Abstract
The most common source of Salmonella infections in humans is food of poultry origin. Salmonella enterica serovar Enteritidis has a particular affinity for the contamination of the egg supply. In this study, the medium-chain fatty acids (MCFA), caproic, caprylic, and capric acid, were evaluated for the control of Salmonella serovar Enteritidis in chickens. All MCFA were growth inhibiting at low concentrations in vitro, with caproic acid being the most potent. Contact of Salmonella serovar Enteritidis with low concentrations of MCFA decreased invasion in the intestinal epithelial cell line T84. By using transcriptional fusions between the promoter of the regulatory gene of the Salmonella pathogenicity island I, hilA, and luxCDABE genes, it was shown that all MCFA decreased the expression of hilA, a key regulator related to the invasive capacity of Salmonella. The addition of caproic acid (3 g/kg of feed) to the feed of chicks led to a significant decrease in the level of colonization of ceca and internal organs by Salmonella serovar Enteritidis at 3 days after infection of 5-day-old chicks. These results suggest that MCFA have a synergistic ability to suppress the expression of the genes required for invasion and to reduce the numbers of bacteria in vivo. Thus, MCFA are potentially useful products for reducing the level of colonization of chicks and could ultimately aid in the reduction of the number of contaminated eggs in the food supply.
Salmonella enterica serovar Enteritidis is the leading cause of human food-borne infections associated with the consumption of chicken eggs and meat (10, 18).
As with serotypes of carcass-contaminating Salmonella, measures are needed that confer protection on chickens, starting from the early posthatch period and lasting until slaughter. In addition to control measures such as vaccination and the use of competitive exclusion flora, the supplementation of feed with acidic compounds has also been proposed to combat Salmonella in chicks (21). Currently, short-chain fatty acids (SCFA; formic, acetic, propionic, and butyric acid) are commonly used in the poultry industry for this purpose. SCFA decrease fecal shedding and the levels of colonization of the ceca and internal organs of chickens by Salmonella (12, 20). Propionic and butyric acid decrease the invasion of intestinal epithelial cells, whereas acetic acid and formic acid do not have this effect (23).
Medium-chain triglycerides have been shown to be good alternatives for nutritional antibiotics in piglets, due to the high antibacterial activity of the medium-chain fatty acids (MCFA) (6, 7). Free MCFA (C6:0 to C12:0) have been shown to be more bactericidal to numerous gram-negative and gram-positive bacteria than the SCFA (17). However, it is not known whether these acidic compounds have any effect on the invasion of intestinal epithelial cells by Salmonella, and no studies have been carried out to evaluate their possible protective effects against Salmonella infections in chickens in vivo.
In this study, Salmonella serovar Enteritidis was evaluated for its growth characteristics and its invasive qualities in human cells in the presence of one of three MCFA, namely, caproic, caprylic, or capric acid. Finally, caproic acid was used as a feed additive to evaluate its efficacy for reducing the abundance of Salmonella serovar Enteritidis in the intestinal tract and in some internal organs in young chickens. While this investigation does not directly assess whether feed supplementation can be used to decrease the egg contamination by Salmonella serovar Enteritidis that results from infection of the laying hen, the findings do suggest that this strategy for control could aid in reducing the level of Salmonella serovar Enteritidis infection in chickens.
MATERIALS AND METHODS
Bacterial strain.
Salmonella serovar Enteritidis phage type 4, strain 76Sa88 Nalr, a well-characterized strain isolated from a poultry farm (4, 5, 22), was used in the experiments.
Growth curves.
Bacteria were grown for 20 h in Luria-Bertani (LB) medium consisting of 10 g of Bacto Tryptone (Oxoid, Basingstoke, England), 5 g of yeast extract (Oxoid), and 5 g of NaCl (Merck, Leuven, Belgium) per liter of distilled water. The suspension was then diluted 1:50 in LB medium supplemented with caproic (C6), caprylic (C8), and capric acid (C10) (all products from Sigma, St. Louis, Mo.). For each MCFA, five nutrient broths were made at concentrations of 0, 2, 5, 10, and 15 mM. The pH values of all the solutions were brought to pH 6 with 10 M HCl, and the osmolarities of all the solutions were adjusted to the same values by adding NaCl to the solutions with lower osmolarities. Osmolarity was measured in a Fiske One-Ten osmometer (Indumed, Dendermonde, Belgium). The osmolarity values of the solutions were adjusted because differences in osmolarity influence the invasion of Salmonella (9). After a 1:1,000 dilution of the bacteria in these media, the suspensions were statically incubated at 37°C. The number of CFU in these suspensions was determined by titration each hour for 6 h of growth. This step was carried out by making 10-fold serial dilutions of 20 μl of the bacterial suspensions for each time interval. Then six 20-μl samples of each dilution were inoculated on brilliant green agar (BGA) and incubated for 20 h at 37°C, after which the colonies were counted. Statistical analysis was performed by comparing the mean times needed to increase the bacterial number with 1 log unit by using analysis of variance methods and SPSS software, version 11.5.
Invasion assay.
Cells of the human colon carcinoma cell line T84 were seeded in 96-well cell culture plates (Greiner, Frickenhausen, Germany) at a density of 5 × 105 cells/ml of culture medium (Dulbecco's modified Eagle's medium, 10% fetal calf serum, and 2% l-glutamine, without antibiotics) and grown for 24 h. Bacteria were grown for 20 h in LB medium, after which the suspension was diluted 1:1,000 in the MCFA solutions, the compositions of which are described above. After 4 h of incubation at 37°C, the suspensions were centrifuged and resuspended in Dulbecco's modified Eagle's medium with 10% fetal calf serum. The number of CFU per milliliter was determined by plating six 20-μl samples of a dilution series of the suspensions on BGA, after which the plates were incubated for 20 h at 37°C. The suspensions were kept at 4°C until they were used in the assay. The bacterial suspensions were diluted to a density of 5 × 106 CFU/ml. From these diluted suspensions, 200 μl was transferred to cells. This sample was centrifuged for 10 min at 514 × g rpm to make close contact between the bacteria and the cells. The plates were then incubated for 1 h at 37°C and 5% CO2. The cells then were rinsed three times with Hanks' balanced salt solution (Life Technologies, Paisley, Scotland). Cell culture medium with gentamicin (50 μg/ml) was added, and the plates were incubated for 1 h at 37°C and 5% CO2. The cells were then rinsed three times with phosphate-buffered saline and lysed with 1% Triton X-100 (Sigma) in distilled water. From this lysate, a 10-fold dilution series was made. From each dilution, six 20-μl samples were inoculated on BGA to determine the number of CFU of Salmonella serovar Enteritidis per milliliter. Statistical analysis was performed by an analysis of variance using SPSS version 11.5 software.
Construction of transcriptional fusions.
The pCS26 plasmid was used for the construction of Salmonella serovar Enteritidis 76Sa88 carrying transcriptional fusions between the promoters of the regulatory gene of the Salmonella pathogenicity island I, hilA, and luxCDABE (14). This low-copy-number plasmid contains luxCDABE genes as a reporter system. Sequences 500 bp upstream from the hilA gene start codon were searched for promoter by using the algorithm available at http://www.fruitfly.org/seq_tools/promoter.html. The predicted promoter sequence was amplified by PCR. The primer sequences were TCCTCTCGAGTTGACGCTATAACTGAAGGGAG (forward primer) and GTAGGATCCAGCCGTCCATGTTGAGTATGA (reverse primer), leading to a PCR product of 704 bp, starting 592 bp upstream of the hilA start codon. To enable cloning into the pCS26 cloning site, the 5′ ends of both primers were modified with XhoI and BamHI restriction sites, respectively, as indicated by the underlining in the sequences above. The amplification product containing the promoter sequence was digested with the XhoI and BamHI restriction endonucleases, purified with a QIAquick gel extraction kit (QIAGEN, Hilden, Germany), and ligated with pCS26, digested and purified in the same way. The ligation mixture was used for electroporation of Salmonella serovar Enteritidis 76Sa88 by using an Escherichia coli Pulser (Bio-Rad, Hercules, Calif.). Kanamycin-resistant colonies (selection marker of pCS26) were tested by PCR to contain a promoter-plasmid junction by using the forward primer derived from the promoter sequence and the reverse primer from the luxC gene of plasmid pCS26. The sequence of the promoter-plasmid junction was confirmed by DNA sequencing with an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, Calif.).
Measurement of hilA expression.
A FluoroScan Ascent fluorometer (Labsystems, Helsinki, Finland) was used to quantify light production (luminescence) by Salmonella serovar Enteritidis 76Sa88 carrying the plasmids containing the hilA-luxCDABE transcriptional fusion. Bacterial cultures were grown in microplates in 200 μl of LB medium, supplemented with 2 mM concentrations of caproic acid, capric acid, and caprylic acid, and in nonsupplemented LB medium at 37°C. Light production was measured automatically every 2 min. In control experiments, the bacteria were grown in the different nutrient broths, and all the cultures were confirmed to grow with the same generation time under the given experimental conditions. As the growth curves in all the experiments did not differ significantly, the total light produced is presented as a function of time of cultivation in all the figures. Statistical analysis was performed by an analysis of variance using SPSS version 11.5 software.
Chickens.
Lohmann White specific-pathogen-free chicks (Lohmann Tierzucht, Cuxhaven, Germany) were hatched and housed in isolation. Before the start of the experiment, 20 chickens were euthanized, and serum samples were taken for the detection of maternal antibodies against Salmonella serovar Enteritidis by means of a previously described anti-Salmonella serovar Enteritidis enzyme-linked immunosorbent assay (3). All birds were seronegative. Chicks were given autoclaved drinking water and irradiated feed (25 kGy of γ-irradiation) ad libitum. The experiment was performed under the supervision of the ethical committee of the Faculty of Veterinary Medicine, Ghent University.
In vivo trial.
Chicks were randomly divided in two groups of 20. From the day of hatch, one group received feed supplemented with 3 g of caproic acid per kg of feed, while the other group received nonsupplemented feed. The animals were infected orally with 3 × 103 CFU of Salmonella serovar Enteritidis 76Sa88 at day 5. At day 6, cloacal swabs were taken for the detection of Salmonella. At day 8, chicks were euthanized by intravenous T61 (Intervet, Mechelen, Belgium) injection. Samples of cecum, liver, and spleen were taken for bacteriological analysis.
Bacteriological analysis.
Cloacal swabs were directly inoculated on BGA (Oxoid) plates with 20 μg of nalidixic acid/ml of culture medium and incubated for 20 h at 37°C. When negative after direct inoculation, the samples were preenriched in buffered peptone water for 24 h at 37°C, after which the samples were enriched by the addition of 1 ml of this suspension to 9 ml of brilliant green tetrathionate broth. After incubation for 20 h at 37°C, one drop of this suspension was plated on BGA.
Samples of ceca, liver, and spleen were homogenized and 10-fold dilutions were made in buffered peptone water starting from 5-, 10- and 20-fold dilutions for ceca, liver, and spleen, respectively. For each dilution, six 20-μl samples were inoculated on BGA. After incubation for 20 h (at 37°C), the number of CFU/g of tissue was determined by counting the bacterial colonies. For samples which were negative after titration, preenrichment and enrichment were performed, as described above. Samples that were negative after titration but positive after Salmonella enrichment were presumed to contain 5 (ceca), 10 (liver), or 20 (spleen) CFU/g of tissue. Samples that were negative after enrichment were presumed to have 0 CFU/g. The mean number of CFU per gram of tissue was calculated for each group. SPSS version 11.5 software was used for statistical analysis. A nonparametric Mann-Whitney test was used to determine significant differences between the treatment groups. P values below 0.01 denote highly significant statistical differences, P values between 0.011 and 0.05 denote significant differences, and P values between 0.05 and 0.1 denote marginal trends. Differences with P values above 0.1 are not significant.
RESULTS
Growth curves.
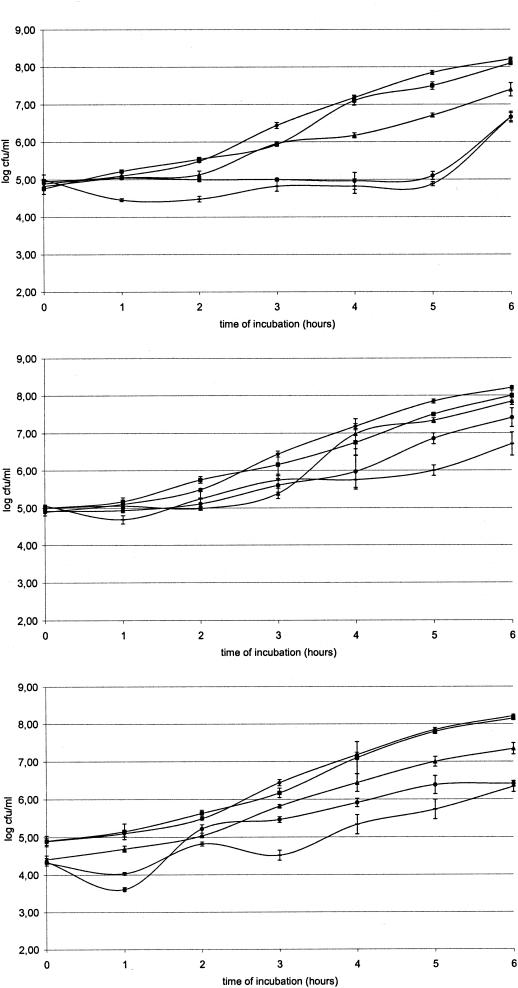
Figure 1 shows that all MCFA are bacteriostatic for Salmonella at certain concentrations. Significant differences (P < 0.05) were found between the control group and capric acid (at concentrations of 5, 10, and 15 mM), caproic acid (5, 10, and 15 mM) and caprylic acid (10 and 15 mM). For further in vitro experiments, an MCFA concentration of 2 mM was selected as it essentially does not influence bacterial growth.
FIG. 1.
Growth curves of Salmonella serovar Enteritidis 76Sa88 in LB supplemented with 2 (▪), 5 (▴), 10 (•) and 15 (−) mM concentrations of caproic (top), caprylic (middle), and capric acid (below) and in unsupplemented LB medium (♦, in all panels).
Invasion assay.
The mean percentage (± standard deviation) of invasion of Salmonella serovar Enteritidis bacteria, grown in nonsupplemented medium, in T84 cells was 5.75% (± 0.50%). Preincubation in LB medium, supplemented with 2 mM concentrations of capric, caproic, and caprylic acid for 4 h, resulted in mean percentages of invasion of 0.15% (± 0.03%), 0.59% (± 0.17%), and 0.26% (± 0.05%), respectively. Preincubation of Salmonella serovar Enteritidis 76Sa88 in LB medium (pH 6) supplemented with 2 mM concentrations of the MCFA in all cases resulted in a significant decrease in invasion of Salmonella serovar Enteritidis 76Sa88 in T84 cells, relative to preincubation in nonsupplemented LB medium (all P values were <0.000). Differences between the acids were not significant. P values were 0.841 (comparison between capric and caprylic acid), 0.329 (comparison between capric and caproic acid), and 0.758 (comparison between caproic and caprylic acid). When the same tests were done with bacteria grown in LB medium with or without MCFA at pH 7, similar results were obtained.
Measurement of hilA expression.
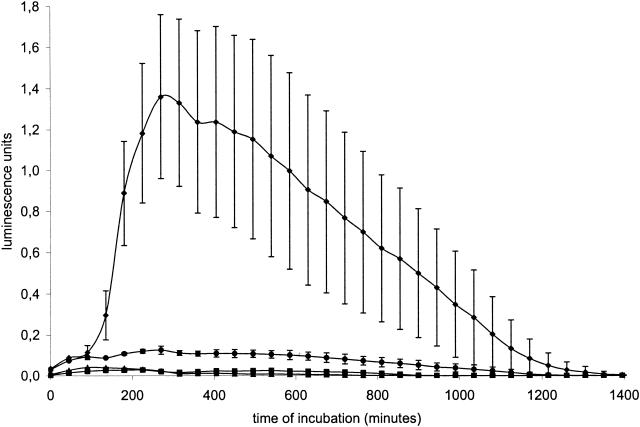
Figure 2 shows that the incubation of Salmonella serovar Enteritidis 76Sa88 with a 2 mM concentration of capric, caproic, or caprylic acid in each case decreased hilA expression relative to the level of expression measured in bacteria grown in nonsupplemented LB medium (all P values were equal to 0.001). No overall significant differences were observed between the different MCFA.
FIG. 2.
Expression of the regulator of SPI-1 gene expression, hilA, in Salmonella serovar Enteritidis 76Sa88 bacteria grown in LB medium supplemented with 2 mM concentrations of capric (▪), caproic (▴), and caprylic acid (•) and in nonsupplemented medium (♦). Expression was measured as the luminescence of Salmonella serovar Enteritidis 76Sa88 transformed with a plasmid in which the hilA promoter was cloned upstream of the reporter genes luxCDABE. Luminescence (and expression of the virulence genes) was analyzed in real time during incubation of these strains at 37°C in a luminometer.
In vivo trial.
Cloacal swabs taken 1 day postinfection from the challenge control group (infected with Salmonella bacteria but no caproic acid as a feed additive) found 15 animals positive after direct plating, 7 animals positive after enrichment, and 3 animals negative. In the group fed caproic acid-supplemented feed, 7 animals were positive after direct plating, and 7 animals were positive after enrichment, while 11 animals were negative. The differences between both groups were statistically significant (P = 0.008).
The mean (± standard deviation for all values) log CFU of Salmonella serovar Enteritidis 76Sa88 per g of ceca was 8.07 (± 0.58) for control animals, while animals that received caproic acid as a feed supplement had a mean log CFU/g of ceca of 6.41 (± 2.49). In the control group, 13 animals had a bacterial number of more than 8 log CFU/g of ceca, 5 animals had a bacterial count of between 7 and 8 log CFU/g, and 2 animals had a bacterial count of between 6 and 7 log CFU/g. In the group that received caproic acid as a feed supplement, only four animals had a bacterial number of more than 8 log CFU/g of ceca, nine animals had a bacterial count of between 7 and 8 log CFU/g, three animals had a bacterial count of between 6 and 7 log CFU/g, and four animals had a bacterial count of less than 6 log CFU/g.
The mean log CFU of Salmonella serovar Enteritidis 76Sa88 per g of liver was 2.84 (± 1.48) in the control group, while the group of animals that received caproic acid as a feed supplement had a mean log CFU of 1.86 (± 1.21) per g. In the control group, five animals had a bacterial count of more than 4 log CFU/g of liver, six animals had a bacterial count of between 3 and 4 log CFU/g, two animals had a bacterial count of between 2 and 3 log CFU/g, while seven animals had a bacterial count of less than 2 log CFU/g. In the group that received caproic acid as a feed supplement, no animals had a bacterial count of more than 4 log CFU/g of liver, 6 animals had a bacterial count of between 3 and 4 log CFU/g, 2 animals had a bacterial count of between 2 and 3 log CFU/g, and 12 animals had a bacterial count of less than 2 log CFU/g.
The mean log CFU of Salmonella serovar Enteritidis 76Sa88 per g of spleen was 3.77 (± 1.3) in the control group, while the caproic acid group had a mean log CFU of 2.42 (± 2.04) per g. In the control group, five animals had a bacterial count of more than 5 log CFU/g, five animals had a bacterial count of between 4 and 5 log CFU/g, four animals had a bacterial count of between 3 and 4 log CFU/g, four animals had a bacterial count of between 2 and 3 log CFU/g, and two animals had a bacterial count of less than 2 log CFU/g. In the group that received caproic acid as a feed supplement, no animals had a bacterial count of more than 5 log CFU/g of spleen, eight animals had a bacterial count of between 4 and 5 log CFU/g, one animal had a bacterial count of between 3 and 4 log CFU/g, two animals had a bacterial count of between 2 and 3 log CFU/g, and nine animals had a bacterial count of less than 2 log CFU/g.
Statistical analysis performed on the values of the mean log CFU/g of tissue shows significant differences between the control and the group receiving caproic acid in terms of cecal (P = 0.005) and liver (P = 0.043) colonization levels, while differences in the levels of spleen colonization were marginal (P = 0.060).
DISCUSSION
MCFA have some antimicrobial activity against Salmonella, even at low concentrations. Indeed, growth curves show that MCFA concentrations as low as 10 mM already have a bacteriostatic effect. There seems to be some specificity in the action of the MCFA, since capric acid is the most potent antibacterial MCFA for many gram-positive bacteria, while the MIC of caprylic acid is the lowest for E. coli (13, 17, 19). The antibacterial activity of the MCFA appears higher than that of published data on the activity of the SCFA (formic, acetic, propionic, and butyric acid) against both gram-positive and gram-negative bacteria, as well as against Salmonella (17). Indeed, against Salmonella serovar Enteritidis 76Sa88 colonies at pH 6, concentrations of SCFA below 25 mM have a very limited effect on growth of bacteria (23).
The invasion of Salmonella serovar Enteritidis in intestinal epithelial cells is decreased after incubation of the bacteria in growth medium supplemented with caproic, caprylic, or capric acid. The regulation by organic acids of the invasion of Salmonella has already been reported for SCFA, with acetic acid leading to an increase in invasion while propionic and butyric acid decrease invasion 2- to 10-fold (8, 15, 23). MCFA seem to decrease invasion at least to the same extent as butyric acid but at lower concentrations. The invasion-decreasing properties of MCFA, as for SCFA, are serotype independent, since the same observations were made for S. enterica serovar Typhimurium, S. enterica serovar Paratyphi B, and S. enterica serovar Hadar strains (unpublished data).
Nonbacteriostatic concentrations as low as 2 mM considerably decreased hilA expression. This gene is a regulator of the Salmonella pathogenicity island I and is directly involved in the invasion of intestinal epithelial cells (1, 2, 16). Mutations in this gene result in a drop in invasion due to the impairment of the expression of invasion effector genes, such as the SipC gene. This protein promotes internalization of the pathogen when injected into the eukaryotic cell (11). The expression of hilA and the invasion of intestinal epithelial cells are good indicators of the in vivo colonization of Salmonella, but hilA may or may not have a direct role in egg contamination. Indeed, butyric acid decreases hilA expression and the associated invasion of the intestinal epithelial cells as well as decreasing the levels of colonization of ceca and internal organs by Salmonella when it is added to poultry feed (15, 23, 24). In contrast, acetic acid supplementation does not decrease the colonization level and appears to increase the invasion of intestinal epithelial cells (23, 24). Therefore, based on information from the present study and from earlier observations, MCFA supplementation of poultry feed may decrease the colonization of chicks by Salmonella serovar Enteritidis. However, mature chickens can acquire infection on farms, so it is not yet clear what the relative impact of MCFA supplementation could be in regard to directly reducing egg contamination.
In conclusion, to our knowledge, this is the first report demonstrating the possible use of MCFA in controlling Salmonella in poultry. MCFA have demonstrated antibacterial action against Salmonella and decrease virulence gene expression and invasion in intestinal epithelial cells. Caproic acid was shown to be effective in decreasing the levels of colonization of ceca and internal organs when given as a feed supplement to poultry.
Acknowledgments
We thank Venessa Eeckhaut and Marleen Foubert for their skillful technical assistance and Phraselift (Ghent) for editing services.
This work was funded by grant 6035/1 from the Ministry of Public Health, Belgium, and by grant LN000A016 from the Ministry of Education of the Czech Republic.
REFERENCES
- 1.Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella Typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22:703-714. [DOI] [PubMed] [Google Scholar]
- 2.Darwin, K. H., and V. L. Miller. 1999. InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J. Bacteriol. 181:4949-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmidt, M., R. Ducatelle, F. Haesebrouck, P. A. De Groot, M. Verlinden, R. Wijffels, M. Hinton, J. A. Bale, and V. M. Allen. 1996. Detection of antibodies to Salmonella enteritidis in sera and yolks from experimentally and naturally infected chickens. Vet. Rec. 138:223-226. [DOI] [PubMed] [Google Scholar]
- 4.Desmidt, M., R. Ducatelle, and F. Haesebrouck. 1997. Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens. Vet. Microbiol. 56:99-109. [DOI] [PubMed] [Google Scholar]
- 5.Desmidt, M., R. Ducatelle, and F. Haesebrouck. 1998. Immunohistochemical observations in the ceca of chickens infected with Salmonella enteritidis phage type four. Poult. Sci. 77:73-74. [DOI] [PubMed] [Google Scholar]
- 6.Dierick, N. A., J. A. Decuypere, K. Molly, E. Van Beek, and E. Vanderbeke. 2002. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition. I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Livest. Prod. Sci. 75:129-142. [Google Scholar]
- 7.Dierick, N. A., J. A. Decuypere, K. Molly, E. Van Beek, and E. Vanderbeke. 2002. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition. II. In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance. Livest. Prod. Sci. 76:1-16. [Google Scholar]
- 8.Durant, J. A., V. K. Lowry, D. J. Nisbet, L. H. Stanker, D. E. Corrier, S. C. Ricke. 1999. Short-chain fatty acids affect cell-association and invasion of Hep-2 cells by Salmonella Typhimurium. J. Environ. Sci. Health B 34:1083-1099. [DOI] [PubMed] [Google Scholar]
- 9.Galan, J. E., and R. Curtiss. 1990. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect. Immun. 58:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guard-Petter, J. 2001. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 3:421-430. [DOI] [PubMed] [Google Scholar]
- 11.Hayward, R. D., and V. Koronakis. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinton, M., and A. H. Linton. 1988. Control of Salmonella infections in broiler chickens by the acid treatment of their feed. Vet. Rec. 123:416. [DOI] [PubMed] [Google Scholar]
- 13.Kabara, J. J., D. M. Swieczkowski, A. J. Conley, and J. P. Truant. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalir, S., J. McClure, K. Pabbaraju, C. Southward, M. Ronen, S. Leibler, M. G. Surette, U. Alon. 2001. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science 292:2080-2083. [DOI] [PubMed] [Google Scholar]
- 15.Lawhon, S. D., R. Maurer, M. Suyemoto, and C. Altier. 2002. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46:1451-1464. [DOI] [PubMed] [Google Scholar]
- 16.Lostroh, C. P., and C. A. Lee. 2001. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 3:1281-1291. [DOI] [PubMed] [Google Scholar]
- 17.Nakai, S. A., and K. J. Siebert. 2002. Validation of bacterial growth inhibition models based on molecular properties of organic acids. Int. J. Food Microbiol. 2678:1-7. [DOI] [PubMed] [Google Scholar]
- 18.Olsen, S. J., R. Bishop, F. W. Brenner, T. H. Roels, N. Bean, R. V. Tauxe, and L. Slutsker. 2001. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987-1997. J. Infect. Dis. 183:753-761. [DOI] [PubMed] [Google Scholar]
- 19.Sun, C. Q., C. J. O'Connor, and A. M. Robertson. 2002. The antimicrobial properties of milkfat after partial hydrolysis by calf pregastric lipase. Chem. Biol. Interact. 140:185-198. [DOI] [PubMed] [Google Scholar]
- 20.Thompson, J. L., and M. Hinton. 1997. Antibacterial activity of formic acid and propionic acid in the diet of hens on Salmonellas in the crop. Br. Poult. Sci. 38:59-65. [DOI] [PubMed] [Google Scholar]
- 21.Van Immerseel, F., K. Cauwerts, L. A. Devriese, F. Haesebrouck, and R. Ducatelle. 2002. Feed additives to control Salmonella in poultry. World's Poult. Sci. J. 58:501-513. [Google Scholar]
- 22.Van Immerseel, F., J. De Buck, I. De Smet, J. Mast, F. Haesebrouck, and R. Ducatelle. 2002. Dynamics of immune cell infiltration in the caecal lamina propria of chickens after neonatal infection with a Salmonella enteritidis. Dev. Comp. Immunol. 26:355-364. [DOI] [PubMed] [Google Scholar]
- 23.Van Immerseel, F., J. De Buck, F. Pasmans, P. Velge, E. Bottreau, V. Fievez, F. Haesebrouck, and R. Ducatelle. 2003. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 85:237-248. [DOI] [PubMed] [Google Scholar]
- 24.Van Immerseel, F., J. De Buck, F. Pasmans, I. Rychlik, J. Volf, M. Sevcik, F. Haesebrouck, and R. Ducatelle. 2003. The choice of volatile fatty acids for supplementation of poultry feed to reduce Salmonella, p. 160-161. In Proceedings of the 14th European Symposium on Poultry Nutrition, Lillehammer, Norway.