Abstract
Objectives
Circadian rest-activity rhythms (CARs) have been cross-sectionally associated with depressive symptoms, however no longitudinal research has examined whether CARs are a risk factor for developing depressive symptoms.
Methods
We examined associations of CARs (measured with actigraphy over a mean of 4.8 days) with depressive symptoms (measured with the Geriatric Depression Scale) among 2892 community-dwelling older men (mean age: 76.2 +/− 5.5) from the MrOS Sleep Study who were without cognitive impairment. Among 2124 men with minimal (0–2) symptoms at baseline, we assessed associations between CAR parameters and increases to mild (3–5) or clinically significant (≥6) symptoms after an average of 1.2 (+/− 0.32) years later.
Results
Cross-sectional associations between rhythm height parameters were independent of chronic diseases, lifestyle, sleep, and self-reported physical activity covariates. For example, men in the lowest mesor quartile had 2 times the adjusted odds (Adjusted Odds Ratio (AOR)=2.04, 95% CI 1.36–3.04, p=0.0005) of having prevalent clinically significant symptoms (compared to minimal). Longitudinally, low CAR robustness (being in the lowest quartile of the pseudo-F statistic) was independently associated with increasing odds of developing symptoms (i.e. AOR for having clinically significant depressive symptoms at follow-up=2.58, 95% CI 1.11–5.99, p=0.03).
Conclusion
CAR disturbances are indicative of depressive symptomology. Low CAR robustness may independently contribute to the risk of worsening depression symptomology.
Keywords: Aging, circadian rest-activity rhythm (CAR), depression, epidemiology
Sleep disturbances1 and a lack of daytime physical activity2,3 are risk factors for the development of depression, however the pattern of rest and activity has received less attention in psychiatric research. Rest and activity exhibit diurnal variation known as the circadian rest-activity rhythm (CAR). This rhythm can be measured with actigraphy (which reliably distinguishes the sleep/wake period4) and actigraph-identified bedtime, wake-time, mid-sleep time, acrophase5 and sleep period6,7 generally correlate with that of urinary 6-sulphatoxymelatonin secretion. Therefore, the actigraph-measured CAR reflects the biologically entrained circadian rhythm, and such methods may help clarify relationships between the circadian system and health in aging.
The robustness of the CAR has been previously associated with all-cause and cardiovascular mortality among older men8. Rhythm amplitude, robustness and timing of peak activity have also been shown to predict incident dementia among older women9. A recent cross-sectional study demonstrated that older women with greater levels of depressive symptoms were more likely to have circadian rhythm disturbances, specifically lower amplitude and less robust CARs10. No previous research has reported cross-sectional associations of CARs and depression in older men. In addition, no previous research in any population has examined whether CAR characteristics contribute to depression risk over time.
Since characteristics of the CAR may be modifiable (i.e. using bright light therapy11), the CAR may be a viable target for interventions aimed at preventing the development of depression among at risk older adults. We report here cross-sectional associations between CAR disturbances and depressed mood among community dwelling older men. Further, we examine whether baseline CAR disturbances are a risk factor for future increases in depressive symptoms.
Methods
Participants
The Osteoporotic Fractures in Men (MrOS) Sleep Study, conducted between December 2003 and March 2005, included 3,135 participants recruited at six clinical centers in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Monongahela Valley, Pennsylvania; Portland, Oregon; and San Diego, California)12,13. The parent MrOS study included community-dwelling men ≥65 years who could walk without assistance and were without bilateral hip replacements (Figure 1). Men were excluded from the Sleep Study if they regularly used overnight nocturnal oxygen therapy, positive pressure or oral appliances for treatment of sleep apnea (n=150). Other reasons for non-participation were: death (n=349), terminated study participation (n=39), declined sleep study (n=1997), or because MrOS Sleep Study recruitment goals had already been met (n=324).
Figure 1.
Study Sample
Participants were included in the analytic cohort if they had ≥3, 24 hour periods of technically adequate actigraph data (excluded n=134). The validity of self-report depression screening among older adults, including the Geriatric Depression Scale (GDS), depends in part on the cognitive status of the participant14; therefore we excluded 105 participants with a Modified Mini-Mental State Exam (3MS)15 <80 (often used to indicate cognitive impairment16) at baseline. Of these individuals, 4 were missing outcome data at the Sleep Visit resulting in a cross-sectional sample of 2892 men.
Follow-up outcome data was obtained from the MrOS Visit 2 (conducted between March 2005 and May 2006) 1.2 (+/− 0.32 standard deviation (SD)) years after the first Sleep Visit. The prospective sample included only participants without baseline depression (GDS scores ≤2, n=2219). Of these men, we excluded those with cognitive impairment (3MS<80) at follow-up (n=45) and of the remaining men 50 were missing follow-up outcome data. Therefore the prospective sample included 2124 men free of cognitive impairment and baseline depression. All men provided written informed consent, and the study was approved by the Institutional Review Board at each clinic site. Missing covariate data was minimal (<1%) with the exception of sleep disordered breathing (SDB) variables which were measured using polysonomnography (PSG). Altogether covariate data were missing in less than 7% of cases resulting in a minimum cross-sectional multivariable model including 2696 men (86% of Sleep Study participants).
Measures
Circadian rest-activity rhythm (CAR)
The Octagonal Sleep Watch actigraph (SleepWatch-O; Ambulatory Monitoring, Inc, Ardsley, NY) was used to estimate rest/activity rhythms. Participants were asked to wear actigraphs on the non-dominant wrist for a minimum of 5 consecutive 24-hour periods except when bathing or during water sports. The actigraph measures movement using a piezoelectric biomorph-ceramic cantilevered beam, which generates a voltage each time the actigraph is moved, generating reliable estimate of sleep-wake patterns17. These voltages are gathered continuously and stored in one minute epochs, with activity measured in units of counts per minute. Data collected in digital integration mode were used for this analysis. ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to score the actigraphy data. Inter-scorer reliability for scoring of this data has been previously found to be high in our group (intra-class coefficient = 0.95)18 and this measure has been shown to have good concordance with total sleep time from polysomnography19.
A five-parameter extended sigmoidally transformed cosine model with an antilogistic function was used to compute CAR parameters from the activity data gathered by the actigraph. This method has been shown to fit CAR data better than a standard cosine model as the CAR in humans typically exhibits a more “squared” rhythm during the daytime20. Modeled parameters included measures of rhythm height, timing, and robustness.
Rhythm height parameters were: amplitude (peak-nadir difference) and mesor (approximate middle of the fitted curve). To determine if these associations of rhythm height were driven by overall activity level rather than relative peak-nadir differences, standardized amplitude was computed as amplitude divided by mesor.
Rhythm timing measures were: acrophase (time of day of peak activity level), up-mesor (time of day when activity passes up through mesor, also known as left-half detection point representing the time the participant gets going in the morning), down-mesor (time of day when activity passes down through mesor, also known as right-half detection point, representing the time of day the participant settles down for the night).
The extended cosine model also provides a measure of rhythm robustness, called the pseudo-F statistic, reflecting how well the modeled rhythm fits the observed data20. Lower values indicate poorer model fit which suggests that the rhythm may be erratic, and/or variable.
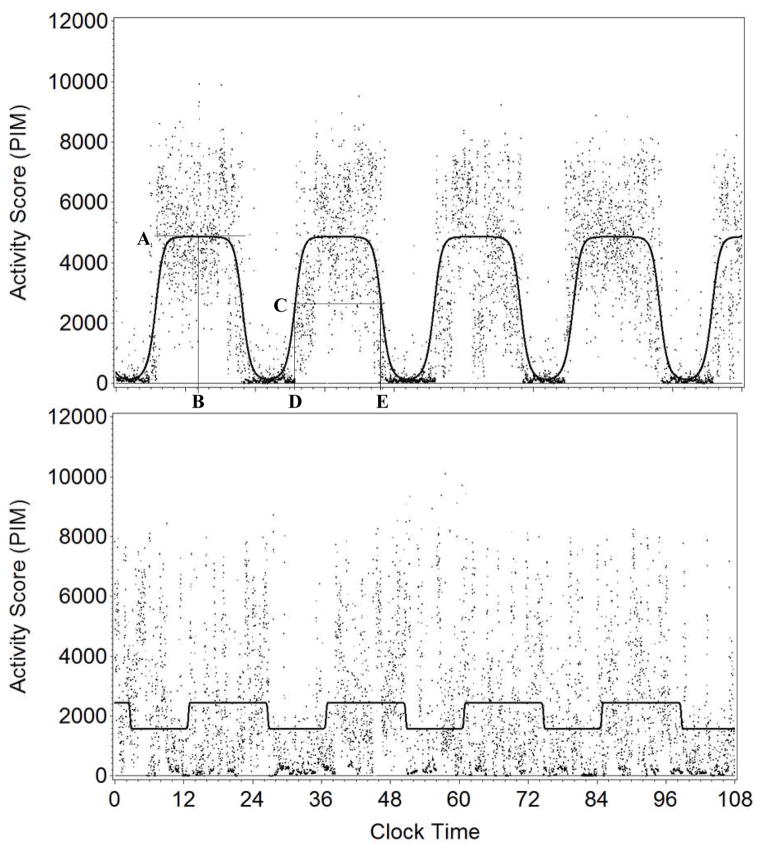
These model parameters are visually depicted along with raw data from two participants (one more and one less robust) for illustrative purposes (Figure 2).
Figure 2.
Raw actigraphy data with modeled CAR from two participants (Top: Robust CAR; Bottom: Less robust CAR)
Circadian Activity Rhythms (CARs) measured over 4 days from two subjects. Dots correspond to raw activity counts.
Top: A single participant with a robust CAR (high pseudo-F statistic). Lines are approximately placed for illustrative purposes. Line A indicates the amplitude of the rhythm, and line B corresponding time of the rhythm’s peak (acrophase). Line C corresponds to the mesor (middle of modeled rhythm), with lines D and E corresponding to the times when the participant passes through their mesor with increasing (up-mesor) decreasing (down-mesor) activty.
Bottom: A participant with a less robust CAR rhythm (low pseudo-F statistic). In contrast with a robust rhythm, this participant’s modeled rhythm has a worse fit to the raw data; a pattern of consolidated rest and activity periods is not clearly evident.
Depressive symptoms
The Geriatric Depression Scale–15 (GDS)21–23 was used to measure depressive symptoms at baseline (MrOS Sleep Visit) and follow-up (MrOS Visit 2). At a standard cut point of ≥6 this instrument has a sensitivity of 90.9% and specificity of 64.5% for detecting Major Depressive Disorder (MDD) compared to a DSM-IV diagnosis of MDD23. Participants were categorized, as previously24, into minimal depressive symptoms (≤2), some depressive symptoms (3–5), or clinically significant depressive symptoms (≥6). The prospective cohort included men in the “minimal” category at baseline, and we examined whether CAR parameters were associated with symptom category increases at follow-up.
Covariates
Other measures included demographic information, educational achievement, smoking status, caffeine (see25 for details on consumption calculation) and alcohol use. Body weight and height measurements were used to calculate a standard body mass index (BMI).
Mental health
Cognitive function was measured using the 3MS15 expressed as continuous variable. Anxiety symptoms were measured using the validated Goldberg Anxiety Scale26 which was modeled as a continuous score indicating the number of symptoms (range: 0–9).
Sleep
Actigraph-computed sleep variables were not included in the main analysis because the nighttime activity data used to compute sleep variables is part of the modeled CAR parameters (an examination of these sleep parameters and depression are presented in a prior publication24). We focused on determining whether the CAR was associated with depression scores independent of subjective sleep disturbances. A standard cut-point of >5 on the Pittsburgh Sleep Quality Index (PSQI)27 (range 0–21) was used to indicate poor sleep quality. Participants also completed the Epworth Sleepiness Scale (ESS) (range 0–24), a self-reported questionnaire measuring daytime sleepiness with a standard cut-point of >1028,29 used to indicate excessive daytime sleepiness.
Sleep disordered breathing (SDB) may also be associated with CAR and depressive symptoms and was measured using one night of in-home polysomnography (PSG). Detailed PSG methods for the MrOS Sleep Study have been published previously30. The apnea-hypopnea index (AHI), calculated as the total number of apneas and hypopneas per hour of sleep, was used to measure SDB. Apnea was defined as complete or near complete cessation of airflow for >10 seconds, and hypopneas were scored if clear reductions in breathing amplitude (at least 30% below baseline breathing) occurred and lasted >10 seconds31; only apneas and hypopneas that were associated with a 3% or greater desaturation were included.
Physical activity
The Physical Activity Scale for the Elderly (PASE), a validated measure of physical activity32,33, was used to assess total physical activity.
Chronic diseases
Participants were asked if they had ever received a physician diagnosis of the medical conditions listed in Table 1, and we examined the number of medical conditions as a potential confounder.
Table 1.
Covariate characteristics by baseline depressive symptom categories
| % total (n) | Minimal depressive symptoms (GDS score < 3) | Some depressive symptoms (GDS score 3–5) | Clinically significant depressive symptoms (GDS score ≥ 6) | p value | χ2 (or F) | df |
|---|---|---|---|---|---|---|
|
| ||||||
| 76.73 (2219) | 17.15 (496) | 6.12 (177) | ||||
| Age | 75.85 (5.28) | 77.60 (5.96) | 77.39 (5.72) | <0.0001 | 25.29 | (2, 2889) |
| BMI | 0.04 | 13.35 | 6 | |||
| <20 | 0.81 (18) | 1.41 (7) | 0 (0) | |||
| 20–25 | 29.17 (647) | 27.82 (138) | 26.14 (46) | |||
| 25–30 | 50.86 (1128) | 45.97 (228) | 50 (88) | |||
| 30+ | 19.16 (425) | 24.80 (123) | 23.86 (42) | |||
| Race | 0.97 | 0.56 | 4 | |||
| White | 90.76 (2014) | 90.73 (450) | 90.96 (161) | |||
| Black | 3.11 (69) | 3.63 (18) | 3.39 (6) | |||
| Other | 6.13 (136) | 5.65 (28) | 5.65 (10) | |||
| Highest level of education | 0.01 | 12.73 | 4 | |||
| Less than high school | 4.46 (99) | 5.04 (25) | 6.21 (11) | |||
| High school | 14.47 (321) | 20.16 (100) | 18.08 (32) | |||
| Some college | 81.07 (1799) | 74.80 (371) | 75.71 (134) | |||
| Anxiety symptoms | 0.55 (1.32) | 1.76 (2.35) | 3.87 (2.88) | <0.0001* | 473.92 | 2 |
| 3MS Score | 93.76 (4.41) | 92.57 (4.63) | 92.02 (5.10) | <0.0001* | 35.99 | 2 |
| PASE score | 154.24 (70.75) | 126.02 (67.88) | 109.56 (66.51) | <0.0001 | 59.39 | (2, 2889) |
| Alcohol use (drinks per week) | 0.0002 | 21.96 | 4 | |||
| <1 | 44.03 (973) | 54.36 (268) | 51.98 (92) | |||
| 1–13 | 49.59 (1096) | 41.99 (207) | 3.65 (18) | |||
| 14+ | 6.38 (141) | 3.65 (18) | 4.52 (8) | |||
| Smoking status | 0.02 | 12.28 | 4 | |||
| Current | 1.67 (37) | 2.02 (10) | 5.08 (9) | |||
| Former | 58.09 (44.57) | 59.88 (297) | 61.02 (108) | |||
| Never | 40.24 (893) | 38.10 (189) | 33.90 (60) | |||
| Caffeine consumption, mg/d | 239.94 (247.61) | 234.60 (253.62) | 210.64 (246.08) | 0.18* | 3.47 | 2 |
| Antidepressant use | 5.18 (115) | 12.10 (60) | 24.43 (43) | <0.0001 | 104.49 | 2 |
| Benzodiazepines use | 3.56 (79) | 5.04 (25) | 15.20 (22) | <0.0001 | 31.93 | 2 |
| Non-benzodiazepine non- barbiturate sedatives /hypnotics | 1.62 (36) | 3.43 (17) | 3.41 (6) | 0.02 | 8.36 | 2 |
| >1 IADL disability | 14.51 (322) | 35.08 (174) | 53.67 (95) | <0.0001 | 233.58 | 2 |
| CVD | 17.89 (397) | 27.82 (138) | 28.81 (51) | <0.0001 | 33.28 | 2 |
| Diabetes | 12.35 (274) | 16.53 (82) | 14.69 (26) | 0.04 | 6.55 | 2 |
| Parkinson’s | 0.81 (18) | 2.22 (11) | 3.39 (6) | 0.0008 | 14.20 | 2 |
| Stroke | 2.48 (55) | 6.65 (33) | 9.60 (17) | <0.0001 | 39.42 | 2 |
| COPD | 4.24 (94) | 7.06 (35) | 11.30 (20) | <0.0001 | 21.18 | 2 |
| Hypertension | 47.45 (1053) | 56.45 (280) | 57.63 (102) | 0.0001 | 17.96 | 2 |
| Osteoarthritis | 21.27 (472) | 27.68 (137) | 42.37 (75) | <0.0001 | 45.75 | 2 |
| Rheumatoid Arthritis | 6.31 (140) | 12.12 (60) | 16.38 (229) | <0.0001 | 37.26 | 2 |
| Self-reported poor sleep (PSQI > 5) | 37.31 (828) | 61.69 (306) | 76.84 (136) | <0.0001 | 180.80 | 2 |
| Excessive daytime sleepiness (EPSS > 10) | 10.23 (227) | 18.15 (90) | 25.42 (45) | <0.0001 | 51.87 | 2 |
| AHI | 0.0019 | 17.07 | 4 | |||
| <15 | 57.62 (1202) | 52.24 (245) | 53.09 (86) | |||
| 15–29 | 26.51 (553) | 24.52 (115) | 24.69 (40) | |||
| ≥ 30 | 15.87 (331) | 23.24 (109) | 22.22 (36) | |||
Percent (n) or mean +/− SD shown; CVD = MI or CHF;
Kruskal Wallis test; χ2 test used for categorical variables and analysis of variance used for continuous variables (unless otherwise noted)
Instrumental Activity of Daily Living (IADL) impairment
IADL disability was defined as impairment with any 5 IADLs (heavy housework, preparing own meals, shopping for groceries or clothing, walking 2–3 blocks, climbing ten stairs)34,35.
Medications
Participants were asked to bring all medications used within the last 30 days to the sleep examination. Medications were entered into an electronic database and matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA)36. Number of psychoactive medications (antidepressants, benzodiazepines, and non-benzodiazepine non-barbiturate sedatives/hypnotics) was entered as a covariate.
Statistical analysis
Univariate comparisons of covariates across levels of baseline depressive symptoms categories were made using ANOVA or Kruskal Wallis tests for continuous variables and chi-squared homogeneity tests for categorical variables. CAR parameters may have non-linear relationships with depression, for example both earlier and later timing of peak activity (acrophase) could potentially may be associated with depression. To determine whether our analysis would need to accommodate potential non-linear associations of CAR parameters and depression, we first inspected quartiles of CAR parameters by depressive symptom categories, applying Jonckheere–Terpstra tests to examine statistical trends. In subsequent analyses we contrasted the extreme (highest or lowest) quartile of CAR parameters associated with depressive symptoms to the others in order to represent CAR disturbances.
In both cross-sectional and prospective analyses, two models were constructed: first adjusting for age and study site (Model 1) then adjusting for the other covariates listed above (Model 2). Multinomial logistic regression models were used to examine associations between CAR parameters and depressive symptom categories (compared to the men in 0–2 “minimal depressive symptoms” reference group). The longitudinal application of multinomial regression estimated the odds of symptomology increasing into the higher symptom categories, compared to remaining in the 0–2 “minimal” referent group. Similar results were obtained using continuous and log-transformed CAR variables, and we report results using CAR parameters categorized as the extreme quartile vs. the rest. All significance levels reported were two-sided and all analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Participants with missing covariate data (cross-sectional missing n=196; longitudinal missing n=141) did not differ in terms of the primary predictors or outcomes, although they tended to drink more, and were more often non-white. When dropping AHI as a covariate, missing data was minimal in both cross-sectional (n=23 with missing) and longitudinal models (n=14) and there were no associated differences in predictors, outcomes, or covariates. Because findings were similar whether or not we added the AHI to the models, to maximize generalizability we report findings excluding this covariate.
The majority of the sample (76.7%) reported minimal depressive symptoms (0–2) at baseline (Table 1). Some depressive symptoms (3–5) were reported by 17.2% of the sample, and clinically significant depressive symptoms (≥6) were reported by 6.1% of the sample. Participants with higher levels of depressive symptoms tended to be older, less educated, with more chronic diseases, poorer subjective sleep quality (PSQI), and lower cognitive and physical activity scores. Men in the higher depressive symptom categories also reported more anxiety symptoms and a greater proportion reported utilizing psychoactive medications.
Cross-sectional associations between CARs and level of depressive symptoms
Bivariate tests for trends indicated that being in lower quartiles of rhythm height measures were associated with greater levels of depressive symptoms (using Jonckheere-Terpstra Test; p trend<0.0001, Z value: 8.3884; mesor p trend<0.0001, Z value: −7.2002; standardized amplitude p trend<0.0001, Z value: −3.7965, p<0.0001). Being in higher quartiles of acrophase and up-mesor tended to be associated with membership in the higher depressive symptoms categories (acrophase p trend =0.0021, Z value: −3.08; mesor p trend=0.0001, Z value: 3.8578, p=0.0001). No statistically significant trend was detected between quartiles of down-mesor and depressive symptom levels (p trend=0.10, Z value: 1.6656; therefore this CAR characteristic was not examined further). Men in lower quartiles of the pseudo-F statistic (rhythm robustness) tended to report higher number of depressive symptoms (p trend<0.0001, Z value: −8.1294). The detected associations remained significant after adjustments for age and site (Table 2, Model 1) and mostly showed a gradient where these measures of CARs were associated with increasing odds of being in higher symptom level categories.
Table 2.
Odds ratios (95% CI) of depressive symptom levels at baseline by circadian rhythm measures
| Minimal symptoms (GDS score 0–2)
|
Some symptoms (GDS score 3 – 5)
|
Clinically significant symptoms (GDS score ≥6)
|
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | Wald χ2, 1 d.f. | p-value | OR (95% CI) | Wald χ2, 1 d.f. | p-value | ||
|
|
|
|
|||||
| Rhythm Height
|
|||||||
| Lowest amplitude (peak to nadir difference), counts/min. | |||||||
| Model 1: <2926 vs. >=2926 | 1.00 (ref) | 1.88 (1.51, 2.33) | 32.8615 | <.0001 | 2.35 (1.70, 3.25) | 26.7634 | <.0001 |
| Model 2: <2926 vs. >=2926 | 1.00 (ref) | 1.37 (1.07, 1.75) | 6.2727 | 0.01 | 1.40 (0.93, 2.11) | 2.6268 | 0.11 |
| Lowest modeled means (mesor), counts/min, | |||||||
| Model 1: <1856 vs. >=1856 | 1.00 (ref) | 1.70 (1.37, 2.12) | 22.9351 | <.0001 | 2.49 (1.80, 3.43) | 30.7206 | <.0001 |
| Model 2: <1856vs. >=1856 | 1.00 (ref) | 1.44 (1.13, 1.84) | 8.5664 | 0.003 | 2.04 (1.36, 3.04) | 12.0731 | 0.0005 |
| Low standardized amplitude (amplitude/mesor) | |||||||
| Model 1: <1.57 vs. >=1.57 | 1.00 (ref) | 1.50 (1.20, 1.88) | 13.559 | 0.0002 | 1.43 (1.01, 2.04) | 7.525 | 0.0061 |
| Model 2: <1.57 vs. >=1.57 | 1.00 (ref) | 1.13 (0.88, 1.44) | 0.9129 | 0.34 | 0.93 (0.61, 1.41) | 0.1172 | 0.73 |
| Rhythm Timing
|
|||||||
| Latest timing of rhythm peak (acrophase), hours:minutes | |||||||
| Model 1: ≥2:59 pm vs. <2:59 pm | 1.00 (ref) | 1.25 (1.00, 1.56) | 3.6553 | 0.06 | 1.73 (1.24, 2.41) | 10.5496 | 0.0012 |
| Model 2: ≥2:59 pm vs. <2:59 pm | 1.00 (ref) | 1.12 (0.87, 1.43) | 0.7715 | 0.38 | 1.17 (0.78, 1.76) | 0.5673 | 0.45 |
| Latest timing of up-mesor (left half- deflection), hours:minutes | |||||||
| Model 1: ≥7:44 am vs. <7:44 am | 1.00 (ref) | 1.18 (0.94, 1.47) | 1.9672 | 0.16 | 1.78 (1.28, 2.48) | 11.7059 | 0.0006 |
| Model 2: ≥7:44 am vs. <7:44 am | 1.00 (ref) | 0.94 (0.73, 1.21) | 0.2422 | 0.62 | 1.11 (0.74, 1.66) | 0.2398 | 0.62 |
| Rhythm Robustness
|
|||||||
| Least robust fit (pseudo F-statistic) | |||||||
| Model 1: <703.67 vs. >=703.67 | 1.00 (ref) | 1.56 (1.25, 1.94) | 15.7505 | <.0001 | 2.50 (1.81, 3.45) | 31.129 | <.0001 |
| Model 2: <703.67 vs. >=703.67 | 1.00 (ref) | 1.05 (0.81, 1.35) | 0.3955 | 0.53 | 1.48 (0.97, 2.25) | 4.0677 | 0.04 |
Odds ratios and 95% confidence intervals estimated using multinomial logistic regression
Model 1 (n=2892): Adjusted for age and study site
Model 2 (n=2869): Adjusted for study site, age, race, baseline anxiety symptoms, education, BMI, alcohol consumption, smoking status, caffeine consumption, physical activity score (PASE), cognitive performance (3MS), poor subjective sleep (PSQI), excessive daytime sleepiness (ESS), any IADL disability, number of medications (antidepressant, benzodiazepine, non-benzodiazepine, non-barbiturate sedative/hypnotics), and number of medical conditions (physician diagnosis of osteoarthritis, rheumatoid arthritis, stroke, Parkinson’s disease, diabetes mellitus, COPD, hypertension, CHF and MI).
In the fully adjusted models, compared to being in the minimal symptom category, men in the lowest quartile of mesor had 44% higher odds of having some depressive symptoms (95% CI 1.13–1.84) and 2.04 times the odds (95% CI 1.36–3.04) of having clinically significant depressive symptoms (Table 2). Being in the lowest amplitude quartile was associated with being in the “some symptoms” group after adjustment, but the association with the clinically significant symptom level was attenuated to non-significance. Associations of being in the lowest standardized amplitude with being in a higher depressive symptom category from Model 1 were completely attenuated after adjustments in Model 2. Unadjusted associations of standardized amplitude with depression appeared to be explained predominately by the following (significant associations with depressive symptom category): age, anxiety symptoms, physical activity, medical conditions, IADL disability, psychotropic medication use, poor sleep quality, and excessive daytime sleepiness.
Associations with rhythm timing parameters (later onsets and peaks of activity) were also strongly attenuated to non-significance after adjustments (Table 2). The same covariates which attenuated associations between standardized amplitude and depressive symptom levels (listed above) also appeared to explain associations with a shift towards later timing of these two parameters.
Association between rhythm robustness (the pseudo-F parameter) and prevalent depression symptom levels were also attenuated by the above-listed covariates, however the odds of being in the highest symptom group remained elevated among men in the lowest pseudo-F quartile (Table 2).
Prospective analysis
Participants were followed an average of 1.2 +/− 0.32 SD years after the Sleep Visit. Of the 2124 men in the prospective analysis, 235 (11%) developed some symptoms (GDS score 3–5), and 27 (1.27%) developed clinically significant symptoms (GDS score ≥6).
Tests for trends indicated that men in lower amplitude, standardized amplitude, and pseudo-F quartiles tended to more often experience an increase to the mild or clinically significant symptom categories (using Jonckheere-Terpstra Test; amplitude: p trend=0.0002, Z value: −3.68; standardized amplitude: p trend <0.0001, Z value: −3.76; pseudo-F: p<0.0001, Z=−5.28). No other associations were detected at the bivariate level; therefore, the remaining CAR characteristics were not examined further. After adjustments in the base model these CAR parameters remained associated with higher odds of experiencing increases into either symptom category (Table 3); standardized amplitude, however, was associated with increased odds of being in the “some symptom” but this relationship was attenuated among the clinically significant symptom group.
Table 3.
Odds ratios (95% CI) for elevated depressive levels at follow-up by baseline circadian rhythm measures
| Minimal symptoms (GDS score 0–2)
|
Some symptoms (GDS score 3 – 5)
|
Clinically significant symptoms (GDS score ≥6)
|
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | Wald χ2, 1 d.f. | p-value | OR (95% CI) | Wald χ2, 1 d.f. | p-value | ||
|
|
|
|
|||||
| Rhythm Height
|
|||||||
| Lowest amplitudes (peak-nadir difference) | |||||||
| Model 1: <3038 vs. ≥3038 | 1.00 (ref) | 1.51 (1.13, 2.04) | 7.5167 | 0.006 | 2.49 (1.14, 5.45) | 5.1930 | 0.03 |
| Model 2: <3038 vs. ≥3038 | 1.00 (ref) | 1.07 (0.78, 1.49) | 0.1855 | 0.67 | 2.18 (0.93, 5.08) | 3.2490 | 0.07 |
| Low standardized amplitude (amplitude/mesor) | |||||||
| Model 1: <1.58 vs. ≥1.58 | 1.00 (ref) | 1.49 (1.11, 2.00) | 6.9080 | 0.009 | 1.87 (0.84, 4.16) | 2.3295 | 0.13 |
| Model 2: <1.58 vs. ≥1.58 | 1.00 (ref) | 1.19 (0.86, 1.63) | 1.0966 | 0.30 | 1.58 (0.67, 3.69) | 1.0997 | 0.30 |
| Rhythm Robustness
|
|||||||
| Least robust fit (pseudo F-statistic) | |||||||
| Model 1: <741.93 vs. ≥741.93 | 1.00 (ref) | 1.90 (1.42, 2.54) | 18.8263 | <0.0001 | 2.51 (1.15, 5.48) | 5.3780 | 0.02 |
| Model 2: <741.93 vs. ≥741.93 | 1.00 (ref) | 1.45 (1.06, 2.00) | 5.2631 | 0.02 | 2.58 (1.11, 5.99) | 4.8493 | 0.03 |
Odds ratios and 95% confidence intervals estimated using multinomial logistic regression
Model 1 (n=2124): Adjusted for age and study site
Model 2 (n=2110): Adjusted for study site, age, race, baseline anxiety symptoms, education, BMI, alcohol consumption, smoking status, caffeine consumption, physical activity score (PASE), cognitive performance (3MS), poor subjective sleep (PSQI), excessive daytime sleepiness (ESS), apnea-hypopnea index, any IADL disability, number of medications (antidepressant, benzodiazepine, non-benzodiazepine, non-barbiturate sedative/hypnotics), and number of medical conditions (physician diagnosis of osteoarthritis, rheumatoid arthritis, stroke, Parkinson’s disease, diabetes mellitus, COPD, hypertension, CHF and MI).
After adjustments for all covariates, associations of both remaining rhythm height measures were attenuated to non-significance (Table 3). This attenuation appeared to be explained by significant associations of the following with depressive symptom levels: age, anxiety symptoms, IADL disability, and poor sleep quality.
Associations of being in the lowest pseudo-F quartile with increases to either depressive symptom category remained robust after adjustments (Table 3). While remaining statistically independent of covariates, pseudo-F associated increases into the some symptoms were markedly reduced.
Adding actigraph measured sleep variables to Model 2 did not attenuate these estimates.
Discussion
Community-dwelling older men with lower amplitude, later activity peaks, and less robust CARs were more likely to have prevalent clinically significant depressive symptoms. We also found that men with less robust rhythms had higher odds of experiencing symptom increases from minimal to both mild and clinically significant levels. This association was independent of multiple confounders including chronic diseases, self-reported physical activity, sleep disturbances, and other relevant covariates. CAR robustness may therefore capture risk of worsening depression above and beyond previously established risk factors especially over a short follow up period..
Mesor was a strong, independent predictor of prevalent depressive symptomology. This finding suggests that overall reductions in locomotor activity are associated with prevalent depression. Standardized rhythm height parameters later peaks of rhythm timing were not associated with depression independent of covariates. Therefore, the dampening of activity rhythms and apparent delayed activity onsets may be attributable to co-occurring health problems. In any case, this observed “flatness” may be a useful indicator of even sub-threshold symptomology. Tranah et al.9 found that later peaks of activity were related to incident dementia, so regardless of attribution to other disease processes and lifestyle factors, later activity peaks among older adults may indicate generally poor or worsening health.
Rhythm robustness was independently related to higher odds of increasing from minimal depressive symptomology to both mild (3–5 symptoms) and clinically significant symptomology. The current as well as other37 studies demonstrate associations between, for example, subjective sleep disturbances and future worsening of depression; however these associations did not account for the relationship between CAR robustness and future depression. Strengths of our study include the large sample with objective CAR measures and a wide range of relevant covariates. To our knowledge this is the first study to prospectively investigate relations between CAR rhythms and the development of depressive symptoms over time. Considering that rhythm height (and timing) measures were not independently associated with symptom worsening, our results show that the pseudo-F statistic captures depression risk beyond that of overall reductions in locomotor activity.
It is important to again note that covariate adjustment accounted for a substantial portion of associations between CAR robustness and the milder increases in symptomology, and we cannot comment on the temporal relations between CARs and covariates in our study. Neither can the current analysis conclusively demonstrate any particular role for CAR robustness on the causal pathway to depression. Among older adults, less robust rhythms may be cause or consequence of reduced physical activity, disability, or an inability to maintain states of sleep/wakefulness. While we found that CAR robustness was related to depression risk over time independent of these factors, we also found substantial attenuation of this associated risk. Future research is required to elucidate this potential mechanism and determine where along this putative pathway is most amenable to preventative intervention. Any examination of temporal relations between CARs and covariates included is not possible, and future research is required to understand the ontogeny of CAR rhythms robustness (or lack thereof) among older adults.
Recent research demonstrates associations between circadian patterns of gene expression and depression38. Several prior studies (i.e. 39,40) have found associations between depression and the circadian rhythms of cortisol and norepinephrine. Patients with bipolar disorder reporting less regular social rhythms (subjective reports of the regularity of behaviors, i.e. sleeping, eating, and socializing) have been shown to have faster episode recurrence41. Therefore relations between circadian rhythms and mood disorders may span from genes, to hormones, to patterns of rest, activity, and social interaction. Future research is needed to investigate these multi-level influences and their contributions to the development of late-life psychiatric disorders.
Several limitations must be noted. Our study’s sample consisted of only older men free from cognitive impairment who were mostly white. These results may not generalize to younger men, women, or some minority groups, or adults with cognitive impairment. The relatively short follow-up period (just over 1 year) excludes assessment of the durability of this risk, and these effects may be observed only acutely. We are continuing to follow these men so that a future analysis can address this. Unmeasured confounders including incident stressful events, such as bereavement, may have biased our results by influencing both CARs and future depression. The CAR is indifferent to psychological qualities of rest and activity which may moderate the effects observed. Although the CAR reflects the activity of the master biological keeper in the suprachiasmic nucleus, the CAR can be readily “masked” by voluntary behavior17. The CAR is thus not a direct indicator of circadian biology.
Nevertheless, we have demonstrated that CAR is relevant to changes in depressive symptoms over time in a sample of community-dwelling older men. Although replication is needed in other samples, our findings suggest that CAR robustness is involved in the etiology of late-life depression. Prevention techniques which regularize CARs may curtail the development or worsening of late-life depressive disorders.
Acknowledgments
Funding Support: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. SFS is supported by T32 AG000181.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011 Aug;34(8):1103–1110. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. American journal of epidemiology. 2002 Aug 15;156(4):328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- 3.Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. American journal of epidemiology. 1991 Jul 15;134(2):220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 4.Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001 Dec 15;24(8):957–965. doi: 10.1093/sleep/24.8.957. [DOI] [PubMed] [Google Scholar]
- 5.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. Journal of pineal research. 2001 Oct;31(3):264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 6.Middleton B, Arendt J, Stone BM. Complex effects of melatonin on human circadian rhythms in constant dim light. Journal of biological rhythms. 1997 Oct;12(5):467–477. doi: 10.1177/074873049701200508. [DOI] [PubMed] [Google Scholar]
- 7.Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. Journal of sleep research. 1996 Jun;5(2):69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 8.Paudel ML, Taylor BC, Ancoli-Israel S, et al. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiology international. 2010 Jan;27(2):363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Annals of neurology. 2011 Nov;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive Symptoms and Circadian Activity Rhythm Disturbances in Community-Dwelling Older Women. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013 Mar 26; doi: 10.1016/j.jagp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002 Feb;50(2):282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005 Oct;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005 Oct;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.McGivney SA, Mulvihill M, Taylor B. Validating the GDS depression screen in the nursing home. J Am Geriatr Soc. 1994 May;42(5):490–492. doi: 10.1111/j.1532-5415.1994.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 16.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003 Jan-Feb;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003 May 1;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 18.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005 Dec;28(12):1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell T, Ancoli-Israel S, Redline S, Stone KL Osteoporotic Fractures in Men Study G. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011 Aug 15;7(4):357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Statistics in medicine. 2006 Nov 30;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology. 1986;5:165–173. [Google Scholar]
- 22.Aikman G, Oehlert M. Geriatric Depression Scale. Clinical Gerontologist. 2001;22(3):63–70. [Google Scholar]
- 23.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International journal of geriatric psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Paudel ML, Taylor BC, Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008 Jul;56(7):1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barone JJ, Roberts HR. Caffeine consumption. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1996 Jan;34(1):119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ (Clinical research ed) 1988 Oct 8;297(6653):897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992 Aug;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991 Dec;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011 Dec;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012 Oct 15;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. Journal of clinical epidemiology. 1997 May;50(5):541–546. doi: 10.1016/s0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 33.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 34.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital and health statistics. Ser. 1, Programs and collection procedures. 1987 Jun;(21):1–115. [PubMed] [Google Scholar]
- 35.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis and rheumatism. 1983 Nov;26(11):1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 36.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. European journal of epidemiology. 1994 Aug;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 37.Paudel M, Taylor BC, Ancoli-Israel S, et al. Sleep Disturbances and Risk of Depression in Older Men. Sleep. 2013;36(7):1033–1040. doi: 10.5665/sleep.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 11;110(24):9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenigsberg HW, Teicher MH, Mitropoulou V, et al. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. Journal of psychiatric research. 2004 Sep-Oct;38(5):503–511. doi: 10.1016/j.jpsychires.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Lee MA, Taylor MA. Cortisol suppression and circadian rhythm in endogenous depression: a preliminary report. Biological psychiatry. 1983 Oct;18(10):1127–1132. [PubMed] [Google Scholar]
- 41.Shen GH, Alloy LB, Abramson LY, Sylvia LG. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar disorders. 2008 Jun;10(4):520–529. doi: 10.1111/j.1399-5618.2008.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]