Abstract
Background
The critical events in clearance or persistence of hepatitis C virus (HCV) infection are unknown, but likely to be determined early in acute infection.
Methods
Type 1 and type 2 cytokine production was assessed by HCV peptide ELISpot and multiplex in vitro cytokine production assays in longitudinally collected samples from 20 untreated participants enrolled in the Australian Trial in Acute Hepatitis C (ATAHC); a prospective cohort of acute HCV infection (77% injecting drug users, IDU).
Results
Significantly higher interleukin-10 (IL-10) production (p=0.048), in the relative absence of interferon-gamma (IFN-γ) and IL-2 production, was present early in HCV infection in those who progressed to chronic infection. In contrast, viral clearance was associated with a greater magnitude and broader specificity of IFN-γ (magnitude p<0.001, breadth p=0.004) and IL-2 responses, in the relative absence of IL-10. Early IL-10 production was correlated with higher HCV RNA level at baseline (p=0.046) and week 12 (p=0.018), while IFN-γ and IL-2 production was inversely correlated with HCV RNA level at baseline (IFN-γ p=0.020, IL-2 p=0.050) and week 48 (IFN-γ p=0.045, IL-2 p=0.026). Intracellular staining (ICS) indicated the HCV-specific IFN-γ response was primarily from CD8+ T cells and NK cells, whereas IL-10 production was predominantly from monocytes, with a subset of IL-10 producing CD8+ T cells present only in those who progressed to chronic infection.
Conclusion
IL-10, an immunoregulatory cytokine, appears to play a key role in progression to chronic HCV infection.
Keywords: cytokine balance, cellular immunity, HCV infection, injection drug use, IL-10
INTRODUCTION
Primary HCV infection is asymptomatic in the majority of cases, with 50-80% of individuals developing chronic infection [1]. The largely asymptomatic course of acute HCV infection combined with the marginalized nature of those individuals at greatest risk of HCV (IDU) [1] has limited the number of studies examining the early natural history and cellular immune responses of HCV infection [2].
Clearance of HCV has been associated with early, multi-specific and sustained CD4+ [3-6] and CD8+ [7-9] responses directed against HCV epitopes [10-12], and depletion of either CD4+ or CD8+ cells in the chimpanzee model promoted viral persistence [7, 13, 14]. Studies of chronic HCV infection have demonstrated a low frequency of HCV-specific CD4+ and CD8+ cells both in the peripheral blood and liver [4]. By contrast, no clear correlation has been found as yet between anti-HCV humoral responses and viral clearance [15-17].
The innate immune response is a key component in the activation and maintenance of anti-viral immunity, through induction of cytokines and initiation of the adaptive immune response. IFNs in particular, have been shown to play a critical role in response to viral infections. Recently clearance of HCV has been linked to genetic variation in IL-28B for both spontaneous [18] and treatment induced clearance [19, 20]. Polymorphisms approximately 3 kilobases upstream of the IL28B gene, which encodes IFN-λ-3, have been associated with a 2 fold difference in response to HCV treatment [20] and spontaneous viral clearance [18]. In addition, IL-28A (IFN-λ-2) has been shown to promote antiviral activity by suppressing HCV IRES-mediated translation [21]. Type III IFNs (IL-29, IL-28A, IL-28B) share structural similarity with the IL-10 family of cytokines and share functional characteristics with type I IFNs as they signal through the JAK-STAT pathway and are induced by viral infections. Several HCV proteins, however, have been shown to impair the induction of type 1 IFNs, which then suppresses or delays subsequent adaptive T cell responses critical for viral clearance [22].
Helper CD4+ T cells are important for the control of viremia by promoting the effector function of virus-specific CD8+ T cells, particularly via IL-2 production [23, 24]. For instance, Missale et al (1996) detected higher magnitude HCV-specific T cell responses in those with viral clearance compared to viral persistence [25], suggesting the strength and timing of the T cell response is critical in determining the outcome of infection. Thus, functional impairment, suppression or deletion of antigen-specific T cells appears to be a key determinant of progression to chronicity [11, 26, 27].
During persistent viral infections specific T cells can become functionally inert, incapable of cytotoxicity, and producing IL-2 or IFN-γ [28, 29]; described as the ‘stunned’ phenotype in HCV infection [4]. Interference in the priming environment in HCV infection can reduce the effectiveness of the adaptive immune response, as low IL-2 concentrations caused expansion of memory cells, devoid of effector functions, whilst higher IL-2 concentrations during priming led to fully differentiated effector CD8+ T cells [30].
The importance of the cytokine milieu in determining viral clearance has been emphasized by studies using the murine LCMV model: early and high level IL-10 production was associated with viral persistence [31, 32]. In this context, excess IL-10 has the ability to suppress cytokine production and proliferation by CD4+ and CD8+ T cells, and to alter the function of antigen presenting cells [33-35].
The objective of this study was to analyze the affect of recent HCV infection upon the induction of effective cellular immune responses, in a cohort where the majority of subjects were IDU. The hypothesis was that the type and level of key cytokines produced early in HCV infection is a critical determinant of viral persistence. This study demonstrates that early IL-10 predominant responses are found in those that progress to chronic HCV infection.
MATERIALS AND METHODS
Study subjects
Untreated subjects with HCV infection were recruited from the Australian Trial in Acute Hepatitis C (ATAHC) study - a multi-centre, prospective longitudinal cohort. The study design has been previously described in detail [36]. In brief, eligible subjects had recently acquired HCV infection, defined by an initial positive anti-HCV antibody test within 6 months of enrolment, and either a negative anti-HCV antibody test in the two years prior, or acute clinical HCV within 12 months of the initial positive. All subjects were aged 16 years or above and provided written informed consent. The study protocol was approved by the institutional review board.
The time of HCV infection was estimated as the mid-point between the last negative and first positive anti-HCV antibody tests (for asymptomatic cases) or as 6 weeks prior to onset of acute clinical symptoms or alanine aminotransferase (ALT) level greater than 400 U/L (for clinical cases). The majority of subjects were IDU (77%) and median estimated duration of HCV infection at screening was 25 weeks. Thus, around 50% had acute HCV infection, while the remainder had early chronic HCV infection (6 to 18 months infection duration).
Immunological assays were performed on longitudinally collected samples (baseline, week 12 [wk12], wk24, wk48) from untreated subjects, who were subsequently categorised into clearers (two consecutive negative qualitative HCV RNA assessments) and persisters (two consecutive positive qualitative HCV RNA assessments, Table 1).
Table 1.
Demographic and clinical characteristics of untreated subjects from the ATAHC cohort
| Outcome, (study number, age in years, sex) | Genotype | Duration of HCV infection (weeks) | Baseline HCV RNA IU/mL | Baseline ALT (Peak ALT) IU/mL | Clinical symptoms, type | Risk factor * recent IDU |
|---|---|---|---|---|---|---|
| Clearance | ||||||
| C1 (105, 25, M) | 1a | 32 | 10-600 | 33 (60) | No | Piercing |
| C2 (106, 25, M) | 3a | 7 | 109 804 | 1235 (1235) | No | IDU |
| C3 (203, 35, F) | - | 30 | <10 | 19 (37) | Yes, 2 | IDU* |
| C4 (206, 31, F) | - | 31 | <10 | 11 (361) | No | IDU* |
| C5 (305, 47, F) | - | 30 | <10 | 15 (17) | Yes, 1, 2 | Sexual |
| C6 (307, 28, F) | 1a/b | 11 | 2 941 | 39 (694) | No | IDU |
| C7 (308, 25, M) | 1 | 14 | 10-600 | 104 (104) | Yes, 1-3 | IDU* |
| C8 (607, 39, M) | - | 65 | <10 | 20 (48) | No | IDU* |
| C9 (615, 35, M) | - | 22 | <10 | 32 (32) | Yes, 1, 2 | IDU* |
| C10 (641, 30, M) | - | 26 | <10 | 27 (27) | Yes, 1-3, 5 | IDU |
| C11 (1003, 44, M) | - | 20 | <10 | 110 (110) | Yes, 1-4 | Assault |
| C12 (1004, 21, F) | - | 19 | <10 | 280 (280) | Yes, 1, 2 | IDU |
|
| ||||||
| Mean age 32+/-8, Male 58% | Genotype 1 25% | Mean 25+/-14 | Mean 9 502+/- 31 598 | Baseline Mean 160+/-346 | Symptoms 58% | IDU 75%, Recent 41% |
|
| ||||||
| Persistence | ||||||
| P1 (104, 30, F) | 2a/c | 12 | 1 118 | 223 (561) | Yes, 2 | Sexual |
| P2 (201, 48, M) | 3a | 17 | 2 549 020 | 155 (155) | No | IDU* |
| P3 (204, 25, F) | 2b | 28 | 10-600 | 17 (203) | No | IDU* |
| P4 (303, 24, M) | 3a | 10 | 10-600 | 136 (205) | Yes, 1-3 | IDU* |
| P5 (602, 18, F) | 3a | 61 | 961 | 76 (224) | No | IDU |
| P6 (614, 24, F) | 3a | 24 | 11 103 | 139 (198) | No | IDU* |
| P7 (702, 39, M) | 3a | 8 | 1 396 691 | 446 (446) | No | IDU* |
| P8 (1403, 24, M) | 3a | 56 | 90 496 | 130 (130) | No | IDU |
|
| ||||||
| Mean age 29+/-9, Male 50% | Genotype 1 0% | Mean 27+/-20 | Mean 506 323+/-956 605 | Baseline Mean 165+/-128 | Symptoms 25% | IDU 87%, Recent 62% |
|
| ||||||
| Age p=0.229 Gender p=0.720 | Genotype 1 p=0.018 | Duration p=0.643 | HCV RNA p=0.004 | Baseline p=0.076 Peak p=0.123 | Symptoms present p=0.197 | IDU p=0.619 Recent p=0.633 |
C indicates clearance and P indicates persistence from the untreated longitudinal cohort. Dashes indicate untypable samples. Subjects with HCV RNA 10-600 IU/mL were qualitative positive. The type of clinical symptom is indicated by numbers (1 jaundice, 2 nausea, 3 abdominal pain, 4 fever, and 5 hepatomegaly).
Recent IDU denoted by asterisks on IDU indicates subject has injected in the last month. All subjects were Caucasian.
Virological testing
Qualitative HCV RNA was tested by transcription-mediated amplification assay (TMA, lower limit of detection of 50 copies/mL, Siemens Medical Solutions Diagnostics, New York, USA). In all qualitative HCV RNA positive samples, viral load (VL) was measured using the VERSANT HCV RNA 3.0 Assay (bDNA, lower limit of detection of 3200 copies/mL). Genotyping was performed using the VERSANT HCV Genotype Assay (LiPA).
Immunological assays
Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque density gradient centrifugation (Amersham–Pharmacia, Uppsala, Sweden), washed three times with phosphate-buffered saline (GIBCO BRL) and cyropreserved in media consisting of 90% heat inactivated fetal calf serum (FCS; JRH Biosciences, Kansas, USA) and 10% DMSO (Sigma-Aldrich).
HCV peptides
ELISpot and multiplex in vitro cytokine production assays were performed using peptides (18 amino acids in length, overlapping by 11 amino acids) based on the HCV genotype 1a sequence (NIH AIDS Reference and Reagent Program, Division of AIDS, NIAID, NIH: HCV 1a H77 Peptides). When used in IFN-γ and IL-2 ELISpot assays, peptides were grouped into 10 pools (Core, E1, E2, p7, NS2, NS3, NS4a, NS4b, NS5a and NS5b) covering the entire HCV coding region. Due to restrictions in available cell numbers, three peptide pools (Core-p7, NS2-3 and NS4-5), were used in the multi-array cytokine production assays. Peptides were titrated prior to use, with optimal concentration being 1 μg/mL (final concentration of each peptide). All peptides had an endotoxin level of <0.4 EU/mL (QC-1000 LAL assay was performed following manufacturer’s protocol, Lonza, Melbourne, Australia).
ELISpot IFN-γ and IL-2 assays
ELISpot assays were performed following manufacturer’s protocols (Mabtech, Nacka Sweden) with the exception of the use of coating antibody concentration of 5 μg/mL. Antigens included HCV peptides (10 peptide pools, 1 μg/mL), positive controls (phytohemagglutinin, 5 μg/mL, Sigma-Aldrich, Sydney), Cytomegalovirus, Epstein-Barr virus, Influenza [CEF] peptides, (9 amino acids, 2 μg/mL manufacturer’s recommendation, Mabtech), and anti-CD3 antibody (2 μg/mL, Mabtech) and a negative control (RPMI with 10% FCS and 0.8% DMSO [> maximum DMSO concentration in the peptide pools]). PBMCs were added to triplicate wells at 1 × 105 cells/well (similar to methods used by Ruys et al [37]). Plates were incubated at 37°C, 5% CO2 for 24 hours. Spot forming cells (SFC) were evaluated using an automated ELISpot reader (AID version 3.2.3, Strasberg, Germany). The thresholds for a positive response, ≥ 50 SFC/106 PBMC (after subtraction of negative control values) in both IFN-γ and IL-2 ELISpot assays, were determined using 15 low risk, seronegative blood donors (designated by doubling the mean plus three standard deviations (SD, mean plus 3SD was <25 SFC) of the responses in this control group (Supplementary Figure 1). Positive responses were always at least twice background responses.
Multiplex in vitro cytokine production
PBMC were cultured in 384-well microplates (7.5 × 105/well), stimulated with HCV peptide pools at 1 μg/ml. Positive controls included PHA (5 μg/mL) and CEF peptides (2 μg/mL), and a negative control (RPMI with 10% FCS and 0.8% DMSO). Cells were incubated at 37°C for 48 hours, before 65 μl of supernatant was collected and stored at -80°C until assayed for cytokine levels. A bead-based enzyme linked immunosorbent assay, containing dyed microspheres conjugated with a monoclonal antibody specific for each target cytokine, was performed according to the manufacturer’s protocol (Bio-plex Human Cytokine Assay; BioRad, CA, USA). The following Type 1 and 2 cytokines were measured: IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IFN-γ, TNF-α and granulocyte-monocyte colony stimulating factor (GM-CSF).
IFN-γ and IL-10 intracellular staining
PBMC from samples previously identified as positive for IFN-γ or IL-10 production were cultured for three days with HCV peptide pools (1 μg/mL), PHA (5 μg/mL), or media (RPMI with 10% FCS and 0.8% DMSO). Without the three day culture the frequency of the HCV-specific positive responses was below the limit of detection of the assay. After re-stimulation, PBMCs were incubated with HCV peptides or controls for six hours for IFN-γ and 24 hours for IL-10 staining. Golgi-Stop (BD Biosciences) was added for the last four hours of stimulation. Cells were blocked with 10% human serum (Sigma-Aldrich) and stained for CD markers (CD3, CD4, CD8, CD14, CD19, CD56, or appropriate isotype control, BD Biosciences), permeabilized (Cytofix/cytoperm kit, BD Biosciences), and stained with anti-IFN-γ APC or anti-IL-10 APC (BD Biosciences). Cells were fixed overnight with FACS lysing solution (BD Biosciences), acquired on a FACSCalibur (~200 000 events) and analyzed using BD FACSDiva software (version 4.1.2). The threshold for a positive response was defined as the mean plus 2SD of no antigen stimulation (media alone). For IFN-γ staining, this background threshold ranged between 1.1 to 2.0%, dependent upon the cell type examined (CD4 1.1%, CD8 1.6%, CD19 1.2%, CD56 1.8%, CD14 2.0%), and between 0.7 to 3.1% for IL-10 staining (CD4 0.7%, CD8 0.7%, CD19 1.0%, CD56 0.8%, CD14 3.1%).
Statistical Analysis
Non-parametric analyses were performed using Mann-Whitney tests, Fisher’s exact test was used for categorical analyses, and correlations were performed with Spearman’s statistic (Stata/IC 10.0 for Windows, USA).
Analysis of longitudinal data was performed using a mixed effects model. Prior to the application of the mixed effects regression, each dataset was assessed for skewness and kurtosis. Significantly skewed data was square root transformed. Mann-Whitney tests were also performed on longitudinal data assessing individual timepoints (baseline, wk12, wk24 and wk48). Logistic regression was used to investigate independent predictors of virological outcome between clearers and persisters. A significance level of 0.05 was used for all analyses.
RESULTS
Study subjects
The first 20 untreated subjects with longitudinal samples recruited into ATAHC included 12 with spontaneous viral clearance (clearers) and 8 with progression to chronic HCV infection (persisters, Table 1). The sub-groups were similar in gender, (clearers 58% males, persisters 50% males), age (clearers mean 32 years, range 21-47, persisters mean 29 years, range 18-48) and duration of infection (clearers mean 25 weeks, range 7-65, persisters mean 27 weeks, range 8-61). The subjects were all Caucasian and the most frequent mode of HCV infection was via IDU (clearers 75%, persisters 87%).
HCV-specific IFN–γ and IL-2 ELISpot responses
Early in infection (baseline), significantly more clearers had HCV-specific IFN-γ responses compared to persisters (clearers 10/12, persisters 1/8 p=0.005) and a greater number of clearers had IL-2 responses (clearers 5/12, persisters 1/8), with frequent recognition of NS3 and E2 peptide pools (Supplementary Figure 2).
Clearers had a significantly higher magnitude of IFN-γ responses (summed HCV-specific responses) at both baseline (clearers mean 370 +/- 270 SFC, persisters mean 66 +/- 62 SFC, p<0.001) and wk12 (clearers mean 291 +/- 222 SFC, persisters mean 62 +/- 62 SFC, p=0.019) compared to persisters, which then decreased after viral clearance (post wk24, Figure 1). The breadth of HCV-specific IFN-γ responses (number of the peptide pools positive) was also significantly broader in clearers early in infection at baseline (clearers mean 2 +/- 1 pools, persisters mean 0 +/- 0 pools, p=0.004) and wk12 (clearers mean 1 +/- 1 pool, persisters mean 0 +/- 0 pools, p=0.007) compared to persisters whose responses were narrow in specificity. In addition the magnitude and breadth of IL-2 responses were also higher among clearers (Figure 1).
Figure 1.
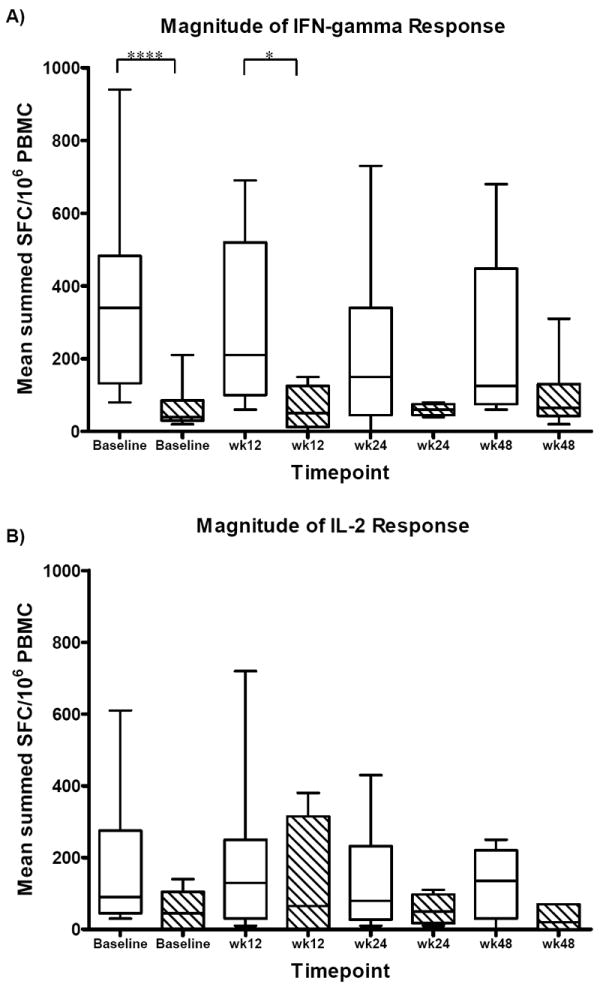
Longitudinal analysis of the magnitude (A-B) and breadth (C-D) of HCV-specific IFN-γ and IL-2 responses in clearers (n=12, white bars) and persisters (n=8, hatched bars) from baseline to week 48. A) A significantly higher magnitude of IFN-γ production was detected in clearers compared to persisters at baseline (p<0.001) and week 12 (p=0.019) by Mann-Whitney. B) Clearers maintained their HCV-specific IL-2 response with higher magnitude of IL-2 production compared to persisters screening to week 48. C) Clearers of HCV infection had significantly broader HCV-specific IFN-γ responses compared to persisters at baseline (p=0.004, Mann-Whitney) and week 12 (p=0.007, Mann-Whitney), and maintained a boarder response to week 48. D) Clearers demonstrated a trend of a broader IL-2 response baseline to week 48 compared to persisters. The horizontal solid lines in the box and whisker plots represent the median response. Four asterisks represent p<0.001, two asterisks represent p<0.01 and one asterisk represents p<0.05.
Longitudinal analysis of cytokine production using the mixed effects model, demonstrated the magnitude of both IFN-γ and IL-2 production was significantly higher in clearers (by 68 SFC p<0.001 and 27 SFC p<0.001 respectively) compared to persisters. Longitudinally, the breadth of the IFN-γ and IL-2 responses was also significantly greater in clearers compared to persisters (by one peptide pool for both cytokines IFN-γ p<0.001, IL-2 p=0.014).
Analysis of subjects with a duration of infection of 24 weeks or less (6 clearers C2, C6, C7, C9, C11, C12 and 5 persisters P1, P2, P4 P6, P7) revealed clearers had a significantly higher magnitude of IFN-γ production at baseline (mean 378 +/- 253 SFC p=0.017) and wk24 (mean 223 +/- 152 SFC p=0.033) compared to persisters (baseline mean 68 +/- 80 SFC, wk24 mean 60 +/- 20 SFC). The breadth of IFN-γ responses were also significantly broader in clearers and were maintained after viral clearance (baseline mean 2 +/- 1 pools p=0.042, wk12 mean 2 +/- 1 pools p=0.046, wk24 mean 1 +/- 1 pool p=0.034) compared to persisters (baseline, wk12, wk24 all 0 +/- 0 pools positive). In addition, clearers had significantly higher magnitude and breadth of IL-2 responses (magnitude wk24 mean 143 +/- 109 SFC p=0.046, breadth baseline mean 2 +/- 2 pools p=0.034 and wk 24 mean 1 +/- 0 pools p=0.034) compared to persisters (magnitude wk24 mean 36 +/- 25 SFC, breadth baseline and wk24 both mean 0 +/- 0 pools).
Of further note is that the genotype 1a peptide set was cross-reactive between several genotypes with positive cytokine responses detectable from genotype 1, 2, 3 and untypeable subjects. For example, at baseline persister P1 who is genotype 2a/c had an IFN-γ response of 210 SFC with 2 peptide pools positive and clearer C2 who is genotype 3a had an IFN-γ response of 380 SFC with 3 pools positive. Responses from the untypeable subjects ranged from 120 SFC with one pool positive (C8) to 940 SFC with 3 pools positive (C5). Those who were genotype 1 had responses ranging from 100 to 800 SFC with positive pools ranging from zero to four. Even though the majority of the clearers were not genotype 1, there was significantly higher magnitude and breadth of Th1 cytokine responses compared to those with viral persistence.
Multiplex in vitro cytokine production
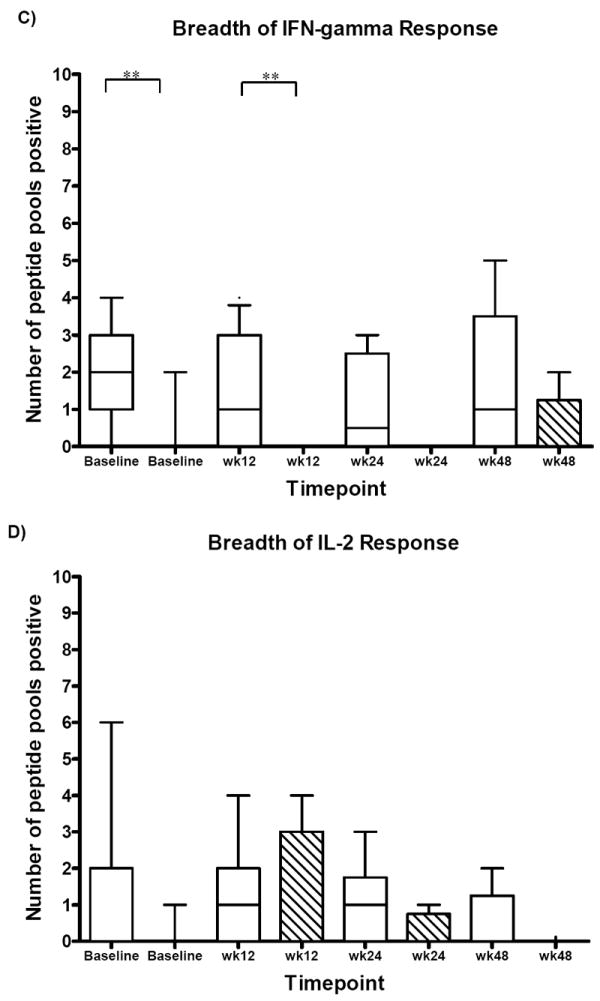
At baseline, clearers had greater IFN-γ (mean 512 pg/mL +/- 673, p=0.04) and IL-2 (mean 334 pg/mL +/- 918) production in response to HCV antigens, and lower levels of IL-10 (mean 126 pg/mL +/- 172, p=0.048) compared to persisters. In contrast, persisters had an early, high level IL-10 production (mean 357 pg/mL +/- 377), in particular to structural HCV proteins, and lower production IFN-γ (mean 27 pg/mL +/- 46) and IL-2 (mean 131 pg/mL +/- 246). This trend was also seen in the cohort of subjects with a duration of infection of 24 weeks or less (baseline clearers IFN-γ mean 206 pg/mL +/- 264, IL-10 mean 134 pg/mL +/- 212 compared to persisters IFN-γ mean 11 pg/mL +/- 15, IL-10 mean 404 pg/mL +/- 425). Representative multiplex cytokine profiles for clearers and persisters are shown in Figure 2.
Figure 2.
Multiplex cytokine production at baseline in a representative example of A) a clearer (duration of infection 25 weeks) and B) a persister (duration of infection 28 weeks) after stimulation with the three HCV peptide pools (Core-p7, NS2-3, NS4-5), control CEF peptides or PHA. Strong Type 1 cytokine responses (IFN-γ/IL-2) to HCV antigens are present in the clearer and absent in the persister. The dotted black line at 10 pg/mL represents the background cytokine production (media alone) in the assay.
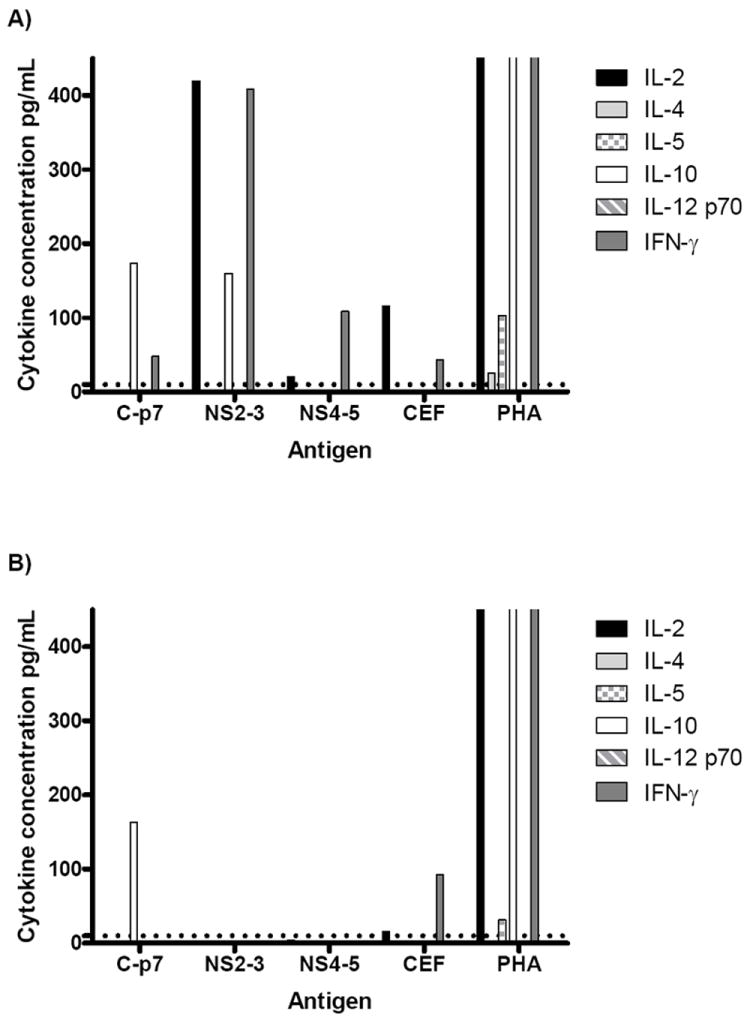
In addition to persisters having a significantly higher magnitude of IL-10 early in infection (as above, baseline p=0.048), they also had a significantly higher ratio of IL-10 to IFN-γ production (ratio IL-10:IFN-γ clearers mean 126:512 pg/mL, persisters mean 357:27 pg/mL, p=0.002, Figure 3) at baseline.
Figure 3.
Longitudinal analysis of HCV-specific A) IL-10 and B) IFN-γ production in clearers (baseline n=12, white bars) and persisters (baseline n=8, hatched bars) by multiplex cytokine assay. A) A significantly higher magnitude of IL-10 production was detected in persisters at baseline (p=0.048) compared to clearers. IL-10 production was maintained in persisters longitudinally from baseline to week 48. B) In contrast, a significantly higher magnitude of IFN-γ production was detected in clearers at baseline (p=0.04), which was maintained in clearers longitudinally from baseline to week 48. C) The ratio of IL-10 production to IFN-γ production at baseline was significantly higher in persisters (p=0.002). In graphs A to C the horizontal line indicates the median and in graph C, each dot represents a subject in the study. Three asterisks represent p<0.005 and one asterisk represents p<0.05.
Clearers had a higher level of both IFN-γ (clearers mean 1340 pg/mL +/- 1591, persisters mean 1008 pg/mL +/- 2080) and IL-10 (clearers mean 656 pg/mL +/- 694) persisters mean 558 pg/mL +/- 819) at wk48 compared to persisters. The elevated IL-10 in the clearers could possibly be a mechanism to downregulate the immune response after viral clearance and reduce inflammation in the liver.
The level of IL 10 and IFN-γ in the clearers was also compared with an unselected cohort of chronic hepatitis C subjects (n=10, chronic infection for >2 years). The magnitude of IL-10 production was significantly higher in the chronics (mean 686 pg/mL +/- 425) compared to the clearers (baseline mean 126 pg/mL +/- 172, p<0.001), while the production of IFN-γ was significantly lower in chronics (mean 183 pg/mL +/- 558) compared to clearers (baseline mean 512 pg/mL +/- 673, p=0.04, Supplementary Figure 3). Thus supporting the pattern of increased IL-10 production, in the relative absence of IFN-γ, being associated with viral persistence.
IFN-γ and IL-10 intracellular cytokine staining (ICS)
ICS was performed on baseline samples from four persisters (P1, P5, P7 and P8) and four clearers (C4, C6, C7, and C8). IFN-γ and IL-10 production was assessed using two HCV peptide pools, Core to p7 and NS4/5 pool. The majority of HCV-specific IFN-γ production was from clearers by both CD8+ T cells and CD56+ cells, with little to no production from persisters. IFN-γ production was also detected more frequently when PBMC were stimulated with the HCV antigen NS4/5 (Figure 4). IL-10 production, however was more frequently detected from the Core to p7 HCV antigen where it was produced mainly by persisters. In persisters, CD8+ T cells were a source of IL-10, with both persisters and clearers having CD14+ IL-10 producing cells (Figure 4).
Figure 4.
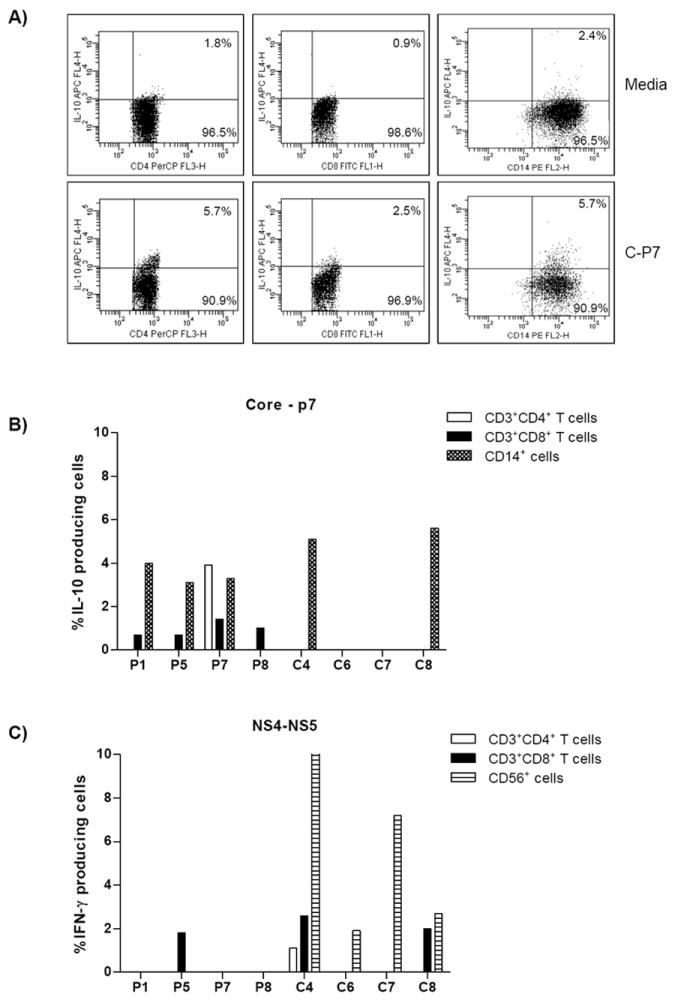
Characterization of HCV-specific IFN-γ and IL-10 producing cells by ICS. PBMCs from 4 persisters and 4 clearers were stimulated with media alone or HCV antigens (Core-p7 or NS4/5) for 3 days prior to ICS. A) IL-10 producing cells from subject P7 are presented where cells were stimulated with media alone (top row) or HCV antigen C-P7 (bottom row). Dot plot analysis of IL-10 producing cells revealed a low background in the media control, whereas C-P7 stimulated CD3+CD4+, CD3+CD8+ and CD14+ IL-10 producing cells were 3.1, 2.7 and 2.3 fold higher in number respectively. B) IL-10 production was more frequently detected following stimulation with Core-P7 antigens and from persisters. HCV-specific IL-10 production was from CD14+ cells in both persisters and clearers, while only persisters had a subset of IL-10 producing CD8+ and CD4+ T cells. C) IFN-γ producing cells were detected following stimulation with NS4/5 antigens. HCV-specific IFN-γ production was mainly from CD8+ T cells and CD56+ cells. The white bars represent CD4+ T cells, the speckled bars represent CD8+ T cells, CD56+ cells are represented by horizontally striped bars and the CD14+ cells by speckled bars. To determine the percentage of cytokine producing cells, viable cells were first gated on either CD14+ cells, CD56+ cells or CD3+ cells. The CD3+ cells were then gated on CD3+CD4+ and CD3+CD8+ T cells.
Immunological correlations with clinical parameters
Clearers and persisters did not differ in their demographic and clinical characteristics including age, gender, ethnicity, symptoms, mode of transmission, duration of infection nor ALT at baseline (p>0.05). Persisters had a significantly higher HCV RNA level at baseline compared to clearers (p=0.004).
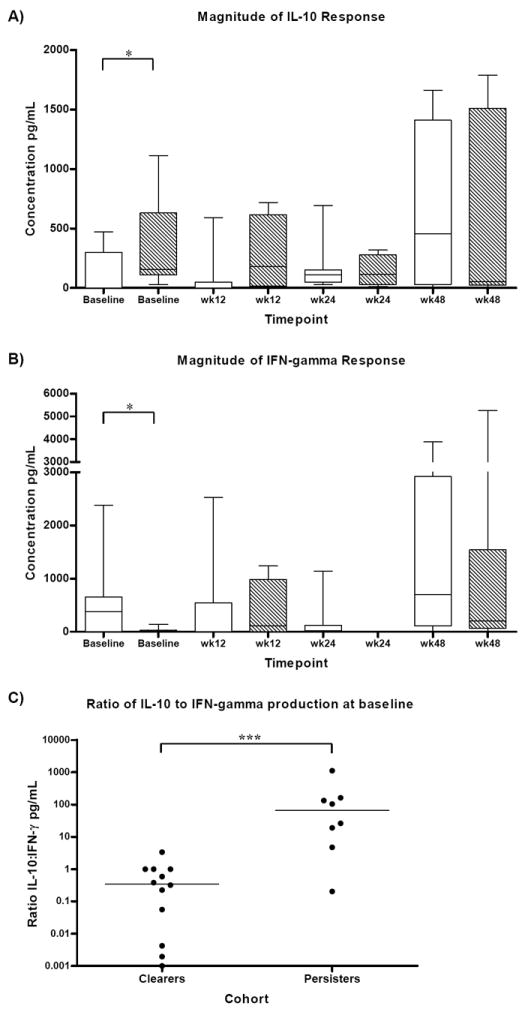
Spearman correlation analysis of the entire cohort (n=20) revealed an inverse correlation between HCV RNA level and both IFN-γ (ELISpot magnitude baseline rho= -0.51 p=0.02 and wk48 rho= -0.59 p=0.045) and IL-2 (ELISpot magnitude baseline rho= -0.44 p=0.05 and wk48 rho= -0.64 p=0.026) production at baseline and wk48. In addition, at baseline and wk12 there was a correlation between high HCV RNA level and high IL-10 production (multiplex assay baseline rho= 0.45 p=0.046, Figure 5, wk12 rho= 0.60 p=0.018). At wk48 an inverse correlation between ALT and IL-2 production (ELISpot magnitude rho=-0.65 p=0.029, multiarray rho=-0.61 p=0.047) was present. Other clinical characteristics were not associated with the cytokine profile (p>0.05).
Figure 5.
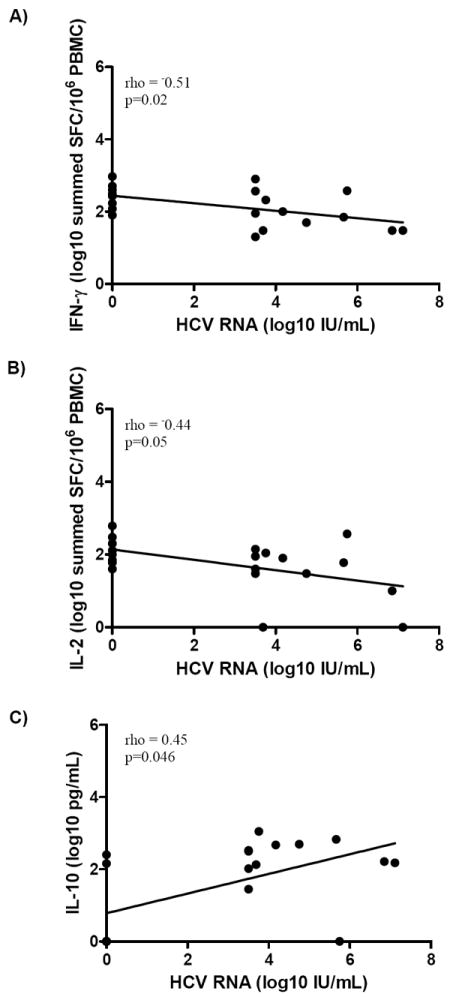
Correlation of HCV-specific cytokine production with HCV RNA levels from A) IFN-γ ELISpot assay B) IL-2 ELISpot assay and C) IL-10 production from the multiplex in vitro cytokine assay at baseline. A) High IFN-γ production was inversely correlated with HCV RNA (Spearman correlation rho=-0.51 p=0.02) B) The magnitude of IL-2 was also inversely correlated with HCV RNA (Spearman correlation rho=-0.44 p=0.05) C) In the multiarray assay, high IL-10 production was positively correlated with high HCV RNA levels (Spearman correlation rho=0.45, p=0.046).
DISCUSSION
This is the first longitudinal study of recent HCV infection in a cohort who were predominantly IDU to demonstrate high IL-10 production is associated with progression to chronic infection. This finding together with the differential patterns of IFN-γ and IL-2 production between clearers and persisters demonstrates that the magnitude and type of cytokines produced early in HCV infection are likely to be critical in determining the outcome of infection.
Previous studies that have measured cytokine levels in the sera of chronically infected subjects have been inconsistent, with some finding increased levels of IFN-γ and IL-2 [38] while others found higher levels of IL-4 and IL-10 in chronics [39, 40] compared to healthy control subjects. However, the significance of serum levels of cytokines in relation to intra-hepatic production and activity is unknown.
In the present study an early, broad and higher magnitude IFN-γ response and sustained IL-2 production were detected in clearers. In contrast, significantly higher magnitude of IL-10 production was present in persisters. The IL-10 production was largely produced in response to HCV structural antigens, in particular core peptides. Core and other HCV proteins have been shown to interfere with IFN production in vitro, disrupting the cytokine balance in favour of chronicity [22]. IL-10, therefore, appears to have a particularly important role in viral persistence, with a low level required for immune regulation, but higher levels detrimental for viral clearance [31, 34].
Previous human studies have investigated this balance using neutralizing antibodies against IL-10 in vitro. HCV-specific T cell responses in chronically infected patients were restored by blocking IL-10, resulting in increased IFN-γ production [41, 42]. By contrast, administration of recombinant IL-10 down-regulated the existing HCV-specific T cell responses and enhanced viral replication [43]. In combination with the data presented here, these studies provide evidence for the central role of IL-10 in the suppression of antiviral immunity in recent and chronic HCV infection, in particular via the ability of IL-10 to inhibit IFN production from effector cells.
The mechanisms of IL-10 inhibition of IFN-γ production include the rapid induction of suppressors of cytokine signaling (SOCS)-3 and SOCS-1 [44]. IL-10 also inhibits IFN-γ-induced gene transcription, particularly via inhibition of STAT-1 activation [44]. Interestingly this inhibition can be overcome by higher concentrations of IFN-γ relative to IL-10, suggesting IL-10 and IFN-γ-induced intracellular mechanisms are competing or interacting [44]. This finding may be pertinent in early HCV infection, where higher magnitude IFN-γ in the relative absence of IL-10 was predominant in clearers, and suggests the ratio of IFN-γ to IL-10 production early in acute infection is particularly important.
Excess IL-10 can be detrimental to viral clearance by suppressing the expression of MHC class II antigens on monocytes [44] and reducing DC ability to prime CD4+ T cells [31]. The early high magnitude IL-10 in this study is likely to promote viral persistence by suppressing cytokine production and proliferation by CD4+ and CD8+ T cells [34, 35], downregulating the CD4+ T cell response and inhibiting or altering the effector function of CD8+ T cells, promoting viral persistence.
IL-10 can also drive naïve CD4+ T cells to become regulatory T cells, enhancing IL-10 production and the likelihood of viral persistence [45]. The importance of IL-10 producing Tregs in HCV infection is not clear. Classical Treg (FoxP3+CD4+CD25+) immune suppression was been demonstrated to be higher in acute infection compared to healthy controls, although there was no difference early in infection between those with viral clearance or persistence [46]. A higher proportion of CD4+CD25+IL-10+ Treg was found in chronic HCV infection compared to recovered and normal control donors [47], although a cross-sectional study in chronic HCV infection found no difference in IL-10 production between CD4+CD25+ and CD4+CD25- T cells, with IL-10 production from peripheral CD4+ and CD8+ cells [42]. While there was no evidence for increased IL-10 producing CD4+ T cells in persisters, they demonstrated a novel CD3+CD8+ IL-10 producing population that may represent dysfunctional effector T cells.
Preliminary ICS experiments presented here in early HCV infection have indicated both CD4+ and CD8+ T cells and monocytes, as sources of IL-10 production, in response to HCV antigens. Interestingly, only in persisters was there a subset of IL-10 producing CD8+ T cells. Whether these CD4+ and CD8+ T cells are Tregs or whether the IL-10 production from monocytes is induced by antigen-specific T cells remains to be investigated.
IL-10 producing CD3+CD8+ cells have also been detected in the liver in chronic HCV infection [48, 49]. The presence of IL-10 in the liver may have a role in reducing immunopathology, but excess IL-10 may be at the cost of viral persistence. Interestingly, IL-10 production has also been demonstrated by monocytes from chronic HCV patients stimulated with Core and NS3 proteins [50-52], and HCV cell culture models have demonstrated that exposure of DCs to HCVcc (H77 and/or JFH1) inhibited maturation, impaired stimulation of CD4+ and CD8+ T cells and induced IL-10 production [53]. Similarly, in HIV infection, monocytes have been shown to be high producers of IL-10 [54]. IL-10 has several cellular sources including mast cells, eosinophils, B cells, monocytes, macrophages, DCs, CD4+ and CD8+ T cells [45], however the factors driving the production of IL-10 in acute HCV infection are not yet clear [55].
Of further significance is the finding that the magnitude of the HCV VL correlated with IL-10 production, while low HCV RNA level was associated with high IL-2 and IFN-γ production. The direct mechanism linking high HCV replication to increased IL-10 production remains to be elucidated, but is likely to be via inhibition of the induction of HCV-specific effector T cell responses.
It is also important to note some potential limitations of this study. Firstly PBMC were used to investigate and compare the magnitude of Th1 and Th2 cytokine responses in clearers and persisters, with ICS experiments indicating the cell sources of cytokine production. This assessment of HCV-specific responses is not directly from the site of infection, the liver, and speculates the trend of cytokine production and effector cell function to be relative to that observed in the periphery. Secondly genotype 1a peptides were used as this was all that was available at the time this study was conducted. The recent availability of peptide sets from other genotypes will allow more precise definition of cytokine production and cross genotype responses in future.
Recent studies revealing a link between IL-28B polymorphisms and viral clearance are of interest for subjects enrolled in the ATAHC study. In relation to this study type III IFNs are of particular interest as they are structurally related to the IL-10 family of cytokines (using the heterodimeric receptor IL-10Rβ and IL-28Rα), with functional characteristics of type I IFNs signaling through the JAK-STAT pathway [19].
In conclusion, subjects who resolved HCV infection had a strong, broad Th1 immune response in the relative absence of IL-10. In contrast, those who developed chronic infection had an early and high magnitude production of IL-10 in the relative absence of IFN-γ. Thus the ability of IL-10 to inhibit Th1 cytokine production and antigen presentation, affecting the activation and maintenance of cellular immune responses and the balance of cytokine production, appears to be a key feature promoting viral persistence. An increased understanding of the cytokine environment early in HCV infection is important for the development of novel therapeutics to facilitate viral clearance.
Supplementary Material
Acknowledgments
We thank Geza Paukovics (Burnet Institute) for assistance with flow cytometry and Tim Spelman (Burnet Institute) for assistance with statistical analysis. We also thank the subjects enrolled in the ATAHC study. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, University of New South Wales.
Financial Support:
This study was funded by NIH IROIDA 15999-01.
JF was supported by an NHMRC PhD Dora Lush scholarship and MH was supported by NHMRC and VicHealth Fellowships. RF was supported by NHMRC Industry Fellowship. GD was supported by a NHMRC Practitioner Fellowship.
List of Abbreviations
- HCV
Hepatitis C virus
- ATAHC
Australian Trial in Acute Hepatitis C
- IDU
injection drug users
- IL-10
Interleukin-10
- IFN-γ
Interferon-gamma
- IL-2
Interleukin-2
- IFN-λ
Interferon-lamda
- JAK-STAT
Janus kinases (JAKs) and Signal Transducers and Activators of Transcription (STATs)
- LCMV
Lymphocytic choriomeningitis virus
- ICS
intracellular staining
- TNF-α
tumor necrosis factor-alpha
- APC
antigen presenting cell
- Treg
classical regulatory T cells (FoxP3+CD4+CD25+)
- VL
viral load
- PBMC
peripheral blood mononuclear cell
- FCS
fetal calf serum
- SFC
spot forming cells
- SD
standard deviation
- C
Clearers
- P
Persisters
- wk
week
Footnotes
Previous Presentations:
14th International Meeting on Hepatitis C Virus & Related Viruses, Glasgow, Scotland, 9-13th September 2007 - Early predominant IL-10 production without IFN-γ in individuals with acute HCV that progress to chronic infection
Flynn J, Dore G, Hellard M, Yeung B, Crooks L, Rawlinson W, White P, Kaldor J, Lloyd A, and Ffrench R on behalf of the Australian Trial in Acute HCV (ATAHC) Study Group. Abstract number: O-19
Author Statement:
No author has a commercial or other association that might pose a conflict of interest.
Contributor Information
Jacqueline K Flynn, Email: jflynn@burnet.edu.au.
Gregory J Dore, Email: gdore@nchecr.unsw.edu.au.
Margaret Hellard, Email: hellard@burnet.edu.au.
Barbara Yeung, Email: byeung@nchecr.unsw.edu.au.
William D Rawlinson, Email: W.Rawlinson@unsw.edu.au.
Peter A White, Email: P.White@unsw.edu.au.
John M Kaldor, Email: jkaldor@nchecr.unsw.edu.au.
Andrew R Lloyd, Email: a.lloyd@unsw.edu.au.
Rosemary A Ffrench, Email: ffrench@burnet.edu.au.
References
- 1.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of Viral Hepatitis. 2006;13(1):34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–5S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 3.Gerlach JT, Diepolder HM, Jung M-C, Gruener NH, Schraut WW, Zachoval R, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology. 1999;117(4):933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 4.Lechner F, Wong DKH, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of Successful Immune Responses in Persons Infected with Hepatitis C Virus. Journal of Experimental Medicine. 2000 May 1;191(9):1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proceedings of the National Academy of Sciences. 2002 Nov 26;99(24):15661–8. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CL, Lauer GM, Robbins GK, McGovern B, Wurcel AG, Gandhi RT, et al. Broad Specificity of Virus-Specific CD4+ T-Helper-Cell Responses in Resolved Hepatitis C Virus Infection. Journal of Virology. 2002 Dec 15;76(24):12584–95. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, et al. Analysis of a Successful Immune Response against Hepatitis C Virus. Immunity. 1999;10(4):439–49. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 8.Gruner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, et al. Association of Hepatitis C Virus-specific CD8+ T Cells with Viral Clearance in Acute Hepatitis C. The Journal of Infectious Diseases. 2000;181(5):1528–36. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 9.Cucchiarini M, Kammer AR, Grabscheid B, Diepolder HM, Gerlach TJ, Gruner N, et al. Vigorous Peripheral Blood Cytotoxic T Cell Response during the Acute Phase of Hepatitis C Virus Infection. Cellular Immunology. 2000;203(2):111–23. doi: 10.1006/cimm.2000.1683. [DOI] [PubMed] [Google Scholar]
- 10.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005 Aug 18;436(7053):946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 11.Wedemeyer H, He X-S, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, et al. Impaired Effector Function of Hepatitis C Virus-Specific CD8+ T Cells in Chronic Hepatitis C Virus Infection. The Journal of Immunology. 2002 Sep 15;169(6):3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 12.Thimme R, Oldach D, Chang K-M, Steiger C, Ray SC, Chisari FV. Determinants of Viral Clearance and Persistence during Acute Hepatitis C Virus Infection. Journal of Experimental Medicine. 2001 Nov 12;194(10):1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, et al. Kinetics of CD4+ and CD8+ Memory T-Cell Responses during Hepatitis C Virus Rechallenge of Previously Recovered Chimpanzees. Journal of Virology. 2003 Apr 15;77(8):4781–93. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woollard DJ, Grakoui A, Shoukry NH, Muthy KK, Campbell KJ, Walker CM. Characterization of HCV-specific Patr class II restricted CD4+ T cell responses in an acutely infected chimpanzee. Hepatology. 2003;38(5):1297–306. doi: 10.1053/jhep.2003.50478. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Sallberg M, Sonnerborg A, Weiland O, Mattsson L, Jin L, et al. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116(1):135–43. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 16.Mizukoshi E, Eisenbach C, Edlin BR, Newton KP, Raghuraman S, Weiler-Normann C, et al. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. Journal of Infectious Diseases. 2008 Jul 15th;198(2):203–12. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proceedings of the National Academy of Sciences. 2004 Jul 6;101(27):10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’hUigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nature Genetics. 2009 Oct;41(10):1100–5. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 20.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Butera M, Nelson D, Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virology Journal. 2005;2(1):80. doi: 10.1186/1743-422X-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freundt EC, Lenardo MJ. Interfering with interferons: Hepatitis C virus counters innate immunity. Proceedings of the National Academy of Sciences. 2005 Dec 6;102(49):17539–40. doi: 10.1073/pnas.0509221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaech SM, Ahmed R. CD8 T cells remember with a little help. Science. 2003 Apr 11;300(5617):263–3. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 24.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. Journal of Virology. 1994 Dec 1;68(12):8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, et al. Different clinical behaviours of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. Journal of Clinical Investigation. 1996 May 17;98(3):706–14. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, et al. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41(5):1019–28. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- 27.Nisii C, Tempestilli M, Agrati C, Poccia F, Tocci G, Longo MA, et al. Accumulation of dysfunctional effector CD8+T cells in the liver of patients with chronic HCV infection. Journal of Hepatology. 2006;44(3):475–83. doi: 10.1016/j.jhep.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Wherry JE, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. Journal of Virology. 2003 Apr 15;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wherry JE, Ahmed R. Memory CD8 T-Cell Differentiation during Viral Infection. Journal of Virology. 2004 Jun 1;78(11):5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francavilla V, Accapezzato D, De Salvo M, Rawson P, Cosimi O, Lipp M, et al. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. European Journal of Immunology. 2004;34(2):427–37. doi: 10.1002/eji.200324539. [DOI] [PubMed] [Google Scholar]
- 31.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MBA. Interleukin-10 determines viral clearance or persistence in vivo. Nature Medicine. 2006;12(11):1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. Journal of Experimental Medicine. 2006 Oct 30;203(11):2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi CM, von Herrath MG. IL-10 and the resolution of infections. The Journal of Pathology. 2008;214(2):224–30. doi: 10.1002/path.2272. [DOI] [PubMed] [Google Scholar]
- 34.Blackburn SD, Wherry JE. IL-10, T cell exhaustion and viral persistence. Trends in Microbiology. 2007;15(4):143–6. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Opal SM, Huber CE. The role of interleukin-10 in critical illness. Current Opinion in Infectious Diseases. 2000;13:221–6. doi: 10.1097/00001432-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Dore GJ, Hellard M, Matthews G, Grebely J, Haber PS, Petoumenos K, et al. Effective Treatment of Injecting Drug Users With Recently Acquired Hepatitis C Virus Infection. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruys TA, Nanlohy NH, van den Berg CHSB, Hassink E, Beld M, van de Laar T, et al. HCV-specific T-cell responses in injecting drug users: evidence for previous exposure to HCV and a role for CD4+ T cells focussing on nonstructural proteins in viral clearance. Journal of Viral Hepatitis. 2008;15(6):409–20. doi: 10.1111/j.1365-2893.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 38.Cribier B, Schmitt C, Rey D, Lang J-M, Kirn A, Stoll-Keller F. Production of cytokines in patients infected by hepatitis C virus. Journal of Medical Virology. 1998;55(2):89–91. doi: 10.1002/(sici)1096-9071(199806)55:2<89::aid-jmv1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Reiser M, Marousis CG, Nelson DR, Lauer G, Gonzalez-Peralta RP, Davis GL, et al. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. Journal of Hepatology. 1997;26(3):471–8. doi: 10.1016/s0168-8278(97)80409-6. [DOI] [PubMed] [Google Scholar]
- 40.Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: Pre- and posttreatment with interferon alfa. Hepatology. 1996;24(1):6–9. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 41.Piazzolla G, Tortorella C, Schiraldi O, Antonaci S. Relationship between interferon-γ, interleukin-10, and interleukin-12 production in chronic hepatitis C and in vitro effects of interferon-α. Journal of Clinical Immunology. 2000;20(1):54–61. doi: 10.1023/a:1006694627907. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, et al. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. Journal of hepatology. 2008;48(6):903–13. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigopoulou EI, Abbott WGH, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor - a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clinical Immunology. 2005;117(1):57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the Interleukin-10 receptor. Annual Review of Immunology. 2001;19(1):683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 45.O’Garra A, Vieira P. TH1 cells control themselves by producing interleukin-10. Nature Reviews Immunology. 2007;7(6):425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 46.Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR, Jr, Tester IA, Wang CC, et al. Functional suppression by FoxP3+CD4+CD25(high) regulatory T cells during acute hepatitis C virus infection. The Journal of Infectious Diseases. 2008;197(1):46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- 47.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004 Nov;40(5):1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 48.Abel M, Sene D, Pol S, Bourliere M, Poynard T, Charlotte F, et al. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006 Dec;44(6):1607–16. doi: 10.1002/hep.21438. [DOI] [PubMed] [Google Scholar]
- 49.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. Journal of Clinical Investigation. 2004 Apr;113(7):963–72. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amaraa R, Mareckova H, Urbanek P, Fucikova T. Production of interleukins 10 and 12 by activated peripheral blood monocytes/macrophages in patients suffering from chronic hepatitis C virus infection with respect to the response to interferon and ribavirin treatment. Immunology Letters. 2002;83(3):209–14. doi: 10.1016/s0165-2478(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 51.Kakumu S, Okumara A, Ishikawa T, Iwata K, Yano M, Yoshioka K. Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clinical and Experimental Immunology. 1997;108(1):138–43. doi: 10.1046/j.1365-2249.1997.d01-987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics LJ, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro-and anti-inflammatory cytokines and inhibit dendritic cell differentiation. Journal of Immunology. 2003;170:5615–24. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 53.Saito K, Ait-Goughoulte M, Truscott SM, Meyer K, Blazevic A, Abate G, et al. Hepatitis C Virus Inhibits Cell Surface Expression of HLA-DR, Prevents Dendritic Cell Maturation, and Induces Interleukin-10 Production. Journal of Virology. 2008 Apr 1;82(7):3320–8. doi: 10.1128/JVI.02547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji J, Sahu GK, Braciale VL, Cloyd MW. HIV-1 induces IL-10 production in human monocytes via a CD4-independent pathway. Int Immunol. 2005 Jun 1;17(6):729–36. doi: 10.1093/intimm/dxh252. [DOI] [PubMed] [Google Scholar]
- 55.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nature Immunology. 2007;8(12):1281–3. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


