Abstract
The phyllosphere of floating macrophytes in paddy soil ecosystems, a unique habitat, may support large microbial communities but remains largely unknown. We took Wolffia australiana as a representative floating plant and investigated its phyllosphere bacterial community and the underlying driving forces of community modulation in paddy soil ecosystems using Illumina HiSeq 2000 platform-based 16S rRNA gene sequence analysis. The results showed that the phyllosphere of W. australiana harbored considerably rich communities of bacteria, with Proteobacteria and Bacteroidetes as the predominant phyla. The core microbiome in the phyllosphere contained genera such as Acidovorax, Asticcacaulis, Methylibium, and Methylophilus. Complexity of the phyllosphere bacterial communities in terms of class number and α-diversity was reduced compared to those in corresponding water and soil. Furthermore, the bacterial communities exhibited structures significantly different from those in water and soil. These findings and the following redundancy analysis (RDA) suggest that species sorting played an important role in the recruitment of bacterial species in the phyllosphere. The compositional structures of the phyllosphere bacterial communities were modulated predominantly by water physicochemical properties, while the initial soil bacterial communities had limited impact. Taken together, the findings from this study reveal the diversity and uniqueness of the phyllosphere bacterial communities associated with the floating macrophytes in paddy soil environments.
INTRODUCTION
Phyllosphere is a special niche harboring diverse species of microbes, especially bacteria (1, 2). There is a general consensus that phyllosphere microbial communities benefit the host plants profoundly and play an important role in the cycles of carbon and nitrogen (2, 3). However, the phyllosphere has gained much less attention for microbiological studies than other plant-related bacterial habitats, such as the rhizosphere (4). With the advent of culture-independent technologies, denaturing gradient gel electrophoresis (DGGE), and high-throughput sequencing, a considerable number of studies have provided insights into the diversities, structures, and even functions of the phyllosphere bacterial communities for terrestrial plants (3, 5–9). However, very limited studies have addressed the microbial diversity of the phyllosphere in aquatic/wetland environments.
Aquatic ecosystems harbor diverse and large microbial populations which drive major biogeochemical processes (10). The surfaces of macrophytes in the water environments can support enormous communities of bacteria (11, 12). Previous studies have focused mainly on the microbial communities of submerged plants with culture-dependent or culture-independent methods such as DGGE and fluorescent in situ hybridization (FISH) (11–13). Aquatic floating macrophytes, however, have received little attention regarding their phyllosphere bacterial communities. Wolffia, in the family Lemnaceae, is a rootless duckweed composed of fronds (or leaves) only. Species of Wolffia are cosmopolitan floating macrophytes and can be frequently found in aquatic environments with static freshwater from tropical to temperate regions (14). Among these diverse aquatic environments, paddy soil ecosystems are prevalent and of great ecological and agricultural significance in China. As the smallest (<3 mm) flowering plant rich in protein, Wolffia has been of great scientific interest (14–16). The simplicity in size and anatomical structure (rootless and without xylem or phloem cells) and high growth rate of Wolffia make it a convenient model plant for biological studies (14, 17). The intimacy of the leaf area to air as well as to water ecosystems makes the phyllosphere of floating macrophytes an interesting subject for microbial exploration with respect to diversity, structure, and function.
Microbial community structuring is another critical aspect for understanding the microbial ecology. For the phyllosphere of terrestrial plants, it is well established that environmental conditions and plant factors modulate the bacterial community structures in the phyllosphere (4). It is suggested that various plant-related habitats actively recruit bacteria from ambient environments, possibly through the expression of specific plant genes in different genotypes (4). This habitat-related species-sorting mechanism was also demonstrated to have an important influence on the bacterial community formation in aquatic environments (13, 18–20). Concerning the floating macrophytes in paddy soil environments, inoculation from water and possible vertical dispersal of bacteria from the sediment (paddy soil) might also play roles in structuring the phyllosphere bacterial communities, given the previous finding that bacterial diversity in surface waters is easily structured by inoculation from soil (20). However, how paddy soils influence the phyllosphere bacterial communities of floating macrophytes remains unclear. Therefore, it is intriguing to investigate the mechanisms involved in the bacterial community structuring in the phyllosphere of floating macrophytes in paddy soils.
Here we present a barcoded 16S rRNA gene sequencing-based study on the phyllosphere bacterial community of one Wolffia species, W. australiana, in a paddy soil ecosystem. Two paddy soils were used to set up the systems for plant incubation. DNA collected from the phyllosphere, water, and soil was subjected to sequencing on the Illumina HiSeq 2000 platform. By analyzing the composition and structure of the phyllosphere bacterial communities of W. australiana, we aimed to address two major questions: (i) what is the core composition of the bacterial community in the phyllosphere of Wolffia and (ii) what controls the bacterial communities of the macrophyte phyllosphere in paddy soil environments.
MATERIALS AND METHODS
Soil preincubation and fertilization.
Two paddy soils sampled from two locations in China (Changde in Hunan Province [designated CD] and Yingtan in Jiangxi Province [designated YT]) (Table 1) were air dried, ground, passed through 2-mm mesh, and stored in covered plastic containers at around 20°C until used in August 2012. Samples (2.5 kg) of the two soils were weighed into plastic cuboid boxes (approximately 28 cm by 18 cm by 18 cm) and incubated with 2.5 liters of freshly prepared deionized water for 2 months in a greenhouse (25 to 32°C) in Xiamen, China. The boxes were wrapped and covered with aluminum foil to avoid algal growth. Four replicates were performed with each paddy soil. The paddy soils were fertilized with CO(NH2)2 (240 mg/kg soil) and K2HPO3 · 3H2O (240 mg/kg soil) (21) 10 days before the experiment and fertilized for a second time with K2HPO3 · 3H2O (100 mg/box) on the same day before the duckweed transplantation to maintain phosphate sufficiency. The water level was kept 5 cm above the soil level in each box, and the water was sampled for physicochemical determination as described below.
TABLE 1.
Characterization of the two paddy soils used for the plant incubation
| Soil parametera | Valueb for soil |
|
|---|---|---|
| CD | YT | |
| Sampling date (yr.mo.day) | 2011.12.22 | 2010.12.30 |
| Sampling location | Changde, Hunan Province, China | Yingtan, Jiangxi Province, China |
| Coordinates | 28°55′N, 111°26′E | 28°12′N, 116°56′E |
| Soil type | Red soil | Red soil |
| Parent material | Quaternary red clay | Quaternary red clay |
| Soil texture (% sand, silt, clay) | 13.23, 75.06, 11.71 | 14.13, 78.28, 7.60 |
| pH | 5.88 (0.14) | 6.08 (0.02) |
| CEC (cmol kg−1) | 5.36 (0.01) | 10.26 (0.02) |
| Cmic (mg kg−1 dry soil) | 1,095.55 (21.35) | 267.43 (2.93) |
| Nmic (mg kg−1 dry soil) | 128.25 (3.19) | 41.26 (6.15) |
| Csoil (%) | 2.09 (0.06) | 1.07 (0.17) |
| Nsoil (%) | 0.22 (0.005) | 0.11 (0.002) |
| NH4+ (mg kg−1 dry soil) | 40.51 (1.26) | 20.67 (0.54) |
| NO3− (mg kg−1 dry soil) | 4.91 (0.55) | 6.47 (0.52) |
| Total P (g kg−1 dry soil) | 1.00 (0.05) | 1.04 (0.09) |
| Total K (g kg−1 dry soil) | 7.64 (0.55) | 9.26 (0.98) |
| Total Fe (g kg−1 dry soil) | 18.90 (1.56) | 24.78 (2.79) |
CEC, cation exchange capacity; Cmic, soil microbial biomass carbon.
Values in parentheses are standard deviations (n = 4).
Sterile culture of the duckweed.
W. australiana was originally provided by Elias Landolt (ETH, Zurich). The process of the plant sterilization has been described elsewhere (17). Briefly, fronds were vigorously washed in Milli-Q water, sterilized with 1% NaOCl for 3 min, and washed several times with sterile water to eliminate NaOCl. The fronds were then placed individually on Hoagland agar (Hoagland solution [17] supplemented with 1% sucrose and 1% agar) in sterile vessels and incubated at 25°C under room light for a few weeks. Several strains of W. australiana without visible bacterial colonies were then transferred into sterilized Hoagland solution supplemented with 1% sucrose and incubated in a plant growth chamber (GZP-450S; Jinghong, Shanghai, China) under the following conditions: 14-h/10-h light/dark cycle with a light intensity of 50 μmol photons m−2 s−1, 30/25°C day/night temperature, and 70% relative humidity. Subcultures with visible turbidity (an indication of microbial growth) were discarded. The remaining fronds were subjected to microscopic examination by field emission scanning electron microscopy (SEM) (S-4800; Hitachi, Japan) with a tension at 5 kV (17). One flask of Wolffia fronds without any microbial growth was obtained and used as sterile material for further incubation. The sterile fronds were maintained in sterile Hoagland solution, and the absence of bacteria was confirmed by checking bacterial growth in LB medium every month when changing the incubation solutions.
Cultivation of duckweed in paddy soils and sample collection.
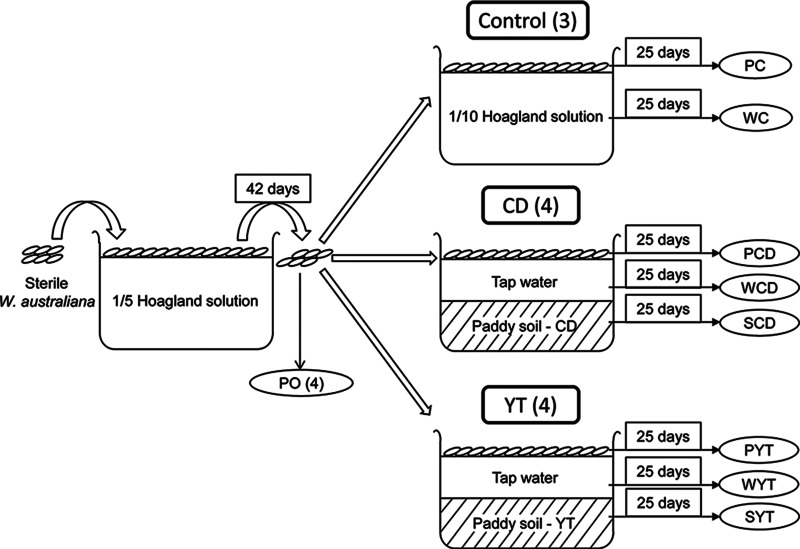
Sterilized W. australiana was grown in 1/5 Hoagland solution prepared with deionized water in a greenhouse (25 to 32°C) for 42 days to form a bacterial community in the phyllosphere (designated PO) (Fig. 1). The nutritional solution was renewed every 2 weeks. Thirty grams (fresh weight [FW]) of the W. australiana was transplanted into each of the incubation boxes. This amount of fronds was sufficient to cover the water surface in order to suppress the algal growth. A control treatment with W. australiana incubated in 4.5 liters of 1/10 Hoagland solution (3 replicates, prepared with tap water) in the same foil-wrapped box was included. Tap water was used to keep the water level 5 cm above the soil level and to sustain the water level in the control. The fronds, water, and soil in each box were sampled after 25 days. An incubation period of 25 days was sufficient to form frond colonies dominated by newly born daughter fronds (14) before total diminishing of the nutrients in the water. Fronds were collected with autoclaved nylon nets, washed thoroughly with freshly prepared deionized water, and dried with autoclaved filter papers. Four sampling replicates of the original duckweed (PO) ready for the transplantation were collected in the same manner. Fresh duckweed fronds were subjected to bacterial examination by SEM as described above. Collection of the phyllosphere bacteria was according to a previous study (17). Briefly, around 10 g (FW) of fronds was transferred into a 250-ml conical flask containing 50 ml of autoclaved 1× phosphate-buffered saline supplemented with 0.02% Tween 20 and was shaken at 200 rpm at 30°C in a shaking incubator (TS-211CGZ; Tunsuc, Shanghai, China) for 2 h. The washing solutions were filtered with nylon nets into 50-ml centrifuge tubes and centrifuged at 9,000 rpm for 30 min. The supernatants were discarded, and the pellets were preserved in the sodium phosphate buffer of the FastDNA spin kit for soil (MP Biomedicals, LLC, Illkirch, France). Water samples were filtered onto 0.22-μm cellulose membranes. Additional water was sampled to determine the physicochemical properties as described below. Soil samples were collected into aluminum foil bags and freeze-dried. All the prepared samples were stored at −80°C until DNA extraction. Thirty-four samples in 9 sample groups were collected, with 4 groups of phyllosphere samples (PO, PC, PCD, and PYT), 3 groups of water samples (WC, WCD, and WYT), and 2 groups of soil samples (SCD and SYT). Each sample group contained 3 (PC and WC) or 4 replicates (Fig. 1).
FIG 1.
Procedure for plant incubation and sampling. Numbers in parentheses indicate the replicate numbers for each treatment. Soil preincubation is not included in this figure. CD, paddy soil sampled from Changde, China; YT, paddy soil sampled from Yingtan, China; PO, phyllosphere bacterial community of the original W. australiana ready for the transplantation; P, phyllosphere; W, water; S, soil; C, control.
Determination of water physicochemical properties.
Water pH and electrical conductivity (EC) were measured with electrodes collected to an Accumet XL60 m instrument (Fisher Scientific, USA). Total phosphate (TP) was measured by molybdenum-ascorbic acid colorimetry (22) after digestion with potassium persulfate. Water samples were passed through 0.45-μm filters before determinations of dissolved nutrient concentrations. Total organic carbon (TOC), total carbon (TC), and total nitrogen (TN) were analyzed by Shimadzu TOC-VCPH analyzer (Shimadzu, Kyoto, Japan). Concentrations of NH4+ and NO3− were determined using ion chromatography (ICS-3000; Dionex, Sunnyvale, CA, USA). Concentrations of K, Ca, Mg, Mn, and Fe were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Optima 7000 DV; Perkin-Elmer, USA).
DNA extraction, PCR amplification, and bacterial community analysis.
The FastDNA spin kit for soil was used following the manufacturer's protocol to extract total DNA from all the samples. The extracted DNA was stored at −20°C until use. The V3 region of the bacterial 16S rRNA gene was amplified using 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 533R (5′-TTACCGCGGCTGCTGGCAC-3′) (23) with sample-identifying barcodes. PCR amplifications were performed in quadruplicate 50-μl reaction mixtures containing 40 to 50 ng of template DNA, 25 μl DreamTaq Green PCR master mix (2×), 20.5 μl H2O, 0.5 μl 1% bovine serum albumin (BSA), and 0.2 μM each primer. The reaction mixtures were subjected to an initial denaturation cycle of 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 30 S, with a final extension step at 72°C for 5 min. PCR amplicons pooled from the quadruplicate reactions were purified using the Universal DNA purification kit (Tiangen, China), and the concentrations were quantified using the Quant-iT PicoGreen double-stranded DNA (dsDNA) assay kit (Invitrogen, USA). The PCR products from all the samples were combined in equimolar amounts and left in 0.1 volume of 3-mol/liter sodium acetate and 3 volumes of 100% ethanol overnight. The pellet of combined DNA was collected by centrifugation at 16,000 × g for 1 h, washed with 75% ethanol, eluted in 50 μl water, and submitted to Beijing Genomics Institute (BGI) (Shenzhen, China) for sequencing (pair end) on the Illumina HiSeq 2000 platform.
Data processing and analysis.
The raw reads were assembled and filtered. Reads with contaminated adapters, an undetermined nucleotide (N), or one base occurring more than 10 times successively were removed. Low-quality reads (containing >20 low-quality bases) were further filtered from the data, and then primer regions were removed. The above processes were conducted by BGI in a prebioinformatics analysis. The resulted sequences were subsequently analyzed using Quantitative Insights Into Microbial Ecology (QIIME) (version 1.6) according to a previous study (24). In brief, operational taxonomic units (OTUs) were picked at 97% sequence similarity on the basis of an open reference by searching reads against the Greengenes database (version 2011) (http://qiime.org/tutorials/index.html). Representative sequences for each OTU were then chosen for subsequent alignment and taxonomic assignment with the RDP classifier. Chimeric sequences, chloroplast and mitochondrial OTUs (around 1%), and singleton OTUs were discarded from the final OTU table. OTUs with the same taxonomy were combined at different taxonomic levels. Rarefaction analysis and α-diversity, and β-diversity calculations were performed based on the OTU table with a random sampling to the minimal sequencing number (58,521) of the samples. Phylogenetic diversity of the whole tree was used as the estimation of α-diversity. For β-diversity analysis, dissimilarity of bacterial communities was calculated using principal-coordinate analysis (PCoA) on pairwise, unweighted UniFrac distances between all samples. In addition, PCoA based on pairwise Bray-Curtis dissimilarity was performed using R (version 2.14.0) (25) with the labdsv package (version 1.6-1). ANOSIM based on 999 permutations was used to compare the dissimilarities between samples by R with the vegan package (version 2.0-7) or by QIIME. Redundancy analysis (RDA) and partial RDA were conducted with the vegan package in R to decipher the contributions of water physicochemical properties (Table 2) (water properties of PO were not available, and thus PO was not included in the analysis), habitat types (phyllosphere and water samples were designated “1” and “2,” respectively), and the presence of soil (PC and WC, without soil, were designated “0”; PCD, WCD, PTY, and WYT, with soil from Changde and Yingtan, were designated “1”) to the structural variation of bacterial communities in the phyllosphere and water samples. Venn diagrams were employed to characterize the shared bacterial communities among sample groups and to generate core microbiome in the phyllosphere. Data variances were analyzed using one-way analysis of variance (ANOVA) with SPSS 16.
TABLE 2.
Water physicochemical properties before plant incubation and at harvest
| Water parameter | Valuea: |
|||||
|---|---|---|---|---|---|---|
| Before plant incubation |
At harvest |
|||||
| Control | CD | YT | Control | CD | YT | |
| pH | 7.24 (0.02) | 7.65 (0.02) | 6.79 (0.08) | 7.54 (0.09) | 6.31 (0.05) | 6.62 (0.08) |
| EC | 397.90 (3.82) | 235.63 (8.59) | 143.45 (5.76) | 262.37 (12.64) | 68.28 (2.40) | 44.96 (5.46) |
| TC (mg liter−1) | 17.58 (0.25) | 52.67 (1.33) | 36.11 (1.66) | 42.77 (1.44) | 73.65 (4.49) | 40.46 (2.94) |
| TOC (mg liter−1) | 10.42 (0.2) | 50.91 (1.32) | 34.65 (1.60) | 16.87 (0.52) | 70.07 (4.45) | 38.26 (2.51) |
| TN (mg liter−1) | 17.43 (0.10) | 16.57 (0.71) | 8.43 (0.24) | 1.68 (0.10) | 8.80 (0.57) | 1.96 (0.14) |
| NH4+ (mg liter−1) | ND | 11.14 (0.96) | 3.50 (0.38) | 0.08 (0.02) | 6.23 (0.62) | 0.18 (0.05) |
| NO3− (mg liter−1) | 40.83 (0.31) | 0.77 (0.25) | 32.00 (1.64) | 2.52 (0.22) | 1.02 (0.19) | 0.99 (0.64) |
| TP (mg liter−1) | 2.93 (0.07) | 4.12 (0.15) | 3.98 (0.39) | 0.17 (0.02) | 0.23 (0.12) | 0.12 (0.02) |
| K (mg liter−1) | 32.52 (0.37) | 16.05 (0.25) | 15.41 (0.68) | 13.53 (4.14) | 0.53 (0.04) | 0.51 (0.21) |
| Ca (mg liter−1) | 24.47 (0.37) | 3.51 (1.33) | 7.33 (0.72) | 22.60 (1.73) | 4.75 (0.19) | 6.41 (1.66) |
| Mg (mg liter−1) | 6.74 (0.05) | 0.75 (0.12) | 1.55 (0.17) | 4.93 (0.29) | 0.51 (0.05) | 0.85 (0.30) |
| Fe (mg liter−1) | 0.06 (0.00) | 1.79 (0.85) | 0.46 (0.26) | 0.02 (0.00) | 1.79 (1.25) | 1.00 (0.48) |
| Mn (mg liter−1) | 0.05 (0.02) | 0.06 (0.02) | 0.17 (0.04) | 0.04 (0.00) | 0.18 (0.02) | 0.05 (0.01) |
Values in parentheses are standard deviations (control, n = 3; CD and YT, n = 4).
Nucleotide sequence accession number.
All the raw sequences after assembling and filtering were deposited in the NCBI SRA database under accession number SRP029929.
RESULTS
Bacteria in the phyllosphere of W. australiana.
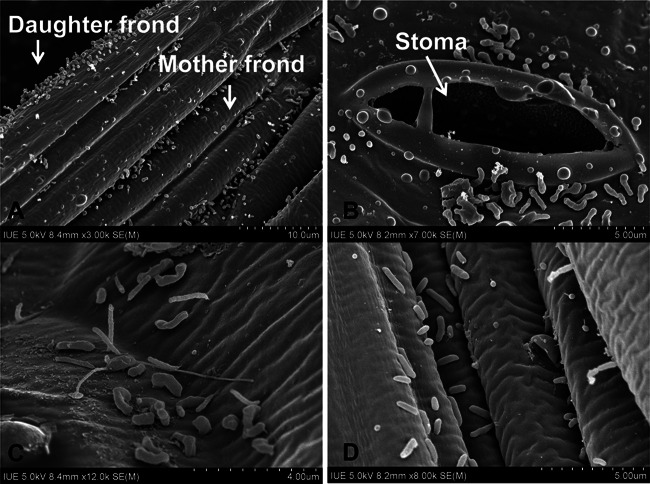
W. australiana from aseptic culture was preincubated in a greenhouse for 42 days to allow bacterial colonization in the phyllosphere before transplantation (Fig. 1). SEM images of W. australiana showed that the bacteria were located mainly around the reproductive pockets (meristematic area), where the daughter fronds attached to the mother fronds (Fig. 2A), and around stomas (Fig. 2B). Wolffia fronds with this bacterial microflora were transplanted into two paddy soil systems (CD and YT) and incubated for 25 days (Fig. 1). The SEM images revealed morphologically diverse bacteria in the duckweed phyllosphere growing in the two paddy soil systems (Fig. 2C and D).
FIG 2.
Representative SEM images of bacteria in the phyllosphere of W. australiana. (A) Bacteria on the reproductive pocket in the phyllosphere of W. australiana ready for transplantation. (B) Bacteria around a stoma in the phyllosphere of W. australiana ready for the transplantation. (C) CD treatment. (D) YT treatment. The pictures were taken by scanning electron microscopy with a tension of 5 kV.
Taxonomic composition of bacterial communities.
A total of 3098,689 pair-end sequences were obtained from 9 sample groups (34 samples) (Fig. 1), ranging from 58,521 to 113,466 reads per sample and resulting in 34,576 OTUs, within which the phyllosphere harbored 6,322 OTUs. The phyllosphere communities in the treatments of CD (PCD) and YT (PYT) harbored the fewest phyla (P < 0.01) compared to those in water and soil communities (Fig. 3A). Proteobacteria and Bacteroidetes predominated in the phyllosphere and water bacterial communities. These two phyla accounted for 97.1% and 91.4% of the total reads in the samples of phyllosphere and water, respectively. The remaining phyla each had a relative frequency <1%. Phylum distributions were relatively even in the soil samples, with Proteobacteria and Bacteroidetes together accounting for only 14.0%. At the class level, Betaproteobacteria was dominant in all the phyllosphere and water samples, followed by Alphaproteobacteria (Fig. 3B). Furthermore, the phyllosphere bacterial communities in paddy soil systems showed a clear compositional shift from the bacterial community of the original phyllosphere (PO) (Fig. 3B).
FIG 3.

Taxonomic distributions of bacterial phyla (A) and classes (B) in the phyllosphere, water, and soil bacterial communities. Each bar represents the average value of three or four replicates in each sample group. Phyla in panel B are distinguished by colors as shown in panel A. Numbers on the right are the counts for the total phyla or classes and the respective standard deviation (SD) in parentheses observed in each sample group. Letters represented results from one-way ANOVA of the phylum or class richness. The same letter indicates no significant difference. P, phyllosphere; W, water; S, soil; O, original duckweed ready for transplantation; C, control.
Patterns of taxonomic distribution became more apparent at the order and family levels (Fig. 4). The phyllosphere bacterial communities showed group distributions divergent from those observed in water samples. PCD and PYT showed compositions that were more similar to each other, which were significantly different from that of the original phyllosphere community (PO). Comamonadaceae was the dominant family in PCD and PYT, accounting for 55.0% and 50.1% of all the reads, respectively, while in PO, Comamonadaceae, Methylophilaceae, and Chitinophagaceae were distributed evenly. The phyllosphere bacterial composition in the control (PC) showed a pattern more similar to that in PO at both taxonomic levels. The distributions of the main classified genera (frequency of >1%) in the phyllosphere were significantly different (P < 0.05) between treatments (see Table S1 in the supplemental material).
FIG 4.

Taxonomic distributions of the most abundant (frequency, >1%) bacterial orders (A) and families (B) in the phyllosphere and the corresponding relative frequencies in water and soil bacterial communities. Other orders/families include all the classified orders/families with relative frequencies of less than 1% in all the phyllosphere samples. Each bar represents the average value of three or four replicates in each sample group. P, phyllosphere; W, water; S, soil; O, original duckweed ready for the transplantation; C, control.
Notably, only around 3% of the total reads in the soil bacterial communities (SCD and SYT) were affiliated with the classified and abundant orders detected in the phyllosphere communities shown in Fig. 4, and the value was 1% at the family level. Community compositions of the two paddy soils showed no significant difference from each other at all the examined taxonomic levels (Fig. 3 and 4).
α-diversity of bacterial communities.
Bacterial diversity, as represented by phylogenetic diversity of the whole tree, varied significantly across the phyllosphere, water, and soil bacterial communities (see Fig. S1 in the supplemental material). The estimate was the highest in the soil samples. Phylogenetic diversity in the phyllosphere was significantly lower (P < 0.05) than those in water samples in the control, CD and YT treatments. Compared between treatments, CD showed the highest phylogenetic diversity in the samples of both phyllosphere and water, while the control treatment had the lowest values. Samples from YT had a higher variability of phylogenetic diversity than the control and CD treatments (see Fig. S1 in the supplemental material).
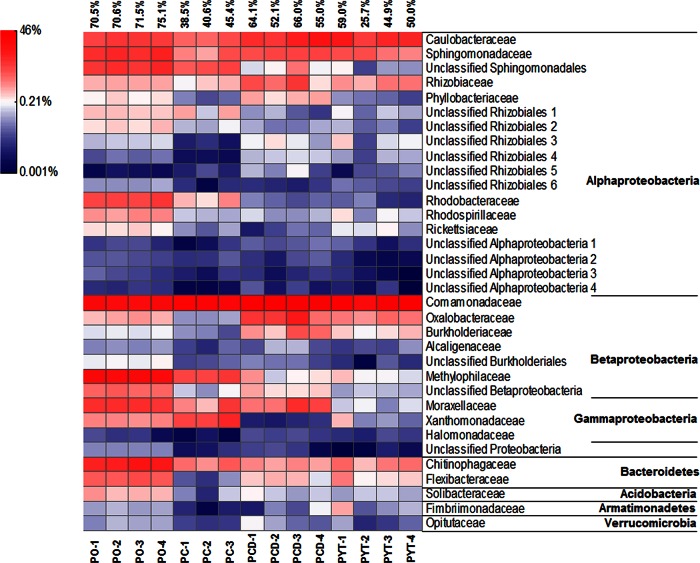
Phyllosphere core microbiome.
In the sample groups of PO, PC, PCD, and PYT, 83 OTUs were obtained as the phyllosphere core microbiome. The core microbiome was composed of OTUs detected in every replicate across all the phyllosphere samples, making up 25.7% to 75.1% (55.3% ± 14.5%) of the total reads in the phyllosphere samples (Fig. 5). The core microbiome represented bacterial groups consistently inhabiting the phyllosphere, regardless of the incubation conditions. Proteobacteria was the dominant phylum (73 OTUs), accounting for 84.6% to 97.8% of the core microbiome. Of the 34 families, 20 were taxonomically classified, with Comamonadaceae, Caulobacteraceae, and Sphingomonadaceae as the most abundant members for all the samples (Fig. 5). Methylophilaceae was detected frequently in PO and PC. At the genus level, Acidovorax, Methylibium, and Asticcacaulis were abundantly detected in all the phyllosphere communities, whereas Methylophilus was distributed mainly in PO and PC (see Table S2 in the supplemental material). These four genera were detected with much lower frequencies in water and soil bacterial communities (see Table S2 in the supplemental material).
FIG 5.
Heat map showing the core bacterial microbiome in the phyllosphere and the relative frequencies for each family. Data above the figure are the total relative frequencies of the chosen taxa in the corresponding phyllosphere communities.
Shared bacterial communities by different sample groups.
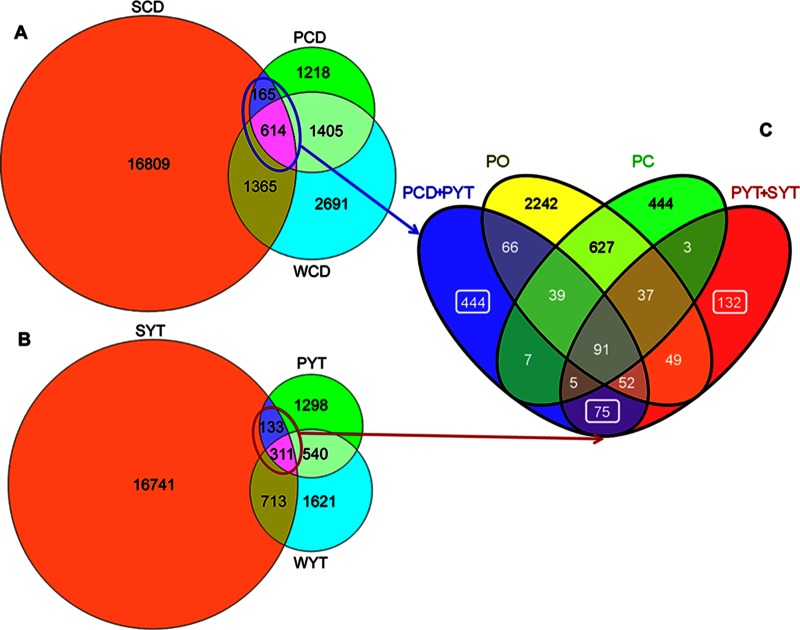
Venn diagrams were used to show shared and unique communities in various sample groups (Fig. 6). In paddy soil treatments, PCD and PYT harbored 1,218 and 1,298 unique OTUs, respectively, while they shared 2,019 and 851 OTUs with the corresponding water samples, respectively. Phyllosphere and soil samples shared 779 and 444 OTUs, accounting for 79.5% and 79.0% of the total reads in PCD and PYT, respectively (Fig. 6A and B; Table 3). However, most parts of the OTUs shared by the phyllosphere and soil (260 and 237 OTUs for CD and YT, respectively) also existed in the original (PO) and the control (PC) phyllosphere communities, accounting for 71.7% and 78.3% of the total reads in PCD and PYT, respectively (Fig. 6C; Table 3). The rest of OTUs (519 and 207 OTUs for the two treatments, respectively) were shared exclusively by the phyllosphere and soil samples. These OTUs were probably newly recruited by the phyllosphere from the soils. However, this portion of OTUs accounted for only 7.8% and 0.7% of the total reads in PCD and PYT, respectively (Fig. 6C; Table 3).
FIG 6.
Venn diagram showing the number of shared and unique OTUs in different sample groups. (A) Shared and unique OTUs in the samples of phyllosphere, water, and soil in the CD treatment. (B) Shared and unique OTUs in the samples of phyllosphere, water, and soil in the YT treatment. (C) OTUs shared by phyllosphere and soil (PCD + SCD and PYT + SYT) compared with the original (PO) and control (PC) phyllosphere communities. The numbers marked by squares are OTUs shared exclusively by phyllosphere and soils. All the observed OTUs were used in the analysis. Circles in panels A and B are proportional to the OTU numbers detected in each sample group.
TABLE 3.
Shared bacterial OTUs between phyllosphere and soil samples in treatments with paddy soilsa
| OTUsb | CD |
YT |
||
|---|---|---|---|---|
| No. of OTUs | Frequency (%)c in PCD | No. of OTUs | Frequency (%)c in PYT | |
| Total shared by phyllosphere and soil samples | 779 | 79.5 ± 7.2 | 444 | 79.0 ± 7.4 |
| Also in PO or PC | 260 | 71.7 ± 5.7 | 237 | 78.3 ± 7.3 |
| Newly recruited from soils | 519 | 7.8 ± 2.9 | 207 | 0.7 ± 0.2 |
All the observed OTUs in the related sample groups were used in the analysis.
PO, phyllosphere community of the original Wolffia for the transplantation; PC, phyllosphere community of the control Wolffia.
Data are the average values and standard deviations from four replicates.
Bacterial community structures as revealed by β-diversity.
The communities clustered strongly by habitat types (phyllosphere, water, and soil) analyzed with the presence/absence-based (unweighted UniFrac distances) and the relative abundance-based (Bray-Curtis dissimilarities) PCoA at the scale of the whole ecosystem (Fig. 7A; see Fig. S2 in the supplemental material). The phyllosphere communities, although they varied considerably, clustered relatively close to each other compared to the water and soil communities, and these communities related more closely to water communities than to soil communities. It was noteworthy that the two soil communities (SCD and SYT) overlapped with each other entirely. PCoA of the relative abundance-based index displayed more distinct clusters of phyllosphere and water communities, in contrast to the analysis with the presence/absence-based index (Fig. 7A; see Fig. S2 in the supplemental material). When analyzed with the phyllosphere and water samples, the PCoA based on Bray-Curtis dissimilarities displayed an interesting pattern (Fig. 7B). The first coordinate (PCO1), explaining 25.0% difference in community variation, separated the phyllosphere samples and the water samples. PCO2, explaining 20.4% dissimilarity, distinguished treatments with (PCD, PYT, WCD, and WYT) or without (PO, PC, and WC) soil. The sample groups exhibited clear differences from one another (P < 0.05 by ANOSIM analysis with R) (Fig. 7B). Replicates of PCD and PYT exhibited greater variations than those in PO and PC (Fig. 7B).
FIG 7.
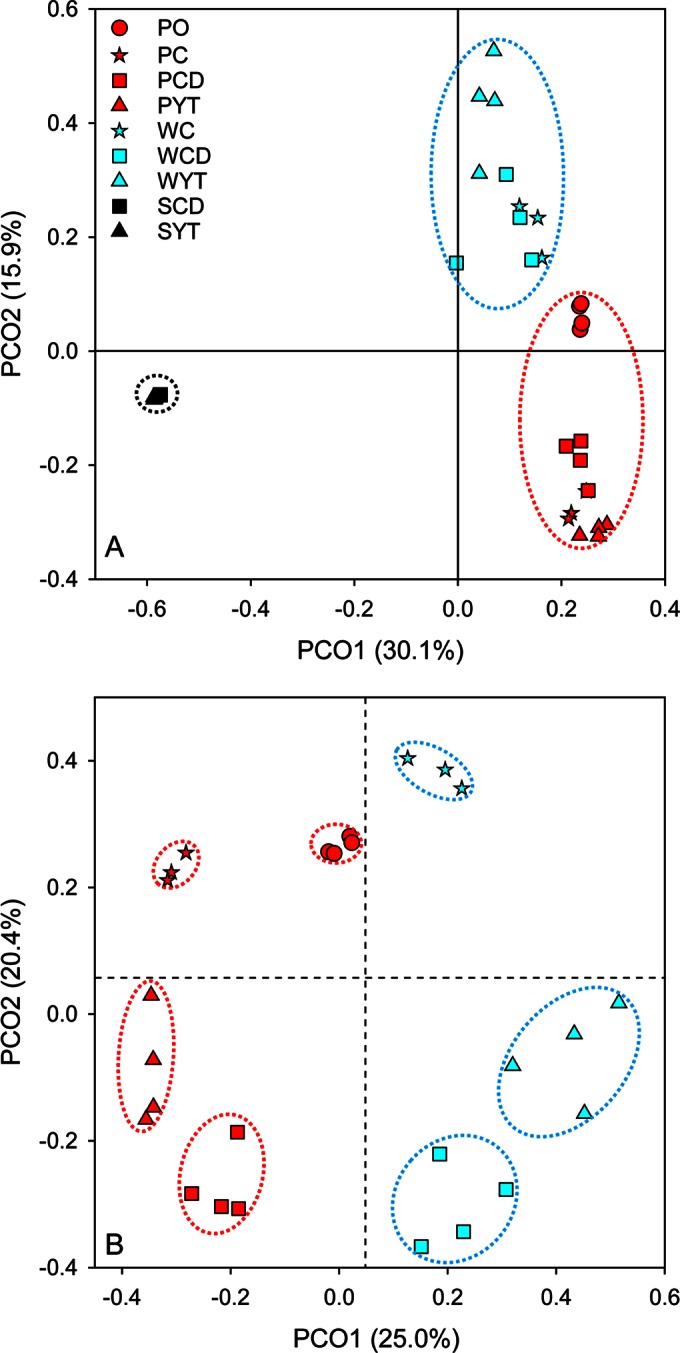
Structures of bacterial communities. (A) Principal-coordinate analysis (PCoA) of pairwise Bray-Curtis dissimilarity between all samples. (B) PCoA of pairwise Bray-Curtis dissimilarity excluding the soil samples. The Bray-Curtis dissimilarity was calculated using R (version 2.14.0) with the labdsv package (version 1.6-1).
Contributions of environmental factors to the bacterial community structures in the phyllosphere.
Partial redundancy analysis (partial RDA) was used to decipher the possible reasons for the distinct cluster distribution of different sample groups observed in the PCoA analysis (Fig. 7B and 8). Water properties, habitat types, and the presence of soil were included as the environmental factors. Water properties and habitat types explained 73.3% in total of the structural dissimilarity among the phyllosphere and water samples, while the presence of soil contributed little to the variation. Contributions of habitat types and water properties were comparable to each other (Fig. 8A). Among the phyllosphere samples, water properties were found as the main contributor (70.2%) to the structural variation, while an effect of the presence of soil was not observed. Five water properties, namely, pH and concentrations of K, NO3−, NH4+, and Fe, significantly influenced the phyllosphere bacterial community (Fig. 8B). Of the five parameters, two groups of properties influenced the communities in different directions, with pH and concentrations of K and NO3− explaining the community variation between the control and paddy soil treatments and concentrations of Fe and NH4+ contributing mainly to the separation of PCD and PYT.
FIG 8.
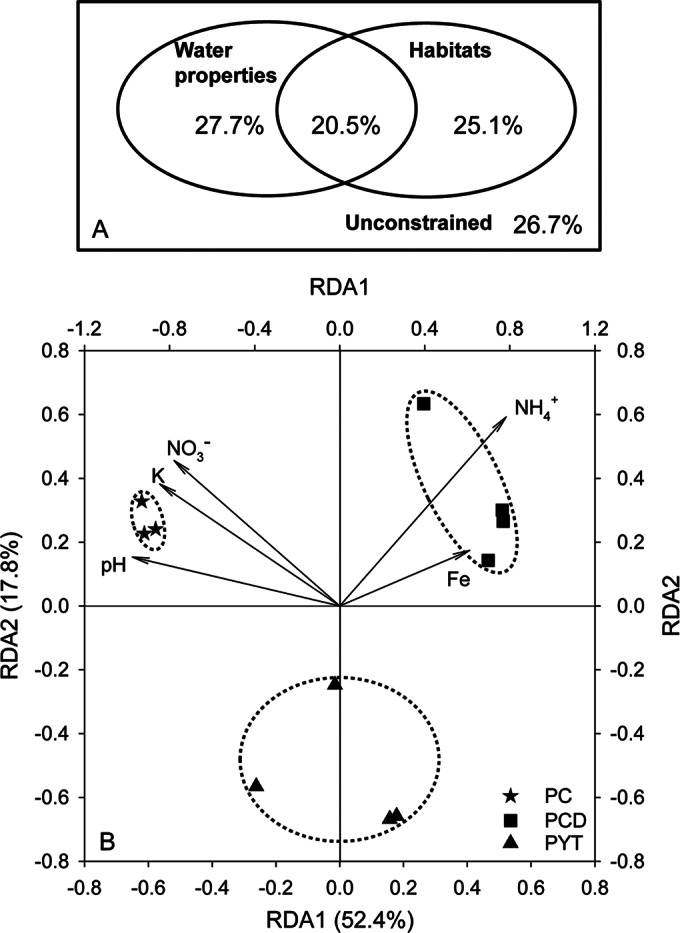
Contributions of environmental factors to the bacterial structuring. (A) Variation partitioning analysis of bacterial diversity among phyllosphere and water samples explained by water properties and habitat types. Water properties at the harvest are shown in Table 2. Habitat types of phyllosphere and water are designated “1” and “2,” respectively. The presence of soil (PC and WC, without soil, are designated “0”; PCD, WCD, PTY, and WYT, with soil from Changde and Yingtan, are assigned “1”) was also included in the environmental factors but with no explanation value observed. (B) Redundancy analysis (RDA) of the relationship between the OTU compositions in the phyllosphere samples and the water properties. The analysis included only PC, PCD, and PYT because the water properties for PO were not available. The length of each arrow indicates the contribution of the parameter to the structural variation.
DISCUSSION
Bacterial taxonomic composition in the phyllosphere of W. australiana.
The composition of the bacterial community in the phyllosphere of W. australiana showed significant uniqueness, in contrast to those found on the leaves of terrestrial plants and submerged macrophytes. For terrestrial plants, Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes are regular colonists, with Proteobacteria and Alphaproteobacteria dominating at the phylum and class levels, respectively (4), while for W. australiana, Proteobacteria and Bacteroidetes together accounted for more than 99% of the total reads, with Betaproteobacteria and Alphaproteobacteria as the most prevalent classes. The phyllospheres of terrestrial plants are able to recruit bacteria from a broad scope of origins, such as soil, air, and other plants (2, 4). In addition to the considerations of bacterial origins and plant species, nutrient limitation and extreme fluctuation of water availability are common variables in shaping the microenvironments for the more diverse bacterial communities in these habitats (4). Phyllosphere bacteria of aquatic plants, however, face less harsh environments, in terms of water and nutrient supplies. The direct exposure of the leaves to the water makes the phyllosphere bacterial population susceptible to the biotic and abiotic water environments, such as the bacterial inoculation and nutritional supplies from the water. However, in contrast to other freshwater macrophytes, W. australiana also harbored unique groups of bacteria. In the phyllosphere of submerged plants, such as Myriophyllum spicatum L and Vallisneria americana, the Cytophaga-Flavobacteria-Bacteroidetes group or Bacteroidetes was detected as the dominant epiphytes over Alphaproteobacteria and Betaproteobacteria (11, 13), which were abundantly associated with W. australiana. The reason for this discrepancy may lie in the distinct plant species and environmental conditions (11). Different species of aquatic plants were demonstrated to secrete a variety of organic compounds (11, 13), having a major determining influence on the epiphyte colonization (1). The distinct environmental conditions can also help to select specific epiphytic bacterial assemblages with best fitness to colonization.
Core microbiome in the phyllosphere of W. australiana.
Our results reveal considerable bacterial richness in the phyllosphere. Plants in the family Lemnaceae are able to secrete various and high levels of organic compounds, such as amino acids and humic substances, which can be utilized by bacteria as substrates for growth (26, 27). In addition, substantial grazing mortality in free water (28) makes the phyllospheres of floating plants ideal shelters for bacterial proliferation. The lower diversity of the phyllosphere bacterial community in comparison with communities in water and soil suggests that the phyllosphere of W. australiana, providing a protective habitat rich in resources, acts as a “habitat filter” (29) selecting unique bacterial assemblages.
The high percentages of core microbiome accounted for in the total reads from the phyllosphere samples demonstrated a profound consistency in the community composition regardless of incubation conditions. The enrichment of Asticcacaulis in the family Caulobacteraceae might be caused by the attraction of the plant exudations, because these bacteria are adhesive and aerobic heterotrophs commonly found in freshwaters (30). Many members of Comamonadaceae, especially the genus Acidovorax (31–33), are frequently detected as denitrifiers coupled with the degradation of organic substrates in wastewater treatment plants (31, 34). Species of Methylibium can also reduce nitrate into nitrite, and more importantly, they are capable of using methanol as a sole carbon source (35). Similar to the case for Methylibium, Methylophilus spp., which were abundant in the phyllosphere exposed to high levels of nitrate (PO and PC), were demonstrated to be denitrifiers able to obligately utilize methanol or methylamine (36). Considerable amounts of methanol can be released as a by-product from plants through demethylation of pectin in cell walls (37). This process of utilizing methanol and other single-carbon organic compounds as the sole sources of carbon and energy by bacteria is termed methylotrophy (38). Methylotrophic one-carbon metabolism was prevalently observed in the phyllosphere of terrestrial plants, linked mainly to the genus Methylobacterium in the class Alphaproteobacteria (3, 7).
The enrichment of bacterial taxa closely related to denitrification and methylotrophy in the phyllosphere of W. australiana implied that the phyllospheres of aquatic floating plants may act as hot spots attracting and supporting these biologically significant microorganisms. The combination of oxic (photosynthesis) and suboxic (half-submerged) microenvironments with abundant organic carbon excreted by the plant may provide an ideal platform for denitrification (39). Coupling of N and one-carbon cycles prevailing in freshwater sediments (40) might also play a key role in the phyllosphere of aquatic floating macrophytes and warrants further investigations under field conditions.
Modulations of the bacterial community in the phyllosphere of W. australiana in paddy soil ecosystems.
In view of the whole ecosystem, habitat type was the dominant factor driving the differentiation of the phyllosphere bacterial communities from those in water and soil samples (Fig. 7 and 8A). Compositions of bacterial communities were more similar within the analyzed phyllosphere samples with different dissimilarity indices (Fig. 7; see Fig. S2 in the supplemental material), strongly suggesting that habitat-related sorting was involved in the compositional structuring in the phyllosphere. Habitat-related species sorting has also been suggested as a key factor in determining bacterial community structures in other aquatic environments (13, 18–20). However, inside the phyllosphere bacterial communities, considerable variations were observed among treatments (Fig. 7B), suggesting that factors besides habitat type participated in the regulation of the bacterial dynamics.
Physicochemical properties of the surrounding water other than the presence of soil were critical drivers of structural variation observed in the phyllosphere bacterial communities (Fig. 8). Phyllosphere bacterial communities of W. australiana were modulated significantly by the concentrations of NO3− and NH4+, implying that the intensive N fertilization in paddy soil ecosystems can considerably influence the microbiology associated with floating macrophytes. In addition to N, K was another important fertilizer in the present study. The pronounced concentration decline from the preincubation period indicated that K actively participated in the biotic processes (Table 2), part of which might be involved in the bacterial metabolism. Both N and K were demonstrated by previous studies to have critical influences on microbiological processes in paddy soil environments (41, 42). pH was a strong driving force of bacterial communities in the phyllosphere of W. australiana, like those found in various environments (43–45). Fe was also found to influence the phyllosphere bacterial structure (Fig. 8B). Genera capable of Fe(II) oxidation, such as Acidovorax, Rhodobacter, and Aquabacterium (46), were detected as abundant members in the phyllosphere (see Table S1 in the supplemental material), since Fe not only is a micronutrient for the bacterial physiology but also functions as an electron source or acceptor depending on the redox conditions in water (46).
The soil bacterial communities in the present study had a marginal effect on the compositions and structures of bacterial communities in the phyllosphere. The standing water between paddy soil and W. australiana, by sorting its own groups of bacteria (19), may serve as a natural and effective barrier to the bacterial dispersion from the paddy soil to the phyllosphere. However, the relatively short time of incubation might not be sufficient for the bacterial dispersion and stable colonization in the phyllosphere, as evidenced by the relatively greater replicate variations in the phyllosphere bacterial communities in the treatments with paddy soil compared to those without (Fig. 7B). Long-term experiments are necessary for the assessment of bacterial movement from paddy soil or sediments to the phyllosphere of floating plants in future studies. Nevertheless, paddy soils with different characteristics could influence the phyllosphere bacterial communities through nutrient retention and release, contributing dramatically to the water properties which modulate the communities greatly as discussed above.
The greater similarities between phyllosphere and water bacterial communities (Fig. 7A; see Fig. S2 in the supplemental material) were possibly due to the half-immersed fronds susceptible to colonization by bacteria in the water. On the other hand, through excreting organic material into the surroundings, W. australiana was capable of shaping not only the compositions of the bacterial communities in the phyllosphere but also the structures of water bacterial communities, given the increment of total organic carbon in the water at the harvest compared to the preincubation period (Table 2).
In summary, we employed W. australiana as a representative aquatic floating plant in paddy soil environments and report here a comprehensive characterization of the phyllosphere bacterial communities of aquatic macrophytes in constructed paddy soil ecosystems. The phyllosphere of W. australiana harbors diverse and unique populations of bacteria with compositions significantly different from those associated with terrestrial and submerged plants. Habitat-related species sorting and physicochemical properties in water controlled mainly by fertilization were the main drivers of bacterial community formation and modulation, while soil bacterial communities had a very limited effect on these processes. The finding of bacterial taxa affiliated with the groups involved in methylotrophy and denitrification in the phyllosphere indicated the importance of aquatic floating plants as a possible hot spot in C and N biogeochemistry. More studies are needed to investigate the phyllosphere bacterial communities of aquatic floating plants in paddy soils under field conditions, where the interactions between aquatic floating plants and other plants (such as rice) and their associated bacterial communities could not be neglected.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (41090282 and 31270153).
We thank Elias Landolt for supplying the duckweed. We are grateful to Laurent Philippot for critically reading the paper and Valerie Gibson for improving the English. We also thank Song-Can Chen and San'an Nie for assistance with the figures.
W.-Y. Xie designed the experiment, conducted the incubation and laboratory work, and wrote the manuscript. W.-Y. Xie and J.-Q. Su performed the bioinformatics and statistical analyses. Y.-G. Zhu participated in the design of the experiments. All of the authors edited and critically reviewed the paper.
We declare no competing financial interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03191-14.
REFERENCES
- 1.Lindow SE, Brandl MT. 2003. Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 3.Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA. 2009. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vorholt JA. 2012. Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 5.Yang CH, Crowley DE, Borneman J, Keen NT. 2001. Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci U S A 98:3889–3894. doi: 10.1073/pnas.051633898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redford AJ, Fierer N. 2009. Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb Ecol 58:189–198. doi: 10.1007/s00248-009-9495-y. [DOI] [PubMed] [Google Scholar]
- 7.Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JH. 2012. Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822. doi: 10.1038/ismej.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knief C, Delmotte N, Chaffron S, Stark M, Innerebner G, Wassmann R, von Mering C, Vorholt JA. 2012. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J 6:1378–1390. doi: 10.1038/ismej.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodenhausen N, Horton MW, Bergelson J. 2013. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One 8:e56329. doi: 10.1371/journal.pone.0056329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinger L, Gobet A, Pommier T. 2012. Two decades of describing the unseen majority of aquatic microbial diversity. Mol Ecol 21:1878–1896. doi: 10.1111/j.1365-294X.2011.05362.x. [DOI] [PubMed] [Google Scholar]
- 11.Hempel M, Blume M, Blindow I, Gross EM. 2008. Epiphytic bacterial community composition on two common submerged macrophytes in brackish water and freshwater. BMC Microbiol 8:doi: 10.1186/1471-2180-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hempel M, Grossart HP, Gross EM. 2009. Community composition of bacterial biofilms on two submerged macrophytes and an artificial substrate in a pre-alpine lake. Aquat Microb Ecol 58:79–94. doi: 10.3354/ame01353. [DOI] [Google Scholar]
- 13.Crump BC, Koch EW. 2008. Attached bacterial populations shared by four species of aquatic angiosperms. Appl Environ Microbiol 74:5948–5957. doi: 10.1128/AEM.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman WS. 1961. The Lemnaceae, or duckweeds—a review of the descriptive and experimental literature. Bot Rev 27:221–287. doi: 10.1007/BF02860083. [DOI] [Google Scholar]
- 15.Stomp AM. 2005. The duckweeds: a valuable plant for biomanufacturing. Biotechnol Annu Rev 11:69–99. doi: 10.1016/S1387-2656(05)11002-3. [DOI] [PubMed] [Google Scholar]
- 16.Xie WY, Huang Q, Li G, Rensing C, Zhu YG. 2013. Cadmium accumulation in the rootless macrophyte Wolffia globosa and its potential for phytoremediation. Int J Phytoremediation 15:385–397. doi: 10.1080/15226514.2012.702809. [DOI] [PubMed] [Google Scholar]
- 17.Xie WY, Su JQ, Zhu YG. 2014. Arsenite oxidation by the phyllosphere bacterial community associated with Wolffia australiana. Environ Sci Technol 48:9668–9674. doi: 10.1021/es501510v. [DOI] [PubMed] [Google Scholar]
- 18.Buesing N, Filippini M, Burgmann H, Gessner MO. 2009. Microbial communities in contrasting freshwater marsh microhabitats. FEMS Microbiol Ecol 69:84–97. doi: 10.1111/j.1574-6941.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones SE, McMahon KD. 2009. Species-sorting may explain an apparent minimal effect of immigration on freshwater bacterial community dynamics. Environ Microbiol 11:905–913. doi: 10.1111/j.1462-2920.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 20.Crump BC, Amaral-Zettler LA, Kling GW. 2012. Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J 6:1629–1639. doi: 10.1038/ismej.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao KQ, Nie SA, Bao P, Wang FH, Bao QL, Zhu YG. 2014. Rhizosphere effect has no effect on marker genes related to autotrophic CO2 fixation in paddy soils? J Soils Sediments 14:1082–1087. doi: 10.1007/s11368-014-0864-x. [DOI] [Google Scholar]
- 22.Zhu YG, Smith FA, Smith SE. 2003. Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 13:93–100. doi: 10.1007/s00572-002-0205-6. [DOI] [PubMed] [Google Scholar]
- 23.Huse SM, Dethlefsen L, Huber JA, Welch DM, Relman DA, Sogin ML. 2008. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet 4:e1000255. doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, VBushman FD, Coastello EK. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 26.Thomas JD, Eaton P. 1996. The accumulation of amino-acids and humic substances in media conditioned by axenic and non-axenic duckweed (Lemna minor L.) and their possible ecological significance. Hydrobiologia 333:121–128. doi: 10.1007/BF00017574. [DOI] [Google Scholar]
- 27.Baker JH, Farr IS. 1987. Importance of dissolved organic matter produced by duckweed (Lemna minor) in a southern English river. Freshw Biol 17:325–330. doi: 10.1111/j.1365-2427.1987.tb01052.x. [DOI] [Google Scholar]
- 28.Šimek K, Pernthaler J, Weinbauer MG, Hornak K, Dolan JR, Nedoma J, Masin M, Amann R. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl Environ Microbiol 67:2723–2733. doi: 10.1128/AEM.67.6.2723-2733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. 2012. The application of ecological theory toward an understanding of the human microbiome. Science 336:1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pate JL, Porter JS, Jordan TL. 1973. Asticcacaulis biprosthecum sp. nov. life cycle, morphology and cultural characteristics. Antonie Van Leeuwenhoek 39:569–583. doi: 10.1007/BF02578901. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino T, Terahara T, Tsuneda S, Hirata A, Inamori Y. 2005. Molecular analysis of microbial population transition associated with the start of denitrification in a wastewater treatment process. J Appl Microbiol 99:1165–1175. doi: 10.1111/j.1365-2672.2005.02698.x. [DOI] [PubMed] [Google Scholar]
- 32.Heylen K, Lebbe L, De Vos P. 2008. Acidovorax caeni sp. nov., a denitrifying species with genetically diverse isolates from activated sludge. Int J Syst Evol Microbiol 58:73–77. doi: 10.1099/ijs.0.65387-0. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Li H, Rensing C, Zhao K, Johnstone L, Wang G. 2012. Genome sequence of the facultative anaerobic arsenite-oxidizing and nitrate-reducing bacterium Acidovorax sp. strain NO1. J Bacteriol 194:1635–1636. doi: 10.1128/JB.06814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan ST, Horiba Y, Yamamoto M, Hiraishi A. 2002. Members of the family Comamonadaceae as primary poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-degrading denitrifiers in activated sludge as revealed by a polyphasic approach. Appl Environ Microbiol 68:3206–3214. doi: 10.1128/AEM.68.7.3206-3214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatsu CH, Hristova K, Hanada S, Meng XY, Hanson JR, Scow KM, Kamagata Y. 2006. Methylibium petroleiphilum gen. nov., sp. nov., a novel methyl tert-butyl ether-degrading methylotroph of the Betaproteobacteria. Int J Syst Evol Microbiol 56:983–989. doi: 10.1099/ijs.0.63524-0. [DOI] [PubMed] [Google Scholar]
- 36.Ginige MP, Hugenholtz P, Daims H, Wagner M, Keller J, Blackall LL. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl Environ Microbiol 70:588–596. doi: 10.1128/AEM.70.1.588-596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galbally IE, Kirstine W. 2002. The production of methanol by flowering plants and the global cycle of methanol. J Atmos Chem 43:195–229. doi: 10.1023/A:1020684815474. [DOI] [Google Scholar]
- 38.Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME. 2009. The expanding world of methylotrophic metabolism. Annu Rev Microbiol 63:477–499. doi: 10.1146/annurev.micro.091208.073600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312. doi: 10.1007/s10021-003-0161-9. [DOI] [Google Scholar]
- 40.Kalyuhznaya MG, Martens-Habbena W, Wang T, Hackett M, Stolyar SM, Stahl DA, Lidstrom ME, Chistoserdova L. 2009. Methylophilaceae link methanol oxidation to denitrification in freshwater lake sediment as suggested by stable isotope probing and pure culture analysis. Environ Microbiol Rep 1:385–392. doi: 10.1111/j.1758-2229.2009.00046.x. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha M, Shrestha PM, Frenzel P, Conrad R. 2010. Effect of nitrogen fertilization on methane oxidation, abundance, community structure, and gene expression of methanotrophs in the rice rhizosphere. ISME J 4:1545–1556. doi: 10.1038/ismej.2010.89. [DOI] [PubMed] [Google Scholar]
- 42.Babu YJ, Nayak DR, Adhya TK. 2005. Potassium application reduces methane emission from a flooded field planted to rice. Biol Fertil Soils 42:532–541. doi: 10.1007/s00374-005-0048-3. [DOI] [Google Scholar]
- 43.Hörnström E. 2002. Phytoplankton in 63 limed lakes in comparison with the distribution in 500 untreated lakes with varying pH. Hydrobiologia 470:115–126. doi: 10.1023/A:1015619921119. [DOI] [Google Scholar]
- 44.Fierer N, Jackson RB. 2006. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Hu M, Xia Y, Wen X, Ding K. 2012. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl Environ Microbiol 78:7042–7047. doi: 10.1128/AEM.01617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber KA, Achenbach LA, Coates JD. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.