Abstract
Subtilin and the closely related entianin are class I lantibiotics produced by different subspecies of Bacillus subtilis. Both molecules are ribosomally synthesized peptide antibiotics with unusual ring structures. Subtilin-like lantibiotics develop strong antibiotic activities against various Gram-positive organisms with an efficiency similar to that of nisin from Lactococcus lactis. In contrast to nisin, subtilin-like lantibiotics partially undergo an additional posttranslational modification, where the N-terminal tryptophan residue becomes succinylated, resulting in drastically reduced antibiotic activities. A highly sensitive high-performance liquid chromatography (HPLC)-based quantification method enabled us to determine entianin and succinylated entianin (S-entianin) concentrations in the supernatant during growth. We show that entianin synthesis and the degree of succinylation drastically change with culture conditions. In particular, increasing glucose concentrations resulted in higher entianin amounts and lower proportions of S-entianin in Landy-based media. In contrast, no succinylation was observed in medium A with 10% glucose. Interestingly, glucose retarded the expression of entianin biosynthesis genes. Furthermore, deletion of the transition state regulator AbrB resulted in a 6-fold increased entianin production in medium A with 10% glucose. This shows that entianin biosynthesis in B. subtilis is strongly influenced by glucose, in addition to its regulation by the transition state regulator AbrB. Our results suggest that the mechanism underlying the succinylation of subtilin-like lantibiotics is enzymatically catalyzed and occurs in the extracellular space or at the cellular membrane.
INTRODUCTION
Lantibiotics are RiPPs (ribosomally synthesized and posttranslationally modified peptide antibiotics) (1) that contain the nonproteinogenic amino acids lanthionine and 3-methyllanthionine (2). They develop strong antimicrobial activities against various Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) (3, 4). Subtilin and the closely related entianin and ericin are produced by Bacillus subtilis ATCC 6633 (B6633), B. subtilis DSM 15029 (B15029), and B. subtilis A1/3 (BA1/3), respectively. They are representatives of the class I lantibiotics, for which nisin from Lactococcus lactis is the first-described, most prominent member (5). Class I lantibiotics have the distinction of an elongated flexible configuration, which is important for their toxic interaction with lipid II and subsequent pore formation (6, 7). Class I lantibiotics are further characterized by a common posttranslational modification mechanism, which is, in contrast to lantibiotics of classes II to IV, catalyzed by two distinct enzymes, LanB and LanC (1, 8, 9). Evidence exists that posttranslational modification of the precursor peptide and its transport out of the cell are mediated by a membrane-associated multimeric synthetase complex, LanBTC, which, in the case of subtilin, consists of dimers of SpaB, SpaC, and SpaT (10, 11).
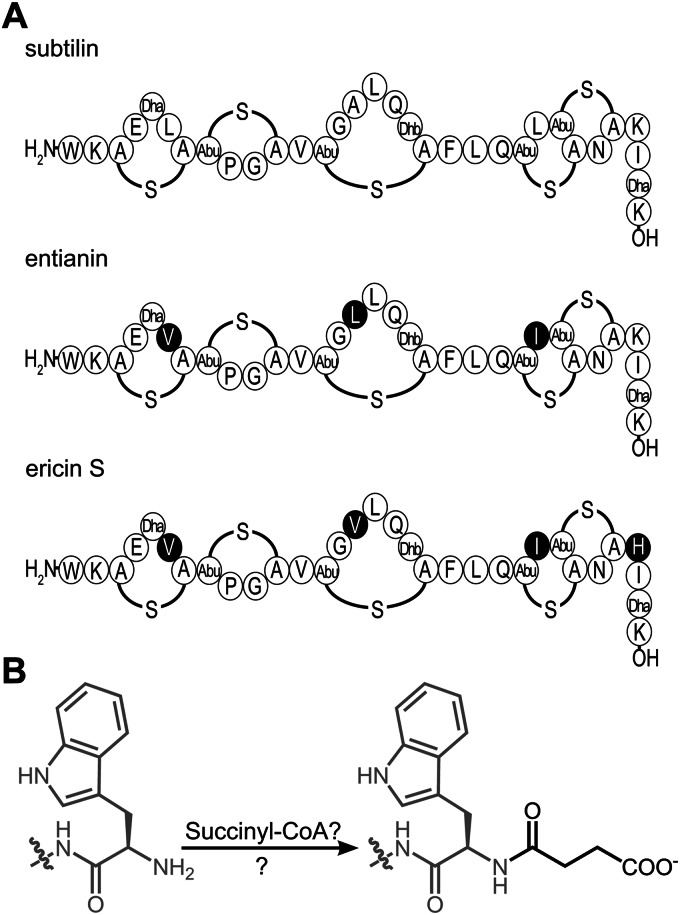
Ericin differs from subtilin at four amino acid positions, and entianin is different from subtilin at three amino acid positions (Fig. 1A); however, these changes are largely conservative. Based on the high similarities of their primary structures, subtilin, entianin, and ericin are grouped together as subtilin-like lantibiotics. Most relevant information on the biological activity of subtilin-like lantibiotics has been obtained with ericin (12) and the recently described entianin (4). The genomic organization for subtilin-like lantibiotics is very similar, with the structural genes spaS, eriS, and etnS (4, 12, 13), the modification and transport gene clusters spaBTC, eriBTC, and etnBTC (4, 8, 12, 14), the immunity genes spaIFEG, eriIFEG, and etnIFEG (4, 12, 15), and the genes spaRK, eriRK, and etnRK encoding two-component regulatory systems (4, 12, 16, 17). All biosynthetic operons of subtilin-like lantibiotics are controlled by three independent promoters that precede, in the case of the subtilin gene cluster, spaB, spaS, and spaI (17).
FIG 1.
Subtilin-like lantibiotics undergo a special posttranslational modification. (A) Strains producing subtilin (B6633), entianin (B15029), and ericin S (BA1/3) share an N-terminal tryptophan residue. Amino acid residues in entianin and ericin S that differ from those in subtilin are highlighted in black. A-S-A, meso-lanthionine; Abu-S-A, 3-methyllanthionine (Abu refers to α-aminobutyric acid); Dha, 2,3-didehydroalanine; Dhb, 2,3-didehydrobutyrine. (B) A succinyl group (black) is added to the N-terminal tryptophan residue (gray), which strongly diminishes antibiotic activity.
The synthesis of subtilin is under the dual control of two independent regulatory systems (17, 18). In the late growth phase, the expression of the major transition state regulator AbrB is repressed by Spo0A (19). AbrB repression causes the derepression of the alternative sigma factor H (σH) (20, 21). Ultimately, σH activates the expression of the two-component regulatory system SpaRK, which consists of the histidine kinase SpaK and the response regulator SpaR. Upon sensing subtilin, the histidine kinase is autoinduced in a quorum-sensing manner and transfers the phosphate group to the response regulator, which in turn induces the expression of the lantibiotic structural gene, the genes of the lantibiotic biosynthesis machinery, and the self-resistance genes (16, 22).
In contrast to other linear lantibiotics, subtilin-like lantibiotics are partially succinylated at the N-terminal tryptophan residue (4, 23). This modification is restricted to this amino acid position and does not occur at any other amino group within the molecule. N-terminal succinylation seems to be a unique feature among class I lantibiotics, which strongly diminishes the antibiotic activity as well as the autoinduction capacity (4, 23). So far the mechanism of lantibiotic succinylation remains unsolved. We previously showed that the deletion of abrB resulted in a strongly increased subtilin synthesis, most of which was succinylated (17, 18).
In the current study, the N-terminal succinylation of subtilin-like lantibiotics was investigated. Succinylation was monitored in different strain backgrounds and under different cultivation conditions. The results show that the concentration of the carbon source has a substantial impact on the degree of succinylation and that the deletion of the transition state regulator AbrB increases the synthesis of subtilin-like lantibiotics. The current data suggest that succinylation of subtilin-like lantibiotics depends on an enzyme whose expression is strongly influenced by the carbon source concentration.
MATERIALS AND METHODS
Bacterial strains used in this study.
For a description of the strains used in this study, see Table 1.
TABLE 1.
Strains used in this study
| B. subtilis strain | Descriptiona | Source or referenceb |
|---|---|---|
| B15029 | Wild type (Ent+) | DSM 15029 |
| B15029.SB1 | amyE::PetnI-lacZ (Cmr, Ent+) | This work |
| B15029.TSp28 | ΔetnS amyE::PspaS-spaS (Neor, Specr, Sub+) | This work |
| B6633 | Wild type (Sub+) | ATCC 6633 |
| B15029.SWF14 | abrB::cat (Cmr) | This work |
Ent+, entianin producer; Sub+, subtilin producer; Cmr, chloramphenicol resistant; Neor, neomycin resistant; Specr, spectinomycin resistant.
DSM, German Resource Centre for Biological Material; ATCC, American Type Culture Collection.
Culture media and culture conditions.
For entianin production and succinylation studies, Landy medium (24) and medium A (13, 25) were used. Landy medium contained 34 mM glutamic acid, 0.1% (wt/vol) yeast extract, 2 mM MgSO4, 6.7 mM KCl, 12 mM phenylalanine, 26 μM FeSO4, 33 μM MnSO4, 1 μM CuSO4 and was adjusted to pH 7.5 with KOH before autoclaving. Fifty ml of sterile 40% (wt/vol) glucose and 5 ml of sterile 20% (wt/vol) KH2PO4 were added to 1 liter autoclaved Landy medium. Medium A contained 10% (wt/vol) sucrose, 0.5% (wt/vol) yeast extract, 61 mM citric acid, 28 mM Na2SO4, 32 mM (NH4)2HPO4, 10 mM KCl, 2 mM MgCl2, 0.3 mM MnCl2, 0.18 mM FeCl3, 0.15 mM ZnCl2 and was adjusted to pH 6.8 with NH4OH before autoclaving.
For liquid cultivation, 5 ml of a fresh preculture of B15029.SB1 was suspended in 50 ml of the respective medium in 500-ml flasks. The cultures were grown with good aeration at 37°C up to 48 h. Samples were taken over time, followed by subsequent separation of the supernatant from cells by centrifugation at 20,400 × g at room temperature for 10 min.
Quantification of subtilin-like lantibiotics.
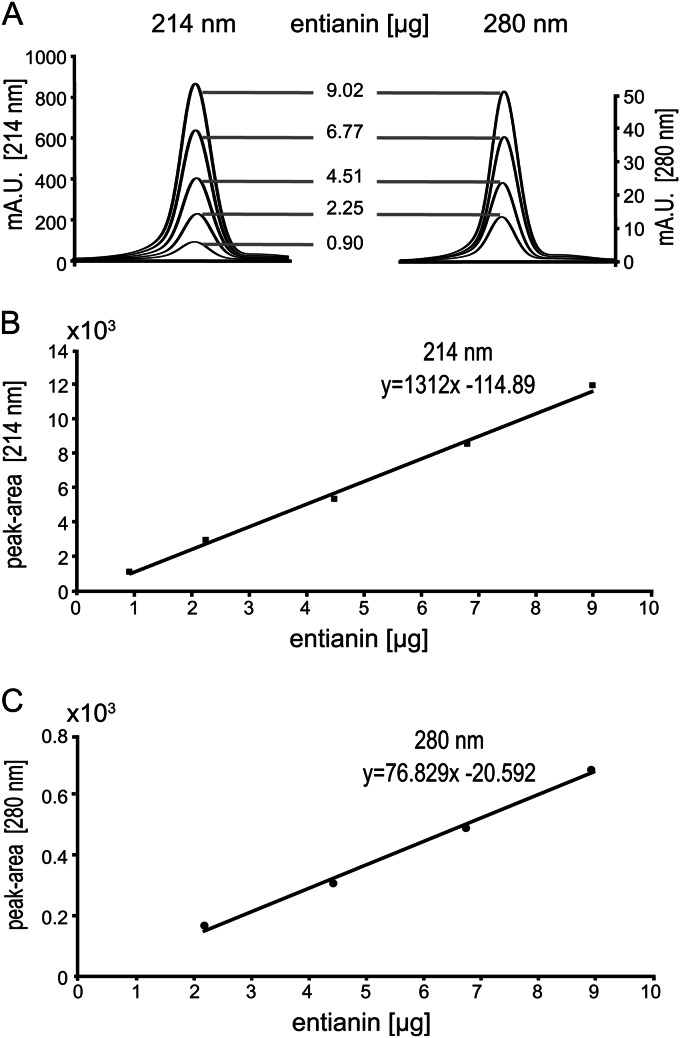
The absorption of various dilutions of a purified entianin stock solution (purified and mass spectrometrically confirmed, as described previously [4]) was monitored at 280 nm. Molarities were calculated using the molar extinction coefficient of entianin (ε280 = 5,750 liter mol−1 cm−1; ProtParam; ExPASy), which is determined mainly by the N-terminal tryptophan residue (ε280 = 5,560 liter mol−1 cm−1). From this stock solution, different dilutions were prepared and loaded onto an analytical reverse-phase high-performance liquid chromatography (RP-HPLC) column (5 μm; 250 by 4.6 mm; Gemini-NX). The samples were recorded at 214 nm and 280 nm. Each corresponding entianin peak area was manually integrated using the Agilent 1200 series ChemStation for LC 3D Systems offline program. The peak areas were recorded in a calibration curve from which the equations y = 1312.4x − 114.51 and y = 76.829x − 20.591 were created for 214 nm and 280 nm, respectively.
Determination of glucose.
The glucose concentrations in various media were determined semiquantitatively by using Diabur-Test 5000 test strips.
Monitoring of entianin and S-entianin production.
The production of entianin and succinylated entianin (S-entianin) was monitored by analytical RP-HPLC. The supernatants were loaded directly onto an analytical RP-HPLC column. A Zorbax Eclipse XDB LC column (C18; 5 μm pore size; 150 by 4.6 mm; Agilent Technologies, Santa Clara, CA) was used for chromatographic separation. Eluent A was 20% acetonitrile and 0.1% trifluoroacetic acid (TFA), and eluent B was 99.9% acetonitrile and 0.1% TFA. Entianin and S-entianin were eluted with a gradient of 17% to 42% eluent B over 18 min. The entianin and S-entianin concentrations were calculated using the equation y = 1312.4x − 114.51, obtained from the entianin calibration.
Promoter activity assay.
The activity of the promoter PetnI(−444–96) was monitored by quantification of the β-galactosidase (β-gal) activity using the reporter strain B15029.SB1 (PetnI-lacZ). Cell pellets were resuspended in working buffer (20 mM β-mercaptoethanol, 60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, pH 7), and cell lysis was achieved by addition of 0.2 mg/ml lysozyme and subsequent incubation at 37°C for 30 min. The β-gal activity was measured as described previously, with normalization to cell density (26).
RESULTS
Succinylation of subtilin(-like) lantibiotics depends on strain background and cultivation conditions.
Subtilin-like lantibiotics (Fig. 1A), such as subtilin from B6633 (18, 23), ericin from BA1/3 (12), and entianin from B15029 (4), are partially succinylated at their N-terminal tryptophan residues. N-terminal succinylation seems to be a unique feature for subtilin-like lantibiotics, and the mechanism of this posttranslational modification (Fig. 1B) still is unknown. Succinylation leads to strongly diminished antibiotic activities (4, 23) compared to those of the unmodified lantibiotics.
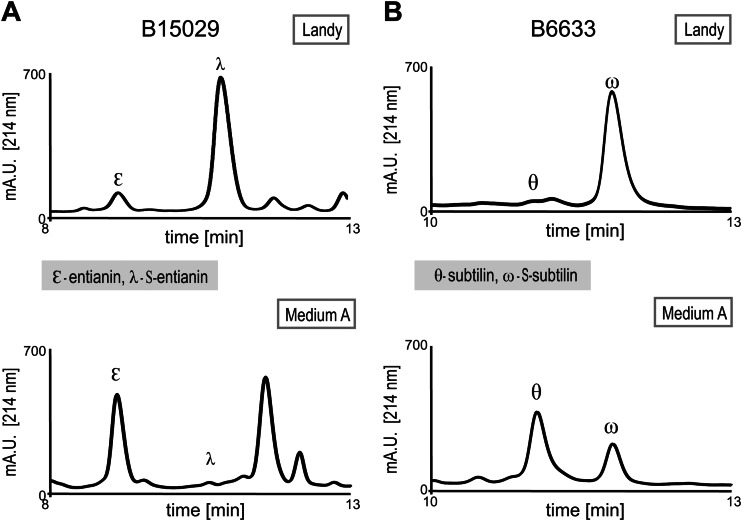
We previously observed that strain B15029 produces a significantly larger amount of entianin and less N-terminally succinylated entianin than the amounts of subtilin and S-subtilin produced by B6633 (4). In Landy medium (24), the ratio of entianin to S-entianin (1:10) was five times increased compared to the ratio between subtilin and S-subtilin (1:50) under the same growth conditions (Fig. 2A and B, top). To confirm that the observed differences in entianin and subtilin succinylation were strain dependent, the entianin structural gene etnS in strain B15029 was deleted and the subtilin structural gene spaS was integrated. The obtained ratio between unsuccinylated and succinylated subtilin upon heterologous expression in B15029 was comparable to that described for entianin (data not shown), strongly indicating that the succinylation amount is strain dependent.
FIG 2.
RP-HPLC chromatograms of supernatants from entianin-producing strain B15029 (A) and subtilin-producing strain B6633 (B). Strains were cultivated in Landy medium (top) or medium A (bottom) for 30 h at 37°C. Unsuccinylated entianin (ε) and subtilin (θ) elute before S-entianin (λ) and S-subtilin (ω), respectively.
Furthermore, we observed for both subtilin-like lantibiotics that the amount of succinylation does not depend solely on the B. subtilis strain but also is strongly influenced by the medium composition (Fig. 2). Cultivation in medium A, a commonly used Bacillus medium (25), resulted in a strong decrease in N-terminal succinylation. Depending on the cultivation conditions, the amount of S-entianin compared to that of entianin (B15029) decreased from 90% in Landy medium to less than 5% in medium A. For subtilin (B6633), the same tendency was observed with 98% succinylation in Landy medium versus 30% in medium A; however, the amount of succinylation still was higher for the subtilin producer B6633. This also confirms our previous findings (4) that among different Bacillus subspecies, the amount of succinylation varies considerably.
Quantification of subtilin-like lantibiotics.
To investigate the influence of cultivation conditions on the succinylation pattern, we established a reliable HPLC-based method to quantify lantibiotic peptides in supernatants. In contrast to other lantibiotics, subtilin-like lantibiotics share an N-terminal tryptophan residue (Fig. 1A), which could be used for quantification due to its absorption at 280 nm. Different dilution samples of an entianin stock solution of known concentration were loaded onto an analytical RP-HPLC column and recorded at 280 nm and 214 nm (Fig. 3A). The linear correlation between peak areas at 280 nm and 214 nm and entianin amounts (in μg loaded onto the HPLC column) are shown in Fig. 3B (for details, see Materials and Methods). The resulting equations are y = 76.829x − 20.592 for 280 nm and y = 1312.4x − 114.51 for 214 nm, respectively. These equations enable a reliable quantification of dissolved subtilin-like lantibiotics based on the absorbance of the N-terminal tryptophan (280 nm) or based on the absorbance of their peptide bonds (214 nm).
FIG 3.
HPLC-based calibration curves to determine concentrations of subtilin-like lantibiotics. (A) Increasing amounts of entianin (concentration was determined via A280 measurement) were loaded onto an analytical C18 RP-HPLC column and recorded at 280 nm and 214 nm, respectively. (B) Calibration curve derived from the peak areas of entianin at 214 nm, resulting in the equation y = 1312.4x − 114.51. (C) Calibration curve derived from the peak areas of entianin at 280 nm, resulting in the equation y = 76.829x − 20.592.
As a control for quantification, we used N-terminally succinylated entianin, and protein determination of entianin and S-entianin confirmed the correct correlation (data not shown). The entianin calibration based on peak integration at 214 nm also now enables the quantification of other linear lantibiotics of similar size, such as nisin, that do not contain aromatic amino acids.
Expression of entianin biosynthesis genes and entianin/S-entianin synthesis.
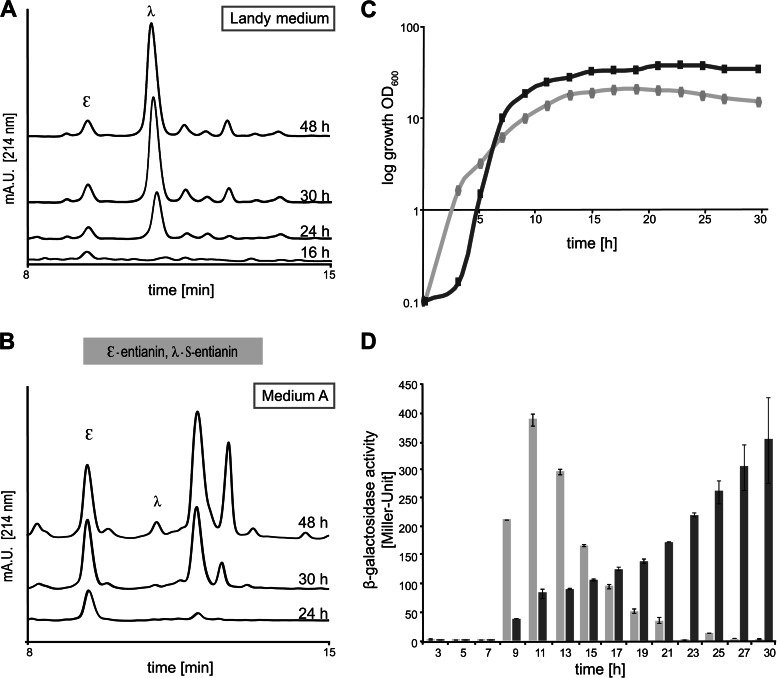
To further investigate the medium-dependent succinylation, we monitored the kinetics of entianin and S-entianin formation and compared them to the expression kinetics of the entianin biosynthesis gene clusters during growth in Landy medium and medium A, respectively. In medium A, no succinylation of entianin occurred until 48 h of growth, whereas in Landy medium S-entianin synthesis coincided largely with entianin synthesis after 16 h of growth (Fig. 4A and B). The entianin biosynthetic gene clusters etnBTC (modification and transport) and etnIFEG (immunity) and the entianin structural gene etnS are under the regulatory control of the EtnRK two-component system (4, 17, 22). After fusion of the respective promoters with the lacZ open reading frame we could demonstrate that, similar to the subtilin gene clusters (22), the regulation of all three promoters (PetnBTC, PetnS, and PetnIFEG) showed a comparable time-dependent expression pattern, although they differed in their strength of transcription. In accordance with previous findings for subtilin, the strongest expression was observed for the promoter of the structural gene etnS, followed by the modification machinery (etnBTC) and the immunity genes (etnIFEG). Based on the β-gal activities, we could calculate an expression ratio of 30:4:1 for PetnS:PetnBTC:PetnIFEG (data not shown). Due to its moderate expression and easy quantification, PetnI was chosen as an indicator for entianin production in the various media.
FIG 4.
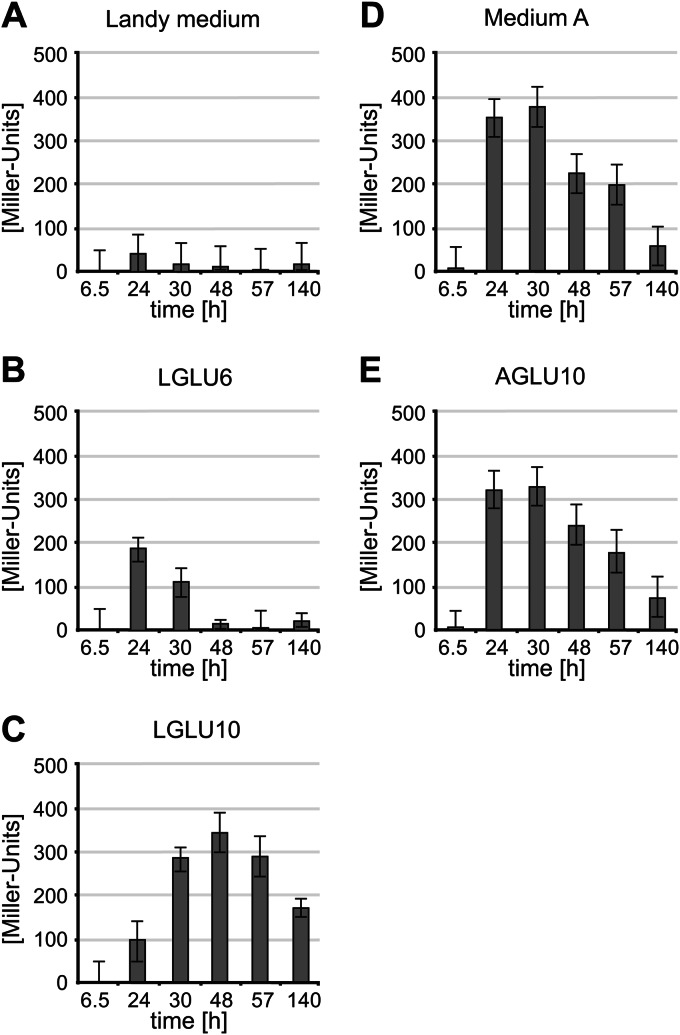
Kinetics of entianin/S-entianin synthesis and PetnI expression during growth in Landy medium and medium A. Shown are comparative RP-HPLC chromatograms of entianin-producing strain B15029.SB1, cultivated in Landy medium (A) or medium A (B). The y axes of all chromatograms display the same intercepts. Depending on the cultivation medium, drastic differences can be observed for the ratio between the highly antimicrobially active lantibiotic entianin (ε) and the N-terminally succinylated S-entianin (λ) with dramatically reduced antibiotic activity. (C) Growth of entianin-producing strain B15029.SB1 in medium A (dark gray) and in Landy medium (light gray). (D) Promoter activity (in Miller Units) of the immunity gene etnI fused with the β-galactosidase open reading frame in entianin-producing strain B15029.SB1 upon growth in Landy medium (light gray bars) and medium A (dark gray bars). Error bars represent the standard deviations between samples from two separate cultures (n = 2), with each measurement carried out in triplicate.
In Landy medium, cells reached the stationary phase earlier than when grown in medium A, and cell densities were 2-fold higher in medium A than in Landy medium (Fig. 4C). In Landy medium, the maximum PetnI expression was detected after 11 h, when cells had reached the early stationary growth phase. Thereafter, PetnI expression decreased continuously and was hardly detectable after 24 h (Fig. 4D). Remarkably, although the biosynthetic genes were expressed to a maximum after 11 h, detectable amounts of entianin first were observed after 16 h, reaching a maximum after 24 to 30 h. S-entianin synthesis was detected first after 24 h, reaching its maximum after 30 h. Our data show that in Landy medium the biosynthetic enzymes remain stable in the stationary growth phase, and that no additional expression of the entianin biosynthetic genes is needed for S-entianin synthesis. In medium A the situation was completely different. PetnI expression started slowly after 9 h during transition from the logarithmic to the stationary growth phase and reached a maximum after 30 h, when cells were in the late stationary growth phase (Fig. 4D). Entianin synthesis first was observed after 24 h and reached a maximum after 30 h (Fig. 4B). No succinylation was detectable during this time period, and minor amounts of S-entianin were observed first after 48 h.
Glucose concentration influences the expression of etn genes and succinylation.
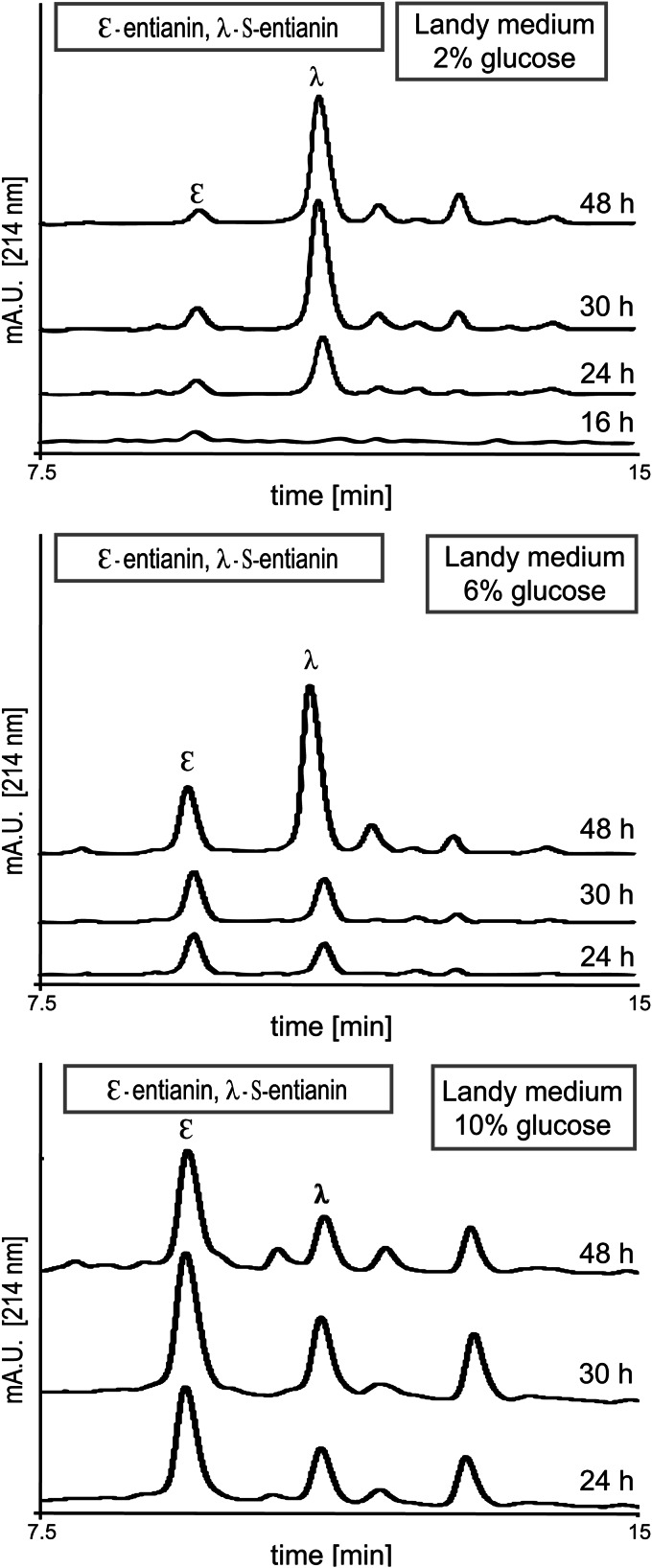
The two cultivation media, Landy and medium A, differ mainly in the carbon source supplied, which is 2% (wt/vol) glucose in Landy medium (24) and 10% (wt/vol) sucrose in medium A (25). This suggested that the sugar and its concentration have a major influence on succinylation. To investigate the role of the carbon sources, we supplemented Landy medium with increasing glucose concentrations (2% in standard Landy, 6% in LGLU6, and 10% in LGLU10), and we replaced the 10% sucrose in medium A with 10% glucose (AGLU10) (Table 2). During the investigation period of 48 h in Landy-based medium, growth was comparable (see Fig. S1 in the supplemental material). This clearly shows that the different sugar concentrations were neither growth limiting nor growth inhibiting. Interestingly, the amount of unsuccinylated entianin increased in correlation with the sugar concentration (Fig. 5).
TABLE 2.
Variations of the culture media Landy and medium A
| Medium | Basic componenta | Sugar source |
|---|---|---|
| Landy | Landy | 2% glucose |
| LGLU6 | Landy | 6% glucose |
| LGLU10 | Landy | 10% glucose |
| AGLU10 | Medium A | 10% glucose |
| Medium A | Medium A | 10% sucrose |
See Materials and Methods for details on the basic components.
FIG 5.
Comparative RP-HPLC chromatograms of supernatants from entianin-producing strain B15029.SB1 cultivated in Landy medium with 2% glucose (top), 6% glucose (middle), or 10% glucose (bottom). The y axes of all chromatograms display the same intercepts. Increasing sugar concentrations led to larger amounts of the N-terminally unmodified lantibiotic entianin (ε), in contrast to S-entianin (λ).
To calculate the amounts of entianin and S-entianin at different time points (Table 3), this experiment was repeated. The time point of sugar depletion was monitored for the three sugar concentrations provided. The amount of 2% glucose was exhausted after 16 h of incubation (early stationary phase), and the maximal amount of unsuccinylated entianin (6 to 7 mg/liter) was reached shortly after this time point (Table 3). Further cultivation increased the entire amount of entianin, reaching a maximum after 30 h; however, all of the additionally synthesized entianin was succinylated (85 mg/liter). At this time point, approximately 90% of entianin was succinylated. In Landy medium with 6% glucose (LGLU6), the carbon source was exhausted after 30 h. The amount of unsuccinylated entianin in the supernatant was 3-fold increased (20 mg/liter) compared to the amount of unsuccinylated entianin obtained with 2% glucose (7 mg/liter). The ratio for entianin versus S-entianin with 6% glucose was 1:1 after 30 h of growth. After glucose consumption the S-entianin proportion further increased, and 70% (64 mg/liter) of the entire amount of entianin (87 mg/liter) was succinylated after 48 h of incubation. In Landy medium with 10% glucose (LGLU10), the amount of unsuccinylated entianin increased even more. With 42 mg/liter entianin, a 2-fold larger amount was obtained upon cultivation in LGLU10 compared to LGLU6 (20 mg/liter). After 48 h the 10% glucose was consumed and a 3:1 ratio for entianin (45 mg/liter) versus S-entianin (18 mg/liter) was measured. Cultivation for more than 48 h did not result in additional entianin synthesis; however, the ratio between entianin and S-entianin slowly shifted toward the succinylated form (data not shown). Although the maximal entire entianin amount with 63 mg/liter in LGLU10 was lower than that of Landy and LGLU6, with approximately 90 mg/liter, the total amounts of unsuccinylated entianin in the supernatant increased 6-fold from 7 mg/liter in Landy to 42 mg/liter in LGLU10 (Table 3). This shows that the succinylation pattern depends on the glucose concentration, with a maximum of succinylation after glucose consumption.
TABLE 3.
Comparison of concentrations for entianin, S-entianin, and total entianin over time in different culture media
| Culture medium and duration (h) | Entianin (mg/liter) | S-entianin (mg/liter) | Entire entianina (mg/liter) |
|---|---|---|---|
| Landy | |||
| 24 | 6 | 52 | 58 |
| 30 | 7 | 85 | 92 |
| 48 | 6 | 80 | 86 |
| LGLU6 | |||
| 24 | 18 | 15 | 33 |
| 30 | 20 | 18 | 38 |
| 48 | 23 | 64 | 87 |
| LGLU10 | |||
| 24 | 39 | 18 | 57 |
| 30 | 42 | 20 | 62 |
| 48 | 45 | 18 | 63 |
| AGLU10 | |||
| 24 | 10 | BD | 10 |
| 30 | 23 | BD | 23 |
| 48 | 62 | 20 | 82 |
| Medium A | |||
| 24 | 10 | BD | 10 |
| 30 | 22 | BD | 22 |
| 48 | 50 | 9 | 59 |
Entire entianin corresponds to the sum of entianin and S-entianin concentrations in the supernatant. Maximal concentrations are indicated in boldface. The values are obtained from one representative experiment, which was repeated at least 3 times with standard deviations between 15 and 20%. BD, below the limit of detection.
With higher concentrations of glucose, the amount of unsuccinylated entianin significantly increased, and maximal entianin amounts were reached after 24 h to 30 h of growth in all three Landy-based media, with concentrations of 6 to 7 mg/liter in Landy with 2% glucose, 20 to 23 mg/liter in Landy with 6% glucose, and 42 to 45 mg/liter in Landy with 10% glucose. Depending on the consumption of glucose, the occurrence of maximum succinylation was shifted from the early stationary phase in Landy with 2% glucose to the late stationary phase in Landy with 10% glucose. This also coincided with the delayed expression of the biosynthetic etn genes, as shown for PetnI expression (Fig. 6A to C). Growth curves were largely the same (see Fig. S1 in the supplemental material), independent from the amount of glucose supplied, indicating that the expression of lantibiotic biosynthesis genes does not depend solely on the transition from logarithmic to stationary growth phase and that lantibiotic biosynthetic gene clusters are influenced by glucose availability. Remarkably, higher glucose concentrations resulted in an increased synthesis of unsuccinylated entianin and less S-entianin. The increase in S-entianin concentrations occurred mainly after glucose consumption, suggesting that succinylation is catalyzed by a glucose-regulated enzyme. The exchange of the 10% sucrose in medium A for 10% glucose (AGLU10) resulted in the comparable expression of the biosynthetic etn genes, as shown for PetnI expression (Fig. 6D and E). Entianin concentrations were almost the same after 30 h of growth (Table 3), whereas after 48 h the amounts of entianin and S-entianin slightly increased, reaching a maximum level of 82 mg/liter with 10% glucose compared to only 59 mg/liter with 10% sucrose. Considering the amount of cells/ml, which is twice as high in medium A and AGLU10 as it is in Landy-based media, the synthesis of entianin per cell number is most effective in LGLU10 medium. However, entianin concentrations in the supernatants are highest in medium A-based media.
FIG 6.
Promoter activity (PetnI) of entianin-producing strain B15029.SB1, cultivated in Landy medium with 2% glucose (A), Landy medium with 6% glucose (LGLU6) (B), Landy medium with 10% glucose (LGLU10) (C), medium A with 10% sucrose (D), or medium A with 10% glucose (E). Error bars represent the standard deviations between samples (n ≥ 2), with each measurement carried out in triplicate.
In summary, our results provide growth conditions for the specific isolation of either entianin or S-entianin, whereby AGLU10 and LGLU10 media are most suitable for entianin purification and standard Landy medium is the preferred source for S-entianin purification (Table 3).
Transition state regulation of entianin synthesis.
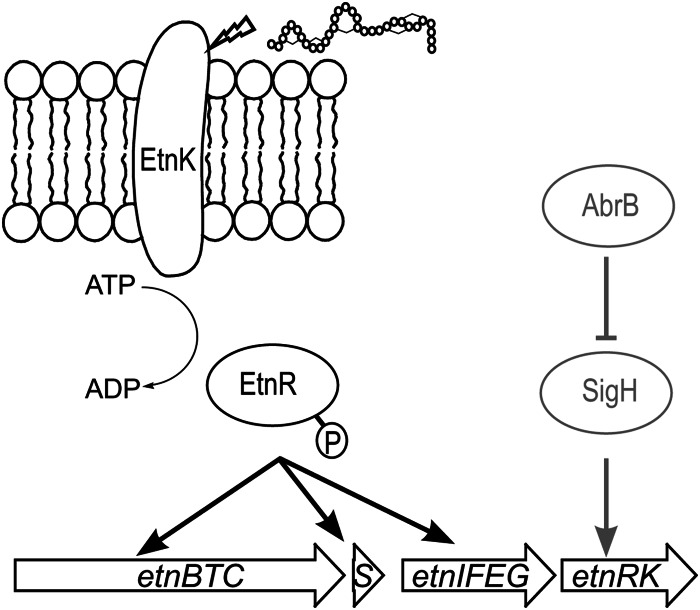
Our results presented here demonstrate that succinylation is influenced by the strain background and the amount of glucose supplied. Using 10% glucose, we could not only reduce the amount of succinylation but also significantly increase the amount of unsuccinylated entianin. As shown here, the transition from logarithmic to stationary growth was not decisive for the expression of entianin biosynthetic genes and entianin synthesis. We previously showed that the expression of subtilin-like lantibiotics is regulated by a dual-control mechanism (17) based on the expression of the two-component system LanRK and a quorum-sensing-like activation of LanRK by the lantibiotic itself. Transcription of LanRK is under the positive control of the alternative sigma factor H (encoded by sigH) (17), which is involved in the transcription of several genes during the transition from exponential to stationary growth phase (20, 27, 28). Sigma H in turn is under the negative control of AbrB (19, 21), a major transition state regulator in B. subtilis (29) (Fig. 7). Previously, we showed that the deletion of abrB in B6633 significantly increased the amount of subtilin produced in Landy medium (17), most of which turned out to be succinylated (18). Based on our findings, we questioned whether the transition state regulator AbrB still influences entianin synthesis if high glucose concentrations are supplied. Therefore, we compared entianin synthesis in an abrB deletion mutant upon growth in medium A (10% sucrose), AGLU10 (10% glucose), and standard Landy medium. As shown in Table 4, the abrB deletion had an additional significant impact on entianin production in all cultivation media. Interestingly, the ratios of S-entianin to entianin described above for abrB+ wild-type cells remained unchanged upon abrB deletion. After 30 h of growth in Landy medium, entianin and S-entianin concentrations increased approximately 2-fold upon abrB deletion. After 30 h of cultivation in medium A, entianin concentrations increased 5-fold in an abrB deletion mutant background, whereas the S-entianin concentration remained comparable in the range of the detection limit. A further increase of 25% was detected for entianin and S-entianin upon growth of an abrB deletion mutant in AGLU10 medium compared to that in medium A. Furthermore, due to the sigma H-driven constitutive expression of the EtnRK two-component regulatory system, entianin synthesis was detected very early, after only 10 h of incubation (Table 4). Remarkably, after 48 h of growth, we observed a strong degradation of entianin in the abrB mutant strain (data not shown), and a maximum yield of entianin was obtained after 30 h of growth.
FIG 7.
Biosynthetic regulation of subtilin-like lantibiotics (shown here for the entianin producer B15029). Until entering stationary growth phase, the transcriptional activation of etnRK by SigH is repressed by AbrB. When repression is reversed in stationary phase, EtnRK can accumulate and be activated by entianin with a quorum-sensing-like mechanism. EtnRK in turn activates the transcription of biosynthetic genes (etnBTC), immunity genes (etnIFEG), and the structural gene (etnS); therefore, it increases entianin production.
TABLE 4.
Amounts of entianin and S-entianin produced by the abrB deletion strain B15029.SWF14 and the B15029 (abrB+) strain in Landy medium, medium A, and AGLU10 at different time pointsa
| Time point | Landy |
Medium A |
AGLU10 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entianin (mg/liter) |
S-entianin (mg/liter) |
Entianin (mg/liter) |
S-entianin (mg/liter) |
Entianin (mg/liter) |
S-entianin (mg/liter) |
|||||||
| abrB mutant | abrB+ | abrB mutant | abrB+ | abrB mutant | abrB+ | abrB mutant | abrB+ | abrB mutant | abrB+ | abrB mutant | abrB+ | |
| 10 h | 8 | BD | 6 | BD | 1 | BD | BD | BD | 9 | BD | BD | BD |
| 20 h | 15 | 6 | 78 | 50 | 78 | 8 | 4 | BD | 100 | 9 | 6 | BD |
| 30 h | 17 | 7 | 156 | 85 | 110 | 22 | 4 | BD | 137 | 23 | 6 | BD |
Maximal concentrations are indicated in boldface. The values were obtained from one representative experiment, which was repeated at least 3 times, with standard deviations between 15 and 20%. BD, below the limit of detection.
DISCUSSION
Linear lantibiotics have a strong antimicrobial potential against Gram-positive bacteria and are of increasing importance for medical treatment and food preservation. Gram-positive producers of linear lantibiotics have established highly sophisticated regulation and immunity systems. In contrast to other linear lantibiotics, such as nisin, epidermin, and Pep5, a characteristic feature of subtilin-class lantibiotics (subtilin, ericin, and entianin) is their N-terminal succinylation (4, 12, 23). This posttranslational modification occurs extensively and strongly reduces antimicrobial activity (4, 23).
Growth conditions in general previously have been described to strongly influence bacteriocin production (30, 31). As shown here, the succinylation of subtilin-like lantibiotics depends not only on the strain background but also on the carbon source provided. In medium A, succinylation of entianin was strongly diminished in strain B15029 until the late stationary growth phase. This is in contrast to Landy medium, where up to 90% of the lantibiotics were succinylated almost concomitantly with entianin synthesis (reference 4 and this work). Cultivation under various conditions revealed that the amount of unsuccinylated entianin could be significantly increased in the presence of high glucose concentrations. More than that, succinylation mainly occurred after glucose consumption.
Until now, the biosynthesis of subtilin-like lantibiotics was considered to be exclusively dependent on the transition to the stationary phase due to its regulation by AbrB (17), a major transition state regulator in B. subtilis (29). However, the results presented in this work showed that the expression of the entianin biosynthetic genes was strongly retarded by increasing glucose concentrations, although the transition to the stationary phase was not affected. The respective maximal promoter activities (PetnI) were reached shortly before glucose consumption, and maximal entire entianin amounts were reached thereafter. These findings indicate that the regulatory effects mediated by the carbon source are independent from the transition state regulation of entianin biosynthesis.
Deletion of AbrB in B. subtilis previously has been shown to increase subtilin biosynthesis (17, 18). This could be verified for entianin biosynthesis. Interestingly, abrB deletion did not affect the ratios observed for entianin and S-entianin in the different cultivation media, although entianin biosynthesis was increased up to 6-fold. Consequently, the concentration of the carbon source rather than the transition state regulator AbrB seems to be important for succinylation.
N-terminal succinylation is highly specific for the N terminus of the peptide and needs to be distinguished from lysine succinylation, which is described for a broad range of organisms and occurs at the ε-amino group of the side chain of lysine (32). The mechanism of lysine succinylation and the succinyl donor in bacteria are not yet clear; however, in yeast the succinylation of lysine residues depends on succinyl-coenzyme A (CoA) originating from the tricarboxylic acid (TCA) cycle. In vitro experiments suggested that lysine succinylation occurs nonenzymatically and correlates with the succinyl-CoA concentration (33). For the N-terminal succinylation of subtilin-like lantibiotics, succinyl-CoA also might be the succinyl donor. However, because none of the three lysines within entianin or subtilin are succinylated, the underlying mechanism is clearly different. As succinylation occurs exclusively at the N-terminal tryptophan, this reaction must take place after the cleavage of the leader peptide. Leader peptide processing can occur prior to (34, 35), concomitantly with (36), or after secretion (37–39) of the precursor peptide. In most cases, this step is mediated by a specific protease (40). This is different for subtilin-like lantibiotics produced by various B. subtilis strains, where cleavage can be performed by one of at least three unspecific extracellular serine proteases (41). Accordingly, succinylation should occur in the extracellular space or attached to the cytoplasmic membrane, which argues against a nonenzymatic reaction. The observed strain- and glucose-dependent differences in the succinylation amount further support an enzymatically catalyzed reaction.
Succinylation provides a negative charge to the N terminus of subtilin-like lantibiotics, which could hinder their interaction with lipid II and support their release from the membrane. Charge changes within the bacterial cell wall or the cytoplasmic membrane have been described to be effective general resistance mechanisms against positively charged antimicrobial peptides (42–44). Similar to these modifications, succinylation could provide a further defense mechanism, independent from the lantibiotic immunity proteins LanI and LanFEG (4, 12, 15). In addition, succinylation drastically reduces the autoinduction capacity (4); therefore, it might prevent the overproduction of toxic lantibiotics.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by DFG (En 134/11-1).
We also acknowledge Goethe University and the state of Hesse for providing laboratory space, basic support, and the necessary equipment, as well as M. Karas for his support during MS analysis. We also thank S. Düsterhus for technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02579-14.
REFERENCES
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney Martin JT, Rebuffat S, Ross RP, Sahl H, Schmidt EW, et al. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep 30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnell N, Entian KD, Schneider U, Götz F, Zähner H, Kellner R, Jung G. 1988. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333:276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- 3.Piper C, Draper LA, Cotter PD, Ross RP, Hill C. 2009. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother 64:546–551. doi: 10.1093/jac/dkp221. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs SW, Jaskolla TW, Bochmann S, Kötter P, Wichelhaus T, Karas M, Stein T, Entian KD. 2011. Entianin, a novel subtilin-like lantibiotic from Bacillus subtilis subsp. spizizenii DSM 15029T with high antimicrobial activity. Appl Environ Microbiol 77:1698–1707. doi: 10.1128/AEM.01962-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers LA, Whittier EO. 1928. Limiting factors in the lactic fermentation. J Bacteriol 16:211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl H, de Kruijff B. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364. doi: 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- 7.Brötz H, Josten M, Wiedemann I, Schneider U, Götz F, Bierbaum G, Sahl HG. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol Microbiol 30:317–327. doi: 10.1046/j.1365-2958.1998.01065.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein C, Kaletta C, Schnell N, Entian KD. 1992. Analysis of genes involved in biosynthesis of the lantibiotic subtilin. Appl Environ Microbiol 58:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelke G, Gutowski-Eckel Z, Hammelmann M, Entian KD. 1992. Biosynthesis of the lantibiotic nisin: genomic organization and membrane localization of the NisB protein. Appl Environ Microbiol 58:3730–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiesau P, Eikmanns U, Gutowski-Eckel Z, Weber S, Hammelmann M, Entian KD. 1997. Evidence for a multimeric subtilin synthetase complex. J Bacteriol 179:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegers K, Heinzmann S, Entian KD. 1996. Biosynthesis of lantibiotic nisin. Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem 271:12294–12301. [DOI] [PubMed] [Google Scholar]
- 12.Stein T, Borchert S, Conrad B, Feesche J, Hofemeister B, Hofemeister J, Entian KD. 2002. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J Bacteriol 184:1703–1711. doi: 10.1128/JB.184.6.1703-1711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee S, Hansen JN. 1988. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem 263:9508–9514. [PubMed] [Google Scholar]
- 14.Gutowski-Eckel Z, Klein C, Siegers K, Bohm K, Hammelmann M, Entian KD. 1994. Growth phase-dependent regulation and membrane localization of SpaB, a protein involved in biosynthesis of the lantibiotic subtilin. Appl Environ Microbiol 60:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein C, Entian KD. 1994. Genes involved in self-protection against the lantibiotic subtilin produced by Bacillus subtilis ATCC 6633. Appl Environ Microbiol 60:2793–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein C, Kaletta C, Entian KD. 1993. Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl Environ Microbiol 59:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein T, Borchert S, Kiesau P, Heinzmann S, Kloss S, Klein C, Helfrich M, Entian KD. 2002. Dual control of subtilin biosynthesis and immunity in Bacillus subtilis. Mol Microbiol 44:403–416. doi: 10.1046/j.1365-2958.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 18.Heinzmann S, Entian KD, Stein T. 2006. Engineering Bacillus subtilis ATCC 6633 for improved production of the lantibiotic subtilin. Appl Microbiol Biotechnol 69:532–536. doi: 10.1007/s00253-005-0023-9. [DOI] [PubMed] [Google Scholar]
- 19.Strauch MA, Perego M, Burbulys D, Hoch JA. 1989. The transition state transcription regulator AbrB of Bacillus subtilis is autoregulated during vegetative growth. Mol Microbiol 3:1203–1209. doi: 10.1111/j.1365-2958.1989.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 20.Weir J, Predich M, Dubnau E, Nair G, Smith I. 1991. Regulation of spo0H, a gene coding for the Bacillus subtilis σH factor. J Bacteriol 173:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauch MA. 1995. Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promote. J Bacteriol 177:6999–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein T, Heinzmann S, Kiesau P, Himmel B, Entian KD. 2003. The spa-box for transcriptional activation of subtilin biosynthesis and immunity in Bacillus subtilis. Mol Microbiol 47:1627–1636. doi: 10.1046/j.1365-2958.2003.03374.x. [DOI] [PubMed] [Google Scholar]
- 23.Chan WC, Bycroft BW, Leyland ML, Lian LY, Roberts GC. 1993. A novel post-translational modification of the peptide antibiotic subtilin: isolation and characterization of a natural variant from Bacillus subtilis A.T.C.C. 6633. Biochem J 291(Part 1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landy M, Warren G, Rosenman S, Colio LG. 1948. Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:539–541. doi: 10.3181/00379727-67-16367. [DOI] [PubMed] [Google Scholar]
- 25.Feeney R, Garibaldi J, Humphreys EM. 1948. Nutritional studies on subtilin formation by Bacillus subtilis. Arch Biochem Biophys 17:435–445. [PubMed] [Google Scholar]
- 26.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 27.Carter HL, Moran CP. 1986. New RNA polymerase sigma factor under spo0 control in Bacillus subtilis. Proc Natl Acad Sci U S A 83:9438–9442. doi: 10.1073/pnas.83.24.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubnau E, Weir J, Nair G, Carter L III, Moran C Jr, Smith I. 1988. Bacillus sporulation gene spo0H codes for σ30 (σH). J Bacteriol 170:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA. 1989. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J 8:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aasen IM, Møretrø T, Katla T, Axelsson L, Storrø I. 2000. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol 53:159–166. doi: 10.1007/s002530050003. [DOI] [PubMed] [Google Scholar]
- 31.Cheigh C, Choi H, Park H, Kim S, Kook M, Kim T, Hwang J, Pyun Y. 2002. Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95:225–235. doi: 10.1016/S0168-1656(02)00010-X. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. 2011. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol 7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C. 2013. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Meyer C, Bierbaum G, Heidrich C, Reis M, Süling J, Iglesias-Wind MI, Kempter C, Molitor E, Sahl HG. 1995. Nucleotide sequence of the lantibiotic Pep5 biosynthetic gene cluster and functional analysis of PepP and PepC. Evidence for a role of PepC in thioether formation. Eur J Biochem 232:478–489. [DOI] [PubMed] [Google Scholar]
- 35.Weil HP, Beck-Sickinger AG, Metzger J, Stevanovic S, Jung G, Josten M, Sahl HG. 1990. Biosynthesis of the lantibiotic Pep5. Isolation and characterization of a prepeptide containing dehydroamino acids. Eur J Biochem 194:217–223. [DOI] [PubMed] [Google Scholar]
- 36.Håvarstein LS, Diep DB, Nes IF. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol 16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 37.van der Meer JR, Polman J, Beerthuyzen MM, Siezen RJ, Kuipers OP, De Vos WM. 1993. Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like serine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J Bacteriol 175:2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Meer JR, Rollema HS, Siezen RJ, Beerthuyzen MM, Kuipers OP, de Vos WM. 1994. Influence of amino acid substitutions in the nisin leader peptide on biosynthesis and secretion of nisin by Lactococcus lactis. J Biol Chem 269:3555–3562. [PubMed] [Google Scholar]
- 39.Geissler S, Götz F, Kupke T. 1996. Serine protease EpiP from Staphylococcus epidermidis catalyzes the processing of the epidermin precursor peptide. J Bacteriol 178:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bierbaum G, Sahl HG. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol 10:2–18. doi: 10.2174/138920109787048616. [DOI] [PubMed] [Google Scholar]
- 41.Corvey C, Stein T, Düsterhus S, Karas M, Entian K. 2003. Activation of subtilin precursors by Bacillus subtilis extracellular serine proteases subtilisin (AprE), WprA, and Vpr. Biochem Biophys Res Commun 304:48–54. doi: 10.1016/S0006-291X(03)00529-1. [DOI] [PubMed] [Google Scholar]
- 42.McBride SM, Sonenshein AL. 2011. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157:1457–1465. doi: 10.1099/mic.0.045997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst CM, Peschel A. 2011. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol Microbiol 80:290–299. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.