Abstract
Objective
Previous research has shown that fluctuations in ovarian hormones (i.e., estradiol and progesterone) predict changes in binge eating and emotional eating across the menstrual cycle. However, the extent to which other eating disorder symptoms fluctuate across the menstrual cycle and are influenced by ovarian hormones remains largely unknown. The current study sought to examine whether levels of weight preoccupation vary across the menstrual cycle and whether changes in ovarian hormones and/or other factors (i.e., emotional eating, negative affect) account for menstrual-cycle fluctuations in this eating disorder phenotype.
Method
For 45 consecutive days, 352 women ages 15–25 provided daily ratings of weight preoccupation, negative affect, and emotional eating. Daily saliva samples also were collected and assayed for estradiol and progesterone levels using enzyme-immunoassay techniques.
Results
Weight preoccupation varied significantly across the menstrual cycle, with the highest levels in the pre-menstrual and menstrual phases. However, ovarian hormones did not account for within-person changes in weight preoccupation across the menstrual cycle. Instead, the most significant predictor of menstrual-cycle changes in weight preoccupation was changes in emotional eating.
Discussion
Fluctuations in weight preoccupation across the menstrual cycle appear to be influenced primarily by emotional eating rather than ovarian hormones. Future research should continue to examine relationships among ovarian hormones, weight preoccupation, emotional eating, and other core eating disorder symptoms (e.g., body dissatisfaction, compensatory behaviors) in an effort to more fully understand the role of these biological and behavioral factors for the full spectrum of eating pathology.
Ovarian hormones (i.e., estradiol and progesterone) have recently been implicated in the etiology of eating disorders in women (1–4). Most research to date has focused on the role of ovarian hormones in risk for binge eating. This is not surprising given findings from animal studies demonstrating that ovarian hormones have direct, causal effects on food intake (5, 6). Specifically, removal of the source of ovarian hormones through bilateral ovariectomy in rats causes increased food intake, and adminstration of estradiol reverses this effect. In contrast, progesterone causes increased food intake, in part, by antagonizing the inhibitory effects of estradiol (6–8).
In humans, food intake, binge eating, and emotional eating (i.e., the tendency to eat when experiencing negative emotions, 9) have been found to be significantly higher during the mid-luteal and pre-menstrual phases of the menstrual cycle as compared to the follicular/ovulatory phases (1, 3, 10–13). Studies that have directly examined estradiol and progesterone levels confirm that within-person changes in ovarian hormones account for these menstrual-cycle fluctuations (1, 3, 13). Specifically, while initial pilot data suggested that lower estradiol and higher progesterone levels were associated with increases in binge eating and emotional eating (1, 3), a recent study indicated that interactions between estradiol and progesterone (i.e., high levels of both) contribute to mid-luteal increases in emotional eating as well (14). Moreover, ovarian hormone/dysregulated eating associations have been shown to be stronger in women with clinically significant levels of binge eating compared to women without binge episodes (13). Importantly, in all previous studies, hormone effects on binge eating and emotional eating were independent of important covariates that also change across the menstrual cycle, including negative affect and body mass index (BMI) (1, 3, 13, 14).
Far fewer studies, however, have examined menstrual cycle changes in other disordered eating variables. Broadening the phenotypes examined is important for developing more complete models of the role of ovarian hormones in the full spectrum of eating pathology. One particularly important set of variables to investigate are those related to weight concerns/preoccupation (i.e., intense preoccupation with weight, dieting, and the pursuit of thinness; 15). Weight concerns have been identified as one of the most robust prospective risk factors for the development of clinically significant eating disorders (16), and weight concerns are directly related to core symptoms (e.g., undue influence of body weight/shape on self-evaluation) of anorexia nervosa and bulimia nervosa (17).
In the only previous report of its kind, Racine et al., (2012) examined the association between menstrual-cycle fluctuations in ovarian hormone and changes in weight concerns in two independent samples of women. In the first sample, robust fluctuations in weight preoccupation were observed across the menstrual cycle, where weight preoccupation levels were highest in the mid-luteal phase (4). Menstrual-cycle changes in weight preoccupation were primarily accounted for by within-person increases in progesterone and, to a lesser extent, decreases in estradiol. However, in a second sample, more modest, non-significant changes in weight preoccupation were observed across the menstrual cycle, and weight preoccupation was highest during the pre-menstrual phase. Reasons for the discrepant results across samples are unclear, although the very small sample sizes of these studies (N = 8 and N = 10, respectively) may have contributed to instability in effects.
Clearly, additional research using larger samples of women is needed to clarify the presence/absence of within-person menstrual cycle fluctuations in weight preoccupation. Moreover, it will be important for these studies to determine whether menstrual cycle changes in weight preoccupation are due to changes in ovarian hormones, changes in psychological factors (e.g., increased negative affect), and/or changes in emotional eating that have been shown to fluctuate across the menstrual cycle in past research (1, 3, 13, 14, 18, 19). For example, emotional eating has previously been linked to ovarian hormones (1, 3, 13, 14), and we might expect weight concerns to increase during certain menstrual cycle phases as a result of emotional eating. Specifically, increased emotional eating during the mid-luteal phase could cause women to be more concerned and/or conscious about their body shape/weight; in this case, weight concerns may be due to eating in the presence of negative emotions rather than to changes in ovarian hormones. Thus, it is important to determine what factors might account for menstrual cycle changes in weight preoccupation: emotional eating, psychological factors (e.g., negative affect) and/or ovarian hormones.
Given the above, the aim of the current study was to investigate within-person changes in weight preoccupation across the menstrual cycle utilizing a large, community-based sample of women. First, we were interested in examining whether levels of weight preoccupation significantly vary across the menstrual cycle. Second, we wanted to investigate whether within-person fluctuations in weight preoccupation across the menstrual cycle are best accounted for by within-person changes in ovarian hormones, negative affect, emotional eating, or a combination of these factors.
METHODS
Participants
Participants included 352 same-sex female twins (194 monozygotic twins; 158 dizygotic twins) between the ages of 15 and 25 years drawn from the Twin Study of Hormones and Behavior Across the Menstrual Cycle (14) within the Michigan State University Twin Registry (MSUTR; 20, 21). All participants completed written informed consent before enrolling in the study. Importantly, a sub-set of these participants (N = 174; 49% of the current sample) was examined in the Klump et al. (2013) study where significant hormone effects for emotional eating were detected. Our analyses extend these initial results by investigating whether similar hormone effects are present for weight preoccupation scores, after controlling for emotional eating and other important covariates.
Twins from the MSUTR are recruited using birth record methods previously described (20; 21). Twins included in the current study were demographically representative of the recruitment region (81.7% Caucasian; 16.2% African American; 1.0 % Asian/Pacific Islander; 1.0 % Native American; http://www.michigan.gov/mdch).
In order to ensure that we captured natural hormonal variations across the menstrual cycle, we developed a variety of participant inclusion/exclusion criteria: 1) regular menstrual cycles (i.e., every 22–32 days) for past 6 months; 2) no hormonal contraceptive use for past three months; 3) no psychotropic or steroid medication use for past 4 weeks; 5) no pregnancy or lactation in past 6 months; and 6) no history of genetic or medical conditions that may influence hormones or appetite/weight. Despite these exclusion criteria, participants from the current study and those from previous MSUTR studies without such criteria did not meaningfully differ on levels of disordered eating (average Cohen’s d = .12, range = .01–.20).
Procedures
All study procedures, methods, and materials were reviewed and approved by the Michigan State University Institutional Review Board. Behavioral and hormone data were provided daily across the 45 days of the study. Salivary samples were collected within the first 30 minutes of waking using previously established methods (3). Questionnaires were completed each evening (after 5:00 PM) using an online data system or pre-printed scantrons. This pattern of morning saliva samples and evening behavioral data collection was to ensure that hormone measurements preceded behavioral ratings each day.
In addition to daily data collection, all participants completed three in-person visits occurring at the start of the study, halfway through the study (~day 23), and at the end of data collection (~day 45). During these in-person assessments, eligibility was re-assessed, height and weight were measured, and completed materials were collected from participants. Between visits, staff contacted participants 1x/week to answer questions and confirm continued protocol adherence. These procedures were effective at identifying individuals who were no longer eligible to participate due to missed periods, medication use, and/or pregnancy during the study (< 3%) as well as minimizing drop-outs. The percentage of participants who were ultimately dropped from the study or whose data were not analyzed due to failure to collect a sufficient number of samples was minimal (< 3% and <6%, respectively).
Measures
Sample characteristics for all study measures are presented in Table 1. Although daily, longitudinal data were examined for most variables (i.e., weight preoccupation, emotional eating, negative affect, ovarian hormones), we present averages across data collection in Table 1 in order to characterize the sample for comparisons with future studies.
Table 1.
Sample Characteristics
| Variable | Mean (SD) | Range |
|---|---|---|
| Age | 18.10 (1.76) | 16–22 |
| Weight Preoccupation | 2.19 (1.98) | .02–7.98 |
| Estradiol (pg/ml) | 2.87 (1.39) | 0.70–12.43 |
| Progesterone (pg/ml) | 124.76 (67.08) | 18.73–397.04 |
| Negative Affect | 15.21 (3.78) | 10–29 |
| Emotional Eating | 0.34 (.41) | 0–3 |
| Body mass index (BMI) | 24.07 (5.67) | 15.81–47.59 |
Note. Values for weight preoccupation, estradiol, progesterone, negative affect, and emotional eating are average values across the 45-day data collection period. Values for BMI are average values across the three study visits.
Weight Preoccupation
Weight preoccupation was assessed daily for 45 days using the Weight Preoccupation Scale of the Minnesota Eating Behaviors Survey (MEBS; 22)1. The MEBS Weight Preoccupation scale consists of 8 true/false questions that ask about a variety of cognitions and behaviors related to weight concerns (e.g., “I am really afraid of gaining weight”; “I often weigh myself to see if I am gaining weight”). Internal consistency for the MEBS Weight Preoccupation scale has ranged from acceptable to excellent in previous studies (α = .71–.85) (22) and in the current study (α = .82). In addition, in support of its criterion validity, women with eating disorders score higher on this scale compared to women without eating disorders (22).
Emotional Eating
Emotional eating was assessed daily for 45 days using the Emotional Eating scale of the Dutch Eating Behavior Questionnaire (DEBQ; 9). The Emotional Eating scale assesses eating in response to negative emotions (e.g., “Did you have desire to eat when you were discouraged?”) on a 5-point scale ranging from not at all to very often. Internal consistencies for the DEBQ Emotional Eating scale are excellent in previous research (α = .93) (3, 4, 9) and in the current study (average α = .90). Importantly, eating in response to negative emotions is thought to be a core feature of binge eating, and the Emotional Eating Scale of the DEBQ has demonstrated validity in differentiating between individuals with bulimia nervosa and/or binge eating, overweight individuals, and college students. Furthermore, the DEBQ Emotional Eating scale is correlated with established measures of binge eating (r’s = .55–.69) (23, 24) as well as with palatable food intake (i.e., ice cream) in a laboratory setting (24).
Negative Affect
The Positive and Negative Affect Schedule (PANAS) Negative Affect scale (25) was used to assess negative affect daily for 45 days. This scale consists of 10 items that assess the full range of daily negative emotions (e.g., distress, nervousness, irritability, fear). The degree to which each emotion was experienced was rated on a 5-point scale ranging from very slightly/not at all to extremely. The PANAS Negative Affect scale has exhibited excellent internal consistency as well as good convergent and discriminant validity (25). Internal consistency in the current study was excellent (average α = .85).
Ovarian Hormones
Estradiol and progesterone were assayed from daily saliva samples. Saliva samples are preferred over other methods (e.g., blood spots) because they represent a less invasive collection method, particularly when repeated samples are needed. Previous research has found that saliva samples are associated with higher compliance and more robust hormone-behavior associations than blood spot sampling (1).
Saliva samples were processed by Salimetrics, LLC (State College, PA, USA) using enzyme immunoassay kits designed specifically for analyzing saliva. These assays show excellent intra- and inter-assay coefficients of variation (estradiol = 7.1% and 7.5%; progesterone = 6.2% and 7.6%), as well as assay sensitivity (measured by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0 pg/mL level; estradiol = 0.10 pg/mL; progesterone = 5 pg/mL) and method accuracy (determined by spike recovery and linearity, estradiol = 104.2% and 99.4%; progesterone = 99.6% and 91.8%). In order to conserve resources, samples were only assayed every other day during menstrual bleeding and early follicular phase when hormones are expected to be low and stable. This process ensured that we captured periods of maximum hormonal change across the menstrual cycle (e.g., mid-late follicular though premenstrual phase) while in turn maximizing the number of participant samples assayed.
Body Mass Index (BMI)
BMI was included as a covariate in analyses given previous research showing its association with weight preoccupation (26), as well as with ovarian hormone levels (27, 28). To determine BMI, participants’ height and weight were measured during the three in-person study visits using a wall-mounted ruler and digital scale, respectively. BMI was calculated using the following formula: (BMI = weight (in kilograms)/height (in meters)2).
Statistical Analyses
Data preparation
Data preparation followed that used in previous studies examining the relationship between ovarian hormones and binge eating/emotional eating across the menstrual cycle (1, 3, 13, 14). For our repeated measures (i.e., hormones, weight preoccupation, negative affect, emotional eating, BMI), five-day rolling averages were calculated and standardized within person. Five-day rolling averages were calculated by averaging the scores of the variable (e.g., weight preoccupation) across a chosen day, the two days prior, and the two days after the chosen day. For example, weight preoccupation levels on day 8 were calculated as the average from days 6 to day 10 (11). Previous research has used rolling averages, and they are preferred because of their ability to minimize random variation that is present in behavioral data due to environmental circumstances (10). In order to accommodate the fact that BMI was assessed at only three time points across the study, rolling averages were calculated using visit 1 BMI for days in-between the first and second in-person assessments, visit 2 BMI for days in-between the second and third in-person assessment, and the visit 3 BMI for the last day of the study. These rolling averages were then converted to within-person standardized scores that were based on each individual participant’s overall standard deviation across the study. This standardization allowed for examination of the degree to which changes in a woman’s ovarian hormones, relative to her equilibrium, predict changes away from the woman’s equilibrium in weight preoccupation.
Statistical models
Similar to previous analyses of this data set (14, 29), mixed linear models (MLMs) were used to examine the possible influence of menstrual cycle phase, ovarian hormones, negative affect, and emotional eating on within-person changes in weight preoccupation. These models were well suited for testing our hypotheses as they could examine the effects of predictors while controlling for the non-independence of the repeated measures and twin data. Specifically, we allowed residual errors for weight preoccupation to correlate between members of a twin pair, and we estimated a time-specific dyadic correlation that allowed twin’s residual errors to correlate from day-to-day. Each time-varying predictor was included as a random effect in order to model random slopes and the relationship between these slopes within twin pairs. However, there was no evidence that random slopes were correlated across twins, so an identity covariance matrix, which estimates a single variance for both twins in a pair, was used.
We first examined whether weight preoccupation significantly varied across the menstrual cycle in order to then investigate whether hormones and/or other factors (i.e., negative affect, emotional eating) accounted for any observed fluctuations. Similar to previous studies, menstrual cycle phase was coded based on dates of menstrual bleeding and increases/decreases in ovarian hormone levels for each participant (1, 3, 14). Specifically, each woman’s menstrual cycle(s) was categorized into five primary cycle phases (i.e., follicular, ovulatory, mid-luteal, pre-menstrual, and menstrual) and three transition phases (i.e., follicular to ovulatory, ovulatory to mid-luteal, mid-luteal to pre-menstrual). In analyses examining weight preoccupation changes across menstrual cycle phase, the follicular phase included the days immediately after the end of menstrual bleeding, during which time progesterone is low and estradiol slowly begins to increase; the ovulatory phase included the days during which estradiol has its peak, as well as the transition phases preceding and following ovulation, during which time estradiol is rising and falling, respectively; the mid-luteal phase included the mid-luteal phase and the mid-luteal to pre-menstrual transition phase, a time characterized by the highest levels of progesterone and a secondary peak in estradiol; the peri-menstrual phase included both the pre-menstrual and menstrual phases when both hormone levels are low. Using MLM, fluctuations in weight preoccupation across the menstrual cycle were tested by including phase as a predictor of weight preoccupation.
Next, we investigated whether significant fluctuations in weight preoccupation across the menstrual cycle were accounted for by within-person changes in ovarian hormones, negative affect, emotional eating, or a combination of these factors. We first fit a model (“Model 1”) that tested the main effects of estradiol, progesterone, as well as the estradiol x progesterone interaction, while controlling for any effects of BMI. In Models 2 and 3, negative affect and emotional eating, respectively, were individually added as predictors in order to examine whether they were significantly associated with within-person changes in weight preoccupation across the menstrual cycle and whether they accounted for any effects of ovarian hormones on weight preoccupation. Finally, Model 4 included both negative affect and emotional eating as predictors of weight preoccupation in order to examine which of these psychological factors might be most strongly associated with changes in weight preoccupation.
RESULTS
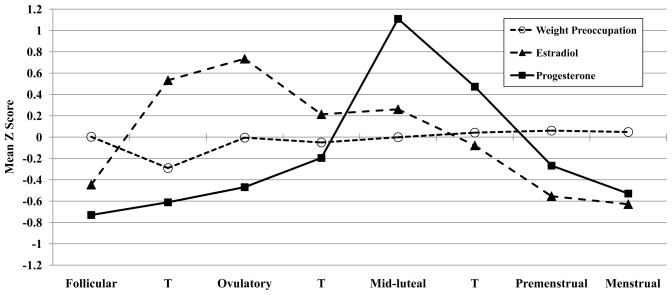
Results from the MLM examining menstrual phase as a predictor of within-person changes in weight preoccupation indicated that levels of weight preoccupation varied significantly across the menstrual cycle (F(3,12,674) = 3.36, p = .02). The highest levels of weight preoccupation were observed during the peri-menstrual phase (i.e., pre-menstrual and menstrual phases; M(S.E.) = .05 (.02)). Importantly, peri-menstrual weight preoccupation scores were significantly higher than scores in all other menstrual cycle phases (follicular, (M(S.E.) = .003 (.02); ovulatory, M(S.E) = −.02 (.02); mid-luteal, M(S.E.) = .005 (.01); t’s = 2.02–3.22; p’s =.002–.04). There were no significant differences among the follicular, ovulatory, and mid-luteal menstrual phases (t’s = 0.08–1.28; p’s = .20–.94), but weight preoccupation scores appeared to be lowest in the ovulatory phase (see Figure 1).
Figure 1.
Changes in Weight Preoccupation, Estradiol, and Progesterone across the Menstrual Cycle
Note. T = transition days that are in-between phases. Mean Z Score = the mean of the 5-day rolling averages calculated within participants, then averaged across participants. Mean values within each phase are included for descriptive purposes only, as the daily z scores were included in the hierarchical linear models for each phase contrast. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = −1): Follicular = +6 to +11; Transition from Follicular to Ovulatory = +12 to +13; Ovulatory = −15 to −12; Transition from Ovulatory to Mid-luteal = −11 to −10; Mid-luteal = −9 to −5; Transition from Mid-luteal to Premenstrual = −4; Premenstrual = −3 to +1; Menstrual = +2 to +5
The second set of MLMs considered whether changes in ovarian hormones, negative affect, and/or emotional eating account for observed menstrual cycle fluctuations in weight preoccupation (see Table 2). Across all models, estradiol, progesterone, and the estradiol-progesterone interaction did not significantly predict within-person changes in weight preoccupation. In contrast, negative affect and emotional eating were both significant predictors of weight preoccupation when entered individually in the MLMs, suggesting that these psychological/behavioral factors may have a stronger effect on menstrual-cycle changes in weight preoccupation than ovarian hormones. Interestingly, when negative affect and emotional eating were included together in Model 4, only emotional eating (and not negative affect) emerged as a significant predictor of weight preoccupation. Thus, of the factors examined in the current study, the strongest predictor of within-person changes in weight preoccupation across the menstrual cycle was emotional eating2.
Table 2.
Ovarian Hormones, Negative Affect, and Emotional Eating Predicting Within-Person Changes in Weight Preoccupation
| Model | b (SE) | t | df | p |
|---|---|---|---|---|
| Model 1: Hormones and BMI Only | ||||
| Estradiol | −.006 (.02) | −0.30 | 315 | .76 |
| Progesterone | .007 (.02) | 0.30 | 326 | .77 |
| Estradiol*Progesterone | .002 (.01) | 0.12 | 314 | .90 |
| BMI | −.02 (.03) | −0.74 | 302 | .46 |
| Model 2: Controlling for NA | ||||
| Estradiol | −.01 (.02) | −0.52 | 309 | .60 |
| Progesterone | .01 (.02) | 0.42 | 327 | .67 |
| Estrogen x Progesterone | −.001 (.01) | −0.01 | 317 | .99 |
| BMI | −.03 (.03) | −0.95 | 302 | .34 |
| Negative Affect | .05 (.02) | 2.42 | 313 | .02 |
| Model 3: Controlling for EE | ||||
| Estradiol | −.01 (.02) | −0.62 | 309 | .53 |
| Progesterone | .01 (.02) | 0.63 | 313 | .53 |
| Estrogen x Progesterone | −.005 (.01) | −0.38 | 306 | .70 |
| BMI | −.02 (.03) | −0.84 | 301 | .40 |
| Emotional Eating | .12 (.02) | 5.07 | 314 | <.001 |
| Model 4 Controlling for NA and EE | ||||
| Estradiol | −.01 (.02) | −0.70 | 305 | .48 |
| Progesterone | .01 (.02) | 0.67 | 315 | .50 |
| Estrogen x Progesterone | −.004 (.01) | −0.29 | 312 | .77 |
| BMI | −.02 (.03) | −0.93 | 301 | .35 |
| Negative Affect | .02 (.02) | 1.23 | 307 | .22 |
| Emotional Eating | .11 (.03) | 4.67 | 307 | <.001 |
Note. BMI = body mass index; NA = negative affect; EE = emotional eating.
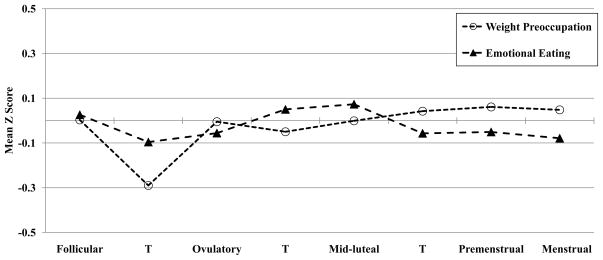
Given this pattern of results, we were interested in examining whether emotional eating episodes earlier in the menstrual cycle may drive later increases in weight preoccupation. Figure 2 depicts mean emotional eating and weight preoccupation scores across the menstrual cycle, and examination of menstrual cycle fluctuations in these symptoms suggests that changes in emotional eating are followed by changes in weight preoccupation. In order to further investigate this hypothesis, we conducted time-lagged prospective analyses that investigated whether emotional eating scores on one day predict weight preoccupation scores during later days in the menstrual cycle. We considered both proximal (i.e., 1, 2, and 3 day time-lagged associations) and more distal (i.e., 5 and 10 day time-lagged associations) relationships between emotional eating and weight preoccupation. To account for within-person stability over time, same-day weight preoccupation was entered as a covariate in analyses. Results indicated that emotional eating significantly predicted within-person changes in weight preoccupation one, two, and three days later, and associations in 5- and 10-day time-lagged analyses were marginally significant (see Table 3). Importantly, analyses examining the reverse relationship (i.e., whether weight preoccupation predicted emotional eating later in the cycle) supported the hypothesis that the direction of effects is from emotional eating to weight preoccupation, as weight preoccupation did not significantly predict lagged emotional eating scores, with the exception of 1-day lagged analyses (see Table 3).
Figure 2.
Changes in Weight Preoccupation and Emotional Eating across the Menstrual Cycle
Note. T = transition days that are in-between phases. Mean Z Score = the mean of the 5-day rolling averages calculated within participants, then averaged across participants. Mean values within each phase are included for descriptive purposes only, as the daily z scores were included in the hierarchical linear models for each phase contrast. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = −1): Follicular = +6 to +11; Transition from Follicular to Ovulatory = +12 to +13; Ovulatory = −15 to −12; Transition from Ovulatory to Mid-luteal = −11 to −10; Mid-luteal = −9 to −5; Transition from Mid-luteal to Premenstrual = −4; Premenstrual = −3 to +1; Menstrual = +2 to +5.
Table 3.
Time-lagged Analyses Examining the Direction of the Association between Emotional Eating and Weight Preoccupation
| Time-lag | Emotional Eating to Weight Preoccupation | Weight Preoccupation to Emotional Eating | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| b (SE) | t (df) | p | b (SE) | t (df) | p | |
| 1 day | .02 (.005) | 2.94 (340) | .003 | .03 (.01) | 2.27 (338) | .02 |
| 2 days | .02 (.009) | 2.82 (322) | .005 | .02 (.02) | 1.35 (346) | .18 |
| 3 days | .03 (.01) | 2.13 (332) | .03 | −.02 (.02) | −1.42 (333) | .16 |
| 5 days | .03 (.02) | 1.81 (334) | .07 | −.02 (.02) | −1.09 (331) | .27 |
| 10 days | .03 (.02) | 1.77 (322) | .08 | −.01 (.02) | −0.51 (308) | .61 |
DISCUSSION
The current study used a large, community-based sample of women to examine whether weight preoccupation, a core eating disorder symptom, significantly varies across the menstrual cycle and whether ovarian hormones, psychological factors (i.e., increased negative affect), and/or emotional eating might account for these within-person changes. The importance of this study is highlighted by the fact that most previous studies examining the influence of the menstrual cycle and ovarian hormones on disordered eating symptoms have focused solely on binge eating and emotional eating. The only previous report to investigate menstrual cycle effects for weight preoccupation examined two very small samples (N’s=8–10) and generated discrepant results (4).
Results from the current study suggest that levels of weight preoccupation vary significantly across the menstrual cycle. Specifically, weight preoccupation scores were highest in the peri-menstrual (i.e., pre-menstrual and menstrual) phase of the menstrual cycle, and weight preoccupation scores in this phase were significantly higher than weight preoccupation scores in all other menstrual cycle phases (i.e., follicular, ovulatory, mid-luteal). Our findings are in line with almost all previous studies examining disordered eating symptoms (i.e., binge eating, emotional eating, body dissatisfaction) in suggesting that risk for disordered eating is higher in the post-ovulatory versus the pre-ovulatory half of the menstrual cycle (1, 3, 10–12). Most importantly, in the one previous report to investigate menstrual-cycle changes in weight preoccupation (4), findings pointed to both mid-luteal and pre-menstrual increases in weight preoccupation, although only the mid-luteal peaks were significant. These results are consistent with the significant peaks in weight preoccupation during the pre-menstrual and menstrual phases and the second highest levels of weight preoccupation during the mid-luteal phase observed in the present study. Given that our large sample provided ample power to detect fluctuations in weight preoccupation across the menstrual cycle, the current findings significantly contribute to our understanding of peak periods of risk for weight concerns across the menstrual cycle.
In considering whether changes in ovarian hormones, emotional eating, or negative affect accounted for menstrual cycle fluctuations in weight preoccupation, we found that within-person changes in negative affect and emotional eating significantly predicted changes in weight preoccupation. However, when both emotional eating and negative affect were included in the model as predictors of weight preoccupation, only changes in emotional eating were significantly associated with changes in weight preoccupation. Interestingly, analyses examining the longitudinal relationship between weight preoccupation and emotional eating confirmed that emotional eating significantly predicts later increases in weight preoccupation and that the direction of the effect is from emotional eating to weight preoccupation and not the reverse (i.e., from weight preoccupation to emotional eating). In contrast, there were no significant effects of estradiol, progesterone, or the estradiol-progesterone interaction on weight preoccupation changes. Therefore, unlike previous research that has demonstrated direct effects of ovarian hormones on other disordered eating symptoms (e.g., binge eating, emotional eating) (1, 3), weight preoccupation appears to be more strongly related to emotional eating than to ovarian hormone changes. Taken together, within the broader construct of disordered eating, specific symptoms may be differentially related to ovarian hormones. In particular, while ovarian hormones significantly interact to predict menstrual-cycle changes in emotional eating, ovarian hormones were not associated with weight preoccupation in our large, community sample.
Although it remains unclear why emotional eating is the strongest predictor of weight preoccupation, a few tentative hypotheses can be put forth. The mid-luteal phase is associated with the highest levels of emotional eating, and previous research has shown that elevated emotional eating is due to higher progesterone and estradiol levels during this phase (14). Increased emotional eating due to changes in ovarian hormones may lead to subsequent increases in concerns about the effect of these eating behaviors on body weight and shape. Indeed, in our data, the highest peaks in weight preoccupation scores occurred during the peri-menstrual phase, following high levels of emotional eating during the mid-luteal phase (see Figure 2), and additional analyses confirmed that emotional eating significantly predicts later increases in weight preoccupation. These findings map on well to previous research using daily diary and ecological momentary assessment designs demonstrating that binge episodes are often followed by an increase in guilt and dietary restraint, constructs that are significantly related to weight preoccupation (30–32). Taken together, our findings suggest that mid-luteal increases in emotional eating (as a result of ovarian hormone effects) may trigger weight concerns and motivate women to more closely monitor their body weight/shape during the subsequent peri-menstrual phase.
Alternatively, an additional set of factors that might explain peaks in weight preoccupation during the peri-menstrual phase are the physical changes that occur prior to and during menstruation (e.g., water retention), as these physical changes could lead to greater concern and preoccupation with body weight. Indeed, research has shown that body dissatisfaction and appearance-related anxiety are associated with water retention and other symptoms of menstrual distress during the peri-menstrual phase (33). Although we controlled for changes in BMI across the cycle, we did not assess peri-mentrual physical symptoms and could therefore not investigate whether increases in weight preoccupation during the peri-menstrual phase were associated with these physical symptoms. Further, body weight/BMI was not measured during the peri-menstrual phase for all participants, as the three study visits during which height and weight were measured did not correspond to specific menstrual cycle phases While changes in body weight across the study period were minimal in our sample (M = 0.40 lb change; SD = 3.80), daily or weekly measurements of body weight may provide additional information with regards to mechanisms for increased weight preoccupation during the peri-menstrual phase. Therefore, future research should combine more frequent weight measurements with an examination of the effects of water retention and other physical changes on fluctuations in weight preoccupation across the menstrual cycle in order to help identify additional factors that may account for these changes.
Finally, it is possible that other biological factors that fluctuate across the menstrual cycle may contribute to changes in weight preoccupation. For example, leptin levels significantly fluctuate across the menstrual cycle (34, 35), and longitudinal data has shown that leptin levels are highest during the luteal phase, which is also associated with increases in BMI (34). Furthermore, other studies have suggested a positive correlation between circulating levels of leptin and BMI in women with eating disorders (e.g., 36). Taken together, it is possible that luteal phase increases in leptin, which correspond to increases in BMI, may result in greater weight preoccupation in the subsequent peri-menstrual phase. While results from the current study did not suggest that ovarian hormones were a significant predictor of weight preoccupation, other biological factors, like leptin, may be important biological candidates to investigate in regard to weight preoccupation changes across the menstrual cycle.
Notably, it is also important for future studies to examine other key symptoms of eating disorders (e.g., body dissatisfaction, dietary restriction, compensatory behaviors) that may fluctuate across the menstrual cycle and that may (or may not) demonstrate associations with ovarian hormones. Information about these symptoms could contribute to a more comprehensive understanding of the role of ovarian hormones in the full spectrum of eating pathology and may point to further differential associations between ovarian hormones and dimensions of disordered eating. Moreover, we might expect peri-menstrual increases in weight preoccupation to confer risk for the subsequent occurrence of eating disorder behaviors aimed at preventing weight gain (e.g., dietary restriction, compensatory behaviors). While data from the current study are not sufficient to investigate these behaviors across the menstrual cycle (i.e., dietary restraint was only measured three times across the 45 day study; the frequency of compensatory behaviors in our community sample is too low), future studies examining relationships between changes in weight preoccupation and changes in these other eating disorder symptoms across the menstrual cycle could help address this possibility. Findings may have important clinical significance for identifying peak periods of risk for specific eating disorder symptoms across a woman’s menstrual cycle.
Primary strengths of the current study include the analysis of longitudinal behavioral and hormone data collected across 45 consecutive days in a large sample of women. Despite these strengths, there are limitations of our study that should be acknowledged. First, given the age range of our sample (15–25 years), many participants were not through the peak period of risk for eating disorders, which extends up until at least age 25 (37). It is important to note that eating disorder attitudinal symptoms, such as weight preoccupation, have been studied in children (38, 39) and have been implicated as risk factors for the later development of full eating disorders (16, 40). Therefore, the age range of our sample is likely appropriate for studying weight preoccupation, a core cognitive correlate of eating disorders. Second, it is unknown if findings for weight preoccupation in our community sample generalize to clinical samples of eating disorder patients, and whether the relationship between objective binge episodes and weight preoccupation would map on to what we observed for emotional eating and weight preoccupation. While these will be important questions for future research, examination of a community sample of women allowed us to investigate the full spectrum of disordered eating severity and to further explore possible etiologic factors contributing to the development of weight preoccupation.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (RO1 MH082054, T32 MH018269, T32 MH070343).
Footnotes
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory (M-EDI)) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmsted, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Given that weight preoccupation varies by BMI (e.g., Baker & Galambos, 2003), analyses were also run to examine possible differences in the predictors of weight preoccupation in participants in the uppermost (BMI > 27.22; N = 71) and lowest quintiles (BMI < 20.02; N = 70) of BMI. Results indicated that neither ovarian hormones nor psychological factors predicted weight preoccupation changes in the lowest BMI quintile group. In the uppermost quintile group, results suggested that, similar to in the full sample, only emotional eating scores were significant predictors of weight preoccupation (b (S.E) = .13 (.06); p = .04).
The content is solely the responsibility of the authors and does not necessarily represent the official views of Michigan State University or the National Institute of Mental Health.
Parts of this manuscript were presented at the Eating Disorders Research Society meeting, Bethesda, Maryland, September 19-21, 2013.
None of the authors have biomedical financial conflicts of interest or other potential conflicts of interest to disclose.
References
- 1.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37:131–41. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt T, Alfano L, Tricamo M, Pfaff DW. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clin Psychol Rev. 2010;30:655–68. doi: 10.1016/j.cpr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klump K, Keel P, Culbert K, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008;38:1749–57. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord. 2012;45:333. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–63. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm Behav. 1989;23:235–50. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 7.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–8. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 8.Klump KL, Suisman JL, Culbert KM, Kashy DA. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav. 2011;59:585–93. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- 10.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144:1592–5. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 11.Lester NA, Keel P, Lipson S. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- 12.Price WA, Torem MS, Dimarzio LR. Premenstrual exacerbation of bulimia: 60% increase in the mean number of binge episodes. Psychosomatics. 1987;28:378–9. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- 13.Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, et al. Ovarian hormone influences on dysregulated eating: a comparison of associations in women with versus without binge episodes. Clin Psychol Sci. 2014 doi: 10.1177/2167702614521794. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klump K, Keel P, Racine S, Burt A, Neale M, Sisk C, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122:131–7. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. J Abnorm Psychol. 2000;109:239–51. [PubMed] [Google Scholar]
- 16.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Wade TD, Zhu G, Martin NG. Undue influence of weight and shape: is it distinct from body dissatisfaction and concern about weight and shape? Psychol Med. 2011;41:819–28. doi: 10.1017/S0033291710001066. [DOI] [PubMed] [Google Scholar]
- 18.Dennerstein L, Burrows GD. Affect and the menstrual cycle. J Affec Disord. 1979;1:77–92. doi: 10.1016/0165-0327(79)90027-2. [DOI] [PubMed] [Google Scholar]
- 19.Ivey ME, Bardwick JM. Patterns of affective fluctuation in the menstrual cycle. Psychosom Med. 1968;30:336–45. doi: 10.1097/00006842-196805000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An Update. Twin Research and Human Genetics. 2012;1:1–7. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–7. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- 22.von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: A brief measure of disordered eating attitudes and behaviors. Eat Behav. 2005;6:373–92. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Racine SE, Culbert KM, Larson CL, Klump KL. The possible influence of impulsivity and dietary restraint on associations between serotonin genes and binge eating. J Psychiatr Res. 2009;43:1278–86. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Strien T, Cleven A, Schippers G. Restraint, tendency toward overeating and ice cream consumption. Int J Eat Disord. 2000;28:333–8. doi: 10.1002/1098-108x(200011)28:3<333::aid-eat11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 26.Davis C. Body image and weight preoccupation: A comparison between exercising and non-exercising women. Appetite. 1990;15:13–21. doi: 10.1016/0195-6663(90)90096-q. [DOI] [PubMed] [Google Scholar]
- 27.Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145:1–9. doi: 10.1530/eje.0.1450001. [DOI] [PubMed] [Google Scholar]
- 28.Yoo KY, Kim H, Shin HR, Kang D, Ha M, Park SK, et al. Female sex hormones and body mass in adolescent and postmenopausal Korean women. J Korean Med Sci. 1998;13:241–6. doi: 10.3346/jkms.1998.13.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Individual differences in the relationship between ovarian hormones and emotional eating across the menstrual cycle: A role for personality? Eat Behav. 2013;14:161–6. doi: 10.1016/j.eatbeh.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiger H, Gauvin L, Engelberg MJ, Ying Kin NMKN, Israel M, Wonderlich SA, Richardson J. Mood-and restraint-based antecedents to binge episodes in bulimia nervosa: Possible influences of the serotonin system. Psychol Med. 2005;35:1553–62. doi: 10.1017/S0033291705005817. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JG. Toward a better understanding of the development of overweight: a study of eating behavior in the natural environment using ecological momentary assessment. Philidelphia: Drexel University; 2009. [Google Scholar]
- 32.Wegner KE, Smyth JM, Crosby RD, Wittrock D, Wonderlich SA, Mitchell JE. An evaluation of the relationship between mood and binge eating in the natural environment using ecological momentary assessment. Int J Eat Disord. 2002;32:352–61. doi: 10.1002/eat.10086. [DOI] [PubMed] [Google Scholar]
- 33.Carr-Nangle RE, Johnson WG, Bergeron KC, Nangle DW. Body image changes over the menstrual cycle in normal women. Int J Eat Disord. 1994;16:267–73. doi: 10.1002/1098-108x(199411)16:3<267::aid-eat2260160307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 34.Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol. 1997;47:101–6. doi: 10.1046/j.1365-2265.1997.2441017.x. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154:285–92. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 36.Monteleone P, Di Lieto A, Tortorella A, Longobardi N, Maj M. Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry Res. 2000;94:121–9. doi: 10.1016/s0165-1781(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 37.Lewinsohn PM, Striegel-Moore RH, Seeley JR. Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. J Am Acad Child Adolesc Psychiatry. 2000;39:1284–92. doi: 10.1097/00004583-200010000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Lucero LD, Hill FA, Ferraro FR. Body dissatisfaction in young children. Psychology: A Journal of Human Behavior. 1999 [Google Scholar]
- 39.Schur EA, Sanders M, Steiner H. Body dissatisfaction and dieting in young children. Int J Eat Disord. 1999;27:74–82. doi: 10.1002/(sici)1098-108x(200001)27:1<74::aid-eat8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 40.Killen JD, Taylor CB, Hayward C, Wilson DM, Haydel KF, Hammer LD, et al. Pursuit of thinness and onset of eating disorder symptoms in a community sample of adolescent girls: A three-year prospective analysis. Int J Eat Disord. 1994;16:227–38. doi: 10.1002/1098-108x(199411)16:3<227::aid-eat2260160303>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]