Abstract
Adhesion G protein-coupled receptors (aGPCRs) comprise the second largest yet least studied class of the GPCR superfamily. aGPCRs are involved in many developmental processes, immune and synaptic functions, but the mode of their signal transduction is unclear. Here, we show that a short peptide sequence (termed the Stachel sequence) within the ectodomain of two aGPCRs, GPR126 and GPR133, functions as a tethered agonist. Upon structural changes within the receptor ectodomain, this intramolecular agonist is exposed to the 7-transmembrane helix domain, which triggers G-protein activation. Our studies show high specificity of a given Stachel sequence for its receptor. Finally, the function of Gpr126 is abrogated in zebrafish with a mutated Stachel sequence, and signaling is restored in hypomorphic gpr126 zebrafish mutants upon exogenous Stachel peptide application. These findings illuminate a previously unknown mode of aGPCR activation, and can initiate the development of specific ligands for this currently untargeted GPCR family.
Introduction
Adhesion G protein-coupled receptors (aGPCRs) are among the largest proteins in nature and composed of a long extracellular domain (ECD), a seven-transmembrane domain (7TM) and an intracellular C-terminal tail (ICD) (Fig. 1A) (Bjarnadottir et al., 2004; McMillan et al., 2002). A further feature of the class is an autoproteolytic cleavage event that occurs at the GPCR Proteolytic Site (GPS), encompassed within the GPCR Autoproteolysis Inducing (GAIN) domain, which cleaves aGPCRs into an N-terminal fragment (NTF) and a C-terminal fragment (CTF) (Arac et al., 2012) (Fig. 1A). aGPCRs play essential roles in the control of cell and tissue polarity (Lawrence et al., 2007) and can modulate synaptic functions (O’Sullivan et al., 2012; Sudhof, 2001). Although increasing information about aGPCR relevance is available from mutant animal models, human diseases and variant-associated phenotypes, little is known about the molecular function, activation, and signal transduction of this receptor class (Langenhan et al., 2013; Liebscher et al., 2013).
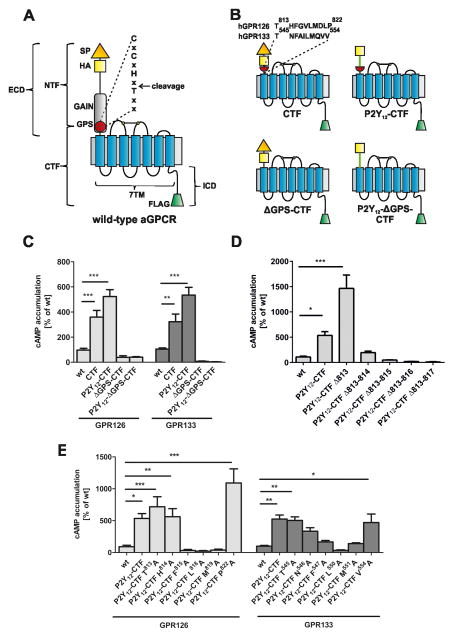
Fig. 1. Identification of a putative agonistic region in GPR126 and GPR133.
(A) Cartoon of a prototypical aGPCR. The extracellular domain (ECD) contains a signal peptide (SP) and the GAIN/GPS domain. aGPCRs also possess a 7TM domain and an intracellular domain (ICD). Autoproteolysis at the GPS yields an N-terminal fragment (NTF) and a C-terminal fragment (CTF). For immunological detection, all constructs were epitope-tagged with an N-terminal HA epitope (yellow square) and a C-terminal FLAG epitope (green trapezoid). (B) hGPR126 and hGPR133 constructs, CTF and ΔGPS-CTF, were generated which lack the NTF and the ECD, respectively. Chimeric constructs were generated by fusing the N terminus of the human P2Y12 receptor (green line) onto the GPR126 and GPR133 mutants. The red half-circle symbolizes the C-terminal portion of the GPS after its cleavage site. See also supp. Table S1. (C–E) cAMP levels from COS-7 cells transfected with wt and mutant GPR126 and GPR133. (C) P2Y12-CTF mutants have increased basal activity compared to wt, which is abolished in ΔGPS-CTF mutants. (D) Constitutive activity of P2Y12-CTF(GPR126) is increased by deletion of Thr813. Receptor activity is abolished when the first three or more aa after the cleavage site are deleted. (E) Single positions within the C-terminal GPS sequence were mutated in GPR126 and GPR133 to alanine as shown. See suppl. Fig. S1C-F for expression studies of all constructs. Data are shown as means ± SEM of three independent experiments each performed in triplicates. EV served as negative control (eV; cAMP level: 3.68 ± 2.54 nM). Statistics were performed by two-way ANOVA and Bonferoni post-hoc test: *p<0.05; **p<0.01; ***p<0.001.
The first indirect functional data for G-protein coupling by aGPCRs came from studies on GPR56 by knockdown experiments of G12/13/p115 RhoGEF pathway components (Iguchi et al., 2008). An intriguing observation was reported in gpr126 mutant zebrafish (zf), which exhibit defects in peripheral myelination (Monk et al., 2009). This phenotype was reversible through forskolin-induced cAMP elevation, suggesting Gs-protein coupling. More direct evidence for Gs-protein coupling was provided by measuring intracellular cAMP levels induced by basal activity of the aGPCRs GPR133 (Bohnekamp and Schoneberg, 2011) and GPR126 (Mogha et al., 2013). Further, experiments with chimeric G proteins, stoichiometric titrations of the Gαs subunit and the receptor as well as Gαs subunit knockdown experiments (Bohnekamp and Schoneberg, 2011) strongly support G-protein coupling for GPR133.
Although it is now clear that aGPCRs couple to G proteins, it remains unclear whether endogenous binding partners can induce activation of aGPCRs. Interestingly, increased aGPCR activity has been described for several aGPCRs when an N-terminal deletion mutant receptor is expressed (Okajima et al., 2010; Paavola et al., 2014; Paavola et al., 2011; Yang et al., 2011) (see Fig. 1A). These observations led to the assumption that the ectodomain functions as an inverse agonist, although at least two scenarios of aGPCR activation have been proposed (Liebscher et al., 2013): 1) the ectodomain contains an inverse agonist that inhibits 7TM signaling; 2) ligand binding at the ECD or NTF removal changes the conformation of an aGPCR and exposes a tethered agonist (suppl. Fig. S1A–B).
To test these two models, we used human (h) GPR126 and GPR133 to analyse the contribution of the ECD to receptor basal activity, since Gs-protein coupling has been experimentally suggested for these aGPCRs (Bohnekamp and Schoneberg, 2011; Gupte et al., 2012; Mogha et al., 2013). Systematic mutagenesis studies revealed tethered peptide sequences within the C-terminal-most part of the ECD that specifically activate G-protein signaling via 7TM interactions in vitro. Finally, we performed loss-of-function and rescue experiments in zf gpr126 mutants to confirm the in vivo and evolutionarily conserved significance of the tethered agonist. Together, our study defines a previously unknown mechanism of aGPCR activation.
Results
ECD deletion activates GPR126 and GPR133
First, we deleted the ECDs of hGPR126 and hGPR133 at their natural GPS cleavage sites and tested the mutants in cAMP assays. In these constructs, termed CTF(GPR126) and CTF(GPR133), the NTF between the signal peptide and the GPS cleavage site was removed but the ECD part located C-terminally to the GPS cleavage site remained attached to the 7TM (CTF in Fig. 1B, suppl. Table S1). All mutants lacking the ECD displayed significantly increased basal activities in cAMP assays (Fig. 1C), consistent with results from other NTF-deficient aGPCRs. Both mutants were poorly detected at the cell surface via HA-tag staining (suppl. Fig. S1C). Accordingly, total ELISA and confocal imaging revealed an absence of the HA-tag in CTF(GPR126) constructs. However, confocal imaging of the C-terminal FLAG-tag showed specific membrane fluorescence (suppl. Fig. S1D). We therefore speculate that the HA tags in the CTF mutant constructs are processed during intracellular protein maturation, thereby precluding detection. Because the N termini of rhodopsin-like receptors can improve cell surface expression and detection of other GPCRs (Bohnekamp and Schoneberg, 2011; Staubert et al., 2010), we added an HA-tagged P2Y12 N terminus to the residual ECD of the CTF mutants. This generated chimeric P2Y12-CTF(GPR126) and P2Y12-CTF(GPR133) receptors (Fig. 1B), which enabled proper plasma membrane detection via HA-tag visualization (suppl. Fig. S1C). As observed for the CTF constructs, P2Y12-CTF(GPR126) and P2Y12-CTF(GPR133) displayed high constitutive activity (Fig. 1C). These results demonstrate that deletion of the NTF activates hGPR126 and hGPR133.
The ECDs of GPR126 and GPR133 contain agonistic domains
We generated GPR126 and GPR133 mutants in which the entire ECD, including all of the GPS motif, was deleted or replaced by the N terminus of P2Y12 (ΔGPS-CTF; P2Y12-ΔGPS-CTF, Fig. 1B). None of the constructs displayed constitutive activity (Fig. 1C), although these chimeras were expressed at the cell surface (suppl. Fig. S1C). These results argue against the inverse agonist model of aGPCR activation because constitutive activity caused by release of an inverse agonist would not depend on the presence of the residual GPS motif. These results point towards an activation model that requires the residual GPS motif, and we hypothesized that the GPS sequence downstream of the cleavage site contains determinants required for receptor activation.
To identify this potential tethered agonist, we sequentially deleted amino acids (aa) C-terminal to the GPS cleavage site in GPR126. Functional analysis showed that while the N-terminal-most aa (Thr813, Fig. 1B) was not essential for receptor activation (Fig. 1D), deletion of the first two as well as larger deletions that removed aa following Thr813 abolished basal receptor activity. This abolishment was not due to expression changes since total and cell surface expression levels were not significantly different between the constructs (suppl. Fig. S1E). To maintain correct aa length C-terminal to the cleavage site, we exchanged several positions with alanine. Again, mutants with an exchange of position 813 retained constitutive activity whereas the exchange of positions 815, 818 and 819 abolished activity in P2Y12-CTF(GPR126) (Fig. 1E), while expression levels were not affected (suppl. Fig. S1F). Mutagenesis studies at corresponding positions in P2Y12-CTF(GPR133) revealed almost identical results (Fig. 1E; suppl. Fig. S1F). These experiments support the existence of a defined agonistic region C-terminal to the GPS.
A tethered peptide activates GPR126
To demonstrate that the aa sequence C-terminal to the GPS cleavage site has agonistic properties, we tested peptides derived from this domain on P2Y12-ΔGPS-CTF(GPR126). Excitingly, systematic truncation of the peptide’s C terminus revealed several agonistic peptides (Fig. 2A). The most efficient peptide, p16 (16 aa long), was used for further structure-function studies. N-terminal deletion of the first two aa abolished agonistic abilities of p16 (p16-1, p16-2; Fig. 2A). This does not contradict the results of Fig. 1D–E, because in the original CTF mutants, the first aa were replaced by the P2Y12 N terminus or by alanine. Thus, these changes are tolerated, whereas the deletions in p16 are not. N-terminal extension beyond the cleavage site by 1 (p16+1) or 2–4 (p16+2 to p16+4) aa showed reduced and no agonistic activity of p16, respectively (Fig. 2A). This indicates that non-cleaved aa upstream of Thr813 are not part of the agonistic structure. In concentration-response curves, p16 displayed low potency (EC50 >400 μM) on both P2Y12-ΔGPS-CTF(GPR126) and wild-type (wt) GPR126 (Fig. 2B), which can be explained by the natural 1:1 stoichiometry of the covalently bound agonist in its natural conformation. The higher cell surface expression of wt GPR126 compared to P2Y12-ΔGPS-CTF(GPR126) (suppl. Fig. S1C) explains the increased efficacy of p16 on wt GPR126 activation. Time course analyses of cAMP accumulation (suppl. Fig. S2A) and GTPγS binding assays (suppl. Fig. S2B) in response to p16 were performed, supporting p16-induced G-protein coupling in GPR126-transfected cells. Note, eV transfected cells showed residual cAMP accumulation (Fig. 2C) and GTPγS binding, indicating endogenous expression of GPR126 in COS-7 cells. This was confirmed by RT-PCR (suppl. Fig. S2C), cAMP assays (Fig. 2C) and kinetic EPIC measurements with siRNA-mediated knockdown of the endogenous GPR126 (suppl. Fig. S2D–E).
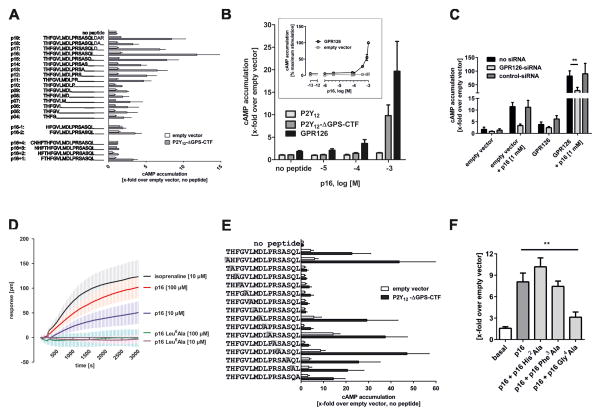
Fig. 2. GPR126 agonistic peptides are derived from the C-terminal part of the GPS.
(A) Application of 1 mM peptides of different lengths derived from the C-terminal part of the GPS beginning at the cleavage site of GPR126 revealed agonistic properties as measured by cAMP accumulation. The highest agonistic efficacy was detected for a peptide containing 16 amino acids (p16). Negative controls: eV, and GPR126-P2Y12-ΔGPS-CTF mutant. Basal cAMP levels were3.8 ± 1.6 nM. ( B) Different p16 concentrations were tested on wt P2Y12, wt GPR126, and P2Y12-ΔGPS-CTF. Inset: concentration-response curve of p16 at wt GPR126 revealed an EC50 value >400 μM. Basal eV levels were 3.2 ± 0.7 nM. (C) COS-7 cells endogenously express low levels of GPR126 (see suppl. Fig S2C). Endogenous and transfected GPR126 are knocked down with primate GPR126-specific siRNA as shown by abolished cAMP formation (x-fold over eV; basal cAMP: 5.5 ± 2.2 nM). This was confirmed by a dynamic mass redistribution assay (Epic Biosensor Measurements) (suppl. Fig. S2D) and reduced cell surface ELISA (see suppl. Fig S2E). (D) The specificity of p16 was confirmed on endogenous GPR126. Mutation of position 6 (Leu6Ala) abolished the response of p16 in EPIC measurements, as indicated by a picometer (pm) shift of the resonant wavelength caused by dynamic mass redistribution within the cell. (E) A systematic alanine-scan within the p16 peptide showed that the six amino acids downstream of Thr813 are required for receptor activation. Basal cAMP levels were 3.8 ± 1.6 nM. (F) p16 Gly4Ala (1 mM) blocked activation of GPR126 by p16 (500 μM). Basal cAMP levels were 18.7 ± 9.4 nM. Data are shown as means ± SEM of three independent experiments each performed in triplicates. Statistics were performed by two-way ANOVA and Bonferoni post-hoc test: *p<0.05; **p<0.01; ***p<0.001.
The endogenous expression of GPR126 and the high sensitivity of EPIC technology enabled us to test p16 apart from heterologous overexpression systems. As shown in Fig. 2D, p16 induced concentration-dependent cellular responses very similar to those found with isoprenaline and β-adrenergic receptor endogenously expressed in COS-7 cells. Mutation of position 6 (Leu6Ala) in p16 abolished the response (Fig. 2D), confirming specificity. To identify functionally relevant positions in the peptide, we performed a systematic alanine scan (Fig. 2E). As expected from our receptor mutagenesis data (Fig. 1D/E), the more N-terminal aa (positions +2 to +7) are required for agonistic activity, whereas positions +8, +10, +12, and +14 to +16 can be replaced with Ala and still show agonistic properties. These data are in line with a high evolutionary conservation of the N-terminal portion of this peptide sequence (suppl. Fig. S2F). Interestingly, the peptide p16 Gly4Ala blocked p16-induced GPR126 activation at double concentration (Fig. 2F), indicating that p16 Gly4Ala can compete with the p16 binding site. Together, these data support the notion that the tethered peptide, p16, activates GPR126.
A tethered peptide activates GPR133
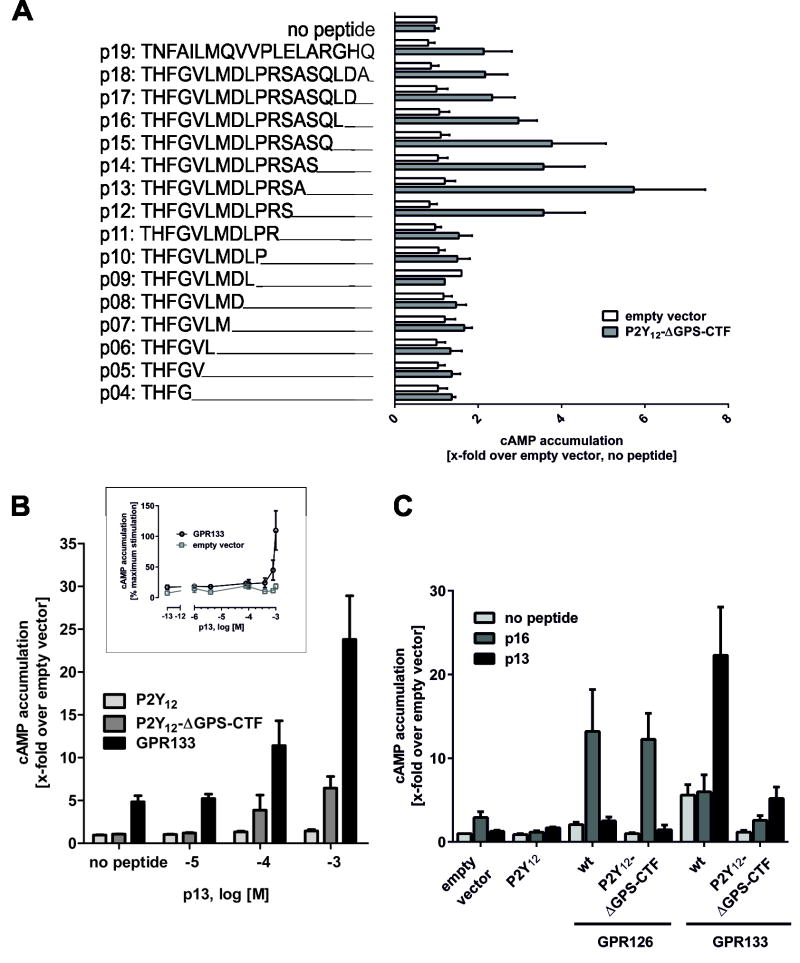
To determine if activation by a tethered peptide is common for aGPCRs, we performed similar studies with GPR133. The P2Y12-ΔGPS-CTF(GPR133) can be activated by a peptide derived from the 13 aa (p13) downstream of the putative cleavage site (Fig. 3A). Concentration-response measurements of p13 revealed specific activity on P2Y12-ΔGPS-CTF(GPR133) and wt receptor (EC50 >400 μM; Fig. 3B). The derived peptides were highly specific for the aGPCR from which they originated: GPR133 p13 did not activate GPR126, and GPR126 p16 did not activate GPR133 (Fig. 3C). Because the importance of GPS cleavage for aGPCR expression and activity has been controversially discussed (Liebscher et al., 2013), we tested two cleavage-deficient mutants, GPR126T841A (Moriguchi et al., 2004) and GPR133H540R (Bohnekamp and Schoneberg, 2011). Both mutants were expressed and activated by their respective peptides (suppl. Fig. S2G–I), indicating that cleavage at the GPS is not required for aGPCR activation by the tethered agonistic peptides. These data demonstrate that the tethered peptide, p13, activates GPR133. Together with our analysis of GPR126, these studies suggest that tethered peptide activation is a common signaling modality for the aGPCR class.
Fig. 3. Tethered agonistic peptides are receptor-specific.
(A) Application of 1 mM peptides of different lengths derived from the C-terminal part of the GPS beginning at the cleavage site of GPR133 revealed agonistic properties as measured by cAMP accumulation. The highest agonistic efficacy was detected for a peptide containing 13 amino acids (p13). Negative controls: eV, and GPR126-P2Y12-ΔGPS-CTF mutant. Basal cAMP levels were 5.2 ± 2.0 nM. (B) Concentration-response curve of the p13 peptide revealed an EC50 > 400 μM. Basal eV levels were 2.9 ± 0.2 nM. (C) Specificity of the p16 (GPR126) and the p13 (GPR133) peptides were verified using wt P2Y12, wt GPR126 and wt GPR133 as controls. p16 peptide activated wt GPR126 and P2Y12-ΔGPS-CTF(GPR126) whereas it exhibited unspecific activity in control receptors due to endogenous expression of GPR126 in COS-7 cells (Fig. 2). The p13 peptide specifically activated wt GPR133 and P2Y12-ΔGPS-CTF(GPR133). Basal cAMP levels were 3.0 ± 0.8 nM. Data are shown as means ± SEM of three independent experiments each performed in triplicates. Statistics were performed by two-way ANOVA and Bonferoni post-hoc test: *p<0.05; **p<0.01; ***p<0.001.
Tethered peptide activation of Gpr126 in vivo
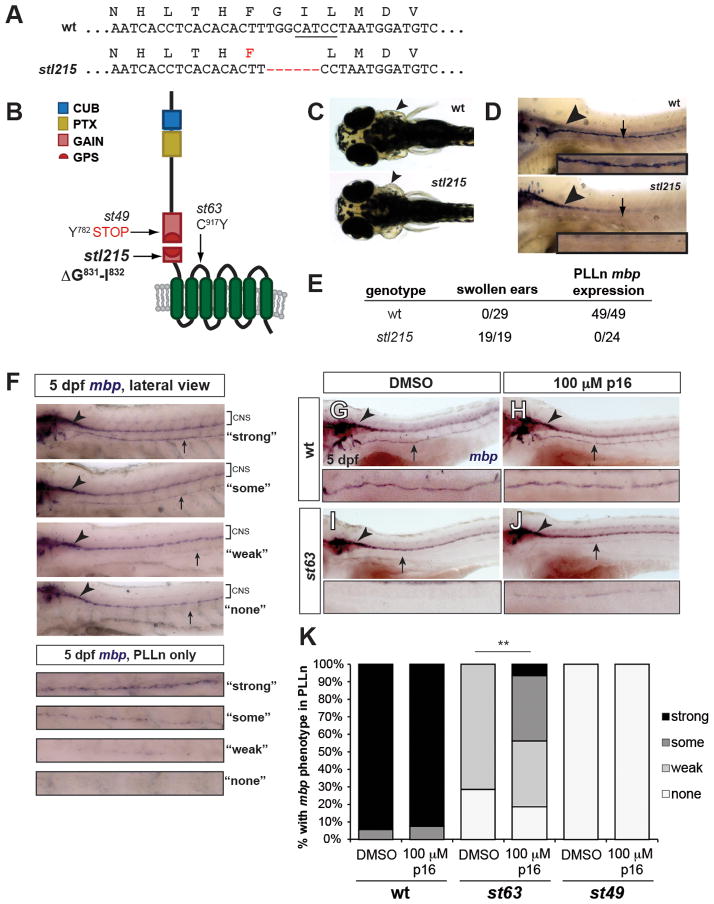
We next sought to test the in vivo relevance of aGPCR tethered peptide activation. For these studies, we used zebrafish because previous mutant analyses demonstrated that Gpr126 is essential for Schwann cell myelination and ear development and that these physiological functions require cAMP elevation (Geng et al., 2013; Monk et al., 2009). Although several zf gpr126 mutant alleles have been recovered in genetic screens (Pogoda et al., 2006), none specifically affect the tethered agonist sequence. Therefore, we utilized Transcription-Activator-Like-Effector-Nucleases (TALENs) to target this region (suppl. Fig. S3A–B); we isolated a mutant, gpr126stl215, which lacks only two codons (Gly831-Ile832) within the tethered agonist sequence (Fig. 4A–B; suppl. Fig. S3C). gpr126stl215 mutants were grossly normal compared to wt animals (suppl. Fig. S3D), but developed swollen ears (Fig. 4C), failed to express myelin basic protein (mbp, a marker of mature Schwann cells) along the posterior lateral line nerve (PLLn) (Fig. 4D–E) and did not myelinate peripheral axons (suppl. Fig. S3E–H). These defects completely phenocopy the previously published gpr126st49 mutant, which has an early stop codon in the GAIN domain upstream of the GPS motif (Fig. 4B) (Monk et al., 2009). Importantly, the Gly831-Ile832 deletion introduced by the gpr126stl215 mutation does not alter cell surface expression of the receptor (suppl. Fig. S4A–B). We therefore conclude that the phenotypes observed in gpr126stl215 mutants are caused by loss of a functional tethered agonist.
Fig. 4. Tethered agonistic peptides function in vivo.
(A) Sequences of wild-type (wt) and stl215 alleles. stl215 is characterized by a 6 base pair (bp) in-frame deletion which results in the removal of amino acids Gly831 and Ile832. The BtsCI restriction enzyme site targeted by the TALEN is underlined. (B) Schematic representation of Gpr126 showing the stl215 allele compared to st49 and st63 alleles. (C) Dorsal view of 4 dpf larvae. Arrowheads indicate normal ear morphology in the gpr126+/+ larva (wt) and swollen ears in the gpr126stl215/stl215 larva (stl215). (D) Lateral view of whole-mount mbp in situ hybridization (WISH) of zf larvae at 4 dpf. The posterior lateral line nerve (PLLn) is marked with an arrow; mbp expression in the central nervous system (CNS) is indicated with an arrowhead. mbp expression can be observed in the CNS but not in the PLLn of gpr126stl215/215 mutant larvae (st215). (E) Quantification of swollen ear phenotype and PLLn mbp expression out of the total number of larvae scored per genotype (wt = gpr126+/+ and gpr126stl215/+). (F–J) WISH of 5 dpf larvae showing mbp expression in CNS (arrowhead) and PLLn (arrow). (F) Scoring rubric for PLLn mbp expression, enlarged panels show PLLn only key. “Strong” = strong and consistent mbp expression, “some” = weak but consistent mbp expression, “weak” = weak and patchy mbp expression, “none” = no mbp expression. wt larvae treated with DMSO (G) or 100 μM p16 (H) have strong PLLn mbp expression. DMSO-treated gpr126st63/st63 mutants have reduced PLLn mbp expression (I), which is significantly rescued with peptide treatment (J). (K) Quantification of WISH experiments. Bars indicate proportion of larvae with each PLLn mbp expression phenotype (as defined in F). **p<0.0001, combined gpr126st63/st63 mutants with “some” and “strong” vs. combined gpr126st63/st63 mutants with “weak” and “none”, Fisher’s Exact Test. wt = gpr126+/+ and gpr126+/st63 siblings of gpr126st63/st63 mutants. N=3 technical replicates, n=105 wt (51 DMSO-treated, 54 peptide-treated), n=53 gpr126st63/st63 (21 DMSO-treated, 32 peptide-treated), n=8 gpr126st49/st49 (4 DMSO-treated, 4 peptide-treated).
Finally, we tested whether p16 serves as an agonist for endogenous Gpr126 in vivo using zf PLLn mbp expression as an assay. The gpr126st63 allele contains a point mutation in the first extracellular loop of the 7TM that converts a conserved cysteine residue to tyrosine (C917Y, Fig. 4B) (Monk et al., 2009). This mutant receptor shows reduced cell surface expression compared to wt (~60% of wt levels; suppl. Fig. S4A) and a concomitant reduction in basal activity (suppl. Fig. S4B). In vivo, mbp expressed is reduced, but not absent, along the PLLn (Pogoda et al., 2006). In contrast, mbp expression is completely absent along the PLLn of the strong loss-of-function gpr126st49 and gpr126stl215 mutants (Fig. 4D–E). We therefore predicted that the gpr126st63 allele produces a hypomorphic Gpr126 protein with reduced signaling capability. Accordingly, our ultrastructural analysis revealed that gpr126st63 mutants can myelinate axons in the PLLn, though fewer axons are myelinated than in wild-type (suppl. Fig. S4C–D) (Petersen et al., in revision).
Because we can infer that gpr126st63 mutants possess a partially functional 7TM, we hypothesized that exogenous addition of p16 could increase the signaling of endogenous hypomorphic Gpr126. This assay is feasible given that small molecules, including peptides, can freely diffuse into the developing larva in the presence of carrier (Morash et al., 2011) and because the functionally important positions in p16 are almost 100% identical between D. rerio and H. sapiens (suppl. Fig. S2F). Indeed, p16 was able to activate wt zf Gpr126 in in vitro cAMP assays (suppl. Fig. S4B). Therefore, we treated gpr126st63 mutants with 100 μM p16 in DMSO from 50–55 hours post-fertilization (hpf); this encompasses a temporal window in which cAMP elevation by forskolin administration can rescue myelination in gpr126st49 mutants (Glenn and Talbot, 2013; Monk et al., 2009). We then qualitatively scored mbp expression in the PLLn (Fig. 4A). As a negative control, we treated siblings with DMSO and observed normal PLLn mbp expression in wt (gpr126+/+ or gpr126st63/+) and reduced or absent mbp in hypomorphic gpr126st63/st63 mutants (Fig. 4F–K). Treatment with 100 μM p16 caused no significant change in wt larvae but significantly rescued mbp expression in gpr126st63/st63 hypomorphs (0% “strong” or “some” in gpr126st63/st63 + DMSO vs. 44% “strong” or “some” in gpr126st63/st63 + p16, Fig. 4H, J, K). To test whether this effect is specific to Gpr126 signaling, we also assayed strong loss-of-function gpr126st49 mutants, which presumably do not express a 7TM (Patra et al., 2013). Exogenous treatment of gpr126st49 mutants with 100 μM p16 did not rescue mbp expression in the PLLn (Fig. 4K), indicating that p16 signals through the 7TM. Together, the loss- and gain-of-function experiments in zebrafish demonstrate the in vivo relevance of tethered peptide activation of aGPCRs.
Discussion
We define a common intramolecular agonistic domain for the aGPCRs GPR126 and GPR133 that comprises a sequence between the GPS cleavage site and TM1. Because of its activating nature and its position at the very C terminus of the ECD, we refer to this agonistic sequence as the “Stachel sequence” (German word for “stinger”). Our analysis of gpr126stl215 suggests that Stachel-mediated activation of Gpr126 is essential for Schwann cell myelination in zebrafish (Fig. 4C–E; suppl. Fig. S3E–G); however, the in vivo mechanisms that unmask this tethered agonistic domain are unknown. GAIN domain crystal structures revealed that the Stachel sequence lies buried between two β-sheets (Arac et al., 2012). We and others have shown that CTF only truncation mutant aGPCRs possess increased basal activity (Fig. 1C–E) (Okajima et al., 2010; Paavola et al., 2014; Paavola et al., 2011; Yang et al., 2011); in all of these studies, the critical GAIN domain β-sheets are deleted along with the rest of the NTF, which presumably exposes the Stachel sequence. Therefore, structural changes in vivo, due to extracellular molecules interacting with the ECD (Langenhan et al., 2013; Liebscher et al., 2013) or even mechanical removal of the NTF may expose the Stachel sequence to activate the 7TM. The low affinity of the Stachel sequence to the 7TM suggests a fast on-off ligand-receptor interaction and supports activation by mechanical signals (Karpus et al., 2013).
Peptide agonists usually bind to their cognate receptor in a sequential two-step mechanism (Monteclaro and Charo, 1996). The first step requires high affinity interactions with extracellular loop regions, whereas the second step is mediated by low affinity interactions with the helix bundle promoting receptor activation. Based on our findings, the first step is not required for aGPCRs, because the activating peptide is part of the receptor’s own ECD and is therefore covalently bound to the 7TM. In the second step of our model of aGPCR activation, the Stachel sequence is predicted to interact with extracellular loops and upper helix bundles as in other peptide/peptide-GPCR pairs (Thompson et al., 2012) which requires a low affinity. This model is also consistent with protease-activated receptors in which thrombin cleaves the receptor’s N terminus and exposes an activating tethered agonist (Vu et al., 1991).
Large ECDs are not unique to the aGPCR family. The ectodomains of glycoprotein hormone receptors (rhodopsin-like GPCR class) are also composed of several hundred aa forming leucine-rich repeat domains. In glycoprotein hormone receptors, a conserved module termed the hinge region (Sangkuhl et al., 2002) connects the ECD to the 7TM in a manner similar to the GPS domain in aGPCRs. Although the interspaced hinge region does not share predicted three-dimensional structural identity with the GPS motif, some features are similar. Namely, both the hinge region and the GPS motif possess multiple disulfide bonds forming at least two loops of the polypeptide chain (Arac et al., 2012). Interestingly, hinge region mutations of glycoprotein hormone receptors can activate these rhodopsin-like GPCRs, suggesting an “intramolecular agonistic unit” (Krause et al., 2012). Similarly, mutations in Cys775, Cys794, Cys807 and Cys809 of GPR126, which normally form disulfide bridges in the GAIN domain, displayed constitutive activity in cAMP assays (suppl. Fig. S4E–F). These data provide further evidence that structural changes in the GPS region promote activation via the Stachel sequence.
Our results are compatible with an activation scenario of aGPCRs in which an intramolecular agonistic domain (the Stachel sequence) is unmasked upon structural changes of the ECD, which subsequently triggers 7TM-mediated activation of G protein-signaling cascades (suppl. Fig. S1B, cis signaling; suppl. Fig. S4G). There is recent evidence that the ECD of GPR126 and of other aGPCRs can mediate biological functions independently of the 7TM (trans signaling) (Patra et al., 2013; Promel et al., 2012). Our discovery opens the possibility for further dissection between trans- and cis-dependent functions; for example, phenotypic perturbations in model organisms through peptide agonists can be attributed to the cis-signaling of the receptor (e.g., Fig 4F–K). Our study defines a previously unknown signaling modality for aGPCRs, and can lay the foundation for rational ligand design that will promote deeper understanding of the physiology and therapeutic usefulness of this emerging class of GPCRs.
Experimental Procedures
aGPCR constructs and functional assays
Epitope-tagged full-length human aGPCR sequences were inserted into pcDps, and mutant aGPCRs were generated by PCR (suppl. Table S1). For functional assays, transfected COS-7 cells were split into 48-well plates and cAMP concentrations were determined with the Alpha Screen cAMP assay kit (PerkinElmer Life Sciences) according to the manufacturer’s protocol. To measure label-free receptor activation, a dynamic mass redistribution (DMR) assay (Corning Epic Biosensor Measurements; Corning Life Sciences, Lowell, MA) with COS-7 cells endogenously expressing GPR126 was performed as described (Schroder et al., 2010). To estimate cell surface and total expression of receptors carrying N-terminal HA and C-terminal FLAG tags, ELISA was used (Schoneberg et al., 1998). Assay data was analyzed using GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA) and statistical details are given in each figure legend.
Peptide synthesis
Solid phase peptide synthesis of the peptides was performed on an automated peptide synthesizer, MultiPep from Intavis AG (Köln, Germany), using standard Fmoc-chemistry.
Zebrafish studies
Adult zebrafish were maintained in the Washington University Zebrafish Consortium facility in accordance with institutional animal protocols (http://zebrafish.wustl.edu/husbandry.htm). Embryos were collected from heterozygous gpr126 mutant adults and mutant larvae were compared to wild-type siblings for all assays. See suppl. methods for details on TALEN mutagenesis, in situ hybridization, transmission electron microscopy, and peptide treatment.
Additional methods
Additional details for all methods are available in suppl. Methods.
Supplementary Material
Suppl. Figure S1. Models of aGPCR activation and expression analysis of wt and mutant GPR126 and GPR133 constructs, Related to Figure 1
Two models of aGPCR activation have been proposed: (A) the Nterminus contains a tethered inverse agonist that inhibits 7TM signaling until ligand binding at the ECD or artificial removal of the ECD. (B) Binding of a ligand at the ECD or removal of parts of the ECD changes its conformation and exposes a tethered agonist. (C) COS-7 cells were transfected with wt and mutant GPR126 and GPR133 constructs. For expression studies, cell surface and sandwich ELISA were used to measure cell surface and total cellular expression levels, respectively. Specific optical density (OD) readings are given as percentage of the human P2Y12. (D) COS-7 cells were transfected with CTF(GPR126), and permeabilized cells were incubated with a monoclonal antibody directed against the C-terminal FLAG-tag. A TRITC-conjugated antibody was used to detect the monoclonal antibody. Nuclei are stained with DAPI. Imaging was performed using a confocal microscope (Zeiss LSM 510). A representative picture shows membrane localization of CTF(GPR126) (red). (E) Sequential aa were deleted in P2Y12-CTF(GPR126) and expression levels in whole cells and at the cell surface were determined through ELISA studies as described under Experimental Procedures. Specific optical density (OD) readings are given as percentage of the human P2Y12. (F) Single positions within the C-terminal GPS sequence were mutated in GPR126 and GPR133 to alanine, and cell surface expression was determined through ELISA measurement. Specific optical density (OD) readings are given as percentage of the human P2Y12. For (C), (E) and (F): Specific optical density (OD) readings (OD value of double HA/FLAG-tagged aGPCR constructs minus OD value of mock-transfected cells) are given as percentage of the human P2Y12 receptor, which served as positive control. For the cell surface ELISA, the non-specific OD value (pcDps) was 0.03 ± 0.03 (set 0%) and the OD value of P2Y12 was 1.30 ± 0.24 (set 100%). OD readings of 0.08 ± 0.04 (set 0%) and 2.22 ± 0.73 (set 100%) were found in sandwich ELISA (total expression) for the empty vector negative (pcDps) and positive control (P2Y12). Data are given as means ± SEM for at least three independent experiments each performed in triplicates.
Suppl. Figure S2. Specification of Stachel peptide mediated activation of GPR126 and GPR133, Related to Figure 2
(A) COS-7 cells were transiently transfected with GPR126 and empty vector plasmid and tested for basal and stimulated cAMP accumulation at several time points shown as x-fold over empty vector. While basal receptor activity remains constant, p16-derived activity increases starting at 10 minutes until it reaches a stable maximum after 30 minutes. Basal cAMP levels were: 10 minutes: 6.01 ± 2.93 nM; 20 minutes: 7.19 ± 2.30 nM; 30 minutes: 7.31 ± 2.66 nM; 60 minutes: 7.43 ± 2.77 nM. The mean ± SEM of three independent experiments performed in triplicate is shown. (B) Membranes prepared from empty vector- and GPR126-transfected cells were used to perform [35S]GTPγS binding assays as described in (Gupte et al., 2012). Incubation was performed for 1 hour in the presence of 0.5 nM [35S]GTPγS, 0.1 nM GDP and 1 mM p16. For control purposes, COS-7 cells were transfected with the Gs-coupled V2 vasopressin receptor and stimulated with 100 nM Arg-vasopressin (Boselt et al., 2009). Non-specific [35S]GTPγS binding was determined in the presence of non-radioactive GTPγS (100 nM). Data are presented as mean ± SEM of two independent experiments, each carried out in triplicates. (C) GPR126 is endogenously expressed in COS-7 cells causing residual activation by p16 of non-transfected cells. Using primers that align to conserved regions of GPR126, transcripts were amplified from COS-7 cell cDNA. Reactions lacking cDNA and containing β-actin specific primers served as negative and positive controls, respectively. (D) For label-free measurements of receptor activation, a dynamic mass redistribution assay (Epic Biosensor Measurements) with COS-7 cells endogenously expressing GPR126 was performed. GPR126-specific siRNA abolished p16-induced responses. Data are presented as mean ± SEM of at least three independent experiments, each carried out in quadruplicates. (E) A cell surface ELISA was used to evaluate the efficiency of GPR126 siRNA knockdown. COS-7 cells were transiently transfected with GPR126 plasmid alone or co-transfected with either control-siRNA or siRNA targeted against GPR126. Specific optical density (OD) readings are given as percentage of the human P2Y12. The non-specific OD value (pcDps) was 0.05 ± 0.03 (set 0%) and the OD value of P2Y12 was 2.23 ± 0.06 (set 100%). Data are presented as mean ± SD of one representative experiment, carried out in triplicates. (F) Alignment of various vertebrate species revealed presence of conserved GPR126 p16 sequence (downstream the GPR126 cleavage site) in all vertebrate classes. Positions that differ from the majority are boxed in black. The tethered agonistic peptide (p16), cleavage site and the beginning of transmembrane helix 1 (TM1) are marked. (G) Because the importance of cleavage for aGPCR expression and activity has been controversially discussed (Liebscher et al., 2013), we tested two cleavage-deficient mutants. Cleavage deficiency has been reported for GPR126T841A (Moriguchi et al., 2004). The lack of autoproteolytic cleavage in GPR133H540R is demonstrated by western blot analysis. wt GPR133 shows a double band at ~100 kDa, which is absent in GPR133H540R, while β-actin controls display equal amounts of protein in both lanes. Human GPR126T841A and mouse GPR133H540R were analyzed for (H) expression in cell surface and whole cell ELISA as percentage of human P2Y12 receptor (non-specific OD value of empty vector was for surface ELISA: 0.06 ± 0.02, whole cell ELISA: 0.04 ± 0.03 each set 0%; and the OD value of P2Y12 in surface ELISA: 1.75 ± 0.29; whole cell ELISA: 2.25 ± 0.05, each set 100%) and (I) basal and agonistic peptide-stimulated cAMP response shown as x-folds over empty vector (empty vector cAMP levels: 3.8 ±1.6 nM). Statistics were performed by applying a two-way ANOVA in combination with a Bonferoni post-hoc test: *p<0.05; **p<0.01; ***p<0.001
Suppl. Figure S3. Agonistic peptide deletion ablates Gpr126 function in vivo, Related to Figure 4 (A) Left and right TALEN recognition sequences in gpr126. The unique restriction enzyme site targeted by the TALEN and used for restriction fragment length analysis is underlined in red. Uppercase letters indicate coding sequence, while lowercase letters represent the intronic region. (B) Restriction fragment length analysis of three representative uninjected control embryos and three embryos injected with TALEN mRNA (25 pg left arm, 25 pg right arm) at 24 hpf. The arrows mark wild-type fragments cleaved by BtsCI, while the asterisk marks the undigested product resulting from TALEN-mediated disruption of the restriction enzyme site. (C) Representative image of the stl215 genotyping assay using restriction fragment length analysis. The wt PCR product has an intact BtsCI site and the digest results in two fragments of 239 bp and 158 bp (arrows). The disrupted BtsCI site in stl215 results in an undigested PCR product (asterisk). (D) Lateral view of the morphology of a gpr126stl215/+ (wt) sibling and a gpr126stl215/stl215 mutant at 5 dpf. (E) Transmission electron microscopy (TEM) of cross-sections through the PLLn. To control for developmental variability along the anterior-posterior axis, all nerves were analyzed at approximately the same body segment (between segments 5–7) of 5 dpf zebrafish larvae. wt PLLn (top) have many myelinated axons (green), whereas Schwann cells in gpr126stl215 sort axons (yellow) but fail to spiral their membranes to form myelin. Scale bar = 500 nm. (F–H) Quantification of TEM images from PLLn of wt (gpr126+/+ and gpr126stl215/+) and gpr126stl215 5 dpf larvae. For all, n=6 PLLn from N=4 wt larvae, and n=6 PLLn from N=4 mutant larvae. Error bars indicate standard deviation, significance determined via Student’s t-test. (F) Average number of sorted axons per PLLn in wt (12.7±1.4) and mutant (8±1.7) larvae. *p<0.001. (G) Average number of myelinated axons per PLLn in wt (11.1±1.5) and mutant (0±0) larvae. **p<10−8. (H) Average number of total axons per PLLn in wt (57.8±7.7) and mutant (61.5±8.1) larvae. No significant difference was observed (p>0.4).
Suppl. Figure S4. Rescue of st63 mutant zebrafish ortholog through p16 and influence of N-terminal positions on GPR126 activity levels, Related to Figure 4
(A) COS-7 cells were transiently transfected with wt, C917Y (gpr126st63) and ΔGly831-Ile832 (gpr126stl215) mutant zebrafish Gpr126 constructs and tested for cell surface expression and (B) basal and stimulated accumulation of cAMP. For the cell surface ELISA, the non-specific OD value (pcDps) was 0.003 ± 0.001 (set 0%) and the OD value of P2Y12 was 1.22 ± 0.33 (set 100%). Data are given as means ± SEM of at least two independent experiments each performed in triplicates. For cAMP assays, empty vector cAMP levels were 1.78 ± 1.12 nM/well. Data are given as means ± SEM of at least two independent experiments performed in triplicates. (C–D) Transmission electron micrographs (TEM) of 5-day post-fertilization (dpf) larvae showing cross-section through the PLLn. To control for developmental variability along the anterior-posterior axis, all nerves were analyzed at approximately the same body segment (between segments 5–7). (C) Myelinated axons are pseudocolored in green in a representative wt larva. (D) Myelinated axons are pseudocolored in green in a representative gpr126st63/st63 mutant larva. For (C–D), melanocytes laden with melanin granules are denoted by “pigment”. Scale bar = 1 μm. (E) To alter GPS structure, disulfide bridge-forming cysteines were systematically mutated to serine in GPR126 (C775S/mutant C→S I, C794S/mutant C→S II, C807S/mutant C→S III and C809S/mutant C→S IV). Interestingly, all mutants displayed constitutive activity in cAMP assay. In all cases, Cys mutations led to a reduction of cell surface expression levels indicating increased intrinsic activity of the mutants. COS-7 cells were transfected with wt and mutant GPR126 constructs. Basal cAMP levels were determined as described under Experimental Procedures. Specific cAMP levels (cAMP value of double HA/FLAG-tagged aGPCR constructs minus cAMP value of mock-transfected cells) were referred to the given wt receptor. Empty vector served as negative control (pcDps; cAMP level: 3.68 ± 2.54 nM/well). For GPR126, basal cAMP levels determined as x-fold over empty vector were 3.70 ± 1.20 (each set 100%). Data are given as means ± SEM of three independent experiments performed in triplicates. Statistics were performed by applying a one-way ANOVA in combination with Bonferoni as post-hoc test: *p<0.05; **p<0.01; ***p<0.001 (F) For expression studies, cell surface and whole cell ELISA were used to measure cell surface and total cellular expression levels, respectively. Specific optical density (OD) readings (OD value of double HA/FLAG-tagged aGPCR constructs minus OD value of mock-transfected cells) are given as percentage of the human P2Y12 receptor, which served as positive control (pC). For the cell surface ELISA, the non-specific OD value (pcDps) was 0.03 ± 0.03 (set 0%) and the OD value of P2Y12 was 1.30 ± 0.24 (set 100%). OD readings of 0.08 ± 0.04 (set 0%) and 2.22 ± 0.73 (set 100%) were found in sandwich ELISA (total expression) for the negative control vector (pcDps) and positive control (P2Y12). Data are given as means ± SEM of three independent experiments each performed in triplicates. (G) Studies with peptides derived from the first 11–19 amino acid positions downstream the GPS of GPR126 and GPR133 (Figs. 2 and 3) revealed positions in the N terminal half of the Stachel sequence that are relevant for its functionality. Many of those positions are highly conserved among aGPCRs. Based on current data, one can speculate that N-terminal cleavage at the GPS, extracellular ligand binding and/or ECD-mediated mechanic signals structurally enable the Stachel sequence to function as a peptide agonist for the 7TM.
Description of human GPR126 and human GPR133 constructs used in this study, Related to Figures 1 and 3
Acknowledgments
We thank Xianhua Piao and Thue W. Schwartz for their very helpful comments and discussion of the paper. We thank Marilyn Levy and Robyn Roth for assistance with TEM. The work was supported by the Deutsche Forschungsgemeinschaft (Sfb 610 to T.S.), the BMBF (IFB Adipositas Diseases Leipzig to J.S. and I.L.), NIH/NINDS (F32NS087786 to S.C.P. and R01NS079445 to K.R.M.), the Boehringer Ingelheim Fonds (MD stipend to N.A.) and the University Leipzig (Formel 1 start-up grant to I.L.).
The abbreviations used are
- 7TM
seven-transmembrane spanning domain
- aa
Amino Acid
- CTF
C-terminal fragment
- ECD
extracellular domain
- ELISA
enzyme-linked immunosorbent assay
- eV
empty vector
- GPCR
G protein-coupled receptors
- GPS
GPCR proteolysis site
- h
human
- HA
hemagglutinin epitope
- ICD
intracellular domain
- mbp
myelin basic protein
- NTF
N-terminal fragment
- PLLn
posterior lateral line nerve
- dpf
days post-fertilization
- hpf
hours post-fertilization
- SP
signal peptide
- TM
transmembrane helix
- wt
wildtype
- WISH
whole mount in situ hybridization
- zf
zebrafish
Footnotes
I.L. and J.S. are joint first authors. I.L., J.S., L.F., K.-U.S., L.M.D. and T.S. performed the in vitro experiments. S.C.P., N.A., A.M. and K.R.M. performed and S.C.P. and N.A. analyzed the zebrafish experiments. I.L., J.S., L.F., T.S. analyzed the data. S.R. synthesized the peptides. I.L., T.S. designed the study and wrote the paper with contributions from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arac D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Sudhof TC, Brunger AT. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. Embo J. 2012;31:1364–1378. doi: 10.1038/emboj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bohnekamp J, Schoneberg T. Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem. 2011;286:41912–41916. doi: 10.1074/jbc.C111.265934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng FS, Abbas L, Baxendale S, Holdsworth CJ, Swanson AG, Slanchev K, Hammerschmidt M, Topczewski J, Whitfield TT. Semicircular canal morphogenesis in the zebrafish inner ear requires the function of gpr126 (lauscher), an adhesion class G protein-coupled receptor gene. Development. 2013;140:4362–4374. doi: 10.1242/dev.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013;140:3167–3175. doi: 10.1242/dev.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte J, Swaminath G, Danao J, Tian H, Li Y, Wu X. Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett. 2012;586:1214–1219. doi: 10.1016/j.febslet.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283:14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- Karpus ON, Veninga H, Hoek RM, Flierman D, van Buul JD, Vandenakker CC, vanBavel E, Medof ME, van Lier RA, Reedquist KA, et al. Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J Immunol. 2013;190:3740–3748. doi: 10.4049/jimmunol.1202192. [DOI] [PubMed] [Google Scholar]
- Krause G, Kreuchwig A, Kleinau G. Extended and structurally supported insights into extracellular hormone binding, signal transduction and organization of the thyrotropin receptor. PLoS One. 2012;7:e52920. doi: 10.1371/journal.pone.0052920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T, Aust G, Hamann J. Sticky signaling--adhesion class G protein-coupled receptors take the stage. Sci Signal. 2013;6:re3. doi: 10.1126/scisignal.2003825. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher I, Schoneberg T, Promel S. Progress in demystification of adhesion G protein-coupled receptors. Biol Chem. 2013;394:937–950. doi: 10.1515/hsz-2013-0109. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Kayes-Wandover KM, Richardson JA, White PC. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J Biol Chem. 2002;277:785–792. doi: 10.1074/jbc.M108929200. [DOI] [PubMed] [Google Scholar]
- Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33:17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- Morash MG, Douglas SE, Robotham A, Ridley CM, Gallant JW, Soanes KH. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis Model Mech. 2011;4:622–633. doi: 10.1242/dmm.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Haraguchi K, Ueda N, Okada M, Furuya T, Akiyama T. DREG, a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Genes Cells. 2004;9:549–560. doi: 10.1111/j.1356-9597.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR, 3rd, Ghosh A. FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron. 2012;73:903–910. doi: 10.1016/j.neuron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima D, Kudo G, Yokota H. Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J Recept Signal Transduct Res. 2010;30:143–153. doi: 10.3109/10799891003671139. [DOI] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Stephenson JR, Ritter SL, Alter SP, Hall RA. The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J Biol Chem. 2011;286:28914–28921. doi: 10.1074/jbc.M111.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra C, van Amerongen MJ, Ghosh S, Ricciardi F, Sajjad A, Novoyatleva T, Mogha A, Monk KR, Muhlfeld C, Engel FB. Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc Natl Acad Sci U S A. 2013;110:16898–16903. doi: 10.1073/pnas.1304837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda HM, Sternheim N, Lyons DA, Diamond B, Hawkins TA, Woods IG, Bhatt DH, Franzini-Armstrong C, Dominguez C, Arana N, et al. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–131. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Promel S, Frickenhaus M, Hughes S, Mestek L, Staunton D, Woollard A, Vakonakis I, Schoneberg T, Schnabel R, Russ AP, et al. The GPS motif is a molecular switch for bimodal activities of adhesion class G protein-coupled receptors. Cell Rep. 2012;2:321–331. doi: 10.1016/j.celrep.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangkuhl K, Schulz A, Schultz G, Schoneberg T. Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J Biol Chem. 2002;277:47748–47755. doi: 10.1074/jbc.M203491200. [DOI] [PubMed] [Google Scholar]
- Schoneberg T, Schulz A, Biebermann H, Gruters A, Grimm T, Hubschmann K, Filler G, Gudermann T, Schultz G. V2 vasopressin receptor dysfunction in nephrogenic diabetes insipidus caused by different molecular mechanisms. Hum Mutat. 1998;12:196–205. doi: 10.1002/(SICI)1098-1004(1998)12:3<196::AID-HUMU7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Schroder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, Muller A, Blattermann S, Mohr-Andra M, Zahn S, et al. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol. 2010;28:943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- Staubert C, Boselt I, Bohnekamp J, Rompler H, Enard W, Schoneberg T. Structural and functional evolution of the trace amine-associated receptors TAAR3, TAAR4 and TAAR5 in primates. PLoS One. 2010;5:e11133. doi: 10.1371/journal.pone.0011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu Rev Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature. 2012;485:395–399. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen G, Mohanty S, Scott G, Fazal F, Rahman A, Begum S, Hynes RO, Xu L. GPR56 Regulates VEGF production and angiogenesis during melanoma progression. Cancer Res. 2011;71:5558–5568. doi: 10.1158/0008-5472.CAN-10-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure S1. Models of aGPCR activation and expression analysis of wt and mutant GPR126 and GPR133 constructs, Related to Figure 1
Two models of aGPCR activation have been proposed: (A) the Nterminus contains a tethered inverse agonist that inhibits 7TM signaling until ligand binding at the ECD or artificial removal of the ECD. (B) Binding of a ligand at the ECD or removal of parts of the ECD changes its conformation and exposes a tethered agonist. (C) COS-7 cells were transfected with wt and mutant GPR126 and GPR133 constructs. For expression studies, cell surface and sandwich ELISA were used to measure cell surface and total cellular expression levels, respectively. Specific optical density (OD) readings are given as percentage of the human P2Y12. (D) COS-7 cells were transfected with CTF(GPR126), and permeabilized cells were incubated with a monoclonal antibody directed against the C-terminal FLAG-tag. A TRITC-conjugated antibody was used to detect the monoclonal antibody. Nuclei are stained with DAPI. Imaging was performed using a confocal microscope (Zeiss LSM 510). A representative picture shows membrane localization of CTF(GPR126) (red). (E) Sequential aa were deleted in P2Y12-CTF(GPR126) and expression levels in whole cells and at the cell surface were determined through ELISA studies as described under Experimental Procedures. Specific optical density (OD) readings are given as percentage of the human P2Y12. (F) Single positions within the C-terminal GPS sequence were mutated in GPR126 and GPR133 to alanine, and cell surface expression was determined through ELISA measurement. Specific optical density (OD) readings are given as percentage of the human P2Y12. For (C), (E) and (F): Specific optical density (OD) readings (OD value of double HA/FLAG-tagged aGPCR constructs minus OD value of mock-transfected cells) are given as percentage of the human P2Y12 receptor, which served as positive control. For the cell surface ELISA, the non-specific OD value (pcDps) was 0.03 ± 0.03 (set 0%) and the OD value of P2Y12 was 1.30 ± 0.24 (set 100%). OD readings of 0.08 ± 0.04 (set 0%) and 2.22 ± 0.73 (set 100%) were found in sandwich ELISA (total expression) for the empty vector negative (pcDps) and positive control (P2Y12). Data are given as means ± SEM for at least three independent experiments each performed in triplicates.
Suppl. Figure S2. Specification of Stachel peptide mediated activation of GPR126 and GPR133, Related to Figure 2
(A) COS-7 cells were transiently transfected with GPR126 and empty vector plasmid and tested for basal and stimulated cAMP accumulation at several time points shown as x-fold over empty vector. While basal receptor activity remains constant, p16-derived activity increases starting at 10 minutes until it reaches a stable maximum after 30 minutes. Basal cAMP levels were: 10 minutes: 6.01 ± 2.93 nM; 20 minutes: 7.19 ± 2.30 nM; 30 minutes: 7.31 ± 2.66 nM; 60 minutes: 7.43 ± 2.77 nM. The mean ± SEM of three independent experiments performed in triplicate is shown. (B) Membranes prepared from empty vector- and GPR126-transfected cells were used to perform [35S]GTPγS binding assays as described in (Gupte et al., 2012). Incubation was performed for 1 hour in the presence of 0.5 nM [35S]GTPγS, 0.1 nM GDP and 1 mM p16. For control purposes, COS-7 cells were transfected with the Gs-coupled V2 vasopressin receptor and stimulated with 100 nM Arg-vasopressin (Boselt et al., 2009). Non-specific [35S]GTPγS binding was determined in the presence of non-radioactive GTPγS (100 nM). Data are presented as mean ± SEM of two independent experiments, each carried out in triplicates. (C) GPR126 is endogenously expressed in COS-7 cells causing residual activation by p16 of non-transfected cells. Using primers that align to conserved regions of GPR126, transcripts were amplified from COS-7 cell cDNA. Reactions lacking cDNA and containing β-actin specific primers served as negative and positive controls, respectively. (D) For label-free measurements of receptor activation, a dynamic mass redistribution assay (Epic Biosensor Measurements) with COS-7 cells endogenously expressing GPR126 was performed. GPR126-specific siRNA abolished p16-induced responses. Data are presented as mean ± SEM of at least three independent experiments, each carried out in quadruplicates. (E) A cell surface ELISA was used to evaluate the efficiency of GPR126 siRNA knockdown. COS-7 cells were transiently transfected with GPR126 plasmid alone or co-transfected with either control-siRNA or siRNA targeted against GPR126. Specific optical density (OD) readings are given as percentage of the human P2Y12. The non-specific OD value (pcDps) was 0.05 ± 0.03 (set 0%) and the OD value of P2Y12 was 2.23 ± 0.06 (set 100%). Data are presented as mean ± SD of one representative experiment, carried out in triplicates. (F) Alignment of various vertebrate species revealed presence of conserved GPR126 p16 sequence (downstream the GPR126 cleavage site) in all vertebrate classes. Positions that differ from the majority are boxed in black. The tethered agonistic peptide (p16), cleavage site and the beginning of transmembrane helix 1 (TM1) are marked. (G) Because the importance of cleavage for aGPCR expression and activity has been controversially discussed (Liebscher et al., 2013), we tested two cleavage-deficient mutants. Cleavage deficiency has been reported for GPR126T841A (Moriguchi et al., 2004). The lack of autoproteolytic cleavage in GPR133H540R is demonstrated by western blot analysis. wt GPR133 shows a double band at ~100 kDa, which is absent in GPR133H540R, while β-actin controls display equal amounts of protein in both lanes. Human GPR126T841A and mouse GPR133H540R were analyzed for (H) expression in cell surface and whole cell ELISA as percentage of human P2Y12 receptor (non-specific OD value of empty vector was for surface ELISA: 0.06 ± 0.02, whole cell ELISA: 0.04 ± 0.03 each set 0%; and the OD value of P2Y12 in surface ELISA: 1.75 ± 0.29; whole cell ELISA: 2.25 ± 0.05, each set 100%) and (I) basal and agonistic peptide-stimulated cAMP response shown as x-folds over empty vector (empty vector cAMP levels: 3.8 ±1.6 nM). Statistics were performed by applying a two-way ANOVA in combination with a Bonferoni post-hoc test: *p<0.05; **p<0.01; ***p<0.001
Suppl. Figure S3. Agonistic peptide deletion ablates Gpr126 function in vivo, Related to Figure 4 (A) Left and right TALEN recognition sequences in gpr126. The unique restriction enzyme site targeted by the TALEN and used for restriction fragment length analysis is underlined in red. Uppercase letters indicate coding sequence, while lowercase letters represent the intronic region. (B) Restriction fragment length analysis of three representative uninjected control embryos and three embryos injected with TALEN mRNA (25 pg left arm, 25 pg right arm) at 24 hpf. The arrows mark wild-type fragments cleaved by BtsCI, while the asterisk marks the undigested product resulting from TALEN-mediated disruption of the restriction enzyme site. (C) Representative image of the stl215 genotyping assay using restriction fragment length analysis. The wt PCR product has an intact BtsCI site and the digest results in two fragments of 239 bp and 158 bp (arrows). The disrupted BtsCI site in stl215 results in an undigested PCR product (asterisk). (D) Lateral view of the morphology of a gpr126stl215/+ (wt) sibling and a gpr126stl215/stl215 mutant at 5 dpf. (E) Transmission electron microscopy (TEM) of cross-sections through the PLLn. To control for developmental variability along the anterior-posterior axis, all nerves were analyzed at approximately the same body segment (between segments 5–7) of 5 dpf zebrafish larvae. wt PLLn (top) have many myelinated axons (green), whereas Schwann cells in gpr126stl215 sort axons (yellow) but fail to spiral their membranes to form myelin. Scale bar = 500 nm. (F–H) Quantification of TEM images from PLLn of wt (gpr126+/+ and gpr126stl215/+) and gpr126stl215 5 dpf larvae. For all, n=6 PLLn from N=4 wt larvae, and n=6 PLLn from N=4 mutant larvae. Error bars indicate standard deviation, significance determined via Student’s t-test. (F) Average number of sorted axons per PLLn in wt (12.7±1.4) and mutant (8±1.7) larvae. *p<0.001. (G) Average number of myelinated axons per PLLn in wt (11.1±1.5) and mutant (0±0) larvae. **p<10−8. (H) Average number of total axons per PLLn in wt (57.8±7.7) and mutant (61.5±8.1) larvae. No significant difference was observed (p>0.4).
Suppl. Figure S4. Rescue of st63 mutant zebrafish ortholog through p16 and influence of N-terminal positions on GPR126 activity levels, Related to Figure 4
(A) COS-7 cells were transiently transfected with wt, C917Y (gpr126st63) and ΔGly831-Ile832 (gpr126stl215) mutant zebrafish Gpr126 constructs and tested for cell surface expression and (B) basal and stimulated accumulation of cAMP. For the cell surface ELISA, the non-specific OD value (pcDps) was 0.003 ± 0.001 (set 0%) and the OD value of P2Y12 was 1.22 ± 0.33 (set 100%). Data are given as means ± SEM of at least two independent experiments each performed in triplicates. For cAMP assays, empty vector cAMP levels were 1.78 ± 1.12 nM/well. Data are given as means ± SEM of at least two independent experiments performed in triplicates. (C–D) Transmission electron micrographs (TEM) of 5-day post-fertilization (dpf) larvae showing cross-section through the PLLn. To control for developmental variability along the anterior-posterior axis, all nerves were analyzed at approximately the same body segment (between segments 5–7). (C) Myelinated axons are pseudocolored in green in a representative wt larva. (D) Myelinated axons are pseudocolored in green in a representative gpr126st63/st63 mutant larva. For (C–D), melanocytes laden with melanin granules are denoted by “pigment”. Scale bar = 1 μm. (E) To alter GPS structure, disulfide bridge-forming cysteines were systematically mutated to serine in GPR126 (C775S/mutant C→S I, C794S/mutant C→S II, C807S/mutant C→S III and C809S/mutant C→S IV). Interestingly, all mutants displayed constitutive activity in cAMP assay. In all cases, Cys mutations led to a reduction of cell surface expression levels indicating increased intrinsic activity of the mutants. COS-7 cells were transfected with wt and mutant GPR126 constructs. Basal cAMP levels were determined as described under Experimental Procedures. Specific cAMP levels (cAMP value of double HA/FLAG-tagged aGPCR constructs minus cAMP value of mock-transfected cells) were referred to the given wt receptor. Empty vector served as negative control (pcDps; cAMP level: 3.68 ± 2.54 nM/well). For GPR126, basal cAMP levels determined as x-fold over empty vector were 3.70 ± 1.20 (each set 100%). Data are given as means ± SEM of three independent experiments performed in triplicates. Statistics were performed by applying a one-way ANOVA in combination with Bonferoni as post-hoc test: *p<0.05; **p<0.01; ***p<0.001 (F) For expression studies, cell surface and whole cell ELISA were used to measure cell surface and total cellular expression levels, respectively. Specific optical density (OD) readings (OD value of double HA/FLAG-tagged aGPCR constructs minus OD value of mock-transfected cells) are given as percentage of the human P2Y12 receptor, which served as positive control (pC). For the cell surface ELISA, the non-specific OD value (pcDps) was 0.03 ± 0.03 (set 0%) and the OD value of P2Y12 was 1.30 ± 0.24 (set 100%). OD readings of 0.08 ± 0.04 (set 0%) and 2.22 ± 0.73 (set 100%) were found in sandwich ELISA (total expression) for the negative control vector (pcDps) and positive control (P2Y12). Data are given as means ± SEM of three independent experiments each performed in triplicates. (G) Studies with peptides derived from the first 11–19 amino acid positions downstream the GPS of GPR126 and GPR133 (Figs. 2 and 3) revealed positions in the N terminal half of the Stachel sequence that are relevant for its functionality. Many of those positions are highly conserved among aGPCRs. Based on current data, one can speculate that N-terminal cleavage at the GPS, extracellular ligand binding and/or ECD-mediated mechanic signals structurally enable the Stachel sequence to function as a peptide agonist for the 7TM.
Description of human GPR126 and human GPR133 constructs used in this study, Related to Figures 1 and 3