Abstract
Rationale
Cocaine and opioids are often co-abused. Laboratory research has focused largely on the reinforcing effects of mixtures of drugs relative to the drugs alone. Less research has examined drug mixing by the subject under concurrent-access conditions.
Objective
Self-administration of various doses of cocaine and remifentanil was examined under concurrent-access conditions. It was hypothesized that if cocaine and opioid combinations were more effective reinforcers than the single drugs, subjects would mix the two drugs by adjusting their responding to cocaine and opioid alternatives to maintain an optimal ratio of cocaine:remifentanil intake.
Method
Three male rhesus monkeys were allowed to self-administer cocaine (0.05–0.2 mg/kg/inj) or saline on one lever and remifentanil (0.05–0.4 µg/kg/inj) or saline on the other lever under concurrent FR 10 schedules. Daily sessions lasted 2-h, and there was a 1-s timeout after every 10-s injection.
Results
When saline and drug were concurrently available, responding on the saline-associated lever was low relative to the drug alternative. When cocaine and remifentanil were concurrently available, both drugs were self-administered above saline levels. Cocaine intake decreased and remifentanil intake increased as a function of the remifentanil dose that was available. Conversely, cocaine intake and remifentanil intake did not change systematically as a function of the cocaine dose that was available.
Conclusion
Monkeys will mix cocaine and an opioid when the two drugs are available concurrently. However, there was no indication that monkeys titrated drug-intake to maintain an optimal ratio of intake of the two compounds.
Keywords: Choice, Cocaine, Polydrug abuse, Remifentanil, Rhesus monkey, Self-administration
Introduction
Among drug abusers, cocaine and opioids are often co-abused concurrently (i.e., at the same time), sequentially (i.e., at different times), or both (Leri et al., 2003). Estimates of cocaine use range from 30–80% among heroin abusers not in treatment (see Leri et al. 2003 for a review), and cocaine use is prevalent among methadone-maintained individuals (Bux et al. 1995; DeMaria et al. 2000). Relative to single drug abuse, cocaine and opioid co-abuse is associated with increased occurrence of drug overdose (Bernstein et al. 2007; Coffin et al. 2003), poorer treatment outcomes (DeMaria et al. 2000; Downey et al. 2000; Magura et al. 1998), and increased incidence of contracting blood-borne diseases (e.g., human immunodeficiency virus; Joe and Simpson 1995; Grella et al. 1995). The prevalence of cocaine and opioid co-abuse and the associated risks highlight the need to understand factors that contribute to their co-abuse.
Anecdotal evidence suggests one reason drug abusers take cocaine and opioids concurrently is that the mixture “feels better” or is more reinforcing than either drug alone (see Leri et al. 2003). This may indicate that the mixture is a more effective reinforcer than either of the single drugs. Laboratory research with human participants has provided mixed results as to whether cocaine and opioid mixtures are more reinforcing than either of the single drugs. Methadone-maintained individuals rated cocaine’s subjective effects more positively than a control group not maintained on methadone (Preston et al. 1996). Conversely, participants’ ratings of the subjective effects of intravenous (i.v.) cocaine and morphine or hydromorphone could generally be predicted by the effects of either drug alone (Foltin and Fischman 1992; Walsh et al. 1996). It should be noted that human self-report measures of subjective effects of cocaine and opioid combinations are not direct measures of reinforcer effectiveness.
Self-administration studies in non-humans have used behavioral economic, progressive-ratio (PR), and choice procedures to directly measure reinforcing potency and effectiveness of cocaine-opioid mixtures relative to the single component drugs. Behavioral economics uses demand-curve analysis to describe reinforcer consumption across a range of response requirements (Hursh and Silberberg 2008). Results from this approach indicate that cocaine-opioid mixtures were not more effective reinforcers than at least one of the single drugs (Mattox et al. 1997; Wade-Galuska et al. 2007; Winger et al. 2006). In PR studies, where the response requirement systematically increases within session, cocaine-opioid mixtures were more potent reinforcers than the single drugs, evidenced by leftward shifts in the dose-response functions for the mixtures (Duvauchelle et al. 1998; Ranaldi and Munn 1998; Rowlett et al. 1998, 2005, 2007; Rowlett and Woolverton 1997; Woolverton et al. 2008, but see Ward et al. 2005). While more potent, cocaine-opioid mixtures were not more effective reinforcers than the single drugs as evidenced by comparable breakpoints for the mixtures and the single drugs (Duvauchelle et al. 1998; Rowlett et al. 1998, 2005, 2007; Rowlett and Woolverton 1997; Ward et al. 2005; Woolverton et al. 2008; but see Ranaldi and Munn 1998).
With drug-vs-food choice, dose-response functions for cocaine-heroin mixtures were shifted leftward relative to either drug alone (Negus 2005). Maximum choice reached similar asymptotes for the mixture and each of the single drugs, and Negus (2005) reported that cocaine-heroin mixtures were more potent, but not more effective reinforcers than either of the drugs alone. With drug-vs-drug choice, where choice was between cocaine-opioid mixtures versus one of the single drugs, the mixtures were generally chosen over the single drugs (Freeman and Woolverton 2011; Ward et al. 2005; Wang et al. 2001). However, choice for the mixture could be overcome by increasing the dose of the single-drug option (Freeman and Woolverton 2011). Thus, cocaine-opioid mixtures were more potent reinforcers than the single drugs, but at maximally effective doses, the mixtures were not more effective reinforcers (Freeman and Woolverton 2011; Negus 2005; Ward et al. 2005; Wang et al. 2001).
Most research that has examined self-administration of cocaine and opioids has compared a mixture of the drugs, where cocaine and an opioid are mixed into one solution, versus one of the single drugs available in a different solution. An exception can be found in the final choice experiment described in Ward et al. (2005) where choice between three cocaine doses (0.75–3.0 mg/kg/inj) and two heroin doses (25.0 and 50.0 µg/kg/inj) were examined under concurrent-access conditions. Responding on one lever resulted in delivery of cocaine and responding on another lever resulted in delivery of heroin. Under these conditions, rats chose cocaine exclusively. Based on the event records shown for two rats, it appears that baseline responding maintained by heroin alone (at 50 µg/kg/inj) was much lower than that for cocaine alone. These doses have been shown to be on the descending limb of the dose-effect curve in rats self-administering heroin alone under a single-operant FR schedule (Beardsley et al. 2004). It is not clear whether different heroin doses would have resulted in more responding on the lever associated with heroin during choice components.
The majority of results described above do not provide evidence that cocaine-opioid mixtures are more effective reinforcers than the single drugs, especially when the doses of the single drugs are high (i.e., approaching the high end of the safe range). However, because humans co-abuse these compounds, modeling and understanding this behavior remains an important objective. In choice studies described above, choices were either mutually exclusive in that choosing a mixture meant forgoing the reinforcer available on the other lever (e.g., food or a single-drug option), and vice versa, or choices of a predetermined mixture were compared to a single-drug or food alternative. A rational next step for an animal model of cocaine-opioid co-abuse may be to create conditions that allow for relatively unconstrained and concurrent intake of each of the single drugs so that “mixing” behavior can be observed and quantified under conditions where choices are not mutually exclusive, and behavior can be allocated to both single-drug alternatives throughout the session. Under such conditions, it may be possible to identify response patterns that reveal an optimal ratio of intake of the two drugs. To that end, the current study examined self-administration of cocaine and remifentanil, a short acting mu-opioid agonist, in rhesus monkeys under concurrent-access conditions where saline or cocaine was delivered following responses on one lever and saline or remifentanil was delivered following responses on a second lever. Choices were not mutually exclusive and animals could respond on both levers throughout the session. The response topography allowed in this procedure may yield novel information about the concurrent use of cocaine and opioids because the subject is able to control the ratio of the mixture of drugs rather than making mixtures with predetermined ratios available for self-administration. It was hypothesized that both drugs would be self-administered during the session. In addition, if there was an optimal ratio of cocaine:remifentanil intake that was maximally effective, it was hypothesized that monkeys would adjust their response allocation and rates to “clamp” the session intake of both drugs at that ratio.
Materials and Methods
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were conducted in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (2011).
Subjects and Apparatus
Four male rhesus monkeys (Macaca mulatta) originally served as subjects. One subject (RO1025) had a profound position bias that affected more than 50% of conditions (data not shown), and this subject was removed from the analysis. For the remaining three subjects, weights ranged from 9.5–10.6 kg at the beginning of the study. DJ9J and DK12 had a history of self-administration of cocaine and remifentanil mixtures (Freeman and Woolverton 2011). RO2050 had a history with drug self-administration under PR and fixed-ratio (FR) schedules, and most recently with cocaine and nicotine mixtures (Freeman and Woolverton 2009). Monkeys had unlimited access to water and were maintained at stable body weights by supplemental feeding (200–350 g/day, Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI). A vitamin supplement was given three times per week, and fresh fruit was given daily. Lights were maintained on a 16/8-h light/dark cycle, with lights on at 0600 h.
As described previously (e.g., Freeman and Woolverton 2009), each monkey was fitted with a mesh jacket (Lomir Biomedical, Malone, NY), attached by a tether to the back wall of the experimental cubicle (1.0 m3, Plaslabs, Lansing, MI). The front door of the cubicle was made of transparent plastic, and the remaining walls were opaque. Two response levers (PRL-001, BRS/LVE, Beltsville, MD) were mounted on the inside of the door with four jeweled stimulus lights (two red, two white) above each lever. Drug infusions were delivered by two peristaltic infusion pumps, one for each lever (Cole-Parmer, Chicago, IL). A Macintosh computer with custom interface and software controlled experimental events and recorded data.
Surgery
Each monkey had a double lumen i.v. catheter implanted according to previous protocols (e.g., Freeman and Woolverton 2011). Monkeys were injected with atropine sulfate (0.04 mg/kg i.m.) and ketamine hydrochloride (10 mg/kg i.m.) followed by inhaled isoflurane. The catheter was implanted into a major vein with the tip terminating near the right atrium. The distal end of the catheter was passed subcutaneously to the mid-scapular region, where it exited the subject’s back. The catheter was threaded through the tether, out the back of the cubicle, and connected to a double-lumen swivel (Lomir Biomedical, Inc., Malone, NY). Each lumen was connected to a separate infusion pump. An antibiotic (Kefzol; Eli Lilly & Company, Indianapolis, IN) was administered (22.2 mg/kg i.m.) twice daily for 7 days to prevent infection. If a catheter became nonfunctional, it was removed, and the monkey was removed from the experiment for 1–2 weeks. After health was verified, a new catheter was implanted (new catheters were needed once for RO2050 and twice for DJ9J). Between sessions, the catheter was filled with 40 units/ml heparinized-saline to prevent clotting at the tip of the catheter.
Procedure
Experimental sessions were conducted 7 days per week, beginning at 12:00 p.m. and were signaled by illumination of white lever lights above one lever. Each lever was associated with a 10-s injection of saline, cocaine (0.05–0.2 mg/kg/inj), or remifentanil (0.05–0.4 µg/kg/inj). The injection period was always 10 s, and the injection volume was 0.1–0.2 ml/s, depending on individual pump speeds. Doses were changed by adjusting the drug concentration in each solution and were calculated based on injection volume and body weight for individual subjects.
Sessions began with four forced trials, with one active lever, signaled by illumination of the corresponding set of white lever lights, and the consequence associated with the active lever was available. The active lever was randomly determined at the start of each session, and strictly alternated between response alternatives for subsequent forced trials. This arrangement ensured exposure to the contingencies programmed for each lever. Following completion of forced trials, free-choice trials began. During free-choice trials, both sets of white lever lights were illuminated, both levers were active, and consequences associated with both levers were available. The number of free-choice trials was not fixed, and sessions lasted two hours.
For all trials, 10 consecutive lever presses (FR 10) had to occur on a single lever to result in delivery of saline or drug associated with that lever. If switching between response levers occurred before the FR 10 was complete, the FR contingency was reset. Following completion of 10 consecutive responses on a single lever, the white lights above the corresponding lever were darkened, and the red lights were illuminated during a 10-s injection of saline or drug associated with that lever. After the injection, all lever lights were darkened for a 1-s timeout. During the injection and timeout, responses on either lever had no programmed consequences.
Dose-response functions were established with an injection of saline or cocaine (0.05–0.2 mg/kg/inj) as the consequence associated with one lever and saline or remifentanil (0.05–0.4 µg/kg/inj) associated with the other lever. The order of conditions were counterbalanced, and doses were examined in an irregular order within and between monkeys (i.e., doses were not presented in ascending or descending order). Each condition was in effect until choice was stable, defined as: 1) the number of reinforcers delivered on each lever were within 10% of the running three-session mean for three consecutive sessions; and 2) there were no upward or downward trends over the three sessions. Once stability was achieved, the injections associated with the two levers were reversed, and stability was re-determined.
Data Analysis
The primary dependent measures were number of injections and drugs intake. Whole-session measures of intake for each compound were not different from data collected at 30-min intervals and, only whole-session data are reported. In control conditions, when saline was concurrently available with cocaine or remifentanil, the number of saline and drug injections were compared using a repeated-measures analysis of variance (ANOVA) for each subject (one ANOVA for cocaine vs. saline and one for remifentanil vs. saline). The purpose of these analyses was to ensure that responding on the drug-associated lever was higher than responding on the saline-associated lever for each subject across the doses tested. For these comparisons, the number of injections in the final three sessions of the initial lever-injection pairing and its reversal were entered in the analysis (i.e., six values for saline and six values for each drug dose).
For analyses conducted on group means, the mean number of injections was calculated for each subject across three stable sessions of each condition for cocaine (0.05–0.2 mg/kg/inj) and remifentanil (0.05–0.4 µg/kg/inj). Mean number of injections was calculated for the original lever-injection pairing and its reversal, and the two values were averaged for overall analyses. A repeated-measures ANOVA was conducted for group means for the number of cocaine and remifentanil injections with cocaine and remifentanil dose as within-subject variables.
Mean cocaine (mg/kg) and remifentanil (µg/kg) intake were calculated for each subject across three stable sessions of a condition for each dose of cocaine (0.05–0.2 mg/kg/inj) and remifentanil (0.05–0.4 µg/kg/inj) tested. Mean intake was calculated for the original lever-injection pairing and its reversal, and the two values were averaged. Repeated-measures ANOVAs were conducted for the group means for cocaine and remifentanil intake with cocaine and remifentanil dose as within-subject variables. If a consistent ratio of cocaine:remifentanil intake was maintained as the doses were changed, it would be indicated by one of two outcomes: 1) both cocaine and remifentanil intake would increase with increases in cocaine and remifentanil dose (i.e., significant main effects of cocaine and remifentanil dose), or 2) both cocaine and remifentanil intake would remain constant with increases in cocaine and remifentanil dose (i.e., no significant main effects of cocaine and remifentanil dose). Significant interactions would indicate that responding was not allocated in a manner that maintained a consistent ratio of cocaine:remifentanil intake. In other words, if increasing the dose of one drug increased intake of that drug while decreasing intake of the other drug, this would indicate that a consistent ratio of cocaine:remifentanil intake was not maintained. Cocaine and remifentanil intake during conditions when one of the levers was associated with saline were not included in these analyses, as this would increase the likelihood of obtaining significant interactions. Greenhouse-Geisser corrections were used for degrees of freedom when sphericity of variance was violated. Generalized eta squared was used to calculate effect size (Bakeman 2005).
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD), and remifentanil hydrochloride was purchased commercially. Final solutions were prepared using 0.9% saline. Doses were expressed as the salt forms of the drugs.
Results
Injections per Session: Drug vs. Saline
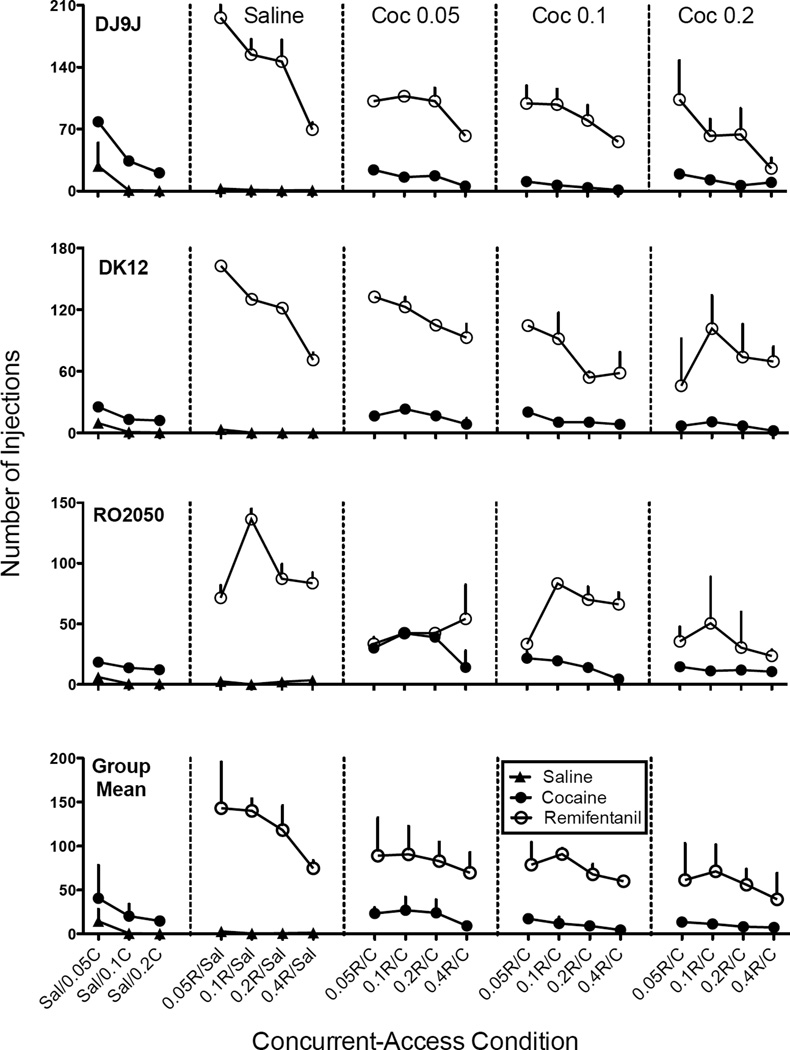
Figure 1 shows the number of saline (triangles), cocaine (solid circles), and remifentanil (open circles) injections as a function of cocaine dose in the leftmost panel and remifentanil dose for saline and each cocaine dose across successive panels. In the leftmost panels, when cocaine and saline were concurrently available, the number of cocaine injections were significantly greater than the number of saline injections for individual subjects at each dose of cocaine (all p’s<0.01 for all subjects and all doses, except 0.05 vs. saline for DJ9J, p<0.05), and saline injections were at or near zero levels when 0.1 or 0.2 mg/kg/inj doses of cocaine were concurrently available with saline. In general, the number of cocaine injections decreased with cocaine dose for individual subjects. However, analyses on the group means did not indicate a significant change in the number of cocaine injections as a function of cocaine dose.
Fig. 1.
Dose-response functions for the number of saline (solid triangles), cocaine (solid circles), and remifentanil (open circles) injections in each of three monkeys and the group means. Each data point is the average of three stable sessions from the initial drug-lever pairing and the average of three stable sessions from the reversed drug-lever pairing. Error bars are one standard error of the mean (+SEM). The x-axis represents each concurrent-access condition beginning in the leftmost panel when different doses of cocaine (C) and saline (S) were concurrently available. The second panel shows conditions when saline and various doses of remifentanil (R) were concurrently available, and subsequent panels show conditions when cocaine (0.5, 0.1, and 0.2 mg/kg/inj, respectively) was concurrently available with various doses of remifentanil. Also note the different y-axes for different subjects.
Similarly, in the second panel of Figure 1, when remifentanil and saline were concurrently available, the number of remifentanil injections were significantly greater than the number of saline injections for each subject (all p’s<0.001 for all subjects and all doses), and at all doses of remifentanil tested, saline injections were at or near zero levels. The number of remifentanil injections decreased with remifentanil dose for DJ9J and DK12 and was biphasic for RO2050. However, analyses on the group means did not indicate a significant change in the number of remifentanil injections as a function of remifentanil dose.
Injections per Session: Drug vs. Drug
Figure 1 also shows the number of cocaine and remifentanil injections across successive panels (beginning with the third column) when both drugs were available. In most cases, the number of cocaine injections decreased with remifentanil dose within each cocaine dose (i.e., within each panel) [F(3,6)=4.6, p<.01, ηG2=.35] but did not significantly change between cocaine doses (i.e., across successive panels). The number of remifentanil injections was either a biphasic or a decreasing function of remifentanil dose. An exception occurred for RO2050 when 0.05 mg/kg/inj of cocaine was available, and remifentanil injections trended upward. For the group means, the number of remifentanil injections did not significantly change as a function of remifentanil or cocaine dose. Overall, subjects tended to obtain more remifentanil injections within a session relative to the number of cocaine injections.
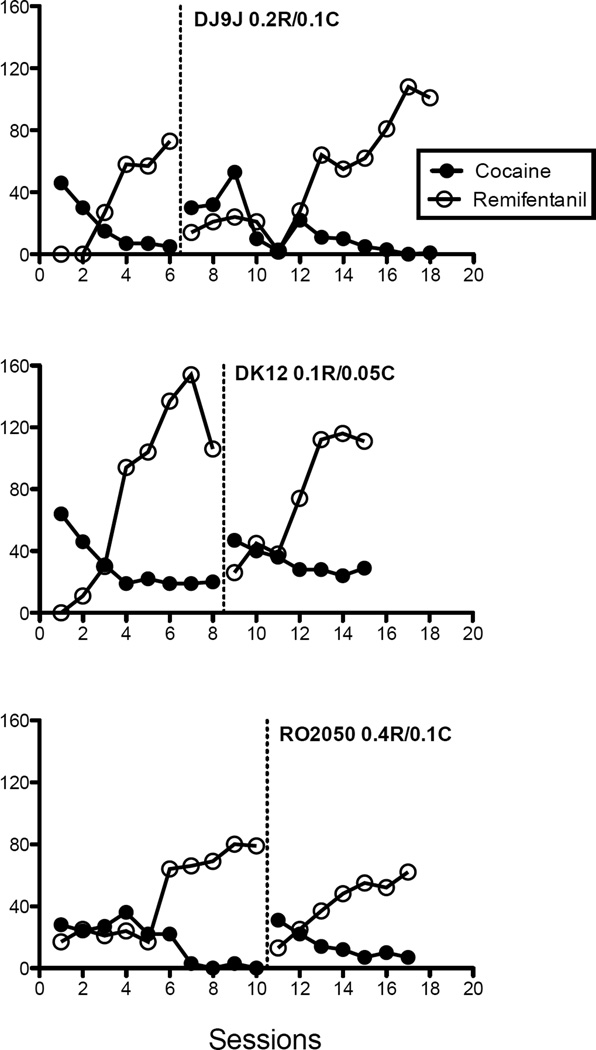
To illustrate the control that the contingencies of reinforcement had over behavior across reversals of lever conditions, Figure 2 shows number of cocaine or remifentanil injections across all sessions for a randomly selected condition for each subject. For all subjects, a pattern of responding was established within the first few sessions and met stability criteria within 6–12 sessions. When the initial lever-injection pairing was reversed for these subjects, responding was initially disrupted but returned to previous patterns of responding within a few sessions. It is possible that position biases influenced the outcome of other conditions, and inspection of individual-subject data for each drug vs. saline and drug vs. drug condition revealed very few instances of an apparent position bias for each subject (DJ9J: Sal/0.05C, DK12: 0.2C/0.05R, and RO2050: 0.05C/0.4R, 0.2C/0.1R, and 0.2C/0.2R). In most cases, the number of injections did replicate across initial lever-injection pairings and the reversal.
Fig. 2.
Number of cocaine (solid circles) and remifentanil (open circles) injections are shown for each session conducted in a randomly selected condition for each subject. Data points before the dotted line show sessions conducted with the initial lever-injection pairing, and data points after the dotted line show sessions conducted in the reversal. For DJ9J (top panel), data are shown for sessions when 0.2 µg/kg/inj remifentanil and 0.1 mg/kg/inj cocaine were concurrently available, for DK12 (middle panel), when 0.1 µg/kg/inj remifentanil and 0.05 mg/kg/inj cocaine were concurrently available, and for RO2050 (bottom panel), when 0.4 µg/kg/inj remifentanil and 0.1 mg/kg/inj cocaine were concurrently available.
Intake per Session: Drug vs. Saline
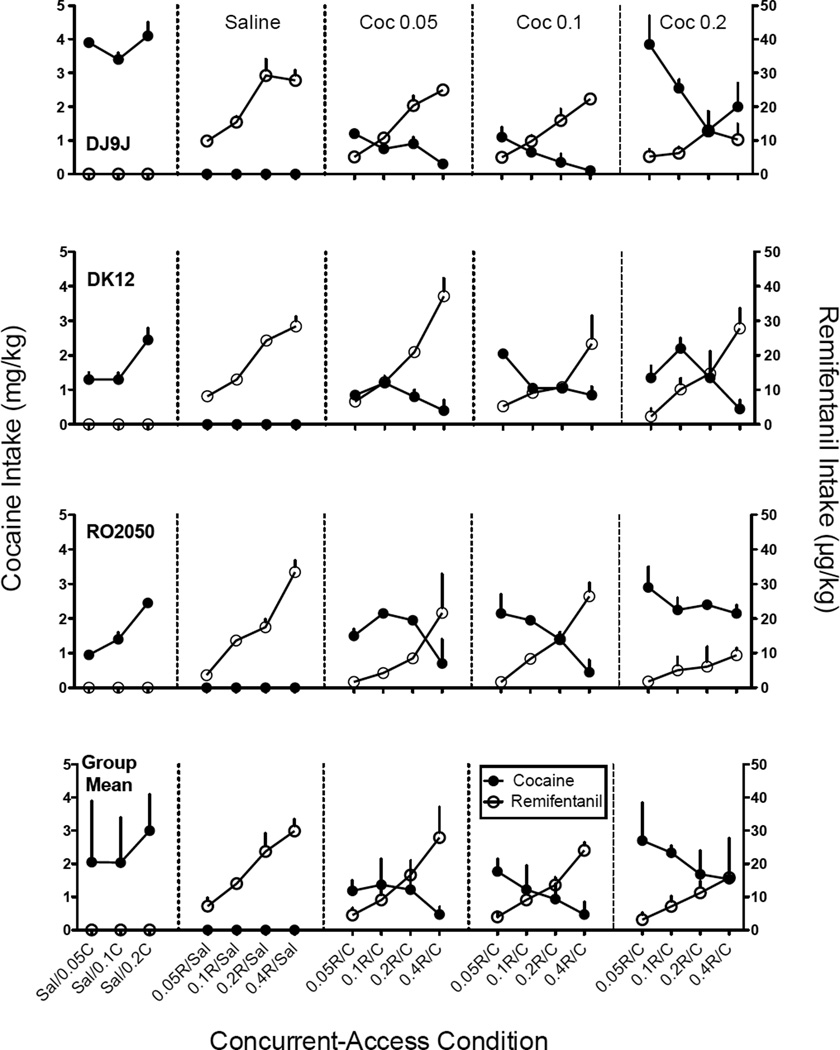
Figure 3 shows cocaine (mg/kg; solid circles) and remifentanil (µg/kg; open circles) intake as a function of cocaine dose in the leftmost panel and as a function of remifentanil dose with saline and each cocaine dose across successive panels. In the leftmost panels, when cocaine and saline were concurrently available, cocaine intake generally increased with cocaine dose for individual subjects (see DJ9J for an exception), and at the group level, cocaine intake significantly increased with cocaine dose [F(2,4)=7.5, p<.05, ηG2=.16]. The increase in intake was most apparent at the 0.2 mg/kg/inj dose of cocaine. In the second panel of Figure 3, when remifentanil and saline were concurrently available, remifentanil intake increased with remifentanil dose for all subjects and the group mean [F(3,6)=20.9, p<.01, ηG2=.89].
Fig. 3.
Dose-response functions for cocaine (solid circles) and remifentanil (open circles) intake in each of three monkeys and the group means. Each data point is the average of three stable sessions from the initial drug-lever pairing and the average of three stable sessions from the reversed drug-lever pairing. Error bars are one standard error of the mean (+SEM). The x-axis represents each concurrent-access condition beginning in the leftmost panel when different doses of cocaine (C) and saline (S) were concurrently available. The second panel shows conditions when saline and various doses of remifentanil (R) were concurrently available, and subsequent panels show conditions when cocaine (0.5, 0.1, and 0.2 mg/kg/inj, respectively) was concurrently available with various doses of remifentanil.
Intake per Session: Drug vs. Drug
Figure 3 also shows cocaine and remifentanil intake across successive panels (beginning with the third column) when both drugs were concurrently available. Under these conditions, cocaine intake for individual subjects was either a decreasing function of remifentanil dose or was biphasic. For the group means, cocaine intake decreased with remifentanil dose within each cocaine dose (i.e., within each panel) [F(3,6)=10.6, p<.01, ηG2=.36] but did not significantly change between cocaine dose (i.e., between each panel). For remifentanil, intake increased with remifentanil dose within each cocaine dose. This finding was consistent across subjects and for the group mean [F(3,6)=26.3, p<.01, ηG2=.75]. For two of three monkeys (DJ9J, RO2050), remifentanil dose-response functions tended to flatten with increases in cocaine dose across successive panels. However, remifentanil intake did not significantly change with cocaine dose.
Overall, cocaine and remifentanil intake were most consistently a function of the dose of remifentanil available (Figure 3). That is, cocaine and remifentanil intake did not significantly change as a function of the dose of cocaine available. For individual subjects, cocaine intake tended to decrease and remifentanil intake tended to increase with remifentanil dose (i.e., a statistical interaction). For the group means (Figure 3, bottom row), significant interactions were obtained for cocaine and remifentanil intake when 0.05 (third panel) and 0.1(fourth panel) mg/kg/inj of cocaine were concurrently available [F(3,6)=28.8, p<.01, ηG2=.64, F(3,6)=44.8, p<.001, ηG2=.92, respectively]. When 0.2 mg/kg/inj cocaine (last panel) was concurrently available with remifentanil, there were no significant main effects of remifentanil or cocaine dose and no interaction. However, the interaction trended toward significance (p=.06).
Discussion
The purpose of the present study was to examine cocaine and remifentanil self-administration under concurrent-access conditions without the constraints of discrete-choice conditions. The design allowed each subject to control the amount and temporal distribution of intake of each drug, which may ultimately provide important information about the manner in which polydrug abusers co-administer their drugs when both are available. It was hypothesized that monkeys would mix cocaine and remifentanil within a session, and that they would adjust their responding to maintain a ratio of cocaine:remifentanil intake reflective of an optimal reinforcement set point. Consistent with previous reports, cocaine and remifentanil functioned as reinforcers in all subjects (e.g., Freeman and Woolverton 2011; Wade-Galuska et al. 2007; Winger et al. 2006), and monkeys responded on both levers when cocaine and remifentanil were concurrently available. While these results indicate that monkeys would co-administer cocaine and an opioid, there was no indication that they would adjust their response rates to maintain an optimal ratio of cocaine:remifentanil intake. Rather, cocaine intake decreased and remifentanil intake increased with remifentanil dose (i.e., a statistical interaction), suggesting that responding within these dose ranges, and under the current experimental arrangement, may have been determined by a preference for remifentanil. This interpretation is consistent with the results of Freeman and Woolverton (2011), who demonstrated that monkeys given a choice between what was deemed to be the highest safe dose of cocaine (0.56 mg/kg/inj) and the highest safe dose of remifentanil (1.7 µg/kg/inj) chose remifentanil in all cases. However, these results differ from those of Ward and colleagues (2005) who reported that rats responded exclusively to a cocaine alternative when cocaine and heroin were concurrently available, suggesting that responding under their experimental arrangement may have been determined by a preference for cocaine. It is also possible that the long half-life and low baseline rates of responding maintained by heroin alone resulted in less responding on the opioid lever in their choice experiments. Ward et al. (2005) also did not include lever-injection reversals and used rats as subjects, either of which could have contributed to the differences between their study and the current one.
Despite the consistent finding that cocaine and opioid combinations are not more effective reinforcers, it is important to note that the combinations are consistently more potent reinforcers than the single drugs (Duvauchelle et al. 1998; Freeman and Woolverton 2011; Negus 2005; Ranaldi and Munn 1998; Rowlett et al. 1998, 2005, 2007; Rowlett and Woolverton 1997; Ward et al. 2005; Woolverton et al. 2008). Increased potency indicates that small amounts of cocaine and opioids combined have reinforcing effects similar to one of the drugs at a larger dose. It is possible, and has been suggested by others (e.g., Winger et al. 2006), that availability of small amounts of cocaine and opioids may facilitate co-abuse of the drugs. The current results support this possibility because some “mixing” behavior (i.e., responding to both alternatives) was observed, and responding was more distributed across the cocaine and remifentanil alternatives when the doses of remifentanil were relatively small (i.e., 0.05 and 0.1 µg/kg/inj). However, the “mixing” behavior observed with small doses of remifentanil essentially disappeared when the dose of remifentanil was relatively large (i.e., 0.4 µg/kg/inj), such that responding was almost exclusive on the remifentanil alternative. This is consistent with previous reports demonstrating that when small and intermediate doses of cocaine and opioids were available, choice was generally for the combination over the single-drug alternative, but choice for the combination was overcome by raising the dose of at least one of the single drugs (Freeman and Woolverton 2011; Ward et al. 2005; Wang et al. 2001). Thus, as previously stated, co-abuse of cocaine and opioids may be a function of the availability of one or both drugs.
With human participants, evidence for the hypothesis that small amounts of cocaine and opioids in combination have a reinforcing effect similar to larger doses of opioids alone is less clear. With methadone-maintained individuals, cocaine use was reduced when higher doses of methadone were administered (Stine et al. 1992). A more recent study found no differences in cocaine use as a function of the dose of methadone available (Epstein et al. 2009). While studies with non-humans suggest that mixing cocaine and opioids may occur more readily when smaller doses of each of the drugs are available (e.g., Freeman and Woolverton 2011; the current study), more research with human participants is necessary to determine whether changes in the amount of cocaine or opioids available result in changes in concurrent use of cocaine and opioids.
Cocaine and remifentanil were concurrently available in the current design. However, the overall pattern of intake for each drug was consistent with previous reports demonstrating unique patterns of self-administration for these two drug classes (see Lynch and Carroll 2001 for a review). Self-administration of stimulants under short-session, unrestricted-access conditions shows a pattern of relatively stable or slightly increasing intake across doses (e.g., Pickens and Thompson 1968; Wilson et al. 1971). This is consistent with the current study where cocaine intake was similar or slightly increased across cocaine dose. Under the same conditions of self-administration (i.e., continuous access during short sessions), opioid intake is less well regulated and tends to increase with dose (e.g., Harrigan and Downs 1978). This is consistent with the current study where remifentanil intake increased with dose in all subjects, across all conditions. Thus, even under conditions of concurrent access, the underpinnings that determine the unique patterns of self-administration for cocaine and opioids appear to be in operation.
In the current study, there were also differences in the amount of behavior that was maintained by each of the drugs. The number of injections obtained for remifentanil was greater than for cocaine. This could have resulted from several aspects of the procedure and the different durations of action for each drug. Remifentanil is relatively short acting (3–5 min half-life in humans; Glass et al. 1999). Since responding to either alternative was not mutually exclusive (i.e., cocaine injections could be followed by remifentanil injections in close proximity, and vice versa), more behavior may have been allocated to the remifentanil alternative to maintain some optimal level of drug intake. Notably, the short timeout (1-s) used in the current study may have allowed for a level of remifentanil accumulation across serial injections that would not have been possible in a previous study that used significantly longer timeouts (Freeman and Woolverton 2011), which could account for the relatively high responding for remifentanil. Cocaine’s duration of action is longer than remifentanil, and less responding may have been allocated to that alternative because fewer infusions were needed to maintain optimal drug intake.
Because lever or position biases have been reported in concurrent-operant procedures that incorporate reversals into the design (e.g., Freeman et al. 2014; Iglauer and Woods 1974; Johanson and Schuster 1974; Woolverton et al. 2012), patterns of behavior across sessions were shown for randomly selected conditions in the current experiment. Inspection of these data and individual-subject data for each condition (data not shown) suggest that in most cases, behavior was sensitive to changes in the contingencies associated with the initial lever-injection pairing and its reversal. Very few of the conditions appeared to be influenced by a position bias.
The procedure used in the current experiment represents a new approach to investigating the underpinnings of co-abuse of cocaine and opioids (or with other co-abused drugs). Most basic research has examined cocaine and opioid self-administration with cocaine-opioid mixtures of predetermined ratios (e.g., Duvauchelle et al. 1998; Freeman and Woolverton 2011; Negus, 2005; Ranaldi and Munn 1998; Rowlett and Woolverton 1997). The general finding is that the mixture is not a more effective reinforcer than the single drugs. However, these studies do not explain the mixing that occurs with cocaine and opioids in drug abusers. By making the single drugs separately but concurrently available, we have shown that monkeys, like humans, will mix cocaine and an opioid when they are able to control the relative intake and temporal delivery of the two drugs. Thus, this procedure could be used to examine whether patterns of cocaine and opioid intake change as a function of a subject’s history or dependence on or withdrawal from one or both of the drugs, any of which could be determinants of polydrug abuse. This procedure could also be used to determine if drug intake changes as a function of session duration or pre-treatment with agonists/antagonists that target receptors of mechanistic interest.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grant R01 DA-019471 to W.L.W. The authors would like to gratefully thank Steven Ross for his technical assistance.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37:379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus,, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharm. 2004;12:163–172. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- Bernstein KT, Bucciarelli A, Piper TM, Gross C, Tardiff K, Galea S. Cocaine- and opiate-related fatal overdose in New York City, 1990–2000. BMC Public Health. 2007;7:31. doi: 10.1186/1471-2458-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Depend. 1995;37:29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, Tardiff K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–1998. Addiction. 2003;98:739–747. doi: 10.1046/j.1360-0443.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- DeMaria PA, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am Acad Addict Psychiatry. 2000;9:145–153. doi: 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharm. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“Speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL. Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacol. 2014 doi: 10.1007/s00213-014-3436-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and remifentanil by monkeys: Choice between single drugs and mixtures. Psychopharmacol. 2011;215:281–290. doi: 10.1007/s00213-010-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and nicotine mixtures by rhesus monkeys. Psychopharmacol. 2009;207:99–106. doi: 10.1007/s00213-009-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass PSA, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesthesia Analgesia. 1999;89:7. doi: 10.1097/00000539-199910001-00003. [DOI] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Cocaine and crack use and HIV risk behaviors among high-risk methadone maintenance clients. Drug Alcohol Depend. 1995;37:15–21. doi: 10.1016/0376-8716(94)01059-t. [DOI] [PubMed] [Google Scholar]
- Harrigan SE, Downs DA. Self-administration of heroin, acetylmethadol, morphine, and methadone in rhesus monkeys. Life Sciences. 1978;22:619–623. doi: 10.1016/0024-3205(78)90342-9. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Iglauer C, Woods JH. Concurrent performance: Reinforcement by different doses of intravenous cocaine in rhesus monkeys. J Exp Anal Behav. 1974;22:179–196. doi: 10.1901/jeab.1974.22-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe GW, Simpson DD. HIV risks, gender, and cocaine use among opiate users. Drug Alcohol Depend. 1995;37:23–28. doi: 10.1016/0376-8716(94)01030-o. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A Choice procedure of drug reinforcers: Cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: Review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky S. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93:51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmacol. 1997;5:113–118. doi: 10.1037//1064-1297.5.2.113. [DOI] [PubMed] [Google Scholar]
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacol. 2005;180:115–124. doi: 10.1007/s00213-004-2133-y. [DOI] [PubMed] [Google Scholar]
- Pickents R, Thompson T. Cocaine-reinforced behavior in rats: Effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Strain EC, Bigelow GE. Enhancement of cocaine’s abuse liability in methadone maintenance patients. Psychopharmacol. 1996;123:15–25. doi: 10.1007/BF02246276. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Munn E. Polydrug self-administration in rats: Cocaine-heroin is more rewarding than cocaine-alone. NeuroReport. 1998;9:2463–2466. doi: 10.1097/00001756-199808030-00007. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Rodefer JS, Spealman RD. Self-administration of cocaine-opioid combinations by rhesus monkeys: Evaluation of the role of μ receptor efficacy using labor supply analysis. J Pharmacol Exp Ther. 2005;312:1289–1287. doi: 10.1124/jpet.104.076646. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: Antagonism by naltrexone. J Pharmacol Exp Ther. 1998;286:61–69. doi: [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacol. 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Stine SM, Freeman M, Burns B, Charney DS, Kosten T. Effect of Methadone dose on cocaine abuse in a methadone program. Amer J Addict. 1992;1:294–303. [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacol. 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Wang NS, Brown VL, Grabowski J, Meisch RA. Reinforcement by orally delivered methadone, cocaine, and methadone-cocaine combinations in rhesus monkeys: Are the combinations better reinforcers? Psychopharmacol. 2001;156:63–72. doi: 10.1007/s002130100731. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DCS. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neurospychopharmacol. 2005;30:286–295. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self administration as a function of dosage per injection in the rhesus monkey. Psychopharmacol. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH. Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 2006;318:223–229. doi: 10.1124/jpet.105.100461. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: Effects of delayed punishment. Psychopharmacol. 2012;220:509–517. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Tallarida R. Self-administration of cocaine-remifentanil mixtures by monkeys: An isobolographic analysis. Psychopharmacol. 2008;198:387–394. doi: 10.1007/s00213-008-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]