Abstract
Objectives
The aims of this study were to examine: (1) the relationship between apathy and disability in late-life depression, and (2) the functional significance of improvement in apathy following escitalopram treatment in terms of its relationship to disability.
Methods
Subjects were 71 non-demented elderly with non-psychotic major depression. After a 2-week single-blind placebo period, subjects who had Hamilton Depression Rating Scale (HDRS) ≥ 18 received escitalopram 10mg daily for 12 weeks. Apathy and disability were assessed with the Apathy Evaluation Scale (AES) and the World Health Organization Disability Assessment Scale II (WHODAS) respectively. These measures and the HDRS were administered at baseline and again following 12 weeks of treatment.
Results
At baseline, 38% of depressed subjects had significant apathy (AES ≥ 36.5). Severity of apathy at baseline significantly correlated with severity of disability. In a multivariate regression model, baseline severity of apathy, but not the overall depressive syndrome (HDRS), significantly correlated with baseline disability. Following escitalopram treatment, improvement in apathy significantly correlated with improvement in disability measures, while change in the rest of the depressive syndrome did not. The overall change in apathy and disability in response to escitalopram treatment was significant but small.
Conclusions
Apathy is common in late-life depression and is associated with disability above and beyond the influence of other depressive symptoms. Given the strong relationship between apathy and disability, understanding the neurobiology of apathy and developing treatments for apathy may improve the functional outcomes of late-life depression.
Keywords: apathy, amotivation, atypical depression, geriatric, function, disability
OBJECTIVE
Late-life depression is challenging to treat. Developing effective treatments calls for the study of meaningful dimensions of this illness that directly affect clinical outcomes such as disability.
Apathy afflicts many older adults who suffer from late-life depression. Its presence predicts poor response of depressive symptoms to treatment and chronicity of depression (1–3). Clinically significant apathy occurs in more than 30% of individuals with major depression, and is most prevalent in depressed older adults (4–8). The syndrome of apathy is defined as a primary motivational impairment that, in depression, results in diminished goal-directed behavior, lack of intellectual interest, and indifference or flattening of affect (9). These clinical signs translate into apathetic, depressed patients being poorly engaged, being more difficult to treat, and posing a greater burden to caregivers (4, 10).
In a previous analysis, we found that escitalopram only modestly improved apathy in a small sample of older depressed individuals (11). Defining the clinical significance of apathy and of its improvement after treatment with escitalopram may contribute further to the identification of apathy as a clinically meaningful dimension of the late-life depressive disorder. Thus, this study examined the contribution of apathy to disability relative to the rest of the late-life depressive syndrome, and the functional significance of change in apathy following escitalopram treatment with respect to its effect on disability outcomes.
METHODS
Subjects
Subjects were 71 depressed older adults (>60 years) from a university geriatric psychiatry clinic recruited from the community through radio and print advertisements for an escitalopram treatment trial. Subjects met DSM-IV-TR criteria for unipolar depression without psychotic features and had a score ≥18 on the 24-item Hamilton Depression Rating Scale (HDRS) (12) after a two-week drug washout/placebo lead-in.
Exclusion criteria were: (1) history of other axis I psychiatric disorders prior to the onset of depression; (2) Mini-Mental State Examination (MMSE) score less than 24 (13) (3) Mild Cognitive Impairment (MCI) according to criteria described by Petersen et al. (14); (4) severe medical illness (i.e., metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke) within the 3 months preceding the study; (5) neurological disorders (i.e., dementia, delirium, history of head trauma, Parkinson’s disease, and multiple sclerosis); (6) diseases often associated with depression (i.e., endocrinopathies other than diabetes, lymphoma, and pancreatic cancer); and (7) treatment with drugs associated with depression (i.e., steroids, α-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine). All subjects signed written and informed consent approved by the Institutional Review Board of Weill-Cornell Medical College.
Treatment
After a 2-week drug washout and single blind placebo lead-in period, subjects who still met DSM-IV-TR criteria for major depression and had a HDRS≥18 received escitalopram 10mg daily for 12 weeks. Subjects were assessed weekly throughout the treatment trial. Assessment consisted of a brief meeting with a research psychiatrist and ratings by a trained research assistant using the HDRS, a medication adherence questionnaire, and a vital signs form. The meeting with the research psychiatrist followed a medication clinical format focusing on psychiatric symptom and side effects evaluation. No participants received psychotherapy.
Measures
Major depressive disorder was diagnosed based on the SCID-R, administered at entry to the study. Depressive symptoms were assessed with the HDRS. Apathy was quantified using the self-rated Apathy Evaluation Scale (AES), a psychometrically validated instrument in older normal individuals and psychiatric patients (15, 16). Overall cognitive impairment was examined in a clinical interview and rated with the MMSE (13) and the Dementia Rating Scale (DRS) (17). Chronic co-morbid medical illness burden was rated with the Charlson Comorbidity Index (18). Disability was rated with the World Health Organization Disability Assessment Schedule version II (WHODAS) (19). All measures were collected at baseline and again following 12 weeks of escitalopram treatment.
Data Analysis
Statistical analysis was performed with SPSS 19.0 (SPSS, Inc.). Mann-Whitney U, t-tests, and paired sample t-tests were used to analyze demographic and clinical aspects of the patient sample, and to quantitatively compare outcome measures before and after antidepressant. Cohen’s d values were calculated to express the difference between means of outcome measures before and after treatment. Cohen’s d values for these repeated measures were calculated using original standard deviations, as opposed to a paired t-test value, according to Dunlop et al (20).
Bivariate analyses included the calculation of correlation coefficients to estimate the relationships between each dependent variable and all continuous independent variables and of t-statistics as a measure of the association between each dependent variable and gender. Relevant covariates were identified and entered along with AES and HDRS in a multivariate linear regression analysis of predictors of disability measures to estimate the independent effects of apathy and depression variables on disability measures. Each regression model was tested for collinearity with close attention paid to differences in zero-order, partial, and part correlations, tolerance, variance inflation factor, eigenvalue, and condition index. All significance tests were two-tailed.
RESULTS
Subjects and Treatment
Seventy-one depressed older adults were studied. Their age range was 60 to 86 years and the female to male ratio was 1.21:1 (Table 1).
Table 1.
Baseline Characteristics of Depressed Elderly Non-Demented Subjects
| N | Mean | Std. Deviation (±) | |
|---|---|---|---|
| Age (Years) | 71 | 70.8 | 6.5 |
| Gender (% Female) | 71 (54.93 %) | -- | -- |
| Education (Years) | 71 | 15.8 | 3.3 |
| Age of depression onset (Years) | 64 | 56.6 | 19.0 |
| Charlson Comorbidity Index | 66 | 1.0 | 1.0 |
| Apathy Evaluation Scalea | 71 | 35.7 | 9.5 |
| Hamilton Depression Rating Scaleb | 71 | 21.34 | 3.8 |
| Mattis Dementia Rating Scale | 71 | 135.23 | 6.1 |
| Mini Mental Status Exam | 71 | 28.3 | 1.4 |
Apathy Evaluation Scale, patient self-rated
24-item Hamilton Depression Rating Scale
Of the 71 depressed elderly subjects, 66 completed the 12-week treatment trial. Five subjects failed to complete the study: two exited because of worsening depression, two exited because they found the treatment ineffective, and one withdrew because of escitalopram-related hyponatremia. We found no significant baseline differences in apathy severity, depression severity, disability or cognitive function between participants who did or did not complete the 12-week treatment trial.
Using a cut-off value of Apathy Evaluation Scale (AES) ≥ 36.5 (15), 38% (27 out of 71) of depressed elders suffered from a clinically significant level of apathy at study entry. Following escitalopram treatment, 16% (11 out of 68) continued to suffer from significant apathy. Only four subjects reported an increase in apathy, all of which were minor increases of two to three AES points out of a total 72-point scale.
Relationship of Apathy to Disability
At baseline, apathy severity was correlated with greater disability (WHODAS), and greater severity of depression (HDRS) (Table 2). Examining subdomains of the WHODAS revealed that baseline apathy was correlated with impaired life activities (household responsibilities, work) and impaired participation in society (community activities), but not with more basic functions such as understanding/communicating, mobility, and self-care. Apathy did not correlate with total or any subdomain (attention, initiation/perseveration, construction, conceptualization, memory) scores of the Mattis Dementia Rating Scale.
Table 2.
Baseline Factor Correlations with Apathy (AESa) in Depressed Subjects
| Factors | Pearson Correlation r-value |
p | N |
|---|---|---|---|
| Age | .052 | .667 | 71 |
| Age of depression onset | .212 | .092 | 64 |
| Gender | −.175 | .143 | 71 |
| Education | −.131 | .276 | 71 |
| Charlson Comorbidity Index | .069 | .583 | 66 |
| WHODASb total score | .283* | .017 | 71 |
| WHODAS domain 1- Understanding and communicating | .221 | .070 | 71 |
| WHODAS domain 2- Mobility | −.011 | .927 | 71 |
| WHODAS domain 3- Self care | −.021 | .866 | 67 |
| WHODAS domain 4- Getting along with people | .224 | .075 | 64 |
| WHODAS domain 5- Life activities | .272* | .025 | 68 |
| WHODAS domain 6- Participation in society | .320** | .007 | 70 |
| Hamilton Depression Rating Scale | .256* | .031 | 71 |
| Mattis Dementia Rating Scale | −.086 | .476 | 71 |
| Mini-Mental Status Exam | −.176 | .143 | 71 |
Apathy Evaluation Scale, patient self-rated
World Health Organization Disability Assessment Scale II
p < 0.05 level (2-tailed);
p < 0.01 level (2-tailed)
To examine the effect of apathy on disability, we performed multivariate regression analysis with baseline measures of apathy (AES), severity of depression (HDRS), and medical burden (Charlson Comorbidity Index) as independent variables and with baseline disability (WHODAS) as the dependent variable. Medical burden was controlled for because bivariate correlation revealed a significant relationship between this variable and disability; other demographic and clinical variables (age, gender, etc.) did not have a significant relationship with disability. This multivariate regression analysis showed that baseline apathy was associated with baseline disability, while depression severity was not (Table 3). Note that baseline apathy did not correlate executive function (initiation/perseveration) measures of the Mattis Dementia Rating Scale (DRS). However, to assess any potentially confounding effect of executive dysfunction on the apparent relationship between apathy and disability, the above multivariate regression analysis was also performed with DRS initiation/perseveration score as a covariate. The addition of this covariate did not significantly alter the model or affect the correlation (standardized beta or p-value) between apathy and disability.
Table 3.
Multivariate Regression Analysis of Baseline Disability †
| Outcome Variable | WHODASa Total | WHODAS Domain 5: Life Activities | WHODAS Domain 6: Participation in society | |||
|---|---|---|---|---|---|---|
|
| ||||||
| β | p | β | p | β | p | |
| Baseline AESb | .242* | .033 | .252* | .023 | .293* | .015 |
| Baseline HDRSc | .151 | .176 | −.081 | .456 | .151 | .200 |
| R2 | .32 | .33 | .29 | |||
| N | 66 | 66 | 66 | |||
Controlling for medical comorbidity covariate (Charlson Comorbidity Index)
World Health Organization Disability Scale II
Apathy Evaluation Scale, patient-rated
24-item Hamilton Depression Rating Scale
p < 0.05 level
Note: The statistical significance of individual regression coefficients were evaluated by t-tests with df = 62 in each regression analysis.
To examine the effect of change in apathy on change in disability, we performed multivariate regression analysis with the following independent variables: baseline measures of apathy (AES), depression (HDRS), and disability (WHODAS); change in apathy; and change in disability. The change in apathy observed following escitalopram treatment was associated with change in disability (Table 4). In contrast, change in overall depressive symptoms (HDRS) was not.
Table 4.
Multivariate Regression of Change in Disabiility Following Escitalopram Treatment†
Controlling for baseline medical comorbidity (Charlson Comorbidity Index), disability ( WHODASa), apathy (AESb), depression (HDRSc)
World Health Organization Disability Scale II
Apathy Evaluation Scale, patient-rated
24-item Hamilton Depression Rating Scale
p < .05,
p < .01
Note: The statistical significance of individual regression coefficients were evaluated by t-tests with df = 59.
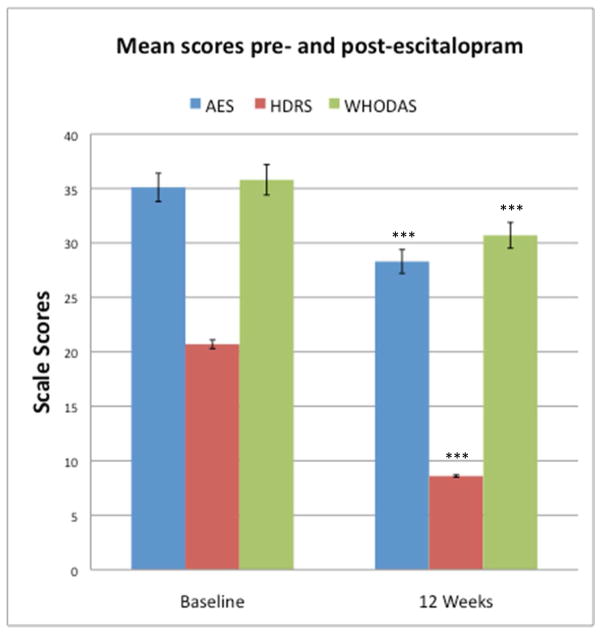
Following escitalopram treatment, mean scores of depression, apathy, and disability all decreased (Mean ΔHDRS 12.1, SD 7.2, t(65) = 13.31, p < .001, d = 2.39, 95% Confidence Interval for d [2.11, 2.66]; Mean ΔAES 6.8, SD 9.3, t(65) = 5.39, p < .001, d = 0.77, 95% Confidence Interval for d [0.50, 1.03]; Mean ΔWHODAS 5.3, SD 8.2, t(65) = 4.90, p < .001, d = 0.56, 95% Confidence Interval for d [0.28, 0.84]) (Figure 1). A comparison of Cohen’s d effect sizes suggested that the improvement in both apathy (AES) and disability (WHODAS) were proportionally smaller than the improvement in depression severity (HDRS) after escitalopram treatment.
Figure 1.
Apathy, depression, and disability in patients before and after 12 weeks of escitalopram treatment. Mean Apathy Evaluation Scale (AES), 24-item Hamilton Depression Rating Scale (HDRS) and World Health Organization Disability Scale II (WHODAS) scores with corresponding standard errors of the mean are depicted. ***p< .001 relative to corresponding baseline measure, paired t-test, N=66 for each measure
CONCLUSIONS
The principal finding of this study is that apathy in late-life depression is persistent and associated with disability above and beyond the influence of the rest of the depressive syndrome. To our knowledge, this is the first study to focus on the unique contribution to disability that apathy confers to the clinical burden of late-life depressive illness. The implication of these findings is that apathy is a clinically meaningful dimension of depression, improved treatment of which could ameliorate disability in depressed older adults.
The frequency (38%) and persistence of apathy in nearly half of all who presented with apathy at baseline is consistent with prior reports. Apathy is one of the most common neuropsychiatric syndromes in the aging population, affecting between 3–11% of community-residing older adults (4, 21, 22) and more than 30% of individuals with late-life major depression (5–7). We observed that 16% of depressed older individuals treated with escitalopram continued to suffer from apathy, consistent with earlier studies documenting that 18.6% of depressed patients who complete a course of selective serotonin reuptake inhibitor (SSRI) treatment report persistent apathy (23).
While apathy is strongly associated with executive dysfunction in patients with MCI and Alzheimer’s disease (24), apathy was not correlated with executive dysfunction in our study sample. Our results are consistent with earlier conclusions made by Marin et al that apathy may in fact be independent of executive dysfunction in elderly with late-life depression (25).
Late-life depression is a leading cause of disability in the aging population (26, 27). An important and frequent contributor to disability in these patients may be apathy. Our finding that apathy in late-life depression is associated with disability above and beyond the influence of the rest of the depressive syndrome is consistent with findings from other fields. In apathetic, depressed, HIV positive patients, apathy more than depression predicted worse every-day functioning (28). In stroke literature, Hama et al suggested that post-stroke depression consists of two core syndromes, i.e. affective/depressive vs. apathetic, in which distinct neuroanatomical mechanisms influence functional recovery. This assertion was based on the observation that post-stroke apathy and depression are frequently comorbid and the severity of apathy, but not that of depression, is inversely correlated with improvement in daily function (29). Subsequent research substantiated the differential effects of depression versus apathy on functional recovery after stroke as well as the different monoamingergic neuroanatomic pathways associated with affective versus apathetic symptoms after stroke (30, 31). Late-life mental health studies have shown that apathy comorbid with depression can be distinguished from depressed mood (32) and preliminary efforts have begun to uncover the unique neuroanatomical substrates of apathy in geriatric depression (33, 34). Our finding that baseline apathy is cross-sectionally associated with disability and that change in apathy, and not severity of depression, is associated with change in disability suggests that apathy is a distinct and meaningful dimension of the late-life depressive disorder.
The changes in both apathy and disability with escitalopram treatment were small. This finding corroborates the inadequacy of SSRIs in improving apathy and disability in late-life depression (35–38). A recent randomized controlled trial of depression with apathy using SSRI or serotonin-norepinephrine reuptake inhibitors (SNRI) documented the persistence of apathy after an initial SSRI course and some improvement in apathy when a switch was made to treatment with either another SSRI or SNRI. However, the difference between the two treatment arms was not appreciable (39). Yet, escitalopram has been shown to be more effective than placebo in preventing new onset of apathy following stroke (40).
Our results are limited in several respects. Treatment was restricted to escitalopram titrated to 10mg daily. It is possible that higher doses may have led to greater changes in apathy and disability. In the absence of a placebo arm, we also cannot rule out that the improvement in apathy and disability may have been a placebo response and not related to escitalopram. We attempted, however, to exclude participants prone to early placebo response by introducing a two-week placebo lead-in phase. Focusing on non-demented, non-MCI depressed elderly individuals may have limited the severity of apathy in our sample and the applicability of our findings to other less cognitively intact populations. However, it permitted us to study apathy related to late-life depression that could not be attributed to diagnosable dementing disorders. Lastly, this is a study of a rather small number of participants, and our findings should be viewed as preliminary.
In conclusion, the results of this study highlight apathy as a common, persistent, and disabling clinical manifestation of late-life depression. Its persistence and its impact on disability suggest that evaluation of apathy should be part of the ongoing clinical assessment of depressed older patients. Study of the neurobiology of apathetic depression may identify appropriate treatment targets since the available pharmacology offers only modest help.
Acknowledgments
Source of Funding: Dr. Alexopoulos received grant support from Forest Pharmaceuticals and has been a member of speakers’ bureaus of Astra Zeneca, Avanir, Forest, Merck, and Lundbeck. He holds equity of Johnson and Johnson.
This work was supported by NIMH grants R01 MH65653, R01 MH079414, P030 MH085943, T32 MH019132 (GSA), R01 MH097735 (FMG) and the Sanchez Foundation. Escitalopram and placebo were provided free of cost by Forest Pharmaceuticals, Inc.
Footnotes
Conflicts of Interest:
No other authors report biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta psychiatrica Scandinavica. 1986;74:183–186. doi: 10.1111/j.1600-0447.1986.tb10603.x. [DOI] [PubMed] [Google Scholar]
- 2.Lavretsky H, Lesser IM, Wohl M, et al. Clinical and neuroradiologic features associated with chronicity in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- 3.Levkovitz Y, Sheer A, Harel EV, et al. Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain stimulation. 2011;4:266–274. doi: 10.1016/j.brs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotoxicity research. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- 5.Forsell Y, Jorm AF, Fratiglioni L, et al. Application of DSM-III-R criteria for major depressive episode to elderly subjects with and without dementia. The American journal of psychiatry. 1993;150:1199–1202. doi: 10.1176/ajp.150.8.1199. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan KR, Hays JC, Tupler LA, et al. Clinical and phenomenological comparisons of late-onset and early-onset depression. The American journal of psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- 7.Mehta M, Whyte E, Lenze E, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. International journal of geriatric psychiatry. 2008;23:238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- 8.Lampe IK, Heeren TJ. Is apathy in late-life depressive illness related to age-at-onset, cognitive function or vascular risk? International psychogeriatrics / IPA. 2004;16:481–486. doi: 10.1017/s1041610204000766. [DOI] [PubMed] [Google Scholar]
- 9.Marin RS. Differential diagnosis and classification of apathy. The American journal of psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Holtta EH, Laakkonen ML, Laurila JV, et al. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. Journal of the American Medical Directors Association. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Yuen GS, Gunning FM, Woods E, et al. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. Journal of affective disorders. 2014 doi: 10.1016/j.jad.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Clarke DE, Reekum R, Simard M, et al. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. The Journal of neuropsychiatry and clinical neurosciences. 2007;19:57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Marin RS. Apathy: a neuropsychiatric syndrome. The Journal of neuropsychiatry and clinical neurosciences. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 17.Mattis S, editor. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Epping-Jordan JA, Ustun TB. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- 20.Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. Meta–analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1:170–177. [Google Scholar]
- 21.Groeneweg-Koolhoven I, de Waal MW, van der Weele GM, et al. Quality of life in community-dwelling older persons with apathy. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22:186–194. doi: 10.1016/j.jagp.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Onyike CU, Sheppard JM, Tschanz JT, et al. Epidemiology of apathy in older adults: the Cache County Study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- 23.Bolling MY, Kohlenberg RJ. Reasons for quitting serotonin reuptake inhibitor therapy: paradoxical psychological side effects and patient satisfaction. Psychotherapy and psychosomatics. 2004;73:380–385. doi: 10.1159/000080392. [DOI] [PubMed] [Google Scholar]
- 24.McPherson S, Fairbanks L, Tiken S, et al. Apathy and executive function in Alzheimer’s disease. Journal of the International Neuropsychological Society : JINS. 2002;8:373–381. doi: 10.1017/s1355617702813182. [DOI] [PubMed] [Google Scholar]
- 25.Marin RS, Butters MA, Mulsant BH, et al. Apathy and executive function in depressed elderly. Journal of geriatric psychiatry and neurology. 2003;16:112–116. doi: 10.1177/0891988703016002009. [DOI] [PubMed] [Google Scholar]
- 26.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 27.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 28.Kamat R, Morgan E, Marcotte TD, et al. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. Journal of affective disorders. 2013;150:1069–1075. doi: 10.1016/j.jad.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hama S, Yamashita H, Shigenobu M, et al. Depression or apathy and functional recovery after stroke. International journal of geriatric psychiatry. 2007;22:1046–1051. doi: 10.1002/gps.1866. [DOI] [PubMed] [Google Scholar]
- 30.Hama S, Yamashita H, Yamawaki S, et al. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. 2011;11:68–76. doi: 10.1111/j.1479-8301.2011.00358.x. [DOI] [PubMed] [Google Scholar]
- 31.Murakami T, Hama S, Yamashita H, et al. Neuroanatomic pathways associated with poststroke affective and apathetic depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:840–847. doi: 10.1016/j.jagp.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 32.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. The Journal of neuropsychiatry and clinical neurosciences. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS, Hoptman MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: a preliminary study. Journal of affective disorders. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavretsky H, Ballmaier M, Pham D, et al. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodela S, Venkata PD. Antidepressant induced apathy responsive to dose reduction. Psychopharmacology bulletin. 2010;43:76–79. [PubMed] [Google Scholar]
- 36.Padala PR, Padala KP, Monga V, et al. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. The Annals of pharmacotherapy. 2012;46:e8. doi: 10.1345/aph.1Q656. [DOI] [PubMed] [Google Scholar]
- 37.Sato S, Asada T. Sertraline-induced apathy syndrome. The Journal of neuropsychiatry and clinical neurosciences. 2011;23:E19. doi: 10.1176/jnp.23.1.jnpe19. [DOI] [PubMed] [Google Scholar]
- 38.Wongpakaran N, van Reekum R, Wongpakaran T, et al. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Annals of general psychiatry. 2007;6:7. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raskin J, George T, Granger RE, et al. Apathy in currently nondepressed patients treated with a SSRI for a major depressive episode: outcomes following randomized switch to either duloxetine or escitalopram. Journal of psychiatric research. 2012;46:667–674. doi: 10.1016/j.jpsychires.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Mikami K, Jorge RE, Moser DJ, et al. Prevention of poststroke apathy using escitalopram or problem-solving therapy. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:855–862. doi: 10.1016/j.jagp.2012.07.003. [DOI] [PubMed] [Google Scholar]