Abstract
Flavobacterium psychrophilum causes bacterial cold-water disease in multiple fish species, including salmonids. An autochthonous Enterobacter strain (C6-6) inhibits the in vitro growth of F. psychrophilum, and when ingested as a putative probiotic, it provides protection against injection challenge with F. psychrophilum in rainbow trout. In this study, low-molecular-mass (≤3 kDa) fractions from both Enterobacter C6-6 and Escherichia coli K-12 culture supernatants inhibited the growth of F. psychrophilum. The ≤3-kDa fraction from Enterobacter C6-6 was analyzed by SDS-PAGE, and subsequent tandem mass spectroscopy identified EcnB, which is a small membrane lipoprotein that is a putative pore-forming toxin. Agar plate diffusion assays demonstrated that ecnAB knockout strains of both Enterobacter C6-6 and E. coli K-12 no longer inhibited F. psychrophilum (P < 0.001), while ecnAB-complemented knockout strains recovered the inhibitory phenotype (P < 0.001). In fish experiments, the engineered strains (C6-6 ΔecnAB and C6-6 ΔecnAB<pET101::ecnAB>) and the wild-type strain (C6-6) were added to the fish diet every day for 38 days. On day 11, the fish were challenged by injection with a virulent strain of F. psychrophilum (CSF 259-93). Fish that were fed C6-6 had significantly longer survival than fish fed the ecnAB knockout strain (P < 0.0001), while fish fed the complemented knockout strain recovered the probiotic phenotype (P = 0.61). This entericidin is responsible for the probiotic activity of Enterobacter C6-6, and it may present new opportunities for therapeutic and prophylactic treatments against similarly susceptible pathogens.
INTRODUCTION
Probiotics are defined as live or dead microbial feed supplements that provide health benefits to the host animal (1). Probiotic strains of Bifidobacterium, Lactobacillus, and Streptococcus (2) have been used successfully in people and terrestrial animals, including poultry (3) and cattle (4), for the past century. Probiotic supplementation has gained attention from the aquaculture industry during the last 2 decades, especially for shrimp production, where antibiotic treatment has been reduced dramatically in favor of supplementing feed with commercially available probiotics composed of Bacillus subtilis and Vibrio alginolyticus (5, 6). In aquaculture, a probiotic is further defined as a live microbial cell that, when administered via the feed or to the rearing water, benefits the host by improving disease resistance, health and growth performance, feed utilization, stress response, and meat quality (7, 8). The mechanism of action of probiotics has been defined vaguely as improving the host's “microbial balance” (9) or the balance of the ambient environment, but molecular mechanisms are rarely investigated further, even though a number of applied studies have demonstrated probiotic efficacy against a variety of Gram-negative and Gram-positive pathogens in fish and shellfish (7).
In the case of salmonid species, such as Atlantic salmon (Salmo salar Linnaeus) and rainbow trout (Oncorhynchus mykiss Walbaum), for which global annual production exceeds 2.9 megatons and $13.7 billion (10), probiotics may have a significant economic benefit while reducing the therapeutic demand for antibiotics. Bacterial cold-water disease (BCWD) (also called rainbow trout fry syndrome [RTFS]), which is caused by Flavobacterium psychrophilum (11), is a significant challenge for salmonid aquaculture. The Gram-negative bacterium F. psychrophilum causes acute and commonly fatal septicemic infections in young salmonid fry (12, 13) and other fish species (14) and severe economic losses in older fish populations, due to reduction of product value, anatomical deformities, and high treatment costs (15–17). Currently, there is no commercial vaccine available to control BCWD (18). Improved rearing conditions (e.g., stress reduction, reduced stocking densities, cleaner raceways, adjusted feeding strategies, and improved disinfection protocols) and antimicrobials are the only practical control measures. In the United States, oxytetracycline and florfenicol are approved for treatment of BCWD, but drug resistance may be emerging, and concerns about the environmental impact of these treatments make it imperative to find new strategies (19–22).
A probiotic strain, Enterobacter sp. strain C6-6, was recently isolated from trout intestines and was shown to inhibit F. psychrophilum on agar diffusion plates (23) while reducing mortality during F. psychrophilum challenge studies (24). The agar diffusion data indicated that the cell-free supernatant of this probiotic strain could inhibit F. psychrophilum growth in vitro. A preliminary study showed that treating the supernatant with proteinase K resulted in a loss of inhibition (see Fig. S1 in the supplemental material). Consequently, we hypothesized that the probiotic Enterobacter strain C6-6 inhibits growth of F. psychrophilum in vitro and reduces mortality in vivo via the activity of a soluble bactericidal protein. The objective of this study was to identify the soluble inhibitor and to determine if it is required to protect fish from in vivo challenge with F. psychrophilum.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Enterobacter strain C6-6 was grown in tryptone yeast extract salts (TYES; 0.4% tryptone, 0.04% yeast extract, 0.05% MgSO4 · 7H2O, 0.05% CaCl2 · 2H2O, pH 7.2) broth (25) at 37°C with shaking for up to 48 h for inhibition experiments, on Luria-Bertani (LB) agar and in LB medium (Difco) at 37°C with shaking for maintenance and bacterial engineering, and in tryptic soy (TS) broth (Difco) at 15°C with shaking for preparation of cells that were used in the in vivo experiments. Supernatant of C6-6 was harvested by first pelleting cells from TYES culture (1,600 × g for 10 min at 15°C), and then the decanted supernatant was immediately filter sterilized (0.22-μm Millex-GP syringe filter units; EMD Millipore). Sterilized supernatant was used immediately in subsequent experiments. Escherichia coli ATCC 29947 (strain K-12) was made nalidixic acid resistant (Nalr) by serial passages on LB agar plates containing increasing concentrations of antibiotic until the strain was capable of growing in the presence of 30 μg/ml nalidixic acid (26). The K-12 strain was maintained and harvested using the same methods that were used to process Enterobacter C6-6. E. coli strain S17 was cultured in LB broth.
F. psychrophilum strains CSF 259-93 (27) and B17 (27) were grown in TYES at 15°C with shaking for at least 24 h or by culture for several days on TYES agar plates. Supernatant of CSF 259-93 was harvested by pelleting the cells at 1,600 × g for 10 min at 15°C and sterilizing them with 0.22-μm syringe filters or Anatop 25 syringe filter units (Whatman) with a 0.1-μm pore size. Harvesting of CSF 259-93 for the infection challenge followed the methods of Burbank et al. (24). CFU per ml were counted using the drop-plate method originally described by Chen et al. (28).
Size-exclusion experiments.
Supernatants of freshly harvested Enterobacter C6-6 and E. coli K-12 were size fractionated by use of 3-kDa centrifugal filtration units (Amicon) at 1,600 × g. The concentrated supernatant composed of the >3-kDa fraction was diluted back to the starting volume before use. Using a Bioscreen-C MBR instrument (Growth Curves USA) and 100-well honeycomb plates, triplicate wells were filled with 100 μl of the flowthrough (≤3 kDa) or the >3-kDa fraction and 100 μl of diluted CSF 259-93 culture and grown at 15°C for 5 days. The CSF 259-93 used for these experiments was grown in 1× TYES to turbidity for 3 to 5 days, and then a 1:1,000 dilution was made in 2× TYES. The Bioscreen-C MBR system measured the optical density at 600 nm every 3 h for 5 days after a brief period of shaking (5 s). Control wells contained 100 μl of CSF 259-93 grown with 100 μl of exhausted supernatant of CSF 259-93.
Protein separation and identification.
Enterobacter C6-6 and E. coli K-12 were cultured in 25-ml volumes for 24 to 48 h in TYES. The respective supernatants were harvested as described above and immediately frozen in liquid nitrogen and stored at −20°C. The frozen samples were then freeze-dried (Thermo Savant MicroModulyo) and eluted with 2 ml of phosphate-buffered saline (PBS). These >10-fold-concentrated supernatants were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (29). Resultant gels were stained using Sypro Ruby protein gel stain (Life Technologies), and the protein band of interest was excised (Enterobacter C6-6 sample only), digested with trypsin, and submitted for liquid chromatography-tandem mass spectrometry (LC-MS/MS) (University of Idaho Environmental Biotechnology Institute, Moscow, ID). Results were analyzed using a Mascot search (Matrix Science).
Generation of ecnAB deletion mutants.
Initial efforts to delete the ecnAB locus, using data from a previously sequenced strain of Enterobacter (NCBI accession no. NC_009436.1), apparently failed due to insufficient sequence identity with Enterobacter C6-6. To circumvent this issue, we sequenced strain C6-6 by using a MiSeq instrument (Illumina) to quickly identify the sequence flanking the ecnAB locus. We were not attempting to produce a closed and annotated sequence for the C6-6 genome. A genomic library was made using a TruSeq DNA LT sample prep kit (Illumina) per the manufacturer's instructions. Briefly, 1 μg of genomic DNA was sheared using a Covaris M220 focused ultrasonicator (peak incident power = 50 W; duty factor = 20%; cycle/burst [count] = 200; 65-s duration). The sheared DNA was then end repaired and adenylated, and Illumina adaptors containing bar-coded sequences were ligated to the fragments. After gel purification, a short 10-cycle PCR was performed to amplify the library. Finally, the purified library was analyzed using a model 2100 Bioanalyzer (Agilent Technologies) and quantified using a ddPCR library quantification kit (Bio-Rad) with a QX100 ddPCR system (Bio-Rad). The library was diluted to 2 nM, hydrolyzed in 0.2 M NaOH, and diluted to a flow cell loading concentration of 500 pM. The library was sequenced using an Illumina MiSeq benchtop sequencer with sequencing-by-synthesis technology per the manufacturer's protocol. The run was set for “Generate FASTQ only” workflow in Illumina Experiment Manager. A 600-cycle MiSeq v.3 reagent cartridge (Illumina) was used to sequence the library with a paired-end 2 × 301 sequencing run. Data were demultiplexed automatically on the MiSeq instrument and imported into CLC Genomics Workbench v. 6.5.1 (Qiagen). A de novo assembly was executed, and the resulting contigs were queried against BLASTn (http://www.ncbi.nlm.nih.gov/BLAST) to locate the ecnA and/or ecnB gene fragments.
An approximately 430-bp region encompassing the ecnAB locus was deleted from both the Enterobacter C6-6 and E. coli K-12 chromosomes, but using two different methods. The method of gene splicing by overlap extension (SOE) (30) was used to generate the Enterobacter C6-6 deletion, with minor modifications. Briefly, primers were designed to amplify approximately 200-bp regions flanking both sides of the ecnAB locus (Table 1) (primer pairs ecnAB_A1F/ecnAB_A1R and ecnAB_A2F/ecnAB_A2R). The resulting products were combined and used as the template in a second round of PCR, using only the ecnAB_A1F and ecnAB_A2R primers, to produce a spliced PCR product of approximately 400 bp. After size confirmation by gel electrophoresis, the PCR product was purified using a QIAquick PCR purification kit (Qiagen). E. coli S17 with plasmid pDM4 (31) was cultured overnight in LB with 34 μg/ml chloramphenicol, and plasmid DNA was isolated using a PureYield plasmid miniprep system (Promega) according to the manufacturer's recommendations. The pDM4 plasmid contains an R6K origin of replication that requires λpir to replicate. This allows development of the genetic construct by use of a λpir strain of E. coli while preventing replication in Enterobacter C6-6. As a consequence, only Enterobacter cells that undergo allelic exchange will be detected during the screening process. The purified PCR template and the suicide vector pDM4 were both digested using the restriction enzymes SacI and XhoI for 3 h at 37°C, purified again, quantified by using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific), and ligated using T4 DNA ligase (New England BioLabs). The ligated product was then transformed into electrocompetent E. coli S17 λpir (32) and selected on LB plates with chloramphenicol. Successful transformants were grown in LB broth with chloramphenicol overnight at 37°C, the plasmid was subsequently isolated, and the insert was verified using PCR and agarose gel electrophoresis (primer pairs pDM4-up/pDM4-down and ecnAB_A1F/ecnAB_A2R) (Table 1). The pDM4 plasmids with inserts of the correct length were sequenced (Eurofins Genomics) and confirmed using Sequencher software (version 4.10.1; Gene Codes Corporation). The plasmids were then transformed into electrocompetent Enterobacter C6-6, using 1,600 V, 25 μF, and 400 Ω, according to the method of Khampang et al. (33). Transformants were recovered on LB plates containing chloramphenicol (34 μg/ml) overnight. Individual colonies were then cultured in LB broth in the presence of 10% sucrose (wt/vol). Sucrose-resistant, chloramphenicol-sensitive colonies were evaluated by PCR for the loss of ecnAB by using primers ecnAB_AIF and ecnAB_A2R (Table 1), and selected clones were designated Enterobacter C6-6 ΔecnAB.
TABLE 1.
Primers used in this study

An E. coli K-12 ΔecnAB mutant was constructed by using site-directed gene deletion as described by Datsenko and Wanner (34) (this procedure did not work with Enterobacter C6-6). Briefly, primers were generated to amplify the chloramphenicol resistance cassette from pKD3 (34). These primers were designed with 5′ extensions that included 40 nucleotides complementary to the flanking regions of the K-12 ecnAB locus (primers K-12_ecnAB_KO_P1 and K-12_ecnAB_KO_P2) (Table 1). The resulting PCR product was column purified (Qiagen), digested with DpnI (New England BioLabs) for 2 h at 37°C, and purified again. E. coli K-12 containing plasmid pKD46 (34) was grown in super optimal broth (SOB; 2% Bacto tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl2, 10 mM MgCl2, 10 mM MgSO4) supplemented with 1 mM l-arabinose and 100 μg/ml ampicillin to an optical density at 600 nm of 0.6 to 0.8 with shaking at 30°C. The Red helper plasmid pKD46 (34) expresses the λ Red recombinase that facilitates allelic exchange. This plasmid can be cured at 37°C due to its temperature sensitivity. Electrocompetency was achieved by washing E. coli K-12<pKD46> twice in ice-cold distilled water and twice in 10% glycerol, with the final cell concentration increasing 100-fold. Electrocompetent cells (80 μl) were pulsed with approximately 150 ng of PCR product, using a Gene Pulsar 1 instrument (Bio-Rad), and immediately resuspended with SOC medium (35), followed by recovery on a shaker at 30°C for 1 h. To select for transformants, the cells were then incubated on LB plates with chloramphenicol at 30°C overnight. Successful replacement of the gene of interest with the chloramphenicol marker was confirmed by PCR amplification using primers for sequences within the chloramphenicol cassette and genomic primers that immediately flanked the gene of interest (primer pair ecnAFScreen/C1 and primer pair C2/ecnBRScreen) (Table 1) (34).
Complementation of ecnAB.
The Enterobacter C6-6 ecnAB locus and flanking sequence were amplified using Pfu Turbo Hotstart DNA polymerase (Agilent Technologies) and primers ecnAB-pET101-F and ecnAB-pET101-R (Table 1). The amplicon was immediately ligated into pET101/D-TOPO-vector (Invitrogen) and transferred into chemically competent TOP10 cells (Invitrogen). Selection was accomplished on LB agar (Difco) containing 50 μg/ml ampicillin (Sigma). Transformants were then cultured in LB broth supplemented with 100 μg/ml ampicillin overnight, and the plasmid was harvested using a PureYield plasmid miniprep system (Promega). The plasmid (100 to 300 ng) with the ecnAB locus was introduced into electrocompetent Enterobacter C6-6 ΔecnAB cells according to the method of Khampang et al. (33), using a Gene Pulsar 1 instrument (1,600 V, 25 mF, and 400 Ω; Bio-Rad). Cells were immediately resuspended with SOC medium (35) and incubated for 1 h at 37°C on a shaker. Finally, transformants were selected on LB plates supplemented with 100 μg/ml ampicillin overnight at 37°C. The complemented strain was designated Enterobacter C6-6 ΔecnAB<pET101::ecnAB>. This vector incorporates a C-terminal His tag into the recombinant protein. We confirmed that the presumptive EcnB protein was present by Western blotting with and without induction using IPTG (isopropyl-β-d-thiogalactopyranoside) (see Fig. S2 in the supplemental material).
An E. coli C6-6 ΔecnAB complement was created using a similar strategy. Briefly, the E. coli K-12 ecnAB locus was amplified using Platinum Taq Polymerase and the primer pair K12ecnABCompR/ecnAFScreen (Table 1). After ligating the resulting amplicon into the pCR2.1-TOPO-vector (Invitrogen), TOP10 cells (Invitrogen) were chemically transformed and selected on LB agar with 100 μg/ml ampicillin. Transformants were cultured in LB broth with 100 μg/ml ampicillin overnight, and the plasmid was isolated using a PureYield plasmid miniprep system (Promega). Approximately 100 ng of plasmid was introduced into chemically competent E. coli K-12 ΔecnAB cells by heat shock at 42°C for 30 s. Cells were immediately resuspended with SOC medium (35) and allowed to recover for 1 h at 37°C on a shaker. Finally, transformants were selected on LB plates supplemented with 34 μg/ml chloramphenicol and 100 μg/ml ampicillin. Successful complements were confirmed using the primer pairs T7F/M13R, ecnAFScreen/C1, and C2/ecnBRScreen (Table 1).
Inhibition plate assays.
Inhibition plate assays were used to detect the inhibitory phenotype of Enterobacter C6-6 against F. psychrophilum CSF259-93 strain B17 (36). Strain B17 was used because it does not exhibit a gliding motility phenotype that would otherwise complicate inhibition plate assays (our unpublished data). B17 was inoculated into 20 ml of TYES broth 5 days prior to the experimental setup, grown at 15°C on a shaker, and adjusted to an optical density at 600 nm of approximately 0.2 in a 50-ml conical tube. The culture (75 μl) was evenly spread onto a TYES plate by use of glass beads to grow a “lawn” of F. psychrophilum on the agar surface. Plates were then allowed to dry briefly at 15°C. Enterobacter and E. coli strains were inoculated into 20 ml of TYES broth 2 days prior at 37°C and pelleted at 1,600 × g for 10 min at 15°C. The pellet was resuspended in 5 ml of TYES. The TYES plates were divided in quarters, and 30 μl of each respective test strain as well as exhausted supernatant of F. psychrophilum B17 was deposited on the agar surface in triplicate locations. Plates were incubated at 15°C for 5 to 10 days, until an F. psychrophilum B17 lawn was visible. The plates were photographed and analyzed with the software ImageJ (http://imagej.nih.gov/ij/). Areas of clearance were normalized by dividing by their respective drop/colony areas, and the means and standard errors were calculated for 7 to 14 independent replicates.
Fish and feed preparation.
Rainbow trout fry were hatched at the Aquaculture Research Institute at the University of Idaho (Moscow, ID) and then transferred to the College of Natural Resources at the University of Idaho (n = 500; ∼4 g each). Upon arrival, five freshly euthanized fish (see below) were tested for bacterial contamination by probing liver, spleen, and kidney tissues with sterile inoculation loops and streaking samples onto TS agar (Difco) and TYES agar. Additionally, a section of hind gut was aseptically dissected and the intestinal mucus squeezed onto a TS agar plate and streaked across the plate surface. All cultures were incubated for 96 h at 15°C. Remaining fish were divided into five groups and acclimated in flowthrough tanks with 500 liters of dechlorinated municipal water (10 to 15°C) and aeration through air stones. Fish were fed a commercial salmonid diet (Bio-Olympic Fry, 1.5 mm; Bio-Oregon) at a rate of 3% of body weight per day for 1 week for acclimation. Fish were assigned to treatment groups (Table 2) and were fed the same commercial salmonid diet with or without the addition of 5% menhaden oil (Table 2). After injection challenge on day 11 (see below), fish were divided into triplicate groups of 25 and transferred to 20-liter flowthrough tank systems (3 separate tanks per treatment group) with dechlorinated municipal water (10 to 15°C) and supplemental aeration by air stones.
TABLE 2.
Treatment groups for the trout challenge trial
| Treatment groupa | Basal diet supplementb | Relative % survival (mean ± SEM)c |
|---|---|---|
| C6-6 wild type | Oil + Enterobacter strain C6-6 | 58.1 ± 5.59 |
| ecnAB knockout | Oil + Enterobacter C6-6 ΔecnAB | 19.4 ± 22.3 |
| ecnAB complement | Oil + Enterobacter C6-6 ΔecnAB<pET101::ecnAB> | 80.7 ± 9.68 |
| No-probiotic control | Oil only | Reference |
| Mock infection | Nothing added | 100 ± 0 |
Fish were fed the treatment diet for 10 days before experimental subcutaneous challenge with Flavobacterium psychrophilum strain CSF 259-93 (1.03 × 107 CFU/fish). Treatment groups were fed the assigned diets and were then divided into three independent replicate tanks at the time of injection challenge with CSF 259-93. The mock infection group was treated with TYES medium. Tanks were subsequently monitored for an additional 28 days.
The basal diet consisted of an amount equivalent to 3% of body weight of Bio-Olympic Fry (1.5-mm pellet size; Bio-Oregon) with 5% (wt/vol) menhaden oil.
Relative percent survival (RPS) was calculated relative to the survival of the no-probiotic control group (see Materials and Methods for the formula). Numbers in bold represent groups with statistically significant differences from the no-probiotic control group (P <0.001).
Feed with the probiotic strains was prepared according to the method of Burbank et al. (24), with minor modifications. Briefly, the quantity of feed (g) was calculated daily, and rations were prepared fresh every day in 50-ml conical tubes. One colony of each strain (Enterobacter C6-6, Enterobacter C6-6 ΔecnAB, or Enterobacter C6-6 ΔecnAB<pET101::ecnAB>) was inoculated into 15 ml of TS broth without antibiotics and cultured on a shaker for 24 h at 15°C. The cells were pelleted at 1,600 × g for 10 min and resuspended in 7.5 ml menhaden oil. A 5% menhaden oil-cell suspension (vol/wt) was mixed into the feed (except for the mock infection control), resulting in 106 to 108 bacterial cells per g as determined by the drop-plate method (28). Feeding started 10 days before infection and ended at 28 days postinfection.
Infection challenge.
CSF 259-93 was grown in TYES broth at 15°C for 72 h before infection and harvested by centrifugation at 1,600 × g for 15 min at 15°C. The supernatant was discarded, and the pellet was resuspended in 1× TYES to an optical density at 525 nm of 0.2. The number of CFU per ml was determined using the drop-plate method (28). Fish were withheld from feed for 24 h and then injected subcutaneously with 25 μl of the virulent strain CSF 259-93 (1.03 × 107 CFU/fish) or with 25 μl 1× TYES (mock-infected groups). Feeding was resumed 24 h after infection. Daily mortalities were recorded, and at least 25% of dead fish per tank were evaluated for signs of BCWD and isolation of yellow-pigmented bacteria by culturing of the liver, spleen, and kidneys on TYES agar at 15°C for at least 96 h. The relative percent survival (RPS) was calculated according to the method of LaFrentz et al. (37), using the following formula: RPS = [1 − (cumulative percent mortality of treatment group/cumulative percent mortality of positive-control group)] × 100. Animal challenge experiments conformed to a protocol approved by the University of Idaho Institutional Animal Care and Use Committee.
Statistical analysis.
SigmaPlot (v.11.0; Systat Software) was used for all statistical analysis. One-way analysis of variance (ANOVA) and Tukey's multiple-comparison test were used to evaluate results from the size-exclusion and inhibition plate assays. Kaplan-Meier curves were plotted for the survival analysis, based on pooled data across replicate treatment tanks (n = 3 per treatment), and the statistical differences were assessed by employing a log rank test and Tukey's multiple-comparison test. Because each treatment tank served as an independent replicate, we also compared relative percentages of survival between treatments by using ANOVA and Tukey's multiple-comparison test. The threshold for significance was a P value of <0.05.
Nucleotide sequence accession number.
The contig identified to contain ecnA and ecnB was annotated and submitted to GenBank under accession no. KM407562.
RESULTS
The inhibitory protein has a mass of ≤3 kDa.
A preliminary proteinase digest indicated that inhibition was likely caused by a soluble protein (see Fig. S1 in the supplemental material). Under the assumption that this putative protein would have a low mass, we fractionated supernatants from Enterobacter C6-6 and E. coli K-12 (≤3 kDa and >3 kDa). These fractions, unfractionated supernatants, and a control that was composed of exhausted supernatant from CSF 259-93 culture were incubated with diluted CSF 259-93. The unfractionated supernatants of Enterobacter C6-6 and the ≤3-kDa fractions inhibited the growth of CSF 259-93 (P < 0.001) (Fig. 1), while the larger fraction from Enterobacter C6-6 (>3 kDa) did not have a significant effect on CSF 259-93 culture (P > 0.05). Unexpectedly, the E. coli K-12 strain produced an identical phenotype, where unfractionated medium and the ≤3-kDa fraction inhibited growth of CSF 259-93 (P < 0.001) (Fig. 1), while the larger fraction (>3 kDa) did not affect growth of F. psychrophilum (P > 0.05).
FIG 1.
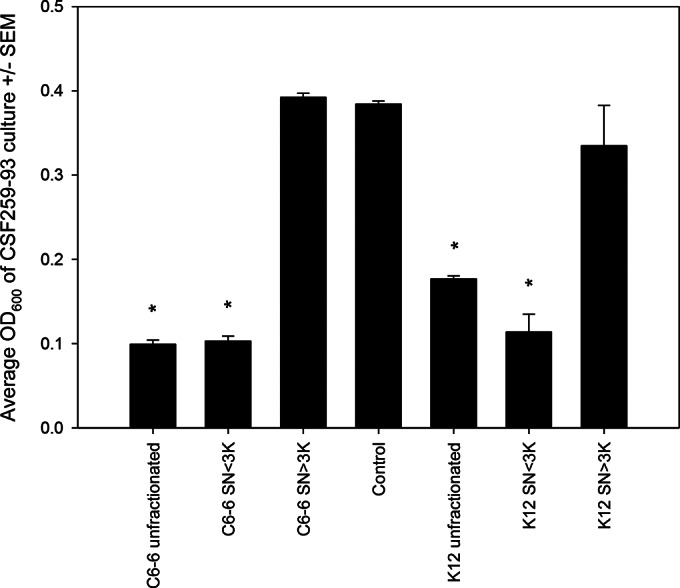
Flavobacterium psychrophilum (CSF 259-93) was cultured in the presence of supernatant from Enterobacter (C6-6) or Escherichia coli (K-12). Unfiltered supernatant and the ≤3-kDa fraction from both species inhibited growth of F. psychrophilum, whereas treatment with the higher-mass fraction (>3 kDa) was indistinguishable from treatment with spent medium from F. psychrophilum (Control). Bars represent means plus standard errors of the means (SEM). Asterisks represent differences from the control (P < 0.05; one-way ANOVA and Tukey's multiple-comparison test).
LC-MS/MS identified entericidin as a candidate protein.
Supernatants of Enterobacter C6-6 and E. coli K-12 were concentrated by freeze-drying and separated using SDS-PAGE (Fig. 2). For both supernatants, a dense band was present in the mass range of approximately 3 kDa. The Enterobacter C6-6 band was excised and submitted to liquid chromatography and tandem mass spectrometry. This analysis identified three hypothetical proteins (Ent638_0594, Ent638_3274, and Ent638_3824), an lpp repeat-containing protein, and an entericidin B membrane lipoprotein. The latter protein has been implicated in cell lysis (38), and consequently, we focused our subsequent analysis on this protein.
FIG 2.
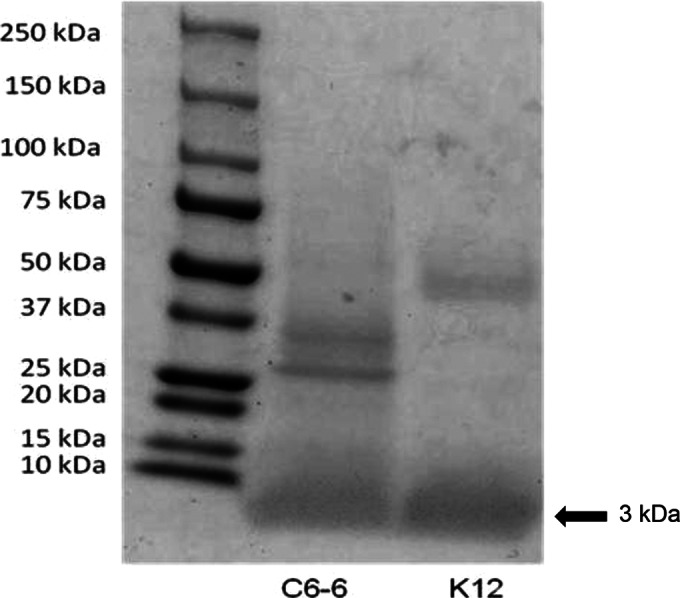
SDS-PAGE (10%) confirmed the presence of proteins of approximately 3 kDa (arrow) from concentrated supernatants that were collected from Enterobacter C6-6 and E. coli K-12. The C6-6 gel band was excised and submitted for analysis by LC-MS/MS.
ecnAB is required for Enterobacter C6-6 to inhibit F. psychrophilum in vitro.
ecnAB knockout and complement strains were engineered to demonstrate the role of the ecnAB locus in inhibiting F. psychrophilum. For these experiments, results from CSF 259-93 were difficult to analyze due to the swarming nature of the strain. Consequently, we report data only for strain B17, because it is deficient in gliding motility. Agar inhibition assays clearly demonstrated that the wild-type Enterobacter C6-6, wild-type E. coli K-12, and complemented ecnAB knockout strains (Enterobacter C6-6 ΔecnAB<pET101::ecnAB> and E. coli K-12 ΔecnAB<pCR2.1::ecnAB>) produced readily detectable areas of clearance against F. psychrophilum strain B17 (P < 0.001) (Fig. 3). No significant zone of clearance was detectable for the ecnAB knockout strains (Enterobacter C6-6 ΔecnAB and E. coli K-12 ΔecnAB).
FIG 3.
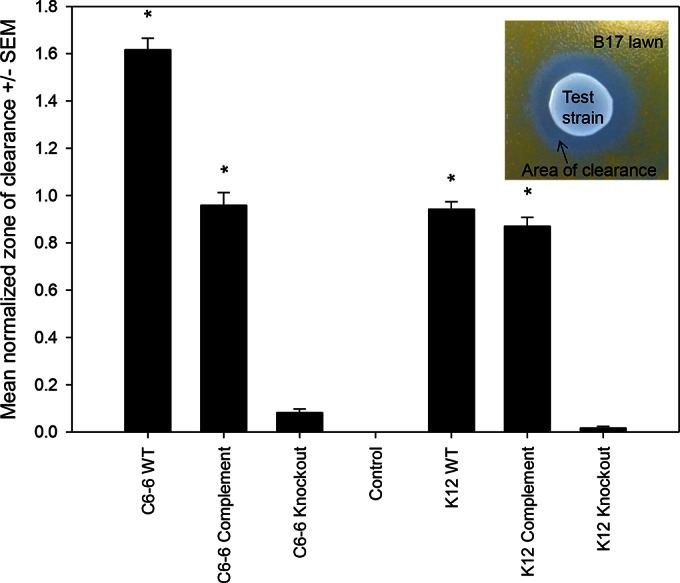
Deletion of the ecnAB locus results in loss of the inhibitory phenotype against F. psychrophilum strain B17, based on an agar inhibition assay. Areas of clearance were measured as total pixels of clearance divided by the total area of the test strain (center of the inset). Mean values were calculated for 7 to 14 independent replicates, and error bars represent the standard errors of the means. C6-6 WT, Enterobacter C6-6; C6-6 Complement, Enterobacter C6-6 ΔecnAB<pET101::ecnAB>; C6-6 Knockout, Enterobacter C6-6 ΔecnAB; Control, exhausted supernatant from F. psychrophilum B17 culture; K-12 WT, E. coli K-12; K-12 Complement, E. coli K-12 ΔecnAB<pCR2.1::ecnAB>; K-12 Knockout, E. coli K-12 ΔecnAB. Statistical differences were assessed using one-way ANOVA and Tukey's multiple-comparison test. Asterisks represent differences from the control (P < 0.001).
ecnAB is required for Enterobacter C6-6 to protect trout fry from BCWD challenge.
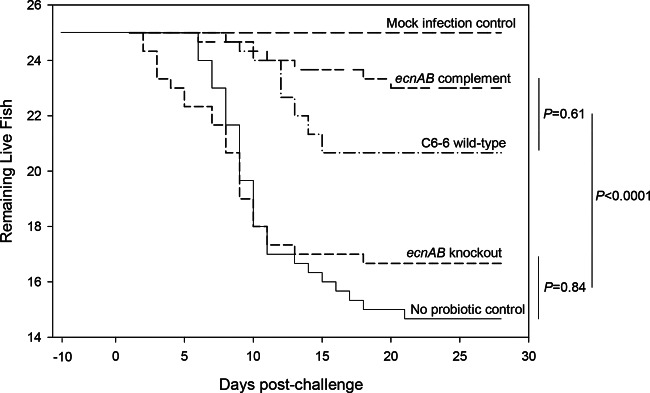
A health check at the onset of the fish trial found no evidence of either Enterobacter or F. psychrophilum from the intestinal contents or liver, spleen, and kidney tissues of selected fish. Three independent groups of trout fry were fed one of several treatment diets (Table 2). As expected, fish that were fed the wild-type Enterobacter C6-6 strain and then challenged with CSF 259-93 experienced fewer mortalities than those of the no-probiotic control group (P < 0.001) (Fig. 4). Consistent with our hypothesis that ecnAB is requisite for the probiotic activity of Enterobacter C6-6, the mortality of fish treated with the ecnAB knockout strain (Enterobacter C6-6 ΔecnAB) was not different from that of the no-probiotic control group (P = 0.84), while the group treated with the complemented strain (Enterobacter C6-6 ΔecnAB<pET101::ecnAB>) was not different from the group treated with wild-type Enterobacter C6-6 (P = 0.61). The RPS values for the groups fed Enterobacter C6-6 and Enterobacter C6-6 ΔecnAB<pET101::ecnAB> were 58.1% and 80.7%, respectively, while the group fed Enterobacter C6-6 ΔecnAB had a 19.4% relative percent survival (Table 2). The mock infection control group experienced no mortalities during the experimental period.
FIG 4.
Deletion of ecnAB eliminates the probiotic benefit of Enterobacter C6-6. Four groups of fish (25 fish per group in triplicate) were fed a standard trout diet with feed supplemented with menhaden oil mixed with “ecnAB complement” (Enterobacter C6-6 ΔecnAB<pET101::ecnAB>), “C6-6 wild-type” (Enterobacter C6-6), “ecnAB knockout” (Enterobacter C6-6 ΔecnAB), or “No probiotic ctrl” (oil only; no added Enterobacter). After 10 days of supplemented feeding, these groups were challenged by subcutaneous injection with a virulent strain of F. psychrophilum (CSF 259-93). One group of fish (25 fish in triplicate) was fed the standard diet without any supplementation and served as a mock infection control. Daily mortalities were recorded for 28 days after infection. For this analysis, data were pooled across replicate treatment tanks (n = 3 per treatment), and statistical analysis was performed using the log rank test.
DISCUSSION
Burbank et al. (23) first showed that Enterobacter C6-6 culture or spent medium inhibited growth of F. psychrophilum in a manner consistent with the presence of a soluble inhibitor. Herein we successfully identified a small protein (entericidin B) in the supernatant of Enterobacter C6-6 cultures that is linked to the inhibitory phenotype in vitro. Burbank et al. (24) showed that when Enterobacter C6-6 was used as an oral probiotic treatment, there was a significant reduction in mortality after injection challenge with the virulent F. psychrophilum strain CSF 259-93. At the time of this work, it was unclear if the putative soluble inhibitor protein found by Burbank et al. (23) was responsible for this protection or if protection was conferred by an alternative mechanism, such as cross-reactive immunity, that is consistent with recent findings (39) (see discussion below). We demonstrated that the soluble protein produced by the ecnAB locus is required for inhibition of F. psychrophilum both in vitro and in vivo. We did not determine if the protein encoded by the ecnAB locus is actively secreted or if this is a passive release mechanism after normal bacterial cell death.
The ecnAB locus encodes two small peptides, designated EcnA and EcnB (44 and 48 amino acids, respectively) (38). ecnA is located upstream from ecnB, with 106 intervening bases that include a putative translation terminator and promoter region. The locus was first described by Bishop et al. (38) as a putative toxin-antitoxin system in E. coli because of hypothesized similarities to a plasmid addiction module, mazEF, that has been studied in E. coli (40). EcnA was designated the antitoxin that neutralizes the cell toxin EcnB based on the observation that overproduction of EcnB resulted in a greater degree of bacteriolysis, while expression of ecnA and ecnB in cis produced only minor bacteriolytic activity. Bishop et al. (38) further suggested that ecnB expression is promoted by a stationary-phase sigma promoter that is active only under hyperosmotic and starvation culture conditions, and consequently this putative toxin-antitoxin system could serve a role akin to programmed cell death. This is a novel but controversial idea because, for instance, deletion of the putative ecnA antitoxin gene alone should be lethal, but this is not the case for the E. coli Keio collection (41).
If the protein produced by the ecnAB locus is secreted and bacteriolytic as we hypothesize, then it clearly has a much broader spectrum of activity than that of a classic bacteriocin (42). For example, sequence homologues of ecnB have been annotated in the chromosomes of diverse species of proteobacteria, indicating that this trait is highly conserved and may play a largely unappreciated role in structuring microbial communities (see Fig. S4 in the supplemental material). As an example of this potential, we originally intended E. coli K-12 to serve as a negative-control strain for our phenotypic assays, but we instead discovered that K-12 also inhibits growth of F. psychrophilum, and we confirmed the role of the E. coli ecnAB locus by using a gene knockout and complementation strategy. Additionally, we also used our inhibition assay to confirm that Enterobacter C6-6 inhibits growth of Flavobacterium columnare (see Fig. S3), the etiologic agent of columnaris disease, which is a devastating disease of cold- and warm-water fish species (43).
While a secreted or passively released toxin could easily explain the in vitro activity against F. psychrophilum, the mechanism by which the toxin functions in vivo is not clear. Our in vitro data suggest that a direct interaction is required for the entericidin to inhibit F. psychrophilum, but this presents a conundrum for our experimental model, whereby the majority of the Enterobacter C6-6 bacteria reside in the gut, while the pathogen challenge occurs through subcutaneous injection of F. psychrophilum. Enterobacter species will colonize the skin of fish (44), possibly via fecal contamination of the water, and subsequently may interact directly with the pathogen at the site of the injection. Our lab experience suggests that inhibition is quickly diluted, and fish skin generally supports low population densities of bacteria (44), so it is unclear if bacteria on the skin surface can play a role in inhibition following injection challenge. It is possible that large quantities of the toxin are released systemically by the gastric Enterobacter C6-6 flora, but our in vitro experiments showed no evidence that large quantities of toxin are produced.
Another scenario might involve coresidency in leukocytes. Professional phagocytic cells in fish, especially those from the spleen, which have less production of reactive oxygen species (ROS), are probably responsible for disseminating F. psychrophilum in vivo (45). If these cells also harbor Enterobacter C6-6, then the proximal condition could be satisfied. Alternatively, de Vries et al. (46) described the ecnAB locus as being essential for Moraxella catarrhalis to adhere to the upper and lower respiratory tracts of humans. Consequently, it is possible that these membrane lipoproteins serve a dual role as lytic peptides and adhesion molecules. If these proteins are freely available in vivo, they may directly affect F. psychrophilum while competing for adhesion sites within the fish host. Testing for the distribution of the toxin (e.g., by Western blotting) in different host tissues might resolve this question.
Indirect mechanisms may also be involved with in vivo protection. For example, as has been speculated for other probiotics (47), it is possible that the entericidin enhances barrier functions and the innate immune response. In this scenario, the entericidin could elicit release of antimicrobial compounds into the mucus or induce production of a cross-reactive IgM that helps to protect against F. psychrophilum in mucus and blood (48). LaPatra et al. (39) recently demonstrated that intraperitoneal injection of live and formalin-killed Enterobacter C6-6 produced a protective immune response against challenge with F. psychrophilum CSF 259-93, while treatment with culture supernatant failed to induce a significant increase in the titer of antibodies against F. psychrophilum.
Regardless of the mechanism, it is clear from the current study that the entericidin locus is requisite for the probiotic activity of Enterobacter C6-6, but further work is needed to understand the mechanism of action in vivo. Given the apparent conservation of entericidin, it is conceivable that it plays a large and unappreciated role in structuring microbial communities, and this is potentially the case for mammalian microbiota as well. The small size of these peptides (∼48 amino acids for EcnB) also means that it may be possible to efficiently produce large quantities for use in different microbiological and industrial applications.
Supplementary Material
ACKNOWLEDGMENTS
Jim Deringer from Washington State University provided early technical assistance with this work.
This work was supported in part by the Western Regional Aquaculture Center, the Idaho State Board of Education, the Department of Veterinary Microbiology and Pathology, the Washington State University College of Veterinary Medicine Agricultural Animal Health Program, and the Washington Agricultural Research Center.
Kenneth D. Cain has applied for intellectual property protection for the probiotic strain C6-6. Ponnerassery S. Sudheesh works as a postdoctoral fellow in the Cain lab. None of the remaining authors have a commercial affiliation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02965-14.
REFERENCES
- 1.Fuller R. 1987. Probiotics—their basis and their benefits. J Appl Bacteriol 63:R11–R12. [Google Scholar]
- 2.Smoragiewicz W, Bielecka M, Babuchowski A, Boutard A, Dubeau H. 1993. Les probiotiques. Can J Microbiol 39:1089–1095. doi: 10.1139/m93-165. [DOI] [Google Scholar]
- 3.Fulton RM, Nersessian BN, Reed WM. 2002. Prevention of Salmonella enteritidis infection in commercial ducklings by oral chicken egg-derived antibody alone or in combination with probiotics. Poultry Sci 81:34–40. doi: 10.1093/ps/81.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Khuntia A, Chaudhary LC. 2002. Performance of male crossbred calves as influenced by substitution of grain by wheat bran and the addition of lactic acid bacteria to diet. Asian-Australas J Anim Sci 15:188–194. [Google Scholar]
- 5.Brunt J, Austin B. 2005. Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 28:693–701. doi: 10.1111/j.1365-2761.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Balcázar JL, Rojas-Luna T, Cunningham DP. 2007. Effect of the addition of four potential probiotic strains on the survival of Pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96:147–150. doi: 10.1016/j.jip.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bogwald J, Castex M, Ringø E. 2010. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18. doi: 10.1016/j.aquaculture.2010.02.007. [DOI] [Google Scholar]
- 8.Gómez GD, Balcázar JL. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154. doi: 10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuller R. 1989. Probiotics in man and animals. J Appl Bacteriol 66:365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 10.Statistics and Information Branch of the Fisheries and Aquaculture Department, FAO. 2014. FAO yearbook. Fishery and aquaculture statistics, 2012. FAO, Rome, Italy: http://www.fao.org/3/478cfa2b-90f0-4902-a836-94a5dddd6730/i3740t.pdf. [Google Scholar]
- 11.Michel C, Antonio D, Hedrick RP. 1999. Production of viable cultures of Flavobacterium psychrophilum: approach and control. Res Microbiol 150:351–358. doi: 10.1016/S0923-2508(99)80061-8. [DOI] [PubMed] [Google Scholar]
- 12.Rucker RR, Earp BJ, Ordal EJ. 1953. Infectious diseases of Pacific salmon. Trans Am Fish Soc 12:209–210. [Google Scholar]
- 13.Wood EM, Yasutake TW. 1956. Histopathology of fish. III. Peduncle (“cold-water”) disease. Prog Fish Cult 18:58–61. doi: 10.1577/1548-8659(1956)18[58:HOF]2.0.CO;2. [DOI] [Google Scholar]
- 14.Lehmann J, Mock D, Stürenberg FJ, Bernardet JF. 1991. First isolation of Cytophaga psychrophila from a systemic disease in eel and cyprinids. Dis Aquat Organ 10:217–220. doi: 10.3354/dao010217. [DOI] [Google Scholar]
- 15.Lorenzen E, Dalsgaard A, From J, Hansen FM, Horlyck V, Korsholm H, Mellergaard S, Olesen NJ. 1991. Preliminary investigations of fry mortality syndrome in rainbow trout. Bull Eur Assoc Fish Pathol 11:77–79. [Google Scholar]
- 16.Rangdale RE. 1999. Rainbow trout fry syndrome. Bull Eur Assoc Fish Pathol 19:295. [Google Scholar]
- 17.Rangdale RE, Richards RH, Alderman DJ. 1999. Histopathological and electron microscopical observations on rainbow trout fry syndrome. Vet Rec 144:251–254. doi: 10.1136/vr.144.10.251. [DOI] [PubMed] [Google Scholar]
- 18.Gómez E, Mendez J, Cascales D, Guijarro JA. 2014. Flavobacterium psychrophilum vaccine development: a difficult task. Microb Biotechnol 7:414–423. doi: 10.1111/1751-7915.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruun MS, Schmidt AS, Dalsgaard I, Larsen JL. 2003. Conjugal transfer of large plasmids conferring oxytetracycline (OTC) resistance: transfer between environmental aeromonads, fish-pathogenic bacteria, and Escherichia coli. J Aquat Anim Health 15:69–79. doi:. [DOI] [Google Scholar]
- 20.Bruun MS, Schmidt AS, Madsen L, Dalsgaard I. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201–212. doi: 10.1016/S0044-8486(00)00310-0. [DOI] [Google Scholar]
- 21.Del Cerro A, Marquez I, Prieto JM. 2010. Genetic diversity and antimicrobial resistance of Flavobacterium psychrophilum isolated from cultured rainbow trout, Onchorynchus mykiss (Walbaum), in Spain. J Fish Dis 33:285–291. doi: 10.1111/j.1365-2761.2009.01120.x. [DOI] [PubMed] [Google Scholar]
- 22.Gordon L, Giraud E, Ganière JP, Armand F, Bouju-Albert A, de la Cotte N, Mangion C, Le Bris H. 2007. Antimicrobial resistance survey in a river receiving effluents from freshwater fish farms. J Appl Microbiol 102:1167–1176. doi: 10.1111/j.1365-2672.2006.03138.x. [DOI] [PubMed] [Google Scholar]
- 23.Burbank DR, LaPatra SE, Fornshell G, Cain KD. 2012. Isolation of bacterial probiotic candidates from the gastrointestinal tract of rainbow trout, Oncorhynchus mykiss (Walbaum), and screening for inhibitory activity against Flavobacterium psychrophilum. J Fish Dis 35:809–816. doi: 10.1111/j.1365-2761.2012.01432.x. [DOI] [PubMed] [Google Scholar]
- 24.Burbank DR, Shah DH, LaPatra SE, Fornshell G, Cain KD. 2011. Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus mykiss). Aquaculture 321:185–190. doi: 10.1016/j.aquaculture.2011.09.004. [DOI] [Google Scholar]
- 25.Holt RA, Rohovec JS, Fryer JL. 1993. Bacterial coldwater disease, p 3–22. In Inglis V, Roberts RJ, Bromage NR (ed), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, United Kingdom. [Google Scholar]
- 26.Eberhart LJ, Deringer JR, Brayton KA, Sawant AA, Besser TE, Call DR. 2012. Characterization of a novel microcin that kills enterohemorrhagic Escherichia coli O157:H7 and O26. Appl Environ Microbiol 78:6592–6599. doi: 10.1128/AEM.01067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudheesh PS, LaFrentz BR, Call DR, Siems WF, LaPatra SE, Wiens GD, Cain KD. 2007. Identification of potential vaccine target antigens by immunoproteomic analysis of a virulent and a non-virulent strain of the fish pathogen Flavobacterium psychrophilum. Dis Aquat Organ 74:37–47. doi: 10.3354/dao074037. [DOI] [PubMed] [Google Scholar]
- 28.Chen CY, Nace GW, Irwin PL. 2003. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J Microbiol Methods 55:475–479. doi: 10.1016/S0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli UK, Favre M. 1973. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol 80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 30.Horton RM, Cai ZL, Ho SN, Pease LR. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535. [PubMed] [Google Scholar]
- 31.Miller VL, Mekalanos JJ. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 33.Khampang P, Chungjatupornchai W, Luxananil P, Panyim S. 1999. Efficient expression of mosquito-larvicidal proteins in a Gram-negative bacterium capable of recolonization in the guts of Anopheles dirus larva. Appl Microbiol Biotechnol 51:79–84. doi: 10.1007/s002530051366. [DOI] [PubMed] [Google Scholar]
- 34.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 36.LaFrentz BR, LaPatra SE, Call DR, Cain KD. 2008. Isolation of rifampicin resistant Flavobacterium psychrophilum strains and their potential as live attenuated vaccine candidates. Vaccine 26:5582–5589. doi: 10.1016/j.vaccine.2008.07.083. [DOI] [PubMed] [Google Scholar]
- 37.LaFrentz BR, LaPatra SE, Jones GR, Cain KD. 2004. Protective immunity in rainbow trout Oncorhynchus mykiss following immunization with distinct molecular mass fractions isolated from Flavobacterium psychrophilum. Dis Aquat Organ 59:17–26. doi: 10.3354/dao059017. [DOI] [PubMed] [Google Scholar]
- 38.Bishop RE, Leskiw BK, Hodges RS, Kay CM, Weiner JH. 1998. The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J Mol Biol 280:583–596. doi: 10.1006/jmbi.1998.1894. [DOI] [PubMed] [Google Scholar]
- 39.LaPatra SE, Fehringer TR, Cain KD. 2014. A probiotic Enterobacter sp. provides significant protection against Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) after injection by two different routes. Aquaculture 433:361–366. doi: 10.1016/j.aquaculture.2014.06.022. [DOI] [Google Scholar]
- 40.Aizenman E, Engelberg-Kulka H, Glaser G. 1996. An Escherichia coli chromosomal “addiction module” regulated by 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A 93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 43.Fish FF, Rucker RR. 1945. Columnaris as a disease of cold-water fishes. Trans Am Fish Soc 73:32–36. doi: 10.1577/1548-8659(1943)73[32:CAADOC]2.0.CO;2. [DOI] [Google Scholar]
- 44.Austin B. 2006. The bacterial microflora of fish, revised. Sci World J 6:931–945. doi: 10.1100/tsw.2006.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nematollahi A, Pasmans F, Haesebrouck F, Decostere A. 2005. Early interactions of Flavobacterium psychrophilum with macrophages of rainbow trout Oncorhynchus mykiss. Dis Aquat Organ 64:23–28. doi: 10.3354/dao064023. [DOI] [PubMed] [Google Scholar]
- 46.de Vries SPW, Eleveld MJ, Hermans PWM, Bootsma HJ. 2013. Characterization of the molecular interplay between Moraxella catarrhalis and human respiratory tract epithelial cells. PLoS One 8:e72193. doi: 10.1371/journal.pone.0072193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. 2009. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 48.Nayak SK. 2010. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.