, Ob and HSC/P. Contacting MΦ induces NF-κB activation in Ob. Activation of FAK, IKK and NF-κB in Ob is induced by macrophages resulting in inhibition of Ob differentiation and Ccl4 expression. NF-κB signaling intermediate levels are controlled by p62-dependent mechanisms of autophagic degradation and in absence of p62, osteogenesis and Ccl4 production are decreased in vitro and in vivo, resulting in loss of BM HSC/P retention.