Abstract
Mycoplasmas of the Mycoplasma mycoides cluster are all ruminant pathogens. Mycoplasma mycoides subsp. mycoides is responsible for contagious bovine pleuropneumonia and is known to produce capsular polysaccharide (CPS) and exopolysaccharide (EPS). Previous studies have strongly suggested a role for Mycoplasma mycoides subsp. mycoides polysaccharides in pathogenicity. Mycoplasma mycoides subsp. mycoides-secreted EPS was recently characterized as a β(1→6)-galactofuranose homopolymer (galactan) identical to the capsular product. Here, we extended the characterization of secreted polysaccharides to all other members of the M. mycoides cluster: M. capricolum subsp. capripneumoniae, M. capricolum subsp. capricolum, M. leachii, and M. mycoides subsp. capri (including the LC and Capri serovars). Extracted EPS was characterized by nuclear magnetic resonance, resulting in the identification of a homopolymer of β(1→2)-glucopyranose (glucan) in M. capricolum subsp. capripneumoniae and M. leachii. Monoclonal antibodies specific for this glucan and for the Mycoplasma mycoides subsp. mycoides-secreted galactan were used to detect the two polysaccharides. While M. mycoides subsp. capri strains of serovar LC produced only capsular galactan, no polysaccharide could be detected in strains of serovar Capri. All strains of M. capricolum subsp. capripneumoniae and M. leachii produced glucan CPS and EPS, whereas glucan production and localization varied among M. capricolum subsp. capricolum strains. Genes associated with polysaccharide synthesis and forming a biosynthetic pathway were predicted in all cluster members. These genes were organized in clusters within two loci representing genetic variability hot spots. Phylogenetic analysis showed that some of these genes, notably galE and glf, were acquired via horizontal gene transfer. These findings call for a reassessment of the specificity of the serological tests based on mycoplasma polysaccharides.
INTRODUCTION
Within the class Mollicutes, the so-called Mycoplasma mycoides cluster (MMC) (1) is unique: all the members of the MMC are pathogenic for ruminants, yet from a phylogenetic point of view, this cluster belongs to the Spiroplasma clade, which includes a number of species isolated from plants or insects (2). The taxonomy of the MMC was modified recently to reflect more precisely the phylogeny of this group. Because all the cluster members are closely related, their 16S rRNA gene sequences did not provide sufficient resolution to discriminate them accurately (3). A multilocus sequence typing approach was used to obtain a more precise phylogeny (4), which led to a simplification of the taxonomy (5) (Table 1). The cluster currently comprises five species or subspecies: Mycoplasma mycoides subsp. mycoides, which was known formerly as the “small colony” (SC) biotype (1), M. mycoides subsp. capri, M. capricolum subsp. capricolum, M. capricolum subsp. capripneumoniae, and M. leachii. In addition, M. mycoides subsp. capri is subdivided in two serovars: the “large colony” serovar (M. mycoides subsp. capri serovar LC) and the Capri serovar (M. mycoides subsp. capri serovar Capri). In the MMC, two subspecies are particularly important, M. capricolum subsp. capripneumoniae and Mycoplasma mycoides subsp. mycoides, which are, respectively, the etiologic agents of contagious caprine pleuropneumonia (CCPP) and contagious bovine pleuropneumonia (CBPP). These two diseases are characterized by unilateral pleuropneumonia in goats and cattle, respectively. Because they are absent or have been eradicated from many regions or continents, they are notifiable to the World Organisation for Animal Health (OIE), and their detection leads to a ban on live-animal trade. By comparison, the other members of the MMC have a worldwide distribution and induce lesions affecting various organs: mastitis, arthritis, keratitis, pneumonia, septicemia, etc.
TABLE 1.
Taxonomy of the Mycoplasma mycoides cluster
| Taxonomic grouping1987 taxonomya | Mycoides subcluster |
Capricolum subcluster |
||||
|---|---|---|---|---|---|---|
| Mycoplasma mycoides subsp. capri | Mycoplasma mycoides subsp. mycoides LC | Mycoplasma mycoides subsp. mycoides SC | Mycoplasma sp. group 7 | Mycoplasma capricolum subsp. capricolum | Mycoplasma capricolum subsp. capripneumoniae | |
| Current taxonomyb | Mycoplasma mycoides subsp. capri | Mycoplasma mycoides subsp. mycoides | M. leachii | Mycoplasma capricolum subsp. capricolum | Mycoplasma capricolum subsp. capripneumoniae | |
| Serovar | Capri | LC | ||||
| Type/reference strain | PG3 | YG | PG1 | PG50 | C.kid | F38 |
Due to the importance of CBPP for international trade, many studies have been performed to try to unravel the virulence factors of Mycoplasma mycoides subsp. mycoides. The release of the whole-genome sequence of Mycoplasma mycoides subsp. mycoides in 2004 (6) did not allow for the identification of any known virulence factors such as genes coding for toxins, adhesins, or secretion systems described in other pathogenic bacteria. Putative phase variation of surface lipoproteins was identified (7) and might play a role in escaping the host immune response, but this phenomenon was certainly not as pronounced in Mycoplasma mycoides subsp. mycoides as in other mycoplasmas (8). Finally, the close association to host cells and the release of H2O2 and other reactive oxygen species through glycerol metabolism were proposed as a virulence mechanism (9) in a way similar to what had been shown to occur in M. pneumoniae (10). However, some Mycoplasma mycoides subsp. mycoides vaccine strains, such as T1/44, KH3J, and V5, possess the ability to metabolize glycerol and produce significant quantities of H2O2 while being attenuated (11). Hence, H2O2 production alone is not the sole virulence mechanism and other factors may be important. One that certainly deserves attention is the synthesis and secretion of polysaccharides. We refer here to polysaccharides that are found in tight association with the mycoplasma membrane, forming a capsule known as capsular polysaccharide (CPS), whereas free, secreted polysaccharides or those only loosely adherent to the membrane are referred to as exopolysaccharide (EPS). This polysaccharide secretion was documented long ago, and EPS was detected as a soluble product in the blood of Mycoplasma mycoides subsp. mycoides-infected cattle at an early stage of the infection with simple techniques such as Ouchterlony immunoprecipitation in agar (12). Polysaccharides were soon regarded as virulence candidates for Mycoplasma mycoides subsp. mycoides, which might be involved notably in adhesion, resistance to desiccation, resistance to complement activity, or direct stimulation of inflammation. As a consequence, immunological reactions to intravenous or subcutaneous inoculations were studied (13) and many efforts were made to characterize these products. The composition of Mycoplasma mycoides subsp. mycoides CPS was determined from concentrated washed mycoplasmas (14), but the characterization of the EPS was more problematic. The main difficulty resulted from the use of complex medium for the growth of Mycoplasma mycoides subsp. mycoides, which hampered the extraction of pure EPS devoid of medium contaminants. More recently, a procedure that included the incubation of washed mycoplasma cells into a defined medium, devoid of extraneous polysaccharide components, was a key factor for the characterization of Mycoplasma mycoides subsp. mycoides EPS (15). Results showed notably that the composition of this EPS matched exactly that of the CPS (i.e., a galactofuranose homopolymer) and that the alternate secretion of CPS or EPS was correlated with the variation of expression of an active glucose permease belonging to a phosphotransferase system (PTS-G). Owing to the importance of the MMC members as pathogens for small and large ruminants worldwide, as well as the possible use of the polysaccharides as diagnostic or vaccine antigens, we decided to characterize these secreted polysaccharides in all mycoplasmas of the MMC. In addition, the recent publication of at least one full genome for each of the MMC members opened new avenues for comparative and evolutionary genomic studies. We searched for genes that could be part of the metabolic pathways for CPS or EPS production to understand why all these species differ in terms of polysaccharide production and secretion while being so closely related from a phylogenetic point of view.
MATERIALS AND METHODS
Mycoplasma strains and culture conditions.
Mycoplasma strains used in this study are listed in Table 2. They were selected to reflect the broad geographical distribution of the species. For biochemical analyses, mycoplasma strains with sequenced and annotated genomes and, whenever possible, few in vitro passages, were selected. Mycoplasmas were cultured in PPLO medium according to the method of Hayflick with a few modifications (PPLO agar base [Difco, 21 g/liter], enriched with fresh yeast extract [10%], horse serum decomplemented for 1 h at 56°C [15%], glucose [1 g/liter], and sodium pyruvate [2 g/liter]) at 37°C, 5% CO2.
TABLE 2.
List of Mycoplasma strains or genome sequences studied
| Species | Strain designationa | Country | Supplier/referenceb | Genome accession no. |
|---|---|---|---|---|
| M. capricolum subsp. capricolum | California kidT (C.kid) | USA | 50 | NC_007633.1 |
| (Mcc) 7714 | France | CIRAD | ||
| 13051 (14232) | France | ANSES-Lyon | ||
| 10074 | Tajikistan | SVIS | ||
| 2007-046 | France | ANSES-Niort | ||
| IPX | France | CIRAD | ||
| 2002-053 (VP28L) | India | IVRI | ||
| 90122 (C1547) | Ivory Coast | LPA | ||
| M. capricolum subsp. capripneumoniae | 9231-Abomsa | Ethiopia | NVI-E | LM995445 |
| 95043 | Niger | CIRAD | ||
| M1601 | China | 51 | NZ_CM001150.1 | |
| 99108 | Eritrea | SVS | JMJI01000000 | |
| M. leachii | PG50T | Australia | 52 | NC_014751.1 |
| 06049-C3 | Nigeria | NVRI-N | ||
| M. mycoides subsp. capri serovar Capri | PG3T | Turkey | 53 | JFAE00000000 |
| 2003-045 | India | CIRG | ||
| 9139 | Turkey | NVI-ET | ||
| N108 | Nigeria | CIRAD | ||
| L | France | CIRAD | ||
| M. mycoides subsp. capri serovar LC | Y-goatR (YG) | Australia | 54 | |
| 95010-C1 | France | CIRAD | NC_015431.1 | |
| Kombolcha | Ethiopia | NVI-ET | ||
| 99045 (55507-1) | Germany | ITiHo | ||
| 99111 | India | IVRI | ||
| 2007-073 | France | ANSES-Niort | ||
| GM12 | USA | NZ_CP001621.1 | ||
| M. mycoides subsp. mycoides | Afadé | Cameroon | CIRAD | |
| 8740-Rita | Cameroon | LANAVET | ||
| PG1T | Not known | NC_005364.2 | ||
| Gladysdale | Australia | NC_021025.1 |
Strains that are underlined were used for EPS extraction.
Abbreviations (name of supplier): ANSES, Agence Nationale de Sécurité Sanitaire, Lyon, France (F. Poumarat), and Niort, France (P. Mercier); CIRAD, Centre de Coopération Internationale en Recherche Agronomique pour le Développement, Montpellier, France (F. Thiaucourt); CIRG, Central Institute for Research in Goats, Mathura, India (R. Rana); IVRI, Indian Veterinary Institute, Izatnagar, India (V. P. Singh); LANAVET, Laboratoire National Vétérinaire, Garoua, Cameroon (A. Yaya); LPA, Laboratoire Central de Pathologie Animale, Bingerville, Ivory Coast (J. Domenech); NVI-ET, National Veterinary Institute, Debre-Zeit, Ethiopia (Y. Laikemariam); NVRI-N, National Veterinary Research Institute, Vom, Nigeria (N. Nwankpa); SVIS, State Veterinary Inspection Services, Dushanbe, Tajikistan (M. Amirbekov); SVS, Senhit Veterinary Service, Asmara, Eritrea (T. Teklegiorgis); TiHo, Tierärztliche Hochschule, Hannover, Germany (R. Schmidt).
EPS production and extraction.
EPS were produced and extracted as previously described (15). Briefly, mycoplasmas were harvested in the late exponential phase of growth (14,000 × g, 30 min, 4°C) and washed once in phosphate-buffered saline (PBS) before being resuspended in CMRL-1066 medium. After 3 days of incubation at 37°C, mycoplasmas were pelleted by centrifugation at 14,000 × g for 1 h at 4°C. Then, trichloroacetic acid (TCA) was added to the supernatant (10%, wt/vol), and the mixture was incubated at 4°C for 2 h. The proteinaceous precipitate was removed after centrifugation (14,000 × g, 1 h, 4°C). Nine volumes of cold acetone was added to the final supernatant, and the mixture was incubated at least 24 h at −20°C to allow precipitation of polysaccharides. After centrifugation (14,000 × g, 1 h, 4°C) and drying, the EPS pellets were dissolved in ultrapure sterile water.
Chemical analyses.
The monosaccharide components were determined by high-performance anion-exchange chromatography (HPAEC) with a pulsed amperometric detector (ICS 3000 system; Dionex) and polysaccharide structure by nuclear magnetic resonance (NMR) as described previously (15).
Briefly, for HPAEC analysis, the EPS were hydrolyzed, and anionic compounds were separated through CarboPac PA1 columns with a multistep gradient elution procedure. Peak analysis was performed using Chromeleon software, version 7.0.
For the NMR analysis, the 1H NMR spectra were recorded, at 80°C, on a Bruker Avance 500 spectrometer equipped with a 5-mm broadband inverse (BBI) probe and Topspin 1.3 software. 1H NMR spectra were accumulated using a 30° pulse angle, a recycle time of 1 s, and an acquisition time of 2 s for a spectral width of 3,000 Hz for 32,000 data points with a presaturation of the HOD signal using a presaturation sequence provided by Bruker. 13C NMR experiments were conducted on the same spectrometer, operating at 125.48 MHz with 2 s as relaxation delay.
The two-dimensional (2D) 1H/1H correlation spectroscopy (COSY), 1H/1H total correlation spectroscopy (TOCSY), 1H/1H nuclear Overhauser effect spectroscopy (NOESY), 1H/13C heteronuclear single quantum coherence (HSQC) spectroscopy, and 1H/13C heteronuclear multiple-bond correlation (HMBC) spectra were acquired with standard pulse sequences delivered by Bruker.
MAb production and characterization.
BALB/c mice were immunized twice intraperitoneally with Mycoplasma mycoides subsp. mycoides EPS extracts mixed with incomplete Freund adjuvant and once intravenously with EPS (50 μg) suspended in water. Three days later, dilacerated spleen cells and SP2/O cells were fused according to standard procedures (16). The protocol (ref. CIRAD-12CBP001) was approved by the regional ethical committee. The hybridoma culture supernatants were screened using Mycoplasma mycoides subsp. mycoides EPS extract as a positive control and a sham extract from noninoculated PPLO medium as negative-control antigens. The reactivity of the monoclonal antibodies (MAbs) was tested according to the method of Harlow and Lane (16). Briefly, these EPS extracts were spotted on nitrocellulose, incubated 30 min with the supernatants, washed, and incubated with anti-mouse horseradish peroxidase (HRP)-conjugated antibody (P0260; Dako). After the final washing, the spots were revealed with diaminobenzidine (DAB)-nickel chloride-H2O2 substrate. Another hybridoma cell line, “4.83,” kept frozen in liquid nitrogen, was retrieved from a previous fusion for the production of M. capricolum subsp. capripneumoniae-recognizing MAbs (17). It was selected because it recognized a soluble product with a distinct smear in dot blot experiments.
The reactivity of the selected MAbs was monitored by Western blotting using antigens harvested after the incubation into CMRL-1066 medium: concentrated and washed mycoplasma pellets, crude CMRL-1066 supernatant, and concentrated EPS. These samples were analyzed on denaturing polyacrylamide gel gradient 4 to 15% (456-1083EDU; Bio-Rad) in 1× SDS-Tris-glycine running buffer (161-0732; Bio-Rad). After electrophoresis, protein and polysaccharide detection was performed with Coomassie brilliant blue and periodic acid-Schiff (PAS) methods, respectively. Immunodetection by Western blotting was performed after transfer to nitrocellulose membranes at 10 V during 1 h and 15 min. Membranes were probed with MAbs 4.83 and 2.1.31, followed by horseradish peroxidase-conjugated secondary antibodies and detection with DAB-nickel-H2O2 substrate. Dot blotting was used to detect the polysaccharides in various mycoplasma cultures. A concentrated and PBS-washed mycoplasma pellet was used to detect CPS, and culture supernatant was used as antigen to detect EPS. Dot blotting was performed as described for Western blotting except that samples (1 to 5 μl) were directly spotted onto nitrocellulose.
In silico analyses.
Genes potentially involved in polysaccharide biosynthetic pathways in MMC members were identified and retrieved by a BLASTP similarity search (18) using the MolliGen database (19) (http://molligen.org) with genes already identified within Mycoplasma mycoides subsp. mycoides genomes (15) and an E value threshold of 10−8. Additional genes were identified by queries on annotated genes within the MolliGen database or through the Carbohydrate-Active enZymes database (CAZy; http://www.cazy.org/). The evolutionary history of biosynthetic pathways in individual subspecies was inferred from phylogenetic analyses. Protein sequences were retrieved and aligned by MUSCLE (20) using the MolliGen database. Protein phylogenies were inferred by the maximum likelihood method in the MEGA6 molecular evolutionary genetic tool package (21). The best-fitting model of evolution was evaluated with ProtTest (22) for each of the alignments and the “LG with frequencies (+F)” model was finally chosen. All positions containing gaps and missing data were eliminated (complete deletion option), and bootstrap analysis was carried out with 500 replicates. When supported by significant bootstrap values, incongruence between protein and species phylogenies was considered indicative of a potential horizontal gene transfer (HGT). A tree with the housekeeping gene fusA was built as a reference tree reflecting the species phylogeny.
RESULTS
Chemical characterization of EPS from Mycoplasma mycoides cluster members by HPAEC and NMR.
EPS was extracted from six mycoplasma cultures representing the MMC as described in Materials and Methods. Despite several attempts, no detectable amount of EPS was obtained with strains 95010 (M. mycoides subsp. capri serovar LC), PG3T (M. mycoides subsp. capri serovar Capri), and C.kidT (M. capricolum subsp. capricolum). In contrast, EPS were obtained with strains 8740-Rita (Mycoplasma mycoides subsp. mycoides), 95043 (M. capricolum subsp. capripneumoniae), and PG50T (M. leachii), and they were subjected to total acid hydrolysis with 4 M trifluoroacetic acid. Sugar analysis revealed the presence of galactose as the only sugar for Mycoplasma mycoides subsp. mycoides strain 8740-Rita, as previously described for Mycoplasma mycoides subsp. mycoides strain Afadé. The 1H NMR and 2D NMR spectra of EPS for Mycoplasma mycoides subsp. mycoides 8740-Rita were identical to those of Mycoplasma mycoides subsp. mycoides strain Afadé (data not shown) and characteristic of β(1→6)-galactofuranose polymers.
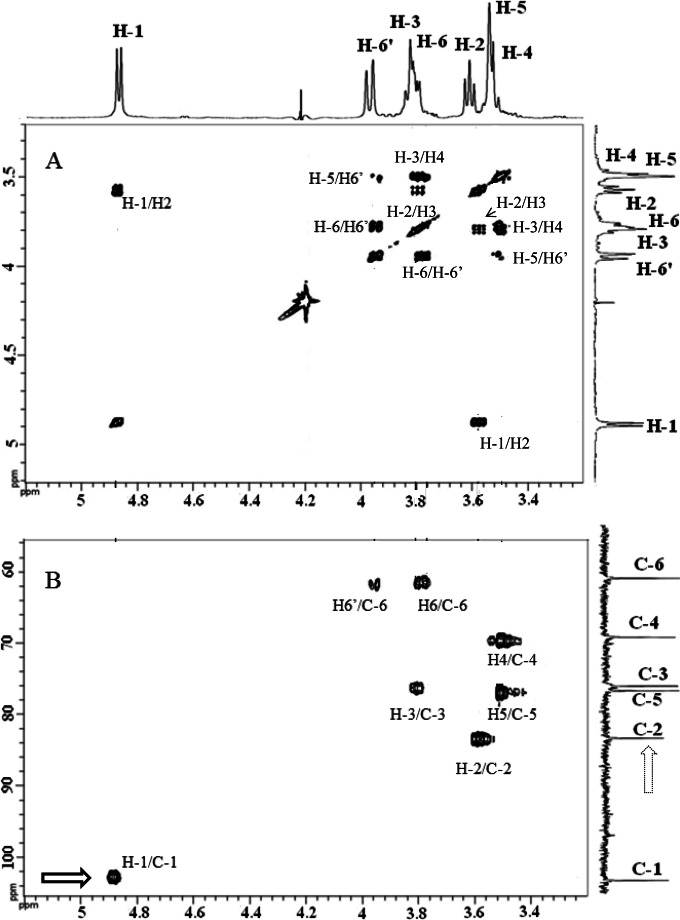
The acid hydrolysate and 1H NMR spectra of EPS from M. capricolum subsp. capripneumoniae 95043 and M. leachii PG50T showed that these polysaccharides were composed only by glucose. Based on correlations observed in 2D NMR COSY spectrum (Fig. 1A), various chemical shifts to proton of glucosyl residues could be attributed: 4.873 (H-1), 3.575 (H-2), 3.793 (H-3), 3.477 (H-4), 3.503 (H-5), 3.774 (H-6), and 3.946 (H-6′) ppm. On the 2D NMR HSQC spectrum (Fig. 1B), the connectivities observed between H-1 and C-1 (103.26 ppm), H-2/C-2 (83.91 ppm), H-3/C-3 (76.82 ppm), H-4/C-4 (70.17 ppm), H-5/C-5 (77.47 ppm), and H-6, H-6′/C-6 (62.17 ppm) were characteristic of a glucan polymer. The H-1 signal at 4.873 ppm (1,2J = 7.83 Hz) and that of C-1 at 103.26 ppm were typical of d-glucose residues with β-pyranoside configuration. H-2 chemical values indicated the presence of a linkage on the C-2 in glucose residues. This linkage was confirmed by the presence of connectivities observed in 1H/1H NOESY between H-1 of glucose (δ 4.873 ppm) and signal at δ 3.575 ppm belonging to H-2 of glucose. The connectivities were also observed in the 1H/13C HMBC spectrum. These results showed that EPS of M. capricolum subsp. capripneumoniae 95043 and M. leachii PG50T were identical and composed of a β(1→2)-glucopyranose polymer. Only one signal for C-2 (83.91 ppm) was present in the 13C spectrum. This was an indication that the polymer was linear, as a cyclic β(1→2)-glucan polymer would have yielded multiple signals (23).
FIG 1.
2D 1H/1H COSY NMR spectrum (A) and 2D 1H/13C HSQC NMR spectrum (B) of EPS produced by M. leachii strain PG50T. The H-1 signal at 4.873 ppm (1,2J = 7.83 Hz) and that of C-1 at 103.26 ppm were typical of d-glucose residues with β-pyranoside configuration (dark arrow). The single signal for C-2 (83.91 ppm) present in the 13C spectrum (light arrow) was indicative of a linear polymer. This spectrum is typical of a linear β(1→2)-glucopyranose polymer. Identical spectra were obtained with M. capricolum subsp. capripneumoniae EPS.
In summary, EPS was successfully extracted and characterized in 3 of 6 strains from the MMC. EPS from 8740-Rita (Mycoplasma mycoides subsp. mycoides) was made of β(1→6)-galactofuranose polymer (galactan), whereas EPS from 95043 (M. capricolum subsp. capripneumoniae) and PG50T (M. leachii) was made of β(1→2)-glucopyranose polymer (glucan).
Monoclonal antibody characterization and polysaccharide detection.
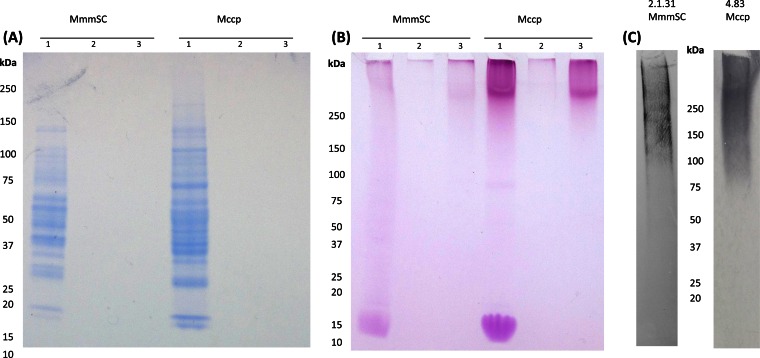
MAbs specific for polysaccharides were produced to allow the detection of these products. More specifically, two MAbs, 2.1.31 (IgG1) and 4.83 (IgM), were selected by dot blot analyses, as they bound to the homologous extracted EPS and PPLO culture supernatants of Mycoplasma mycoides subsp. mycoides 8740-Rita and M. capricolum subsp. capripneumoniae 95043, respectively, but not to PPLO medium control or to the other EPS (see Fig. S1 in the supplemental material). The Coomassie blue staining of the SDS-PAGE gel allowed the detection of protein bands only in the concentrated mycoplasma pellet (Fig. 2A), an indication that the EPS extracts did not contain proteins or peptides at detectable concentrations by this technique, although low levels of protein contamination may have been needed for the migration of the neutral polysaccharide component. The PAS staining, which binds to polysaccharide compounds, revealed a clear difference between the concentrated mycoplasma pellets and the EPS extracts (Fig. 2B). All preparations from both mycoplasmas showed a positive smear at high molecular weight, but the concentrated mycoplasma pellets contained an additional conspicuous spot of low molecular weight, very probably a glycolipid, which was not present in the EPS extracts. The Western blot assay performed with the two MAbs yielded a positive smear of high molecular weight, similar to the PAS-positive smear (Fig. 2C). This ruled out the possibility of recognition by the MAb of any immunoreactive contaminating product that may have been present in the antigen used to immunize the mice. The MAbs 2.1.31 and 4.83 were therefore confirmed to recognize a β(1→6)-galactofuranose polymer (galactan) and a β(1→2)-glucopyranose polymer (glucan), respectively. Owing to the lack of cross-reactivity of each of the two MAbs with the other polysaccharide, we could use these MAbs to detect galactan and glucan in various mycoplasma species using concentrated washed mycoplasma pellets to detect CPS and culture supernatant to detect EPS (Fig. 3).
FIG 2.
Staining of Mycoplasma mycoides subsp. mycoides 8740-Rita and M. capricolum subsp. capripneumoniae 95043 profiles after polyacrylamide gel electrophoresis: Coomassie blue staining (A), Schiff's reagent staining (B), and immunoblotting of their extracted EPS by MAbs (C). Three samples were analyzed for strains 8740-Rita (Mycoplasma mycoides subsp. mycoides [Mmm]) and 95043 (M. capricolum subsp. capripneumoniae [Mccp]): washed mycoplasmas obtained after incubation during 80 h in CMRL supplemented with 3 g/liter of glucose (lanes 1), CMRL supernatant after 80 h of incubation (lanes 2), and extracted EPS from these supernatants (lanes 3). In Western immunoblot analysis, EPS from Mycoplasma mycoides subsp. mycoides and M. capricolum subsp. capripneumoniae were labeled, respectively, with MAbs 2.1.31 and 4.83.
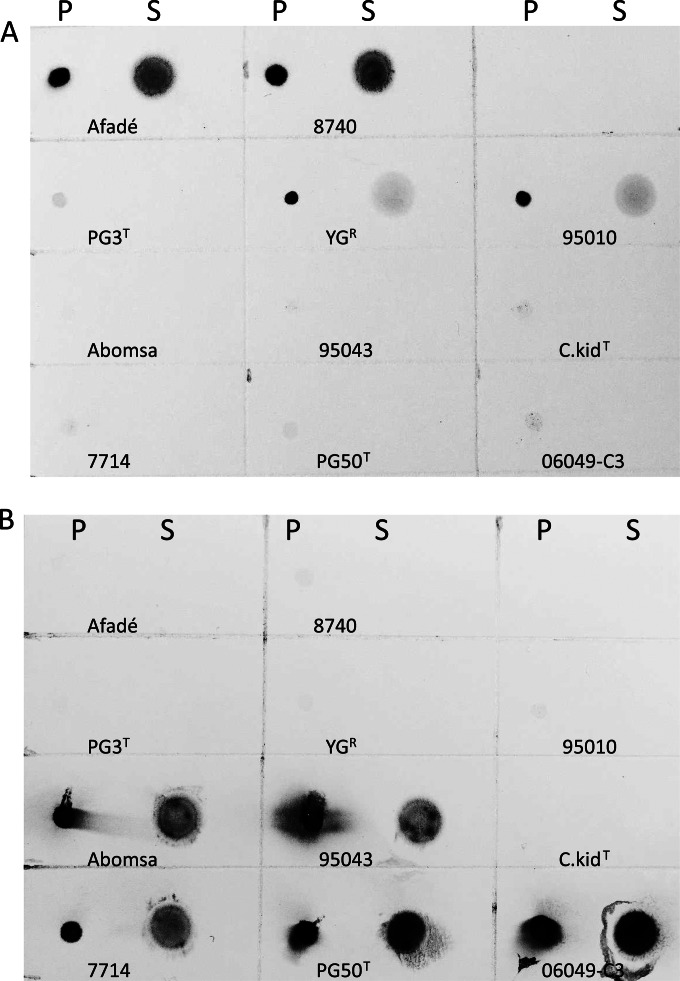
FIG 3.
Dot blot analysis of polysaccharides produced by representative strains of the Mycoplasma mycoides cluster with MAb 2.1.31 (A) and MAb 4.83 (B). P, pelleted mycoplasma cells grown from PPLO medium to detect CPS; S, supernatants from PPLO culture to detect EPS. MAb 2.1.31 yielded positive results with pelleted Mycoplasma mycoides subsp. mycoides strains (Afadé and 8740) and M. mycoides subsp. capri LC (95010 and YGR). Mycoplasma mycoides subsp. mycoides supernatants yielded clearly positive dots, while M. mycoides subsp. capri LC supernatants gave only faint positive dots. No product was detected by this MAb with M. leachii or M. capricolum (M. capricolum subsp. capricolum and M. capricolum subsp. capripneumoniae) strains or with the reference M. mycoides subsp. capri Capri strain PG3. MAb 4.83 yielded positive results with pelleted cells and supernatant from M. leachii (PG50T) and M. capricolum subsp. capripneumoniae (95043). A discrepancy of results was observed with M. capricolum subsp. capricolum reference strain C.kid, which did not yield any positivity, while positive dots were observed for M. capricolum subsp. capricolum strain 7714, both in the pellet and supernatant. No positivity was observed with M. mycoides subsp. capri and Mycoplasma mycoides subsp. mycoides strains with this MAb.
As expected, galactan, recognized by MAb 2.1.31, was detected as CPS and EPS in Mycoplasma mycoides subsp. mycoides strains Afadé and 8740 (Fig. 3A). Galactan was also clearly detected as CPS in M. mycoides subsp. capri LC strains YGR and 95010 but not in M. mycoides subsp. capri Capri strain PG3T, which yielded a very faint reaction. Dots with faint positivity were observed in the supernatants of M. mycoides subsp. capri LC strains YGR and 95010, suggesting a weak secretion of EPS by these strains or the presence of cellular debris in the supernatant. With MAb 2.1.31, very faint or no reactions were observed with M. capricolum subsp. capricolum (strains C.kidT and 7714), M. capricolum subsp. capripneumoniae (strains Abomsa and 95043), and M. leachii (strains PG50T and 06049-C3). Results obtained with 4 additional M. mycoides subsp. capri LC strains were quite homogeneous, with galactan being detected as CPS in the cell pellet and a faint positivity as EPS in the supernatant (see Fig. S2A in the supplemental material). No positivity was detected with any of the 4 additional M. mycoides subsp. capri Capri strains tested (data not shown).
Glucan, recognized by MAb 4.83, was detected in M. capricolum subsp. capripneumoniae strains as well as in M. leachii strains as CPS and EPS (Fig. 3B). Results were more variable with M. capricolum subsp. capricolum strains. No glucan was detected with the reference strain C.kid, while glucan as CPS and EPS was detected for strain 7714. Very faint or no reactivity was observed with MAb 4.83 with M. mycoides subsp. capri and Mycoplasma mycoides subsp. mycoides strains. Results obtained with 5 additional M. capricolum subsp. capricolum strains differed from one strain to another, with glucan being detected as CPS and EPS clearly in some strains (07046, 7714, 02053, and 10074), while it was only faintly detected as CPS in two other strains (IPX and 13051) (see Fig. S2B in the supplemental material). The ability to synthesize and secrete glucan varies greatly among M. capricolum subsp. capricolum strains under these conditions.
In silico analysis of EPS biosynthetic pathways in the Mycoplasma mycoides cluster.
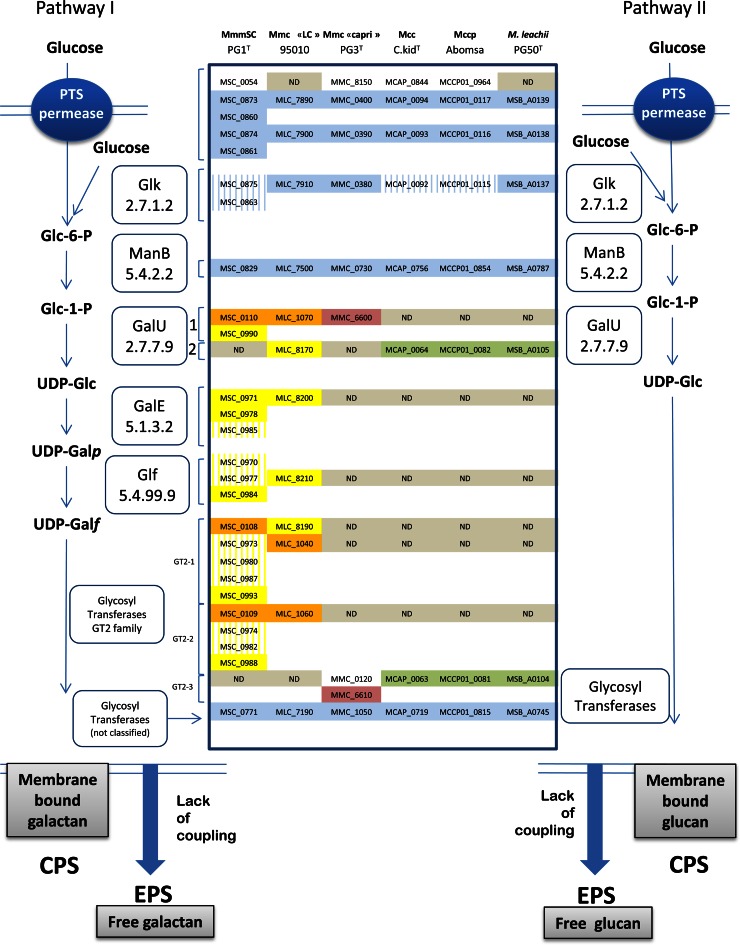
We compared the galactan predicted biosynthetic pathway that was described for Mycoplasma mycoides subsp. mycoides (15) with those found in the other MMC members by performing BLASTP searches as well as data mining using the complete genomes and databases that are now available. Two predicted biosynthetic pathways, possibly leading to the production of either galactofuranose or glucopyranose homopolymers, were evidenced and are presented in Fig. 4. The first steps leading to the formation of UDP-glucose are similar in the two pathways. They involve transport systems predicted to import and phosphorylate glucose (PTS permeases, Glk), a phosphoglucomutase (ManB) to isomerize glucose-6-P into glucose-1-P, and UTP-glucose-1-phosphate uridylyltransferases (GalU) to obtain UDP-glucose. The first pathway, leading to the production of galactan, includes a UDP-glucose 4-epimerase (GalE) and a UDP-galactofuranose mutase (Glf) that are involved in the isomerization of UDP-Glc into UDP-galactopyranose and the conformational change of the pyranose ring into UDP-galactofuranose, respectively. Multiple glycosyltransferases were identified in this pathway. Two of them were identified as belonging to the GT2 family but were clearly segregated into two subfamilies based on similarity comparisons. Another glycosyltransferase could not be classified according to CAZY. This first pathway was observed in Mycoplasma mycoides subsp. mycoides and M. mycoides subsp. capri LC genomes. For the sake of clarity, we refer here to the genes and locus tags observed in the M. mycoides subsp. capri LC genome. The second pathway, which was found in all the other MMC members, differs from the first by the absence of GalE and Glf. Nevertheless, it contains genes leading to the formation of UDP-glucose residues and those coding for glycosyltransferases, which is consistent with the synthesis of a glucan homopolymer. An additional subfamily of glycosyltransferase, GT2-3, was identified in this second pathway. Interestingly, M. mycoides subsp. capri serovar Capri differed from serovar LC, which possesses both galE and glf genes.
FIG 4.
Overview of the polysaccharide biosynthetic pathways in the Mycoplasma mycoides cluster (MMC). The various genes involved in these pathways are represented on the left side for M. mycoides (Mycoplasma mycoides subsp. mycoides [Mmm] and M. mycoides subsp. capri [Mmc]) and on the right side for M. capricolum (M. capricolum subsp. capricolum [Mcc] and M. capricolum subsp. capripneumoniae [Mccp]) and M. leachii. Locus tag numbers are given for a representative genome for each of the species. Genes highlighted in blue were detected in all members of the MMC. Others were found in a limited number of species. Highlighted genes in green, orange, and yellow correspond to genes found in clusters. Genes that show the highest similarity level within the MMC were positioned on the same line. Two galU families were identified as well as three glycosyltransferase type 2 subfamilies. Pseudogenes are represented with vertical stripes in the background. galE, glf, and some glycosyltransferase-coding genes were notably absent from M. mycoides subsp. capri Capri, M. capricolum, and M. leachii. ND, not detected; CPS, capsular polysaccharide; EPS, secreted exopolysaccharide.
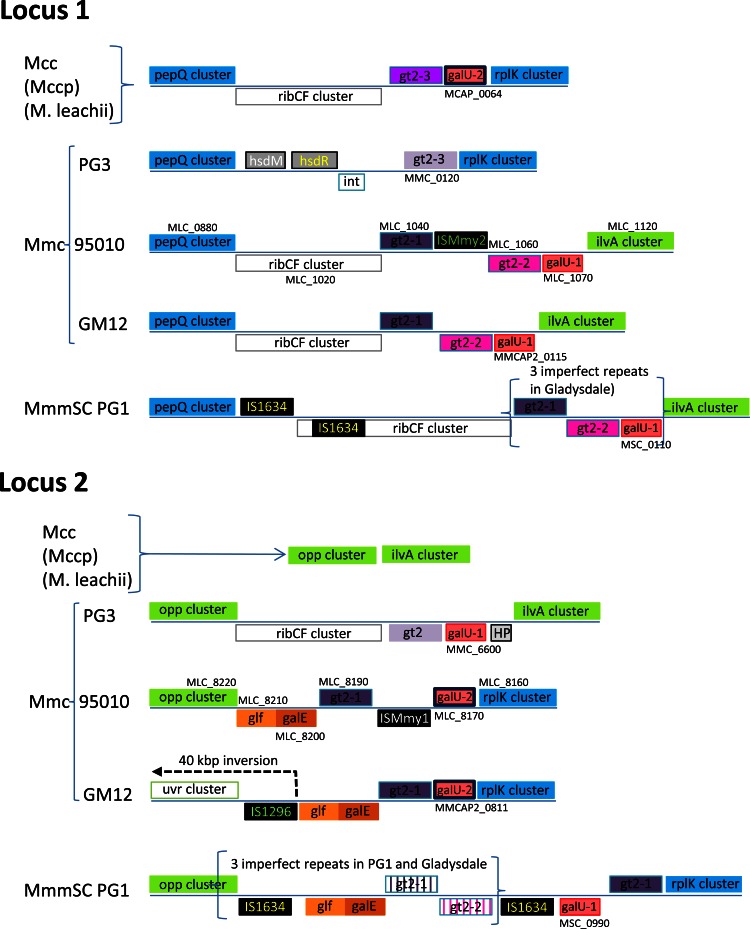
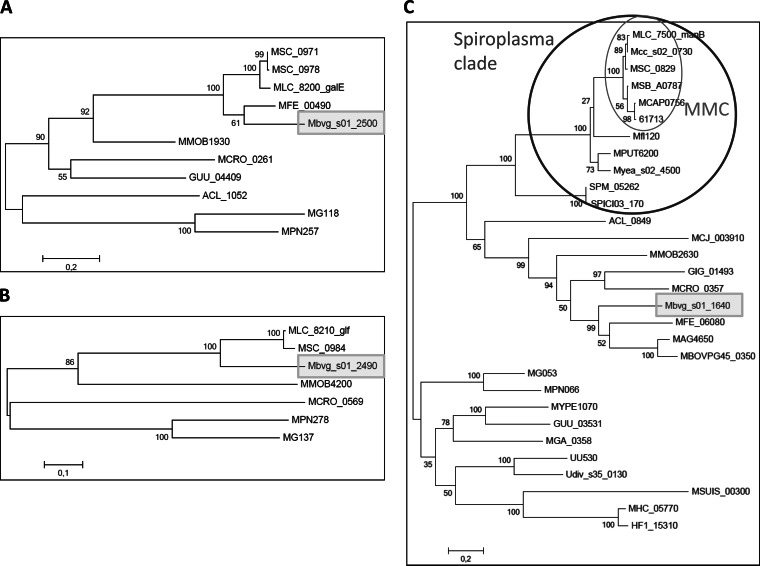
The genes that differentiate the two pathways (galU, galE, and glf-gt2) are organized in two clusters positioned at two loci that show a remarkable degree of polymorphism within the MMC (Fig. 5). A single and well-conserved locus exists among M. capricolum subsp. capricolum, M. capricolum subsp. capripneumoniae, and M. leachii genomes with the glycosyltransferase and galU genes being flanked by the pepQ-ribCF and rplK clusters of genes. However, the two loci in the Mycoplasma mycoides genomes are highly variable. They are hot spots for the insertion of mobile elements such as insertion sequences (IS1296, IS1634, ISMmy1, and ISMmy2, notably in Mycoplasma mycoides subsp. mycoides and M. mycoides subsp. capri LC) and a restriction modification system (hsdR-hsdM) paired with a phage family integrase (int) in M. mycoides subsp. capri Capri. They are also hot spots for horizontal gene transfer. Notably in locus two, the phylogenetic analysis indicated that the pair of genes galE and glf, involved in galactan synthesis, have been exchanged between ancestors of the MMC and M. bovigenitalium (Fig. 6A and B), as the phylogenetic trees differed markedly from that of manB (Fig. 6C) and that of the housekeeping gene fusA (see Fig. S3A in the supplemental material). Within M. mycoides subsp. capri, significant genome rearrangements have occurred with highly different structures of the loci in PG3T compared to 95010 and GM12. The genome of strain PG3T has retained the structure of the loci observed in M. capricolum-related genomes, while a recombination seems to have occurred between the two loci in the M. mycoides subsp. capri LC genomes, with the downstream ilvA cluster instead of the rplK cluster. In addition, a 40-kbp inversion between two IS sequences has occurred just upstream of the galE-glf pair in GM12 locus 2 (Fig. 5). In Mycoplasma mycoides subsp. mycoides genomes, the general organization of the two loci is similar to that of M. mycoides subsp. capri LC genomes but with an increased complexity due to the duplication of the genes related to polysaccharide synthesis. Interestingly, the number of duplications varies from one strain to another, as shown by comparison of the genomes from strains PG1T and Gladysdale.
FIG 5.
Comparison of genome regions including galU, galE, glf, and gt2 genes in the Mycoplasma mycoides cluster. The two loci involved are represented. Only locus 1 is found in M. capricolum subsp. capricolum with highly similar organization in M. capricolum subsp. capripneumoniae and M. leachii. The two loci are flanked by conserved clusters of genes, named pepQ, ilvA, opp, and rplK clusters, and used in the schematic representation as milestones. Homologous genes are represented as boxes with the same color. Pseudogenes are represented with vertical stripes in the background. The locus tag of one M. mycoides subsp. capri LC gene is indicated as a reference for each locus, to facilitate association with the text and other figures.
FIG 6.
Phylogeny of some genes involved in polysaccharide biosynthetic pathways. (A and B) Phylogenetic tree obtained with GalE and Glf, respectively. The trees were generated by the maximum likelihood method, and bootstrap values are indicated at each node of the tree. These genes are found only in a few number of Mollicutes, and the tree topology differs from the species tree. In the Mycoplasma mycoides cluster, galE and glf are found only in M. mycoides subsp. capri LC genomes as well as in Mycoplasma mycoides subsp. mycoides strains. Their closest homologues are found in the M. bovigenitalium genome or in the M. fermentans genome (for galE). This topology and the observation that galE and glf are not found in the other members of the Spiroplasma clade strongly suggest that these genes were acquired by M. mycoides subsp. capri LC and Mycoplasma mycoides subsp. mycoides by horizontal gene transfer from an M. bovigenitalium ancestor. (C) Phylogenetic tree obtained with manB homologues. The topology of this tree is similar to that of the species tree (see Fig. S2A in the supplemental material), except that some branches are missing. Notably, manB is found in all members of the Spiroplasma clade. The tree topology is indicative of a vertical transmission of this gene in all the Mollicutes in which it is found. It may have been deleted from the other genomes.
DISCUSSION
Monosaccharide composition and glycosidic linkages of polysaccharides synthesized by mycoplasmas have been determined for a limited number of species. In M. pulmonis, EPS-I contains equimolar amounts of glucose and galactose, with undetermined linkage (24), while EPS-II, involved in biofilm formation, and another polysaccharide of M. pneumoniae contain also N-acetylglucosamine (25). Members of the MMC differ from M. pneumoniae and M. pulmonis and appear to be diverse in terms of polysaccharide production and secretion in spite of their very close phylogenetic relationship. Two different polysaccharide products were detected, and strains differed in their ability to produce and/or secrete these compounds. Galactan, a galactofuranose homopolymer, was detected as CPS and EPS in Mycoplasma mycoides subsp. mycoides strains. The same compound was detected with the galactan-specific MAb in all M. mycoides subsp. capri serovar LC strains as CPS, in the mycoplasma cell pellets. However, only faint signals were observed in the supernatants, indicating that EPS secretion was very limited or that the positivity was due to cellular debris. No galactan was detected in M. mycoides subsp. capri serovar Capri strains. Our results showing that Mycoplasma mycoides subsp. mycoides and M. mycoides subsp. capri LC share the same type of CPS may explain why cross-reactions were observed between these two mycoplasmas (previously classed as different biotypes of the same subspecies) in the growth inhibition technique, which was formerly considered a reference technique to identify species (26). Strains of M. mycoides subsp. capri serovar Capri, which do not produce galactan but are phylogenetically very closely related to M. mycoides subsp. capri LC, do not produce the same cross-reactions with Mycoplasma mycoides subsp. mycoides. The absence of galactan in M. mycoides subsp. capri serovar Capri strains had already been noticed when the chemical composition of the nucleic acids and macromolecular components was studied by Jones et al. (27). In this previous study, the only polysaccharide detected in the type strain PG3 was composed of glucose in very small amounts (0.5% of the dry weight) compared to galactan, which represented 10% of the dry weight of Mycoplasma mycoides subsp. mycoides. The potential ability of M. mycoides subsp. capri Capri strains to produce a polysaccharide, as suggested by its gene repertoire, remains to be studied, as well as the conditions that would favor such a production.
A linear β(1→2)-glucopyranose homopolymer was detected as CPS and EPS in M. capricolum subsp. capripneumoniae and M. leachii. This composition differed from what had been already described for M. capricolum subsp. capripneumoniae strains (28). In that study, the monosaccharide components of the M. capricolum subsp. capripneumoniae EPS had been identified as glucose, galactose, mannose, fucose, galactosamine, and glucosamine. The difference with our results may be attributed to the crude preparations used in 1987 to precipitate the polysaccharide from the culture supernatant. These preparations were probably contaminated by polysaccharides present in the very rich medium needed to grow these fastidious mycoplasma strains. Serum and yeast extract are often the source of contamination (29) and may have biased some earlier studies. Our concentration procedure ensured that the precipitated product was pure mycoplasma polysaccharide. The linear β(1→2)-glucopyranose homopolymer identified in M. capricolum subsp. capripneumoniae and M. leachii is rarely found in bacteria. Very short linear β-d-(1→2)-glucans were evidenced in the periplasm of Escherichia coli and tropical Rhizobium species (30, 31), and cyclic β-d-(1→2)-glucans found, for instance, in Mesorhizobium species (23, 32) or Brucella (33) are more frequent.
Diverse polysaccharide profiles were observed with M. capricolum subsp. capricolum strains when tested with MAb 4.83. No polysaccharide was detected in the type strain C.kid, while some other M. capricolum subsp. capricolum strains yielded positive results in the pellet only and others in the pellet and supernatant. The genetic basis of this phenotypic difference has not been unraveled yet. The stock of strain C.kidT analyzed could well be a variant that does not produce polysaccharide. Mycoplasma strains, notably those kept as reference cultures, have been passaged and cloned many times to ensure their purity. This cloning procedure may have been responsible for the selection of variants that differ from the original stock.
Within the MMC, there are three species or subspecies that were shown to secrete EPS, i.e., Mycoplasma mycoides subsp. mycoides, M. leachii, and M. capricolum subsp. capripneumoniae, and one species with varying ability to secrete EPS, M. capricolum subsp. capricolum. We could not identify any specific transport system in their genomes that would distinguish them from their nonsecreting relatives of the MMC. However, we observed a characteristic common to the three secreting species: the disruption of the putative lipoprotein gene located immediately upstream of a glycosyltransferase (homologous to MLC_7190) that was shown to be specific to the Spiroplasma clade (see Fig. S4 in the supplemental material). The disruption of the lipoprotein gene was not a strain-specific event, as it was also disrupted in all the M. capricolum subsp. capripneumoniae, M. leachii, and Mycoplasma mycoides subsp. mycoides strains examined. This glycosyltransferase is also flanked downstream by a conserved hypothetical protein (MLC_7180) with four predicted transmembrane segments and an RDD motif that may be associated with a transport activity. This cluster of genes (MLC_7200-7190-7180) may therefore code for a polysaccharide export complex in the MMC. If confirmed, this would imply that polysaccharide secretion in these three species may be altered, a consequence of genome decay, with a reduced functionality, preventing the linkage of the polysaccharide moiety to the membrane-anchored moiety to form a CPS. A previous study had already shown that the level of CPS/EPS production in Mycoplasma mycoides subsp. mycoides was modulated by the expression of a PTS-G gene (15) that depended on phase variations. It is highly probable that CPS/EPS production in the members of the MMC is tightly regulated, considering the importance of these products for bacterial survival in various ecological niches. In many bacteria, CPS/EPS regulation may depend on promoters (34) and external factors such as oxygen availability (35) or quorum sensing (36). Similar studies would be needed to decipher the regulatory mechanisms at stake in mycoplasmas. The polysaccharide-specific MAbs could be used in capture enzyme-linked immunosorbent assays (ELISAs) to monitor the level of polysaccharide secretion in vitro or in vivo and evaluate how these levels are correlated with strain virulence. They could also be used in electron microscopic assays to evaluate CPS thickness. Parallel studies, with in vitro cellular models, could be performed with purified EPS to evaluate their impact on host gene transcription.
The phenotypic variability that we observed in the MMC, as regards polysaccharide production, was paralleled with a marked genetic polymorphism. The candidate genes involved in polysaccharide production are grouped in clusters and positioned within two loci that are hot spots of genetic variability. This variability is highlighted by the presence of insertion sequences of various types, by recombination events, by gene duplication (with duplicated genes being often truncated by frameshift mutations), and by the presence of genes that have been shown to be exchanged by horizontal gene transfer. The importance of HGT has been recognized only recently in mycoplasmas (37, 38). Genes involved in polysaccharide synthesis must be added to the list of genes that can be exchanged by HGT in mycoplasmas. This is no exception to other prokaryotes, as genes for polysaccharide synthesis are often carried by mobile genetic elements, such as plasmids in lactococci (39), or are closely associated to insertion sequences, as in Desulfovibrio (40), Lactococcus (41), or Crocosphaera (42). Moreover, the genes identified in the MMC often do not have any homologue in other mycoplasma genomes (see Fig. S3B in the supplemental material). The other mycoplasmas may not produce any polysaccharide or have completely different polysaccharide gene repertoires, leading to the production of a variety of products. Further work will be needed to elucidate their exact composition and their role in host-pathogen interactions. At the onset of infection, polysaccharides are involved in the resistance to the innate immune responses (43, 44), but further investigations are needed to evaluate the respective roles of CPS and EPS in the induction of the exacerbated lung inflammation, which is the hallmark of CBPP and CCPP. Polysaccharides present as soluble products are T-independent antigens, while CPS, a polysaccharide coupled probably to diglycosyldiacylglycerol residues (45), will trigger a T-dependent immune response (46) and a strong memory response. This type of response, which is directed to a mycoplasma surface-exposed antigen, may be important for protection and may help in designing more-potent vaccines in the future.
Meanwhile, our results may have rapid practical applications for diagnostic tests. The complement fixation test (prescribed by the OIE) and the latex agglutination test for CCPP are based on polysaccharide antigen (47, 48). Their specificity may be jeopardized by the frequent infections of goats with MMC species, and notably M. capricolum subsp. capricolum strains, which are likely to induce false-positive results. M. capricolum subsp. capricolum strains represented 26% of the French mycoplasma strains isolated from goats between 2003 and 2008 (49). The specificity of these tests has to be reevaluated.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Ph.D. grant awarded jointly by ANSES and CIRAD to Clothilde Bertin.
We thank the LABGeM team within the “France Genomics” infrastructure, Evry, France, for their help in the genome annotation and analysis through the Microscope platform as well as the CBiB in Bordeaux, France, for their help in the annotation of the M. mycoides subsp. capri Capri PG3T genome within the EVOLMYCO project.
Finally, we thank L. Lakhdar for her technical help in immunization of mice at the Animal experimentation platform in Lyon as well as M. Dejean for the extraction of the polysaccharides at CIRAD, Montpellier, France.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02892-14.
REFERENCES
- 1.Cottew GS, Breard A, DaMassa AJ, Erno H, Leach RH, Lefevre PC, Rodwell AW, Smith GR. 1987. Taxonomy of the Mycoplasma mycoides cluster. Isr J Med Sci 23:632–635. [PubMed] [Google Scholar]
- 2.Gasparich GE, Whitcomb RF, Dodge D, French FE, Glass J, Williamson DL. 2004. The genus Spiroplasma and its non-helical descendants: phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. Int J Syst Evol Microbiol 54:893–918. doi: 10.1099/ijs.0.02688-0. [DOI] [PubMed] [Google Scholar]
- 3.Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyaizu H, Yang D, Mandelco L, Sechrest J, Lawrence TG, Van Etten J. 1989. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol 171:6455–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manso-Silvan L, Perrier X, Thiaucourt F. 2007. Phylogeny of the Mycoplasma mycoides cluster based on analysis of five conserved protein-coding sequences and possible implications for the taxonomy of the group. Int J Syst Evol Microbiol 57:2247–2258. doi: 10.1099/ijs.0.64918-0. [DOI] [PubMed] [Google Scholar]
- 5.Manso-Silvan L, Vilei EM, Sachse K, Djordjevic SP, Thiaucourt F, Frey J. 2009. Mycoplasma leachii sp. nov. as a new species designation for Mycoplasma sp. bovine group 7 of Leach, and reclassification of Mycoplasma mycoides 0subsp. mycoides LC as a serovar of Mycoplasma mycoides subsp. capri. Int J Syst Evol Microbiol 059: 1353–1358. doi: 10.1099/ijs.0.005546-0. [DOI] [PubMed] [Google Scholar]
- 6.Westberg J, Persson A, Holmberg A, Goesmann A, Lundeberg J, Johansson KE, Pettersson B, Uhlen M. 2004. The genome sequence of Mycoplasma mycoides subsp. mycoides SC type strain PG1T, the causative agent of contagious bovine pleuropneumonia (CBPP). Genome Res 14:221–227. doi: 10.1101/gr.1673304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson A, Jacobsson K, Frykberg L, Johansson KE, Poumarat F. 2002. Variable surface protein Vmm of Mycoplasma mycoides subsp. mycoides small colony type. J Bacteriol 184:3712–3722. doi: 10.1128/JB.184.13.3712-3722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glew MD, Marenda M, Rosengarten R, Citti C. 2002. Surface diversity in Mycoplasma agalactiae is driven by site-specific DNA inversions within the vpma multigene locus. J Bacteriol 184:5987–5998. doi: 10.1128/JB.184.21.5987-5998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilo P, Frey J, Vilei EM. 2007. Molecular mechanisms of pathogenicity of Mycoplasma mycoides subsp. mycoides SC. Vet J 174:513–521. doi: 10.1016/j.tvjl.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hames C, Halbedel S, Hoppert M, Frey J, Stülke J. 2009. Glycerol metabolism is important for cytotoxicity of Mycoplasma pneumoniae. J Bacteriol 191:747–753. doi: 10.1128/JB.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischof DF, Janis C, Vilei EM, Bertoni G, Frey J. 2008. Cytotoxicity of Mycoplasma mycoides subsp. mycoides small-colony type to bovine epithelial cells. Infect Immun 76:263–269. doi: 10.1128/IAI.00938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurotchkin TJ. 1937. Specific carbohydrate from Asterococcus mycoides for serological tests of bovine pleuropneumonia. Proc Soc Exp Biol Med 37:21–22. doi: 10.3181/00379727-37-9438P. [DOI] [Google Scholar]
- 13.Shifrine M, Gourlay RN. 1965. The immediate type allergic skin reaction in contagious bovine pleuropneumonia. J Comp Pathol 75:381–385. doi: 10.1016/0021-9975(65)90018-6. [DOI] [PubMed] [Google Scholar]
- 14.Buttery SH, Plackett P. 1960. A specific polysaccharide from Mycoplasma mycoides. J Gen Microbiol 23:357–368. doi: 10.1099/00221287-23-2-357. [DOI] [PubMed] [Google Scholar]
- 15.Bertin C, Pau-Roblot C, Courtois J, Manso-Silván L, Thiaucourt F, Tardy F, Le Grand D, Poumarat F, Gaurivaud P. 2013. Characterization of free exopolysaccharides secreted by Mycoplasma mycoides subsp. mycoides. PLoS One 8:e68373. doi: 10.1371/journal.pone.0068373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. 1988. Antibodies, a laboratory manual. Cold Spring Harbor Laboratory, New York, NY. [Google Scholar]
- 17.Thiaucourt F, Bolske G, Libeau G, Le Goff C, Lefevre PC. 1994. The use of monoclonal antibodies in the diagnosis of contagious caprine pleuropneumonia (CCPP). Vet Microbiol 41:191–203. doi: 10.1016/0378-1135(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 18.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barré A, de Daruvar A, Blanchard A. 2004. MolliGen, a database dedicated to the comparative genomics of Mollicutes. Nucleic Acids Res 32:D307–D310. doi: 10.1093/nar/gkh114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abascal F, Zardoya R, Posada D. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 23.Choma A, Komaniecka I. 2003. Characterisation of Mesorhizobium huakuii cyclic beta-glucan. Acta Biochim Pol 50:1273–1281. [PubMed] [Google Scholar]
- 24.Daubenspeck JM, Bolland JR, Luo W, Simmons WL, Dybvig K. 2009. Identification of exopolysaccharide-deficient mutants of Mycoplasma pulmonis. Mol Microbiol 72:1235–1245. doi: 10.1111/j.1365-2958.2009.06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons WL, Daubenspeck JM, Osborne JD, Balish MF, Waites KB, Dybvig K. 2013. Type 1 and type 2 strains of Mycoplasma pneumoniae form different biofilms. Microbiology 159:737–747. doi: 10.1099/mic.0.064782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aubaidi JM, Dardiri AH, Fabricant J. 1972. Biochemical characterization and antigenic relationship of Mycoplasma mycoides subsp. mycoides, Freundt and Mycoplasma mycoides subsp capri (Edward) Freundt. Int J Syst Evol Microbiol 22:155–164. [Google Scholar]
- 27.Jones AS, Tittensor JR, Walker RT. 1965. The chemical composition of the nucleic acids and other macromolecular constituents of Mycoplasma mycoides var. capri. J Gen Microbiol 40:405–411. doi: 10.1099/00221287-40-3-405. [DOI] [PubMed] [Google Scholar]
- 28.Rurangirwa FR, McGuire TC, Magnuson NS, Kibor A, Chema S. 1987. Composition of a polysaccharide from mycoplasma (F-38) recognised by antibodies from goats with contagious pleuropneumonia. Res Vet Sci 42:175–178. [PubMed] [Google Scholar]
- 29.Daubenspeck JM, Jordan DS, Dybvig K. 2014. The glycocalyx of Mollicutes, p 131–147. In Browning GF, Citti C (ed), Mollicutes: molecular biology and pathogenesis. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 30.Geiger O, Weissborn AC, Kennedy EP. 1991. Biosynthesis and excretion of cyclic glucans by Rhizobium meliloti 1021. J Bacteriol 173:3021–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissborn AC, Rumley MK, Kennedy EP. 1992. Isolation and characterization of Escherichia coli mutants blocked in production of membrane-derived oligosaccharides. J Bacteriol 174:4856–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaharada Y, Kiyota H, Eda S, Minamisawa K, Mitsui H. 2008. Structural characterization of neutral and anionic glucans from Mesorhizobium loti. Carbohydr Res 343:2422–2427. doi: 10.1016/j.carres.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Guidolin LS, Ciocchini AE, Iñón de Iannino N, Ugalde RA. 2009. Functional mapping of Brucella abortus cyclic β-1,2-glucan synthase: identification of the protein domain required for cyclization. J Bacteriol 191:1230–1238. doi: 10.1128/JB.01108-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shainheit MG, Mulé M, Camilli A. 2014. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun 82:694–705. doi: 10.1128/IAI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geno KA, Hauser JR, Gupta K, Yother J. 2014. Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability. J Bacteriol 196:1992–2003. doi: 10.1128/JB.01545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K-J, Kim J-A, Hwang W, Park S-J, Lee K-H. 2013. Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol Microbiol 90:841–857. doi: 10.1111/mmi.12401. [DOI] [PubMed] [Google Scholar]
- 37.Sirand-Pugnet P, Lartigue C, Marenda M, Jacob D, Barre A, Barbe V, Schenowitz C, Mangenot S, Couloux A, Segurens B, de Daruvar A, Blanchard A, Citti C. 2007. Being pathogenic, plastic, and sexual while living with a nearly minimal bacterial genome. PLoS Genet 3:e75. doi: 10.1371/journal.pgen.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beven L, Charenton C, Dautant A, Bouyssou G, Labroussaa F, Skollermo A, Persson A, Blanchard A, Sirand-Pugnet P. 2012. Specific evolution of F1-like ATPases in mycoplasmas. PLoS One 7:e38793. doi: 10.1371/journal.pone.0038793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forde A, Fitzgerald GF. 2003. Molecular organization of exopolysaccharide (EPS) encoding genes on the lactococcal bacteriophage adsorption blocking plasmid, pCI658. Plasmid 49:130–142. doi: 10.1016/S0147-619X(02)00156-7. [DOI] [PubMed] [Google Scholar]
- 40.Walker CB, Stolyar S, Chivian D, Pinel N, Gabster JA, Dehal PS, He Z, Yang ZK, Yen H-CB, Zhou J, Wall JD, Hazen TC, Arkin AP, Stahl DA. 2009. Contribution of mobile genetic elements to Desulfovibrio vulgaris genome plasticity. Environ Microbiol 11:2244–2252. doi: 10.1111/j.1462-2920.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- 41.Dabour N, LaPointe G. 2005. Identification and molecular characterization of the chromosomal exopolysaccharide biosynthesis gene cluster from Lactococcus lactis subsp. cremoris SMQ-461. Appl Environ Microbiol 71:7414–7425. doi: 10.1128/AEM.71.11.7414-7425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bench SR, Ilikchyan IN, Tripp HJ, Zehr JP. 2011. Two strains of Crocosphaera watsonii with highly conserved genomes are distinguished by strain-specific features. Front Microbiol 2:261. doi: 10.3389/fmicb.2011.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw BM, Daubenspeck JM, Simmons WL, Dybvig K. 2013. EPS-I polysaccharide protects Mycoplasma pulmonis from phagocytosis. FEMS Microbiol Lett 338:155–160. doi: 10.1111/1574-6968.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaurivaud P, Lakhdar L, Grand DL, Poumarat F, Tardy F. 2014. Comparison of in vivo and in vitro properties of capsulated and non-capsulated variants of Mycoplasma mycoides subsp. mycoides strain Afadé: a potential new insight into the biology of contagious bovine pleuropneumonia. FEMS Microbiol Lett 359:42–49. doi: 10.1111/1574-6968.12579. [DOI] [PubMed] [Google Scholar]
- 45.Andres E, Martinez N, Planas A. 2011. Expression and characterization of a Mycoplasma genitalium glycosyltransferase in membrane glycolipid biosynthesis: potential target against mycoplasma infections. J Biol Chem 286:35367–35379. doi: 10.1074/jbc.M110.214148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snapper CM. 2012. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann N Y Acad Sci 1253:92–101. doi: 10.1111/j.1749-6632.2011.06329.x. [DOI] [PubMed] [Google Scholar]
- 47.March JB, Gammack C, Nicholas R. 2000. Rapid detection of contagious caprine pleuropneumonia using a Mycoplasma capricolum subsp. capripneumoniae capsular polysaccharide-specific antigen detection latex agglutination test. J Clin Microbiol 38:4152–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rurangirwa FR, McGuire TC, Kibor A, Chema S. 1987. A latex agglutination test for field diagnosis of contagious caprine pleuropneumonia. Vet Rec 121:191–193. doi: 10.1136/vr.121.9.191. [DOI] [PubMed] [Google Scholar]
- 49.Chazel M, Tardy F, Le Grand D, Calavas D, Poumarat F. 2010. Mycoplasmoses of ruminants in France: recent data from the national surveillance network. BMC Vet Res 6:32. doi: 10.1186/1746-6148-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordy DR, Adler HE, Yamamoto R. 1955. A pathogenic pleuropneumonialike organism from goats. Cornell Vet 45:50–68. [PubMed] [Google Scholar]
- 51.Chu Y, Gao P, Zhao P, He Y, Liao N, Jackman S, Zhao Y, Birol I, Duan X, Lu Z. 2011. Genome sequence of Mycoplasma capricolum subsp. capripneumoniae strain M1601. J Bacteriol 193:6098–6099. doi: 10.1128/JB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons GC, Johnston LAY. 1963. Arthritis in calves caused by Mycoplasma species. Aust Vet J 39:11–14. [Google Scholar]
- 53.Tully JG, Barile MF, Edward DGF, Theodore TS, Ernø H. 1974. Characterization of some caprine mycoplasmas, with proposals for new species, Mycoplasma capricolum and Mycoplasma putrefaciens. J Gen Microbiol 85:102–120. [DOI] [PubMed] [Google Scholar]
- 54.Laws L. 1956. A pleuropneumonia-like organism causing peritonitis in goats. Aust Vet J 32:326–329. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.