Abstract
Human Vibrio infections associated with consumption of raw shellfish greatly impact the seafood industry. Vibrio cholerae-related disease is occasionally attributed to seafood, but V. vulnificus and V. parahaemolyticus are the primary targets of postharvest processing (PHP) efforts in the United States, as they pose the greatest threat to the industry. Most successful PHP treatments for Vibrio reduction also kill the molluscs and are not suitable for the lucrative half-shell market, while nonlethal practices are generally less effective. Therefore, novel intervention strategies for Vibrio reduction are needed for live oyster products. Chitosan is a bioactive derivative of chitin that is generally recognized as safe as a food additive by the FDA, and chitosan microparticles (CMs) were investigated in the present study as a potential PHP treatment for live oyster applications. Treatment of broth cultures with 0.5% (wt/vol) CMs resulted in growth cessation of V. cholerae, V. vulnificus, and V. parahaemolyticus, reducing culturable levels to nondetectable amounts after 3 h in three independent experiments. Furthermore, a similar treatment in artificial seawater at 4, 25, and 37°C reduced V. vulnificus levels by ca. 7 log CFU/ml after 24 h of exposure, but 48 h of exposure and elevated temperature were required to achieve similar results for V. parahaemolyticus and V. cholerae. Live oysters that either were artificially inoculated or contained natural populations of V. vulnificus and V. parahaemolyticus showed significant and consistent reductions following CM treatment (5%) compared to the amounts in the untreated controls. Thus, the results strongly support the promising potential for the application of CMs as a PHP treatment to reduce Vibrio spp. in intact live oysters.
INTRODUCTION
Vibrio species cause a significant proportion of human infections associated with consumption of raw or undercooked shellfish, particularly raw oysters (1). The primary pathogens in the United States are Vibrio vulnificus and V. parahaemolyticus; however, in 2011 the first U.S. outbreak of cholera in recent history was attributed to the consumption of oysters contaminated with V. cholerae O75 (2). Unlike other foodborne pathogens associated with seafood, Vibrio spp. occur naturally in estuarine environments, and their abundance is seasonal (3, 4). During warmer months (when the water temperature is >20°C), nearly all oysters harvested from U.S. Gulf Coast waters harbor V. vulnificus and/or V. parahaemolyticus, with the highest densities periodically exceeding 104 most probable number (MPN)/g (5). Despite extensive efforts employing hazard analysis and critical control point approaches and improved sanitation by the seafood industry, the incidence of seafood-associated cases continues to escalate, particularly during summer months, perhaps as a consequence of increasing global water temperatures (6). Annual reports of the incidence of Vibrio-related disease per 100,000 population increased from 0.09 to 0.28 in the Cholera and Other Vibrio Illness Surveillance system and from 0.15 to 0.42 in FoodNet in the last 15 years (1).
In response to the Vibrio risk assessment, the FDA implemented guidance regarding postharvest processing (PHP) of Gulf Coast oysters harvested during summer months (7). Established PHP methods to reduce Vibrio numbers in oysters include thermal, gamma irradiation, freezing, and high-hydrostatic-pressure treatments (8–10). Although effective in reducing pathogen loads to nondetectable levels (<30 MPN/g), these approved PHP treatments generally kill the shellfish and may lead to undesirable changes in shelf life, color, flavor, and texture (11). Furthermore, a substantial demand for live oysters is apparent (12). Ice immersion (13), depuration (immersion in recirculating, sanitized seawater) (14), and relaying (transport to offshore high-salinity/low-Vibrio sites) (15) methods maintain product integrity but are less effective. Therefore, development of novel PHP alternatives is vital to the seafood industry for alleviating issues of pathogenic Vibrio spp. in raw oysters.
Chitin is the second most abundant natural biopolymer after cellulose and is a component of various marine organisms, such as the shells of crab, lobster, and shrimp (16, 17). Because of the low level of biodegradation of chitin, a large amount of crustacean exoskeleton waste accumulates after seafood processing, accounting for 50 to 90% of the total solid waste landing in the United States (18, 19). In this respect, commercial application of chitin derivatives from inexpensive seafood refuse is both an environmentally acceptable use of an oceanic resource and an economically feasible solution for waste disposal. In recent decades, chitosan has attracted a great deal of attention with a wide range of applications (20). Chitosan is a deacetylated derivative of chitin, and chitosan derived from shrimp was recently approved for generally recognized as safe (GRAS) status as a food additive by the U.S. Food and Drug Administration (21). In addition, Japan and South Korea have approved the use of chitosan as a food additive since 1983 and 1995, respectively (22, 23). Chitosan-mediated systems can significantly improve the bioavailability of drug delivery and are categorized as nanoparticle, microparticle, or macrodelivery systems (17, 24, 25). Furthermore, the antimicrobial activity of chitosan against both Gram-positive and Gram-negative pathogens as well as against food spoilage bacteria has been well demonstrated (26, 27).
Chitosan microparticles (CMs) are derived from chitosan with minor cross-linking modification, and a recent study showed that application of CMs as a feed additive resulted in reduced shedding of Escherichia coli O157:H7 in cattle (28). Chitosan was previously shown to be effective against V. vulnificus in vitro and in mice (29), but the effects of CMs against pathogenic Vibrio species and possible applications to live oysters have not been studied. Therefore, the objective of this study was to investigate the effects of CM treatment on pathogenic Vibrio spp. and evaluate the potential feasibility of the use of CMs for PHP of live oysters.
MATERIALS AND METHODS
Bacterial strains and inoculum preparation.
The three clinical strains of Vibrio spp. used in this study included V. vulnificus CMCP6 (an encapsulated biotype 1 strain with the C genotype commonly found in clinical strains) (30), V. parahaemolyticus TX2103 (serotype O3:K6) (31), and V. cholerae 139 classical O1 (32) and were provided by P. Gulig, A. DePola, and J. Johnson, respectively. Strains were stored as −80°C frozen stock cultures in Luria-Bertani broth with NaCl (LBN; 1.0% tryptone, 0.5% yeast extract, 1.0% NaCl in deionized water, pH 8.4) in 50% glycerol. For each experiment, bacteria were retrieved from the frozen stock and plated onto LBN agar (LA), and individual colonies were used to inoculate LBN for preparation of liquid inocula. All media were from Difco (Sparks, MD), and unless otherwise stated, all other reagents were from Sigma-Aldrich (St. Louis, MO).
CM preparation.
Preparation of CMs followed a previously described protocol (28, 33). Briefly, chitosan was purchased from Sigma-Aldrich (catalog number 448869-250G), and a 1% (wt/vol) chitosan solution was prepared in 2% (vol/vol) acetic acid with 1% (wt/vol) Tween 80. After addition of 2 ml of 10% (wt/vol) aqueous sodium sulfate, the chitosan solution was stirred and sonicated for 20 min to generate microparticles. The chitosan microparticles were collected by centrifugation at 6,000 rpm for 10 min, washed with deionized water three times, and freeze-dried for further use.
In vitro evaluation of effects of CMs on growth of Vibrio spp.
To evaluate growth inhibition, for each experiment, bacteria from frozen stock cultures were streaked onto LA for isolation, and the plates were incubated at 37°C overnight. Inocula of each species were picked from LA plates and were cultured separately overnight (18 to 23 h) in LBN broth at 37°C with shaking (100 rpm). The overnight cultures were serially diluted in phosphate-buffered saline (PBS); enumerated by determination of the absorbance at 600 nm, which was compared to the absorbance on a standard curve; and diluted in LBN (40 ml) at pH 7.4 to ca. 104 log CFU/ml. Each strain was incubated in LBN with different CM concentrations (0.0, 0.1, 0.3, and 0.5%, wt/vol) in 250-ml Erlenmeyer flasks with shaking at 37°C, and the number of CFU/ml was determined by plating on LA plates at 0, 3, 6, 9, and 12 h postinoculation.
For survival studies, sterile artificial seawater (ASW; Instant Ocean sea salt; Aquarium Systems) was prepared in deionized water at a salinity of 20 ppt and pH 7.4. Inocula were prepared as described above, except that they were prepared at levels of ca. 107 log CFU/ml in flasks of ASW (40 ml) with different CM concentrations (0, 0.1, 0.3, and 0.5%) and incubated at 37, 25, or 4°C without shaking. The survival of each species was determined by plate counts on LA after 24 and 48 h incubation. All in vitro results were reported as the number of mean log CFU/ml ± standard deviation from three independent experiments with three replicate flasks for each experiment.
Effects of CM treatment on survival of V. vulnificus and V. parahaemolyticus in artificially inoculated oysters.
Live oysters (Crassostrea virginica) were obtained from a local seafood market, transported in coolers on ice packs, and brought to the laboratory within 2 h. Oysters were acclimated in air at room temperature (25 ± 1°C) for 30 min in order to avoid temperature shock and then cleaned under tap water to remove any dirt or debris. Subsequently, the oysters (up to 30 oysters/tank) were placed in 30-gallon tanks (Nalgene heavy-duty rectangular high-density polyethylene [HDPE] tanks of 24 by 18 by 18 in. with a cover) in 20 liters of ASW (20 ppt, pH 7.4) for 24 h of acclimation at room temperature (25 ± 1°C), using two pumps with charcoal filtration (Tetra Whisper internal power filter 10i). Following acclimation in ASW, tetracycline was used as previously described (34) to reduce the indigenous Vibrio levels prior to experimental inoculation. Oysters (n = 6) were transferred to smaller tanks (Nalgene HDPE pans 21 by 17 by 5 in.) containing 6 liters of ASW with tetracycline (final concentration, 10 μg/ml) and incubated at room temperature without filtration for 24 h. Exposure to antibiotics was discontinued by transferring oysters to fresh ASW in new 6-liter tanks, followed by incubation for 24 h with charcoal filtration to remove residual tetracycline.
Oysters were artificially inoculated by addition of V. vulnificus or V. parahaemolyticus to the ASW (ca. 106 CFU/ml) in fresh tanks, covered with foil, and incubated without filtration for 24 h. Oysters were then transferred to a new tank containing 6 liters of ASW and various concentrations (0.0, 0.1, 0.3, and 0.5%, wt/vol) of CMs and individually evaluated for survival of V. vulnificus or V. parahaemolyticus after 0, 24, and 48 h of exposure to CMs. Oysters were removed from the tanks, transferred to a biological safety cabinet, and shucked under sterile conditions using shucking knives that had been rinsed with ethanol (70%) and flamed. Oyster meats were collected aseptically in 50-ml sterile conical tubes, weighed, and homogenized for 30 s with an equal volume of PBS using a sterile miniblender (Stomacher 80 Biomaster; Seward) to prepare 1:2 dilution sample suspensions. Serial 10-fold PBS dilutions were used to enumerate the Vibrio spp. by spread plating on selective agars, namely, modified cellobiose-polymyxin B-colistin (mCPC) agar for V. vulnificus (35) or Vibrio CHROMagar (CHROMagar Microbiology, Paris, France) for V. parahaemolyticus. Presumptive V. vulnificus isolates (yellow colonies on mCPC agar) or V. parahaemolyticus isolates (mauve colonies on Vibrio CHROMagar) were counted, and the counts are reported as the number of log CFU/g. All experiments were independently conducted three times using 3 oyster replicates for each experimental condition and time point for a total of 9 oysters per treatment.
Effects of CM treatment on survival of indigenous Vibrio spp. in oysters.
Market oysters were obtained in the summer to ensure high levels of vibrios and acclimated to laboratory conditions in holding tanks as described above. Oysters were then transferred to experimental tanks and treated with various concentrations of CMs (0, 0.1, 0.3, and 0.5%) as described above. Oysters were individually evaluated for survival of V. vulnificus, V. parahaemolyticus, V. cholerae, and heterotrophic aerobic bacteria after 0, 24, and 48 h of exposure to CMs by determining plate counts on mCPC agar, Vibrio CHROMagar, thiosulfate-citrate-bile salts-sucrose (TCBS) agar, and LA, respectively, as described above. Typical colonies were assessed by PCR in trial 3, using primers specific for V. vulnificus (36), V. parahaemolyticus (37), and V. cholerae (38). The results represent those from three independent experiments using 3 oysters per experimental condition and time point in the first and second trials and 6 oysters per sample in trial 3 for a total of 12 oysters per treatment.
Statistical analysis.
The results of the microbiological tests were log transformed for statistical analysis. Analyses of variance (ANOVAs) were performed to test the null hypothesis that there were no effects of chitosan treatment on the numbers of CFU of the bacterial population per g in samples. If a null hypothesis was rejected at the 0.05 level, a Tukey's multiple-mean-comparison test was used to identify differences in treatments. Another ANOVA of all the in vivo test results was also performed on the basis of the differences between the counts on either day 1 or day 2 and the pretreatment counts. Student t tests were then used to determine if the mean differences were significantly different from zero. The analysis was run using JMP pro (version 11) software.
RESULTS
Chitosan inhibits the growth of Vibrio spp. in broth culture.
Different CM concentrations (0.1, 0.3, and 0.5) were evaluated for inhibition of growth of the three pathogenic Vibrio spp. under optimal culture conditions. Exposure to 0.5% CMs resulted in growth cessation, and the levels of all three Vibrio spp. were significantly (P < 0.0001) reduced compared to those in untreated control samples (0.0% CMs) and became nondetectable at 3 h posttreatment (Fig. 1). Similar results were obtained with 0.1 and 0.3% CMs for V. vulnificus and with 0.3% CMs for V. parahaemolyticus (P < 0.0001). However, V. cholerae showed a more gradual inhibition at 0.3% CMs compared to that for the control samples, and no inhibition was observed for V. cholerae and V. parahaemolyticus in 0.1% CMs. With 0.3% CMs, the reductions of V. cholerae were significantly less than those of V. vulnificus or V. parahaemolyticus (P < 0.02), and with 0.1% CMs, the reductions of V. parahaemolyticus and V. cholerae were significantly less than those of V. vulnificus (P < 0.03). Thus, the efficacy of CMs for the elimination of these pathogenic vibrios varied among species.
FIG 1.
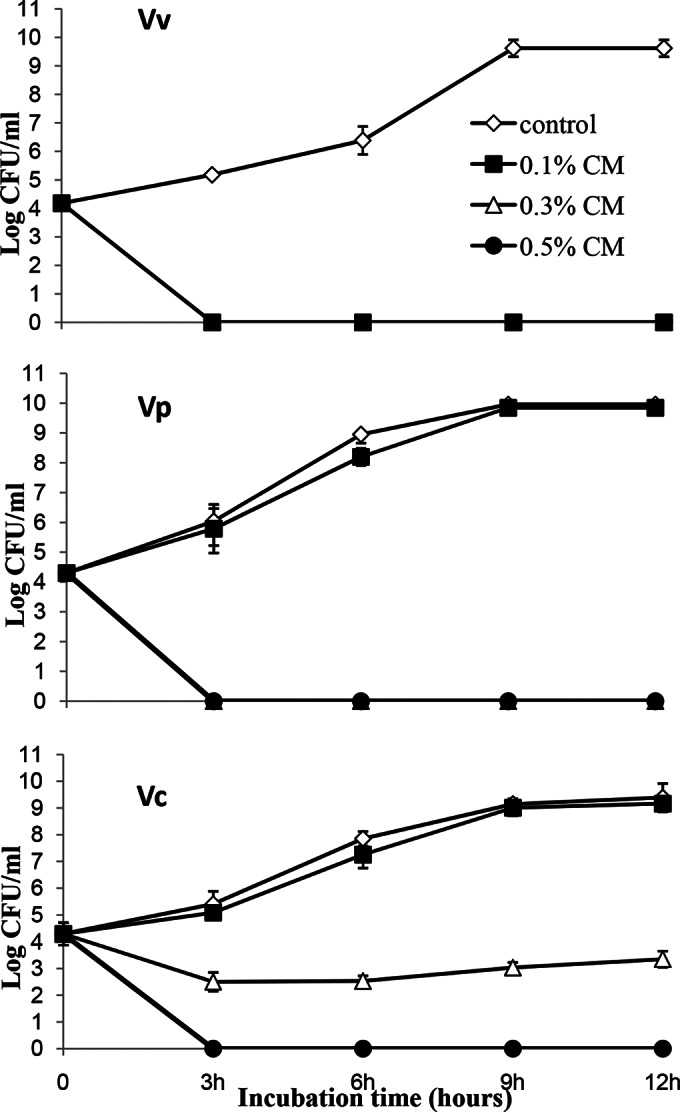
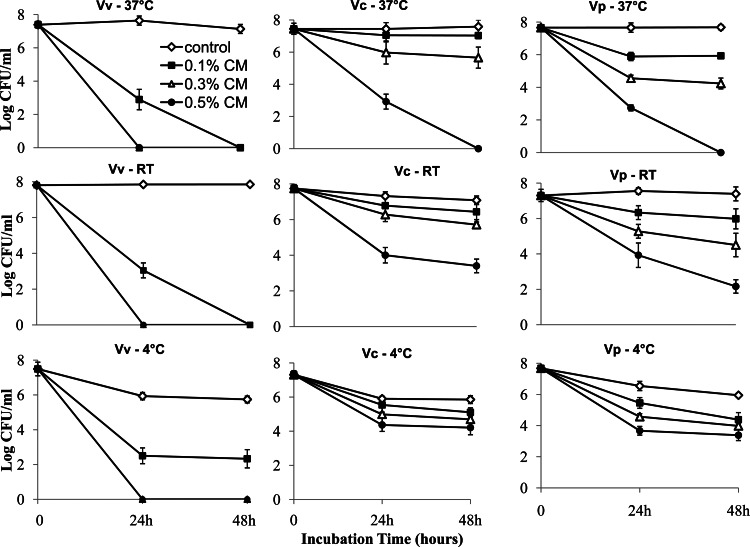
Effects of CMs on growth of Vibrio spp. in broth culture. V. vulnificus (Vv), V. parahaemolyticus (Vp), and V. cholerae (Vc) were cultured in LB with different concentrations of CMs (0, 0.1, 0.3, and 0.5%) at 37°C with shaking, as described in Materials and Methods, and bacterial growth was evaluated by determining plate counts (numbers of mean log CFU/ml). Results are the means from three independent experiments; standard deviations are indicated by error bars.
Effects of CMs on survival of Vibrio spp. in ASW.
The effects of CMs on the survival of pathogenic Vibrio spp. under simplified estuarine conditions, namely, in ASW at 20 ppt and pH 7.4, were investigated using high levels of bacteria (ca. 107 CFU/ml). As shown in Fig. 2, dramatic reductions (mean, >7 log CFU/ml) were observed for all three species in comparison to the levels for untreated control cultures following exposure to 0.5% CMs at 37°C (P < 0.001). V. vulnificus was the most sensitive of the species to the deleterious effects of CMs and was no longer detected in either 0.3% or 0.5% CMs by 24 h at all incubation temperatures examined. V. vulnificus also became nondetectable even in 0.1% CMs by 48 h at 25°C and 37°C but not at 4°C. Thus, sensitivity to CMs also varied with temperature and appeared to increase with increasing temperature, as a reduction to nondetectable levels was not achieved at 4 or 25°C for V. parahaemolyticus and V. cholerae. However, significant effects (P < 0.05) of all CM concentrations compared to the effects for the untreated controls were evident for all temperatures examined for V. vulnificus and V. parahaemolyticus by 48 h of exposure. However, 0.1% CMs did not result in any significant inhibition compared to that of the nontreated controls for V. cholerae at any temperature over the entire experiment. The results showed that sensitivity to CMs in ASW was consistent with growth inhibition in broth culture, in that the same general trend for species sensitivity was observed: V. vulnificus > V. parahaemolyticus > V. cholerae.
FIG 2.
Effects of CMs on survival of Vibrio spp. in ASW. V. vulnificus (Vv), V. parahaemolyticus (Vp), and V. cholerae (Vc) were incubated in ASW (20 ppt, pH 7.4) with different concentrations of CMs (0, 0.1, 0.3, and 0.5%) at either 37°C, ca. 25°C (room temperature [RT]), or 4°C. Bacterial survival (number of mean log CFU/ml) was calculated from three independent experiments; standard deviations are indicated by error bars.
Effect of CM treatment on survival of Vibrio spp. in artificially inoculated live oysters.
Live oyster experiments were conducted for V. vulnificus or V. parahaemolyticus, as these species are the targets of oyster PHP in the U.S. seafood industry. Artificial inoculations were achieved by pretreating the oysters with tetracycline to remove native Vibrio populations and subsequently inoculating the ASW with Vibrios, which allows the oysters to internalize these bacteria via filter feeding, as previously described (34). The survival of Vibrio spp. in individual oysters (n = 3) was evaluated in three independent experiments after 0, 24, and 48 h of exposure to CMs by determining plate counts on selective agars (mCPC agar and Vibrio CHROMagar for V. vulnificus or V. parahaemolyticus, respectively). PCR confirmation was not performed, as prescreening revealed no background Vibrio levels after tetracycline treatment (data not shown).
The pretreatment inocula in oyster meats averaged 4.6 log CFU/g (Fig. 3). All three trials showed significant (P < 0.001) reductions in numbers of CFU of V. vulnificus in oyster tissues compared to the levels for the untreated controls after 24 h of exposure for all concentrations of CMs, and the levels continued to decline at 48 h. By 48 h, the reductions for V. vulnificus averaged >4.0 log CFU/g from three independent experiments following treatment with 0.5% CMs. Even at 0.1% CMs, decreases in V. vulnificus levels were ca. 2 log CFU/g and were significantly (P < 0.0001) different from the levels observed in untreated control oysters. Significant reductions (P < 0.02) were also obtained for V. parahaemolyticus treated with both 0.3% and 0.5% CMs compared to the levels for the untreated controls, resulting in reductions of 2.2 and 3.3 log CFU/g, respectively, after 2 days. No significant differences in V. parahaemolyticus levels in oysters treated with 0.1% CMs from those in the untreated oysters were observed. Thus, the results were consistent with those of the in vitro experiments, in that both species were sensitive to CMs, but the V. parahaemolyticus response was somewhat attenuated compared to that of V. vulnificus.
FIG 3.
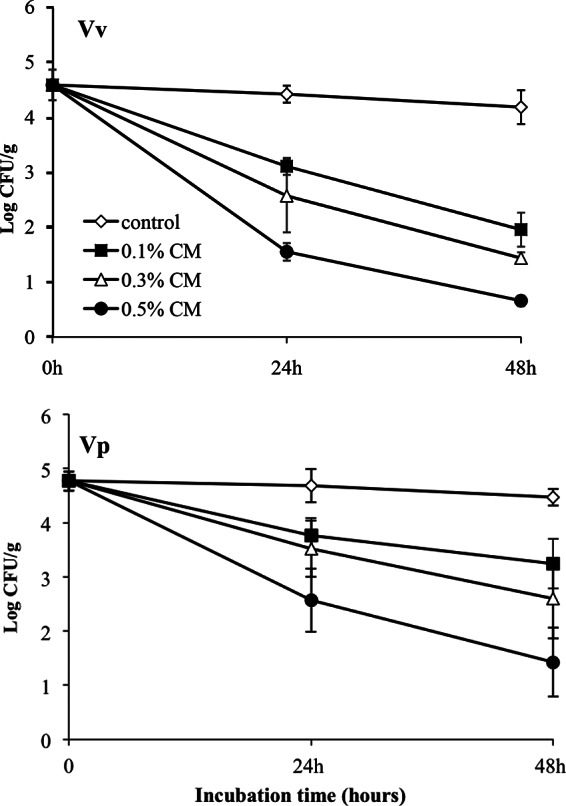
Effects of CMs on survival of Vibrio spp. in artificially inoculated oysters. Oysters were inoculated with V. vulnificus (Vv) or V. parahaemolyticus (Vp) by suspension of bacteria in ASW (20 ppt, pH 7.4, room temperature), as described in Materials and Methods. Inoculated oysters were exposed to different concentrations of CMs (0, 0.1, 0.3, and 0.5%). Vibrio levels (number of mean log CFU/g ± standard deviation) in oysters were determined on selective agars from three independent experiments for a total of nine oysters for each condition and sampling time point at 0, 24, and 48 h posttreatment.
Effect of CM treatment on survival of indigenous vibrios in live oysters.
To further examine the antimicrobial effect of CMs, fresh summer oysters with indigenous populations of Vibrio spp. were subjected to CM treatment in three independent experiments. The numbers of mean log CFU/g for V. vulnificus and V. parahaemolyticus were determined by the plate count method using selective agars. PCR confirmation was performed only in trial 3 and showed >80% agreement with the presumptive identifications (data not shown), which is consistent with the reported accuracy of Vibrio identification on these agars (35, 39). V. cholerae was not detected in these oysters. The heterotrophic aerobic bacteria in oyster homogenates were also enumerated by the standard plate count method using nonselective LA.
As expected, the initial concentrations of V. vulnificus (Table 1) and V. parahaemolyticus (Table 2) before treatment varied more than the concentrations in artificially inoculated oysters, presumably due to distinct conditions at harvest or during storage. Although the results were consistent among the three independent experiments, the data were not averaged due to variations in the initial levels. Significant reductions (P < 0.05) in V. vulnificus levels compared to those for the untreated controls were observed for all CM concentrations by 24 h, and the levels continued to decline after 48 h posttreatment. Exposure to 0.5% CMs was more effective than that to the lower concentrations, and reductions on day 2 posttreatment compared to the levels for the untreated controls ranged from 1.9 to 3.9 log CFU/g for V. vulnificus and from 1.9 to 2.6 log CFU/g for V. parahaemolyticus over the three experiments. Furthermore, greater vibriocidal activity was observed for treated samples, determined by comparison of the levels after treatment to the initial levels, and reached reductions of 4.0 and 4.7 log CFU/g for V. vulnificus and V. parahaemolyticus, respectively, in some experiments. The levels of heterotrophic aerobic bacteria also declined following CM treatment compared to the levels for the untreated controls, and the reductions ranged from 1.4 to 3.4 log CFU/g (Table 3). The results may have been confounded by differences in the initial levels among the three trials, but the results clearly demonstrate the significant effects of CMs on Vibrio species in live oysters. It should also be noted that with 0.5% CMs, levels of V. vulnificus posttreatment were <30 CFU/g for all experiments (Interstate Shellfish Sanitation Conference [ISSC] criteria for validation of a PHP treatment), and the levels of V. parahaemolyticus were all <100 CFU/g (criteria for the harvest) (40–42).
TABLE 1.
Effects of CMs on indigenous V. vulnificus in oysters
| Expt no. | CM treatment (%) |
V. vulnificus concn in oysters (no. of mean log CFU/g ± SD)a |
||
|---|---|---|---|---|
| Pretreatment | Day 1 | Day 2 | ||
| 1 | 0.0 (control) | 4.74 ± 0.16 | 4.96 ± 0.52 | 4.64 ± 0.80 |
| 0.1 | 3.34 ± 0.29b | 2.69 ± 0.33 | ||
| 0.3 | 3.00 ± 0.57b | 1.60 ± 1.39b | ||
| 0.5 | 2.51 ± 0.64b | 0.70 ± 1.22c | ||
| 2 | 0.0 (control) | 3.83 ± 0.15 | 3.80 ± 0.50 | 3.74 ± 0.13 |
| 0.1 | 2.99 ± 0.40 | 2.13 ± 0.22 | ||
| 0.3 | 2.68 ± 0.21b | 1.24 ± 1.07b | ||
| 0.5 | 2.05 ± 0.21b | 0.53 ± 0.92b | ||
| 3 | 0.0 (control) | 4.01 ± 0.38 | 3.69 ± 0.40 | 3.02 ± 0.23 |
| 0.1 | 2.74 ± 0.71 | 1.19 ± 1.00b | ||
| 0.3 | 1.34 ± 1.07b | 0.90 ± 1.07b | ||
| 0.5 | 1.29 ± 1.43b | 1.08 ± 1.19b | ||
The number of mean log CFU/g ± standard deviation (SD) based on plate counts on mCPC agar from three independent experiments with 3 oysters in the first two experiments and 6 oysters in the third experiment for a total of 12 oysters for each experimental condition and time point.
The reduction of the V. vulnificus level from initial the level is <3.52 mean log CFU/g but is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
The reduction of the V. vulnificus level from the initial level is >3.52 mean log CFU/g and is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
TABLE 2.
Effects of CMs on indigenous V. parahaemolyticus in oysters
| Expt no. | CM treatment (%) |
V. parahaemolyticus concn in oysters (no. of mean log CFU/g ± SD)a |
||
|---|---|---|---|---|
| Pretreatment | Day 1 | Day 2 | ||
| 1 | 0.0 (control) | 5.98 ± 0.44 | 5.63 ± 0.10 | 4.32 ± 0.66 |
| 0.1 | 3.94 ± 0.77b | 2.88 ± 0.40 | ||
| 0.3 | 3.93 ± 0.65b | 2.59 ± 0.38 | ||
| 0.5 | 3.26 ± 0.44b | 1.72 ± 0.46c | ||
| 2 | 0.0 (control) | 3.47 ± 0.46 | 3.48 ± 0.54 | 3.46 ± 0.37 |
| 0.1 | 3.33 ± 0.58 | 2.33 ± 0.55 | ||
| 0.3 | 2.53 ± 0.67 | 2.07 ± 0.24 | ||
| 0.5 | 1.73 ± 0.17b | 1.13 ± 0.98b | ||
| 3 | 0.0 (control) | 3.13 ± 0.62 | 2.58 ± 1.47 | 2.69 ± 0.52 |
| 0.1 | 1.88 ± 1.15 | 1.56 ± 1.39 | ||
| 0.3 | 0.84 ± 0.93 | 0.69 ± 1.06b | ||
| 0.5 | 0.68 ± 0.79 | 0.76 ± 0.84b | ||
The number of mean log CFU/g ± standard deviation (SD) based on plate counts on Vibrio CHROMagar from three independent experiments with 3 oysters in the first two experiments and 6 oysters in the third experiment for a total of 12 oysters for each experimental condition and time point.
The reduction of the V. parahaemolyticus level from the initial level is <3.52 mean log CFU/g but is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
The reduction of the V. parahaemolyticus level from the initial level is >3.52 mean log CFU/g and is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
TABLE 3.
Effects of CMs on heterotrophic aerobic bacteria in naturally infected oysters
| Expt no. | CM treatment (%) | Concn of heterotrophic aerobic bacteria in oysters (no. of mean log CFU/g ± SD)a |
||
|---|---|---|---|---|
| Pretreatment | Day 1 | Day 2 | ||
| 1 | 0.0 (control) | 6.96 ± 0.13 | 6.91 ± 0.10 | 6.11 ± 0.30 |
| 0.1 | 4.68 ± 0.28b | 4.33 ± 0.52b | ||
| 0.3 | 4.32 ± 0.69b | 3.89 ± 0.48b | ||
| 0.5 | 4.27 ± 0.41b | 2.75 ± 0.67c | ||
| 2 | 0.0 (control) | 5.69 ± 0.30 | 5.43 ± 0.23 | 5.31 ± 0.21 |
| 0.1 | 4.28 ± 0.33b | 3.89 ± 0.27b | ||
| 0.3 | 3.72 ± 0.27b | 3.01 ± 0.31b | ||
| 0.5 | 3.11 ± 0.43b | 2.16 ± 0.30b | ||
| 3 | 0.0 (control) | 5.40 ± 0.47 | 4.83 ± 0.27 | 4.90 ± 0.36 |
| 0.1 | 3.93 ± 0.61b | 3.66 ± 0.91 | ||
| 0.3 | 3.97 ± 0.39 | 3.41 ± 0.52b | ||
| 0.5 | 3.63 ± 0.73b | 3.49 ± 0.76b | ||
The number of mean log CFU/g ± standard deviation (SD) based on plate counts on LA from three independent experiments with 3 oysters in the first two experiments and 6 oysters in the third experiment for a total of 12 oysters for each experimental condition and time point.
The reduction of the heterotrophic aerobic bacterial level from the initial level is <3.52 mean log CFU/g but is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
The reduction of the heterotrophic aerobic bacterial level from the initial level is >3.52 mean log CFU/g and is significantly different from the level for the control samples treated with 0.0% CMs, as determined by two-tailed, one-way ANOVA (P < 0.05).
DISCUSSION
Currently approved PHP methods effectively lower Vibrio levels but are generally detrimental to maintaining live oyster shell stock and can be expensive (12). Therefore, novel and more economical PHP strategies are required for the successful treatment of oysters harvested from Gulf Coast waters. This study demonstrated that chitosan in the form of microparticles has strong anti-Vibrio activity both on the growth of these bacteria in culture and on their survival in seawater and oysters. In fact, in vitro growth was completely halted, and bacteria were nondetectable after 3 h of exposure to 0.5% CMs. Similar treatment in seawater also reduced the levels of all three species by >7.0 log CFU/ml within 48 h or sooner at 37°C. Anti-Vibrio activity was dependent upon the species, CM concentration, temperature, and exposure time. Among the three species, V. vulnificus exhibited the greatest sensitivity, and the response of all species was attenuated at 4°C, suggesting that increased temperature serves to augment the negative effects of CMs on survival. In contrast, the levels of all species gradually declined somewhat at 4°C without CM treatment compared to those in the untreated samples at higher temperatures, suggesting a shift to a viable but nonculturable (VBNC) state previously described for these species as a response to lower temperatures (43). Induction of a VBNC state as a consequence of chitosan treatment was not investigated, but prior studies demonstrated the rapid loss of membrane integrity and viability in E. coli under similar conditions of CM exposure (44). CM treatment did not appear to induce a VBNC state in Vibrios at low temperature, as bacteria were actually less sensitive to treatment at a lower temperature.
CM treatment was highly effective in reducing Vibrio levels in live oysters for either inoculated or autochthonous populations of V. vulnificus and V. parahaemolyticus. The results suggested that the mitigation of Vibrio spp. in oysters harboring natural populations was somewhat less efficacious than that in artificially infected oysters. However, these differences may reflect the variability of pretreatment bacterial levels in naturally infected oysters, as samples with higher initial concentrations generally exhibited greater reductions following CM treatment. Discrepancies in the results from studies with natural versus artificial populations may also reflect a greater heterogeneity of natural bacterial populations, as it is plausible that various strains are more resistant to CM exposure. Alternatively, the physiological state of the natural Vibrio populations compared to that of the artificial Vibrio populations may provide preadaptation for resisting CMs (14, 45, 46).
Previous examination of the anti-Vibrio vulnificus activity of water-soluble fractions of chitosan (10,000 and 1,000 kDa) found greater activity with the higher molecular mass, 1 to 10 mg/ml (0.1 to 1.0%) of which was required for in vitro growth inhibition. Furthermore, coadministration of 0.1 to 0.5 mg of chitosan with V. vulnificus infections in mice significantly increased survival and decreased dissemination in mice (29). Chitosan contains positively charged molecules that bind to negatively charged structures on cell surfaces and subsequently induce the leakage of intracellular material from bacterial cells (26, 44, 47). Exposure to water-soluble fractions of chitin has been shown to induce competence in V. cholerae and V. vulnificus for uptake of DNA and is used in molecular biology for transformation experiments (48, 49). The metal-binding capacity of chitosan was also considered to block pathogens by disrupting the synthesis of proteins consisting of virulence factors, such as cytolysin, elastase, and metalloproteinase (29, 50, 51). In addition, soluble chitosan was found to inhibit Vibrio cell-to-cell communication through the suppression of intracellular reactive oxygen species generation, which is known to induce cell death (29).
Chitosan microparticles were used in the present study, as Jeon and colleagues (44) demonstrated that they have significant antimicrobial activity at pH 7 to 8, which coincides with the optimum pH levels for both vibrios and oysters. They suggested that hydrophobic interactions contribute to the mechanism of CM antimicrobial activity above neutral pH, and binding of CMs to outer membrane protein OmpA and to lipopolysaccharide (LPS) in E. coli O157:H7 was shown to disrupt membranes, leading to cell death. The mechanism of CM activity against Vibrio spp. has not been investigated and is likely to be complex, due to the diversity of these species. Significant species differences in sensitivity to CMs were reported for Vibrio spp. on the basis of the strains tested, and investigations into the basis for these differences may provide a better understanding of the mechanisms of activity. It is plausible that differences in the compositions of capsular polysaccharide, LPS, or outer membrane proteins among these species contribute to altered surface charge, hydrophobicity, binding properties, etc., that correspond to species-specific differences in CM sensitivity.
Although validation of CM treatment for PHP of oysters will require criteria more exhaustive than those presented herein, these results demonstrate that CM treatment likely meets the standards for oyster PHP validation. PHP validation standards described by the ISSC (40) require a geometric mean reduction of >3.52 log MPN/g from an initial level of 4 log MPN/g to achieve <30 MPN/g following PHP for three independent trials using 10 replicates of 12 pooled oysters for each trial. The Canadian Food Inspection Agency (CFIA) recently added total end product guidelines for raw oysters, limiting V. parahaemolyticus counts so that no more than 1 in 5 samples has counts exceeding 100 total V. parahaemolyticus organisms per gram and no single sample has counts exceeding 10,000 total V. parahaemolyticus organisms per gram (41, 42). In our study, the observed reductions, as determined by plate counting for V. vulnificus in artificially inoculated oysters and in one of three trials using natural populations, attained the reductions that meet the PHP validation criteria, and in all experiments the counts reached <30 CFU/g by day 2 of treatment. The criteria for V. parahaemolyticus based on CFIA guidelines were also realized. These results substantiate the potential for application of CMs against Vibrio spp. in oyster PHP, particularly for the reduction of V. vulnificus. We investigated live oysters, but applications of CMs for use as PHP with other seafood or as a hurdle technology in which the processed product is then subjected to additional PHP treatment(s) may also be effective. Further studies will be needed to optimize the effects of CM treatments and to determine the sensitivities of the different species and of strains within each species, as well as to explore the capacity for scaling up of the process and to investigate possible changes in the sensory attributes and shelf life of the resulting product. Validation of CMs as a PHP treatment for live oysters or other shellfish should provide the first available treatment that effectively eliminates potentially pathogenic vibrios while maintaining the viability of the molluscan shellfish.
ACKNOWLEDGMENTS
This research was funded in part by Florida Sea Grant (87202) and the USDA AFRI (2014-6702-21597).
We greatly appreciate the contributions from Jessica Lepper, Evan Johnson, Stephan Javaheri, and Michael Starr for their laboratory assistance.
REFERENCES
- 1.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin Infect Dis 54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onifade TJM, Hutchinson R, Van Zile K, Bodager D, Baker R, Blackmore C. 2011. Toxin producing Vibrio cholerae O75 outbreak, United States, March to April 2011. Euro Surveill 16(20):pii=19870 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19870. [PubMed] [Google Scholar]
- 3.Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, Chirtel SJ. 1998. Influence of water temperature and salinity on Vibrio vulnificus in northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol 64:1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamplin M, Rodrick GE, Blake NJ, Cuba T. 1982. Isolation and characterization of Vibrio vulnificus from 2 Florida estuaries. Appl Environ Microbiol 44:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook DW, O'Leary P, Hunsucker JC, Sloan EM, Bowers JC, Blodgett RJ, DePaola A. 2002. Vibrio vulnificus and Vibrio parahaemolyticus in U.S. retail shell oysters: a national survey from June 1998 to July 1999. J Food Prot 65:79–87. [DOI] [PubMed] [Google Scholar]
- 6.Paz S, Bisharat N, Paz E, Kidar O, Cohen D. 2007. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res 103:390–396. doi: 10.1016/j.envres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. 2009. Backgrounder on measures to eliminate risk caused by Vibrio vulnificus infection from consumption of raw molluscan shellfish. Food and Drug Administration, Washington, DC: http://www.fda.gov/NewsEvents/Speeches/ucm187014.htm Accessed 15 January 2010. [Google Scholar]
- 8.Mahmoud BSM. 2009. Reduction of Vibrio vulnificus in pure culture, half shell and whole shell oysters (Crassostrea virginica) by X-ray. Int J Food Microbiol 130:135–139. doi: 10.1016/j.ijfoodmicro.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Kural AG, Shearer AEH, Kingsley DH, Chen HQ. 2008. Conditions for high pressure inactivation of Vibrio parahaemolyticus in oysters. Int J Food Microbiol 127:1–5. doi: 10.1016/j.ijfoodmicro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Andrews LS, Park DL, Chen YP. 2000. Low temperature pasteurization to reduce the risk of vibrio infections from raw shell-stock oysters. Food Addit Contam 17:787–791. doi: 10.1080/026520300415336. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Romero M, Kelly AL, Kerry JP. 2007. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov Food Sci Emerg Technol 8:30–38. doi: 10.1016/j.ifset.2006.05.002. [DOI] [Google Scholar]
- 12.Muth MK, Viator CL, Karns SA, Cajka JC, O'Neil M. 2013. Analysis of the costs and economic feasibility of requiring postharvest processing for raw oysters. Compr Rev Food Sci Food Saf 12:652–661. doi: 10.1111/1541-4337.12031. [DOI] [PubMed] [Google Scholar]
- 13.Quevedo AC, Smith JG, Rodrick GE, Wright AC. 2005. Ice immersion as a postharvest treatment of oysters for the reduction of Vibrio vulnificus. J Food Prot 68:1192–1197. [DOI] [PubMed] [Google Scholar]
- 14.Tamplin ML, Capers GM. 1992. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol 58:1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motes ML, DePaola A. 1996. Offshore suspension relaying to reduce levels of Vibrio vulnificus in oysters (Crassostrea virginica). Appl Environ Microbiol 62:3875–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tharanathan RN, Kittur FS. 2003. Chitin—the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr 43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 17.Kurita K. 2006. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol 8:203–226. doi: 10.1007/s10126-005-0097-5. [DOI] [PubMed] [Google Scholar]
- 18.Tan SC, Tan TK, Wong SM, Khor E. 1996. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr Polym 30:239–242. doi: 10.1016/S0144-8617(96)00052-5. [DOI] [Google Scholar]
- 19.Knorr D. 1984. Use of chitinous polymers in food: a challenge for food research and development. Food Technol 38:85–89. [Google Scholar]
- 20.FDA/CFSAN. 2012. Shrimp-derived chitosan GRAS notices. FDA, Washington, DC: http://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices Accessed 16 December 2014. [Google Scholar]
- 21.FDA. 2012. GRAS notices: shrimp-derived chitosan. http://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=443&sort=GRN_No&order=DESC&startrow=1&type=basic&search=chitosan Accessed 6 December 2013. [Google Scholar]
- 22.Korea Food and Drug Administration. 1995. Food additives code. Korea Food and Drug Administration, Seoul, South Korea. [Google Scholar]
- 23.Prashanth KVH, Tharanathan RN. 2007. Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Sci Technol 18:117–131. doi: 10.1016/j.tifs.2006.10.022. [DOI] [Google Scholar]
- 24.Pal K, Behera B, Roy S, Ray SS, Thakur G. 2013. Chitosan based delivery systems on a length scale: nano to macro. Soft Mater 11:125–142. doi: 10.1080/1539445X.2011.586082. [DOI] [Google Scholar]
- 25.Hossain S, Rahman A, Kabir Y, Shams AA, Afros F, Hashimoto M. 2007. Effects of shrimp (Macrobracium rosenbergii)-derived chitosan on plasma lipid profile and liver lipid peroxide levels in normo- and hypercholesterolaemic rats. Clin Exp Pharmacol Physiol 34:170–176. doi: 10.1111/j.1440-1681.2007.04568.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Du YM, Wang XH, Sun LP. 2004. Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol 95:147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Chen YM, Chung YC, Wang LW, Chen KT, Li SY. 2002. Antibacterial properties of chitosan in waterborne pathogen. J Environ Sci Health A Tox Hazard Subst Environ Eng 37:1379–1390. doi: 10.1081/ESE-120005993. [DOI] [PubMed] [Google Scholar]
- 28.Jeong KC, Kang MY, Kang JH, Baumler DJ, Kaspar CW. 2011. Reduction of Escherichia coli O157:H7 shedding in cattle by addition of chitosan microparticles to feed. Appl Environ Microbiol 77:2611–2616. doi: 10.1128/AEM.02587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BC, Kim MS, Choi SH, Kim KY, Kim TS. 2009. In vitro and in vivo antimicrobial activity of water-soluble chitosan oligosaccharides against Vibrio vulnificus. Int J Mol Med 24:327–333. doi: 10.3892/ijmm_00000236. [DOI] [PubMed] [Google Scholar]
- 30.Kim YR, Lee SE, Kim CM, Kim SY, Shin EK, Shin DH, Chung SS, Choy HE, Progulske-Fox A, Hillman JD, Handfield M, Rhee JH. 2003. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect Immun 71:5461–5471. doi: 10.1128/IAI.71.10.5461-5471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DePaola A, Ulaszek J, Kaysner CA, Tenge BJ, Nordstrom JL, Wells J, Puhr N, Gendel SM. 2003. Molecular, serological, and virulence characteristics of Vibrio parahaemolyticus isolated from environmental, food, and clinical sources in North America and Asia. Appl Environ Microbiol 69:3999–4005. doi: 10.1128/AEM.69.7.3999-4005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JA, Salles CA, Panigrahi P, Albert MJ, Wright AC, Johnson RJ, Morris JG. 1994. Vibrio cholerae O139 synonym Bengal is closely-related to Vibrio cholerae El Tor but has important differences. Infect Immun 62:2108–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE. 2001. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials 22:687–694. doi: 10.1016/S0142-9612(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Tucker MS, Gulig PA, Wright AC. 2009. Phase variation, capsular polysaccharide, pilus and flagella contribute to uptake of Vibrio vulnificus by the eastern oyster (Crassostrea virginica). Environ Microbiol 11:1934–1944. doi: 10.1111/j.1462-2920.2009.01916.x. [DOI] [PubMed] [Google Scholar]
- 35.Warner E, Oliver JD. 2007. Refined medium for direct isolation of Vibrio vulnificus from oyster tissue and seawater. Appl Environ Microbiol 73:3098–3100. doi: 10.1128/AEM.02245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner EB, Oliver JD. 2008. Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathog Dis 5:691–693. doi: 10.1089/fpd.2008.0120. [DOI] [PubMed] [Google Scholar]
- 37.Bej AK, Patterson DP, Brasher CW, Vickery MCL, Jones DD, Kaysner CA. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J Microbiol Methods 36:215–225. doi: 10.1016/S0167-7012(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 38.Chun J, Huq A, Colwell RR. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl Environ Microbiol 65:2202–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Pinto A, Terio V, Novello L, Tantillo G. 2011. Comparison between thiosulphate-citrate-bile salt sucrose (TCBS) agar and CHROMagar Vibrio for isolating Vibrio parahaemolyticus. Food Control 22:124–127. doi: 10.1016/j.foodcont.2010.06.013. [DOI] [Google Scholar]
- 40.National Shellfish Sanitation Program. 2013. Guidance documents—naturally occurring pathogens—post harvest processing (PHP) validation/verification guidance for Vibrio vulnificus and Vibrio parahaemolyticus, section IV, chapter IV, part 02. In National Shellfish Sanitation Program guide for the control of molluscan shellfish. National Shellfish Sanitation Program, Washington, DC: http://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.htm Accessed 30 September 2014. [Google Scholar]
- 41.Canadian Food Inspection Agency. 2014. Appendix K: HACCP validation of controls for Vibrio parahaemolyticus, fish products. In Facilities inspection manual. Canadian Food Inspection Agency, Ottawa, Ontario, Canada. [Google Scholar]
- 42.Canadian Food Inspection Agency. 2014. Appendix 2: bacteriological guidelines for fish and fish products (end product). In Standards and methods manual. Canadian Food Inspection Agency, Ottawa, Ontario, Canada. [Google Scholar]
- 43.Oliver JD, Nilsson L, Kjelleberg S. 1991. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol 57:2640–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon SJ, Oh M, Yeo W-S, Galvao KN, Jeong KC. 2014. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS One 9:e92723. doi: 10.1371/journal.pone.0092723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards GP. 1988. Microbial purification of shellfish—a review of depuration and relaying. J Food Prot 51:218–251. [DOI] [PubMed] [Google Scholar]
- 46.Kaspar CW, Tamplin ML, Martin AL. 1990. The effects of temperature and salinity on the survival of Vibrio vulnificus, p 304 Abstr 90th Annu Meet Am Soc Microbiol 1990. American Society for Microbiology, Washington, DC. [Google Scholar]
- 47.Raafat D, von Bargen K, Haas A, Sahl HG. 2008. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74:3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 49.Gulig PA, Tucker MS, Thiaville PC, Joseph JL, Brown RN. 2009. USER friendly cloning coupled with chitin-based natural transformation enables rapid mutagenesis of Vibrio vulnificus. Appl Environ Microbiol 75:4936–4949. doi: 10.1128/AEM.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 51.Schlievert PM. 2007. Chitosan malate inhibits growth and exotoxin production of toxic shock syndrome-inducing Staphylococcus aureus strains and group A streptococci. Antimicrob Agents Chemother 51:3056–3062. doi: 10.1128/AAC.01295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]