Abstract
A two-by-two factorial experiment with pigs was conducted to study the effect of feed grinding (fine and coarse) and feed processing (pelleted and nonpelleted) on physicochemical properties, microbial populations, and survival of Salmonella enterica serovar Typhimurium DT12 in the gastrointestinal tracts of pigs. Results demonstrated a strong effect of diet on parameters measured in the stomachs of the pigs, whereas the effect was less in the other parts of the gastrointestinal tract. Pigs fed the coarse nonpelleted (C-NP) diet showed more solid gastric content with higher dry matter content than pigs fed the fine nonpelleted (F-NP), coarse pelleted (C-P), or fine pelleted (F-P) diet. Pigs fed the C-NP diet also showed significantly increased number of anaerobic bacteria (P < 0.05), increased concentrations of organic acids, and reduced pH in the stomach. In addition, pigs fed the C-NP diet showed increased in vitro death rate of S. enterica serovar Typhimurium DT12 in content from the stomach (P < 0.001). Pigs fed the C-NP diet had a significantly higher concentration of undissociated lactic acid in gastric content than pigs fed the other diets (P < 0.001). A strong correlation between the concentration of undissociated lactic acid and the death rate of S. enterica serovar Typhimurium DT12 was found. In the distal small intestine, cecum, and midcolon, significantly lower numbers of coliform bacteria were observed in pigs fed the coarse diets than in pigs fed the fine diets (P < 0.01). Pigs fed the C-NP diet showed the lowest number of coliform bacteria in these segments of the gastrointestinal tract. Pigs fed the coarse diets showed increased concentration of butyric acid in the cecum (P < 0.05) and colon (P < 0.10) compared with pigs fed the fine diets. It was concluded that feeding a coarsely ground meal feed to pigs changes the physicochemical and microbial properties of content in the stomach, which decreases the survival of Salmonella during passage through the stomach. In this way the stomach acts as a barrier preventing harmful bacteria from entering and proliferating in the lower part of the gastrointestinal tract.
Human food-borne disease outbreaks are a public health concern and have economic importance to the food-producing industry. Subclinical Salmonella enterica infections in pig herds are recognized as an important source of human salmonellosis in Denmark (29). A Danish Salmonella surveillance and control program in pig herds and slaughterhouses has been applied nationally since 1995, and the goal is to reduce the Salmonella prevalence in pork meat to 0.5% (23). In year 2002, the incidence of Salmonella-positive carcass samples in Danish slaughterhouses was 1.4% and the corresponding Salmonella prevalence at herd level monitored by serological testing was 3.2% (1).
There is a growing awareness that it is only possible to reduce the prevalence of Salmonella in pork, and consequently the number of infections in people consuming pork and pork products, through an integration and cooperation of all stages of the pork production chain. This is indicated by several studies. Berends et al. (3) found a strong correlation between the number of live animals that carry Salmonella spp. in their feces and the number of contaminated carcasses in the end of the slaughter line (3). Hurd et al. (12) reported that transport to the slaughterhouse and especially holding in pens at the slaughterhouse increased the prevalence of S. enterica-positive pigs by sevenfold compared with the prevalence among pigs from the same farms but necropsied on the farm (12). These studies also indicate that, if salmonella-free pigs are produced, consumers can be provided with virtually salmonella-free pork products.
There is strong evidence that the physical characteristics of the feed influence the susceptibility of pigs to Salmonella. Feeding a ready-mixed pelleted dry feed increases the risk of Salmonella infection compared with home-mixed, nonpelleted wet feed (17, 28). Coarse grinding of feed also reduces the incidence of Salmonella in pigs compared to fine grinding (15, 30). However, feeding a coarse and/or nonpelleted feed to pigs results in poor growth performance (6, 15, 19, 31, 32) and consequently is an economic disadvantage for the pig producer. To design alternative feeds with similar Salmonella-reducing properties but with improved growth performance, it would be of great importance to determine how coarse feed influences the survival of Salmonella in the gastrointestinal tracts of pigs.
The aim of the present investigation was to study the factors behind the lower incidences of Salmonella in pigs fed a coarse feed. A two-by-two factorial experiment in which pigs were fed a fine or coarse feed given to the animals either as nonpelleted or pelleted feed was carried out. The effect of the experimental diets on physicochemical properties and microbial populations and activity in the gastrointestinal tracts of slaughtered pigs was investigated. An in vitro method was performed to study the survival of S. enterica serovar Typhimurium DT12 in stomach and cecal contents from the pigs fed the different experimental diets. This Salmonella strain is a frequent cause of human salmonellosis and is also common in pig herds in Denmark.
MATERIALS AND METHODS
Animals and feed.
A total of 96 crossbreed Danish Landrace × Yorkshire pigs (average weight, 33 ± 7 kg) were obtained from the herd at the Research Center Foulum, Foulum, Denmark, and assigned to one of four treatment groups in a two-by-two factorial design experiment. The pigs were allocated to the treatment groups with an equal distribution for litter and sex. The experiment was carried out in six replicates of four pigs per group housed together in a pen with no physical or visual contact between animals in different pens. The pigs were fed ad libitum and had free access to water. The feed contained barley (35.9%), wheat (35.9%), soybean meal (12.3%), rapeseed meal (5%), sunflower meal (5%), animal fat (2.3%), dicalcium phosphate (1.2%), calcium carbonate (0.8%), sodium chloride (0.4%), lysine (0.3%), methionine (0.12%), threonine (0.08%), vitamin-mineral mixture (0.2%), and chromic oxide (0.4%). The feed was ground at two levels to obtain a fine or coarse feed and given to the animals as either nonpelleted or pelleted feed.
The four types of experimental feed, fine nonpelleted (F-NP), fine pelleted (F-P), coarse nonpelleted (C-NP), and coarse pelleted (C-P), were analyzed for particle size distribution by a wet-sieve method. Four hundred milliliters of deionized water at 20°C was added to 100 g of feed. The solution was kept for 1 h at room temperature without stirring. The suspension was then sieved for 2 min, at a 3-mm amplitude, with an AS200 control “g” sieve shaker (RETSCH GmbH & Co., Haan, Germany). The sieves (interior dimensions, 200 by 50 mm; DIN ISO 3310/1) were made of stainless steel, and the following mesh sizes were used 3,150, 2,000, 1,000, 500, and 250 mm. The different particle fractions were then dried to constant weight at 100°C and weighed.
Weights of the pigs and feed consumption per pen were registered every week during the experiment. Performance of the pigs (30 to 100 kg) per pen was calculated on the basis of four pigs per pen from day 0 to 28 of the experiment and three pigs per pen for the remaining period until the pigs weighed approximately 100 kg. The pigs were checked daily for signs of diarrhea and given a score on a scale from 0 to 3, with 3 corresponding to aqueous feces, 2 corresponding to thin feces, 1 corresponding to porridge-like feces, and 0 corresponding to normal consistency of feces. The experiment complied with the guidelines of the Danish Ministry of Justice with respect to animal experimentation and care of animals under study.
Collection of intestinal samples.
After 28 days on the experimental diets, one pig per replicate from each experimental group (n = 6) was killed with a lethal injection of pentobarbital sodium (200 g/liter). The gastrointestinal tract was immediately removed and divided into eight segments: stomach, three equal parts of the small intestine, cecum, and three equal parts of the colon including the rectum. The total content from each segment was collected and weighed. Immediately, pH was measured by direct insertion of a pH electrode (Radiometer S.A., Villeurbanne, France) into the matrices, and samples for determination of microbial activity were processed as described below. The intestinal samples were then taken to the laboratory for further analysis.
Examination for gastric ulcer.
At slaughter, the gastric epithelium of the pars oesophagea was visually examined for possible alterations. The scale proposed by Nielsen and Ingvartsen (24) was used, with 0 corresponding to normal epithelium and 10 corresponding to severe constriction due to gastric ulcer.
Physicochemical analyses.
Intestinal samples from all segments were analyzed for dry matter content, determined by freeze-drying, and for the concentration of organic acids (formic acid, short-chain fatty acids [SCFA], lactic acid, and succinic acid) by the method described by Jensen et al. (14). A rheological characterization was done on samples from the stomach. In brief, the samples were centrifuged at 12,000 × g for 20 min at 4°C in capped 50-ml centrifuge tubes. After the tubes were weighed, the supernatant fraction was removed by suction and the centrifuge tubes were turned upside down to drain off residual supernatant fraction for at least 1 h before reweighing. The sediment was subsequently dried at 105°C to constant weight. The water-binding capacity was calculated as the amount of water that was left after centrifugation and removed during the drying process. The sedimentation ability was measured by removing 100 ml of stomach content, placing it in a cylinder tube at 5°C, and subsequently measuring the distribution in fluid and sediment after 24 h.
Microbial determinations.
Feed samples (10.0 g) were mixed with 90 ml of peptone water containing 1% Bacto Peptone (1.07213; Merck, Darmstadt, Germany) and 0.1% Tween 80 and homogenized in a stomacher laboratory blender (Interscience, St. Nom, France) for 2 min. Tenfold dilution series in peptone water were prepared. Total aerobic bacteria were enumerated on plate count agar (Merck; 5463) and coliform bacteria and lactose-negative enterobacteria were enumerated on MacConkey agar (Merck; 5465) after incubation at 38°C for 24 h. Lactic acid bacteria were enumerated on de Man, Rogosa, and Sharpe agar (MRS; Merck; 10660) after anaerobic incubation at 20°C for 48 h. Yeast was enumerated on malt chloramphenicol agar (MCA) containing 10 g of glucose, 5 g of malt extract, 10 g of peptone, 15 g of agar, and 0.050 g of chloramphenicol/liter of distilled water after incubation at 20°C for 72 h.
Gastrointestinal samples (10.0 g) from the stomach, distal third of the small intestine, cecum, and midthird of the colon were transferred under a flow of CO2 into sterile serum bottles containing 90 ml of a prereduced salt medium (11). The suspension was poured into a CO2-flushed plastic bag and homogenized in a stomacher laboratory blender for 2 min. Serial 10-fold dilutions were performed in prereduced salt medium by the technique of Miller and Wolin (21). The number of total culturable anaerobic bacteria was determined with anaerobic roll tubes containing rumen fluid-glucose-cellobiose-agar (11) after incubation at 38°C for 7 days. Coliform bacteria and lactose-negative enterobacteria were enumerated on MacConkey agar after incubation at 38°C for 24 h. Lactic acid bacteria and yeast were enumerated on MRS and MCA, respectively, after anaerobic incubation of MRS plates and aerobic incubation of MCA plates at 38°C for 48 h.
Determination of microbial activity.
Content (5.0 g) from each of the eight segments was extracted with 10.0 ml of perchloric acid (PCA)-EDTA (2 M cold PCA containing 10 mM EDTA) and stored at −80°C. The concentration of ATP in digesta was determined by the luciferin-luciferase method as described by Jensen and Jørgensen (13).
In vitro survival of Salmonella in stomach and cecal content.
Contents from the stomach and cecum (stored at −20°C until use) were thawed, and 10.0-g samples were added to small stomacher plastic bags and inoculated with a 1.0-ml suspension of S. enterica serovar Typhimurium DT12 grown in brain heart infusion broth (Merck; 1.10493). The inoculum was prepared as described by Naughton et al. (22). The stomacher bags were flushed with CO2, sealed airtight, and incubated at 38°C in a shaking water bath. For each sample, four identical subsamples in stomacher bags were prepared, one for each sampling time, at 0, 2, 4, and 6 h of incubation. At sampling, the stomacher bag was cut open and pH was measured by direct insertion of a pH electrode (Radiometer S.A.) into the contents. Then, 90 ml of peptone water was added and the whole solution was poured into a CO2-flushed stomacher bag and homogenized for 2 min. A sample (1 ml) was taken and analyzed for the concentration of organic acids (formic acid, SCFA, lactic acid, and succinic acid) as described by Jensen et al. (14). Then, serial 10-fold dilutions were performed in peptone water. The enumeration of salmonellae was performed on MacConkey agar and brilliant phenol-lysine-sucrose agar (Merck; 1.10747) after aerobic incubation at 38°C for 16 h.
Calculations.
The death rate (μ) of S. enterica serovar Typhimurium DT12 in contents from the stomach and cecum was calculated from semilogarithmic plots of CFU during the 6-h incubation period for stomach contents and 4-h incubation period for cecal contents. The bacterial half-life (T1/2) was calculated from the equation T1/2 = ln 2/μ as described by Knarreborg et al. (16).
Concentrations of undissociated SCFA and lactic acid were calculated from the Henderson-Hasselbach equation: pH = pKa + log10 [A−]/[HA], where [A−] and [HA] represent the concentrations of dissociated and undissociated acids, respectively. The average pH and concentrations of SCFA and lactic acid during the 6- and 4-h incubation periods for stomach and cecal contents, respectively, were used for the calculations. The pKa values are 4.75 for acetic acid, 4.81 for butyric acid, 4.87 for propionic acid, and 3.84 for lactic acid (5).
Statistics.
The effect of grinding (fine versus coarse), form of feed (pelleted versus nonpelleted), and diet (interaction between grinding and form of feed) was analyzed statistically by a two-factorial linear model using the PROC MIXED procedure of SAS (18). Litter was included in the statistical model as a random effect. If the interaction between grinding and form of feed was not significant (P < 0.05), it was omitted from the model. Data obtained from the gastrointestinal tract were analyzed by segment. Statistical analysis of bacterial counts was performed after logarithmic conversion of the data. Results are expressed as least-square means and standard errors of the means (SEM). If the overall effect of a main factor was significant, differences between means were compared by the Fisher's least significant difference and considered significant at P values of <0.05.
RESULTS
Feed.
The distributions of particle sizes in the experimental diets are given in Table 1. The highest percentage of larger particles (>1,000 μm) was found in the C-NP diet, followed by the C-P diet, with an intermediate percentage, and low percentages of particles larger than 1,000 μm in the F-NP and F-P diets. The distribution of bacteria in the feed is also given in Table 1. The numbers of total aerobic bacteria, coliform bacteria, and yeast in the nonpelleted feed were significantly higher than those in the pelleted feed (P < 0.01). There were no differences in the number of lactic acid bacteria among the diets (Table 1).
TABLE 1.
Particle size distribution and microbiological counts of the experimental diets
| Diet | Mean (SD) fraction of particles (g/100 g) of size (μm):
|
Microbiological count (log10 CFU/g) ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| >3,150 | 2,000-3,150 | 1,000-2,000 | 500-1,000 | 250-500 | <250 | Total anaerobic bacteria | Coliform bacteria | Lactic acid bacteria | Yeast | |
| F-NP | 0.0 (0.0) | 0.0 (0.0) | 7.8 (0.6) | 16.1 (0.5) | 9.7 (0.9) | 66.3 (2.0) | 5.64 (0.56)A | 5.10 (0.68)A | 3.29 (0.22) | 4.33 (1.06)A |
| F-P | 0.0 (0.0) | 0.0 (0.0) | 5.0 (0.9) | 16.0 (0.8) | 9.3 (1.1) | 69.7 (1.3) | 4.64 (0.46)B | 3.45 (0.00)B | 3.76 (0.63) | 3.30 (0.47)B |
| C-NP | 3.7 (3.0) | 2.2 (1.5) | 17.9 (1.0) | 12.1 (1.5) | 6.7 (0.9) | 57.5 (3.3) | 5.66 (0.80)A | 4.88 (1.32)A | 3.31 (0.38) | 4.43 (0.99)A |
| C-P | 1.0 (0.5) | 2.0 (1.8) | 13.2 (1.7) | 12.0 (0.6) | 7.3 (0.4) | 64.4 (2.3) | 4.07 (0.98)B | 3.14 (1.46)B | 3.42 (0.07) | 3.14 (1.35)B |
Values within a column that do not have the same superscript letter are significantly different (P < 0.01).
Animal performance.
There were no significant differences in daily weight gain or feed intake between pigs fed the different experimental diets. The feed conversion ratio was significantly better (2.53 versus 2.69 kg of feed/kg of weight gain; P < 0.01) for pigs fed the pelleted feed than for those fed the nonpelleted feed (results not shown). Pigs fed the pelleted feed showed a significantly higher diarrhea score than those fed the nonpelleted feed (0.33 versus 0.02; P < 0.01). There was no effect of grinding (fine versus coarse) on the diarrhea scores (results not shown). The gastric alteration scores are given in Table 2. All pigs fed the C-NP diet had a gastric alteration score of 0, which corresponds to an undamaged epithelium. Both fine versus coarse feed and pelleted versus nonpelleted feed afforded significantly higher scores for altered gastric epithelia of the pigs (Table 2).
TABLE 2.
Gastric alterations and physicochemical properties of stomach content
| Variable | Valuea for diet
|
SEM | Significanceb
|
|||||
|---|---|---|---|---|---|---|---|---|
| F-NP | F-P | C-NP | CP | Grinding | Form | Grinding form | ||
| Gastric alteration score | 2.2 | 4.2 | 0 | 3.2 | 0.7 | * | ** | NS |
| Sedimentation (%) | 72.7 | 64.7 | 100.0 | 77.3 | 5.3 | *** | ** | (*) |
| Water-binding capacity (%) | 40.1A | 33.0A | 80.5B | 42.4A | 4.4 | *** | *** | ** |
| Dry matter (%) | 19.4 | 17.1 | 27.5 | 20.5 | 2.1 | ** | * | NS |
| pH | 3.93AB | 3.7AB | 3.38A | 4.19B | 0.3 | NS | NS | * |
| Acetic acid concnc | 2.6 | 0.0 | 10.8 | 4.5 | 1.6 | *** | * | NS |
| Propionic acid concnc | 0.0A | 0.0A | 5.7B | 0.2A | 1.0 | ** | * | * |
| Butyric acid concnc | 0.0 | 0.0 | 1.6 | 0.2 | 0.7 | * | (*) | (*) |
| Valeric acid concnc | 0.0 | 0.0 | 0.3 | 0.0 | 0.1 | NS | NS | NS |
| Lactic acid concnc | 1.6 | 1.9 | 20.3 | 10.9 | 3.4 | *** | NS | NS |
Values are least-square means (n = 6). Values within a row not having the same superscript are significantly different.
Grinding, fine versus coarse; form, nonpelleted versus pelleted. NS, P > 0.1; (*), P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Units are millimeters per kilogram.
Rheological characteristics of stomach content.
The sedimentation ability and water-binding capacity of stomach content are shown in Table 2. A consistent high sedimentation percentage (100%), corresponding to no separation into fluid and sediment, was observed for stomach contents of pigs fed the C-NP diet. Conversely, the lowest sedimentation percentage, corresponding to the most pronounced separation of stomach content into a watery phase and a precipitate, was observed for pigs fed the F-P diet (Table 2). The coarse versus fine feed and the nonpelleted versus pelleted feed both resulted in significantly higher sedimentation percentages of the stomach content (P < 0.0001 and P < 0.01, respectively). Significantly higher water-binding capacity was observed for stomach contents from pigs fed the C-NP diet (P < 0.01) than for those from pigs fed the other diets (Table 2).
Gastrointestinal digesta, pH, and concentration of organic acids.
The amounts of digesta from the various segments of the gastrointestinal tract were not affected by the experimental diets (results not shown). The dry matter percentage of content from the stomach was significantly higher for pigs fed the coarse feed than for pig fed the fine feed (P < 0.01) and was also higher for pigs fed the nonpelleted feed than for pigs fed the pelleted feed (P < 0.05; Table 2). No differences in dry matter percentages of digesta were observed in the small intestines, ceca, and colons of the pigs (results not shown).
The pH and organic acid concentrations in stomach contents of pigs fed the experimental diets are also given in Table 2. The pH in stomach content was lowest for pigs fed the C-NP diet and differed significantly from the pH of stomach contents found in pigs fed the C-P diet (Table 2). In general, no differences in pH were observed in the small intestines, ceca, and colons of pigs fed the different diets (results not shown).
The concentration of organic acids in the stomach of the pigs was strongly affected by the experimental diet (Table 2). The highest concentrations of the various organic acids in stomach content were observed for pigs fed the C-NP diet, although the effect of diet was not significant for all the individual organic acids (Table 2). The concentration of acetic acid was significantly higher in pigs fed the coarse feed than in pigs fed the fine feed (P < 0.001) and was significantly higher in pigs fed the nonpelleted feed than in pigs fed the pelleted feed (P < 0.05). The concentration of propionic acid was significantly higher in pigs fed the C-NP diet than in pigs fed the other diets (P < 0.05). The concentrations of butyric and lactic acid were significantly higher in stomach contents of pigs fed the coarse feed than in those of pigs fed the fine feed (P < 0.05 and P < 0.001, respectively). Valeric acid was observed in minor concentrations only in pigs fed the C-NP diet (Table 2).
In the other compartments of the intestinal tract, generally little or no effect on the concentrations of organic acids was observed (results not shown). However, the distal small intestines of pigs fed the nonpelleted feed had a significantly higher concentration of formic acid than those of pigs fed the pelleted feed (17.5 versus 12.4 mmol/kg; P < 0.05). The concentration of butyric acid was significantly higher in the ceca (14.8 versus 11.6 mmol/kg; P < 0.05) and almost significantly higher in the midcolons (19.9 versus 18.0 mmol/kg; P < 0.10) of pigs fed the coarse feed than in those of pigs fed the fine feed (results not shown).
Microbial populations and activity.
The numbers of total anaerobic bacteria, lactic acid bacteria, coliform bacteria, and yeast and the concentration of ATP in the gastrointestinal tracts of pigs fed the experimental diets are given in Tables 3 and 4. The number of total anaerobic bacteria in the stomachs of pigs fed the C-NP diet was significantly higher than in those of pigs fed the other diets (P < 0.05). Besides this, the coarse feed resulted in significantly higher numbers of lactic acid bacteria (P < 0.05) in the stomach than fine feed and nonpelleted feed gave significantly higher numbers of yeast (P < 0.05) in the stomach than pelleted feed (Table 3). There were no differences in the numbers of coliform bacteria in the stomachs of pigs fed the different diets. However, in the distal small intestine, cecum, and midcolon, significantly higher numbers of coliform bacteria were observed in pigs fed the fine feed than in pigs fed the coarse feed (Table 3). The midcolons of pigs fed the F-NP diet had a significantly higher (P < 0.01) number of coliform bacteria than those of pigs fed the other diets (Table 3). The number of lactic acid bacteria was significantly higher (P < 0.05) in the ceca of pigs fed the F-P diet than in ceca of pigs fed the F-NP diet. Likewise, the number of total anaerobic bacteria in the midcolons of pigs fed the F-P diet was significantly higher (P < 0.05) than in those of pigs fed the F-NP diet (Table 3).
TABLE 3.
Microbiological counts in gastrointestinal content
| Contentc | Organisms | Counta (log10 CFU/g) for diet:
|
SEM | Significanceb
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| F-NP | F-P | C-NP | C-P | Grinding | Form | Grinding form | |||
| Stomach | Total anaerobic bacteria | 7.06A | 7.16A | 8.53B | 7.59A | 0.28 | *** | (*) | * |
| Lactic acid bacteria | 6.81 | 6.98 | 7.88 | 7.39 | 0.36 | * | NS | NS | |
| Coliform bacteria | 4.98 | 4.55 | 4.35 | 4.73 | 0.34 | NS | NS | NS | |
| Yeast | 4.57 | 3.95 | 4.60 | 4.02 | 0.37 | NS | * | NS | |
| SI 3 | Total anaerobic bacteria | 8.39 | 8.64 | 8.67 | 8.24 | 0.20 | NS | NS | (*) |
| Lactic acid bacteria | 7.89 | 8.42 | 8.50 | 8.31 | 0.25 | NS | NS | (*) | |
| Coliform bacteria | 6.65 | 6.46 | 6.01 | 6.05 | 0.21 | * | NS | NS | |
| Yeast | 5.54 | 4.89 | 5.20 | 5.12 | 0.28 | NS | NS | NS | |
| Cecum | Total anaerobic bacteria | 9.69 | 9.95 | 9.63 | 9.69 | 0.12 | NS | NS | NS |
| Lactic acid bacteria | 8.43A | 9.10B | 9.05AB | 8.64AB | 0.25 | NS | NS | * | |
| Coliform bacteria | 6.84 | 7.25 | 5.92 | 6.33 | 0.25 | *** | NS | NS | |
| Yeast | 5.61 | 5.80 | 5.82 | 5.29 | 0.29 | NS | NS | NS | |
| Co 2 | Total anaerobic bacteria | 9.66A | 10.22B | 9.91AB | 9.91AB | 0.13 | NS | * | * |
| Lactic acid bacteria | 8.88 | 8.85 | 9.42 | 8.94 | 0.34 | NS | NS | NS | |
| Coliform bacteria | 7.53A | 6.44B | 5.87B | 6.45B | 0.32 | ** | NS | ** | |
| Yeast | 5.39 | 4.65 | 5.58 | 5.48 | 0.40 | (*) | NS | NS | |
Values are least-square means (n = 6). Values within a row not having the same superscript are significantly different (P < 0.001).
Grinding, fine versus coarse; form, nonpelleted versus pelleted. Significance: NS, P > 0.1; (*), P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
SI 3, distal one-third of the small intestine; Co 2, mid-one-third of the colon.
TABLE 4.
Concentration of ATP in gastrointestinal contenta
| Content | ATP concnb (μg/g) for diet:
|
SEM | Significance
|
|||||
|---|---|---|---|---|---|---|---|---|
| F-NP | F-P | C-NP | C-P | Grinding | Form | Grinding + form | ||
| Stomach | 0.5A | 0.4A | 2.8B | 0.7A | 0.5 | ** | * | * |
| SI 3 | 5.6 | 4.6 | 7.5 | 3.8 | 1.0 | NS | * | NS |
| Cecum | 21.3 | 13.0 | 17.6 | 14.2 | 2.3 | NS | * | NS |
| Co 2 | 16.9A | 7.1B | 12.3AC | 9.7BC | 2.3 | NS | ** | * |
SI 3, Co 2, NS, and asterisks are as defined for Table 3.
Values are least-square means. Values in a row not having the same superscript are significantly different.
A significantly higher concentration of ATP was found in the stomachs of pigs fed the C-NP diet (P < 0.05) than in those of pigs fed the other diets (Table 4). Higher ATP concentrations were found the distal small intestines, ceca, and colons of pigs fed the nonpelleted than in those of pigs fed the pelleted feed, and this difference was significant in the distal small intestine and cecum (P < 0.05).
In vitro death rate of Salmonella serovar Typhimurium DT12 in contents from the stomach and cecum.
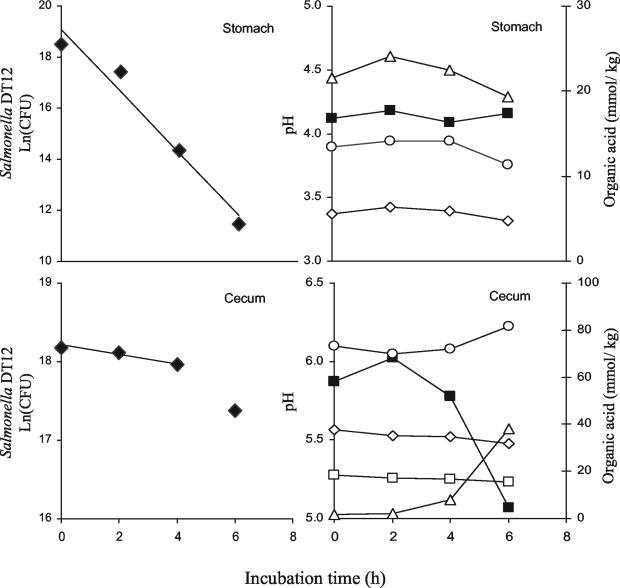
Figure 1 shows an example of typical time courses for the density of the inoculated S. enterica serovar Typhimurium DT12, pH, and the concentrations of SCFA and lactic acid in content from the stomach and cecum during the in vitro incubation. In content from the stomach, the density of S. enterica serovar Typhimurium DT12 declined exponentially during the incubation and the specific death rates of S. enterica serovar Typhimurium DT12 were calculated from the slope of the linear regression for the 6-h incubation period (Fig. 1). The pH and concentrations of SCFA and lactic acid were generally constant during incubation with stomach content, and the results for these parameters given in Table 5 are means of values measured during the 6-h incubation period (Table 5).
FIG. 1.
Typical time courses for the density of S. enterica serovar Typhimurium DT12, pH, and the concentration of organic acids in stomach and cecal contents during the in vitro incubation. ⧫, S. enterica serovar Typhimurium DT12; ▪, pH; ▵, lactic acid; ○, acetic acid; ◊, propionic acid; □, butyric acid.
TABLE 5.
Growth rates of S. enterica serovar Typhimurium DT12 and physicochemical properties measured in vitro in content from the stomachs and ceca of pigs fed the experimental diets
| Content | Parameterc | Valuea for diet:
|
SEM | Significanceb
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| F-NP | F-P | C-NP | C-P | Grinding | Form | Grinding form | |||
| Stomach | Death rate (/h) | 0.30A | 0.44A | 1.90B | 0.19A | 0.18 | ** | *** | *** |
| pH | 4.29AB | 4.26AB | 3.89A | 4.66B | 0.17 | NS | * | * | |
| Acetic acid concn | 6.2 | 4.2 | 12.8 | 8.1 | 1.0 | *** | ** | NS | |
| Propionic acid concn | 0.0A | 0.0A | 6.0B | 0.2A | 0.5 | *** | *** | *** | |
| Butyric acid concn | 0.0 | 0.0 | 0.4 | 0.1 | 0.1 | ** | (*) | (*) | |
| Lactic acid concn | 3.8 | 5.1 | 24.3 | 14.0 | 3.9 | *** | NS | NS | |
| Undissociated lactic acid concn | 0.9A | 0.8A | 10.2B | 1.7A | 0.9 | *** | *** | *** | |
| Undissociated OAd concn | 5.3A | 3.3A | 26.5B | 6.2A | 1.8 | *** | *** | *** | |
| Cecum | Death rate (/h) | 0.10 | 0.25 | 0.43 | 0.21 | 0.10 | NS | NS | NS |
| pH | 5.69 | 5.73 | 5.56 | 5.66 | 0.14 | NS | NS | NS | |
| Acetic acid concn | 78.5 | 82.0 | 82.0 | 77.3 | 3.8 | NS | NS | NS | |
| Propionic acid concn | 32.6 | 39.7 | 35.9 | 34.9 | 3.1 | NS | NS | NS | |
| Butyric acid concn | 8.2 | 9.4 | 13.6 | 11.5 | 1.5 | ** | NS | NS | |
| Lactic acid concn | 5.6 | 1.0 | 11.1 | 4.7 | 3.0 | NS | (*) | NS | |
| Undissociated lactic acid concn | 0.1 | 0.0 | 0.4 | 0.1 | 0.2 | NS | NS | NS | |
| Undissociated OA concn | 17.2 | 19.6 | 22.9 | 16.4 | 5.2 | NS | NS | (*) | |
Values are least-square means. Values within a row not having the same superscript are significantly different.
Grinding, form, NS, and asterisks are as defined for Table 2.
Concentration units are millimoles per kilogram.
OA, SCFA plus lactic acid.
The results showed a significantly higher specific death rate of S. enterica serovar Typhimurium DT12 (P < 0.001) from pigs fed the C-NP diet than from pigs fed the other diets (Table 5). The specific death rate of 1.9/h observed in stomach content from pigs fed the C-NP diet corresponds to a T1/2 of 22 min. This was almost 10 times faster than the T1/2 of 219 min observed for pigs fed the C-P diet (results not shown). The T1/2s were 139 and 95 min for pigs fed the F-NP and F-P diets, respectively (results not shown). The pH was significantly lower for the C-NP diet than for the C-P diet (P < 0.05), and the concentration of propionic acid was significantly higher for the C-NP diet than for the other diets (P < 0.001). The numerically highest concentrations of acetic acid, butyric acid, and lactic acid were observed in stomach contents from pigs fed the C-NP diet (Table 5). The concentrations of SCFA and lactic acid were significantly higher for pigs fed the coarse feed than for pigs fed the fine feed (Table 5). The concentration of undissociated lactic acid was significantly higher for the C-NP diet than for the other diets (P < 0.001).
Figure 1 also shows a typical time course for the density of S. enterica serovar Typhimurium DT12, pH, and SCFA and lactic acid concentrations in cecal content. In cecal content, a drastic increase in the concentration of lactic acid, a decrease in pH, and a decrease in the density of S. enterica serovar Typhimurium DT12 were observed at the end of the incubation period (Fig. 1). For that reason, the specific death rates of S. enterica serovar Typhimurium DT12 were calculated from the slope of the linear regression for the 4-h incubation period (Fig. 1) and the results on pH and concentrations of SCFA and lactic acid are means of values measured during the 4-h incubation period (Table 5). The specific death rates of S. enterica serovar Typhimurium DT12, pH, and concentrations of SCFA in cecal content were not significantly affected by the experimental diets (Table 5). However, the concentration of butyric acid during in vitro incubation was significantly higher (P < 0.01) in cecal content from pigs fed the coarse feed than in that from pigs fed the fine feed (Table 5). Lactic acid accumulated at the end of the in vitro incubations, and the highest lactic acid concentration was observed for the C-NP diet. No significant differences in the concentration of undissociated lactic acid or total organic acids in cecal content were observed (Table 5).
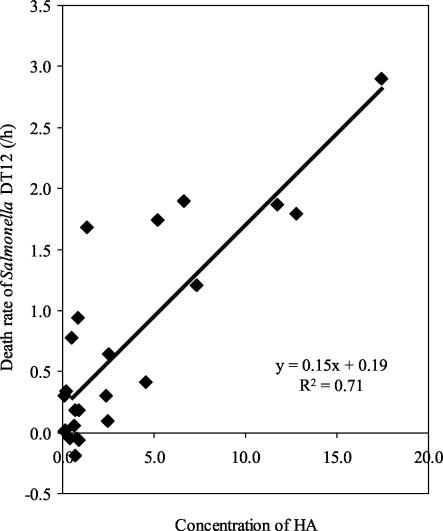
The specific death rates for all observations of the in vitro incubations with S. enterica serovar Typhimurium DT12 in stomach content (regardless of diet) were plotted against the corresponding concentrations of undissociated lactic acid, and the correlation (R2) was calculated by linear regression (y = 0.15x + 0.19) as 0.71 (Fig. 2).
FIG. 2.
Correlation between the death rate of S. enterica serovar Typhimurium DT12 and the concentration of undissociated lactic acid HA (millimolar) in stomach content measured in vitro.
DISCUSSION
The present study shows that pigs fed a nonpelleted feed have less-efficient feed conversion than pigs fed a pelleted feed. This supports data from previous studies (6, 15, 31, 32). The results also show that pigs fed the nonpelleted feed were less likely to experience diarrhea. This effect of feed processing on diarrhea score has not, to our knowledge, been reported before. Furthermore, pigs fed the coarsely ground meal feed all had undamaged tissue of the nonglandular esophageal region in the stomach, whereas pigs fed the other diets showed some degree of lesions and keratinization of this tissue. These results are in accordance with other studies (7, 24, 25, 32). The reason for this prevention of gastric ulcer may be related to the consistency of the stomach content. Stomach content from pigs fed the coarsely ground meal feed had the highest dry matter content and had significantly higher water binding capacity and a more porridge-like consistency (that did not divide into a precipitate and a watery phase on standing) than stomach contents from pigs fed the other diets. These marked effects of the coarsely ground meal feed occurred in spite of only minor differences in the particle size distribution between the coarsely ground meal feed and the coarsely ground pelleted feed. The observed rheological characteristics of stomach content are in accordance with results from other studies (20, 24, 25).
A strong effect of the experimental diets on several microbial parameters was observed in the present study. Pigs fed the coarsely ground meal feed showed significantly increased number of total anaerobic bacteria, significantly higher concentration of ATP, increased concentrations of various organic acids, and lower pH in the stomach than pigs fed the other diets. These results demonstrate that pigs fed the coarsely ground meal feed had much higher microbial fermentation in their stomachs than pigs fed the other diets. Others have reported similar results (15). The coarsely ground meal feed also led to a different profile of organic acids produced, suggesting that the microbiota in the stomach of these pigs had a different composition and/or activity. A slower gastric passage rate when pigs are fed a coarsely versus a finely ground feed has been reported (20, 25). A slower passage rate may be the explanation for the higher microbial activity found in the stomachs of pigs fed the coarsely ground meal feed due to an increase in the time for the microbiota to proliferate in the stomach. In addition, the consistency of the stomach content may influence the growth of the microbiota in the stomach. In this regard, it has been reported that the matrix microstructure in a model porous medium exerts a major influence on the survival of food-borne pathogens (10) and that bacterial survival depends on the formation of microscopic water-rich domains (9). It is possible that the higher water binding capacity and more porridge-like consistency of stomach content assisted microbial growth in the stomachs of pigs fed the coarsely ground meal feed in the present study.
S. enterica serovar Typhimurium DT12 is one of the most frequently isolated strains in human salmonellosis in Denmark and is also common in pig herds (1, 2). The very strong killing effect on S. enterica serovar Typhimurium DT12 in stomach contents from pigs fed the coarsely ground meal feed observed in the present study is in agreement with results showing that the growth of enterobacteria is inhibited at high levels of lactic acid at low pH (16). It is proposed that the undissociated form of lactic acid and other organic acids exerts the antibacterial effect. The undissociated form of the acids can freely cross the bacterial membrane and enter the bacterial cell, where the acids dissociate and cause intracellular pH to drop. As a result, enzymatic processes stop and the proton motive force collapses, causing cell death (26). This is supported by the present results showing a high correlation between the death rate of S. enterica serovar Typhimurium DT12 and the concentration of undissociated lactic acid in the stomach content. The retention time for stomach content in pigs is approximately 3 to 4 h (8). From the present results, it can be estimated that a potential population of Salmonella would be halved about 10 times, resulting in a 1,000-fold reduction in population size, in stomach contents from pigs fed the coarsely ground meal feed. In comparison, the Salmonella population would be halved about one to three times (eightfold reduction) in the stomachs of pigs fed the other diets. Thus, the present results strongly suggest that a combination of low pH and high concentrations of lactic acid in the stomach is important for reducing the number of Salmonella cells entering the small and large intestines of pigs.
A study by Jørgensen et al. (15) has demonstrated a clear positive correlation between the incidence of Salmonella-positive pigs and the density of coliform bacteria in the gastrointestinal tracts of pigs (15). These results indicate that the density of coliform bacteria is a reliable indicator of the population of Salmonella in pigs. In the present study, a significant reduction in the number of coliform bacteria was observed in the distal small intestines, ceca, and colons of pigs fed the coarse feed compared with pigs fed the fine feed. The lowest number of coliform bacteria was observed in pigs fed the coarsely ground meal feed. These results show that the grinding and processing of feed also have an effect on the number of Enterobacteria, such as coliforms and salmonellae, in the lower parts of the intestinal tract.
The most marked effect of grinding and processing of feed was on the microbial activity in the stomachs of pigs in the present study. Less-marked effects in the lower compartments of the gastrointestinal tract were observed. The observed in vitro survival of S. enterica serovar Typhimurium DT12 in cecal content indicates that there is neither an increase nor decrease in Salmonella numbers in the ceca of pigs. The results also demonstrate that the present in vitro method must be applied with some caution when used with cecal content. The calculations of specific death rates, pH, and SCFA concentrations should be based on the 4-h incubation period rather than a 6-h incubation period, due to an accumulation of lactic acid and a decrease in pH in the second 2 h of the incubation period.
Higher concentrations of butyric acid were observed in the ceca and colons of pigs fed the coarsely ground feed than in those of pigs fed the finely ground feed. Stimulation of butyric acid in the hindgut is considered beneficial because it promotes the growth of epithelial cells (27). Thus, the present results are in accordance with a study reported by Brunsgaard, showing significantly larger crypts and epithelial cell proliferation in the colons of pigs fed a coarse diet than in those of pigs fed a fine diet (4).
In conclusion, the present study has demonstrated numerous beneficial effects of feeding pigs a coarsely ground meal feed instead of a finely ground pelleted feed. The coarsely ground meal feed promotes animal welfare by reducing gastric ulcer and diarrhea among the pigs and promotes gastrointestinal health by reducing the number of Salmonella cells and potentially harmful coliforms in the stomach and in the lower parts of the intestinal tract. The present study has put forward an explanation for the Salmonella-reducing properties of coarsely ground meal feed. The coarsely ground meal feed induces physical conditions in the stomach that promote the growth of anaerobic bacteria, accompanied by the production of organic acids, especially lactic acid, and a lowering of the pH. The high levels of lactic acid and low pH in the stomach then kill Salmonella and Escherichia coli and consequently prevent these bacteria from entering, or reduce the numbers bacteria that enter, the parts of the gastrointestinal tract in which they normally proliferate. In this way, the stomach acts as a barrier and breaks the cycle where animals in problematic herds infect each other and are reinfected through the consumption of feces. In addition, the coarsely ground meal feed induces higher concentrations of butyric acid in the hindgut, which may have beneficial effect on gut health by promoting growth of epithelial cells. A drawback of feeding a coarse meal feed to pigs, however, is the poor growth performance and consequently the financial loss to the pig producer. Nevertheless, the present results have contributed to our knowledge regarding the beneficial attributes of the coarse meal feed. This information may be used to design alternative feeds that can reduce the incidences of Salmonella in pig herds without a negative effect on growth performance.
Acknowledgments
This work was financially supported by The Danish Bacon and Meat Council Basic Research Funding Program.
We thank Thomas Rebsdorf, Trine Poulsen, Karin Durup, and Mona Dinsen for technical assistance.
REFERENCES
- 1.Anonymous. 2003. Annual report on zoonoses in Denmark 2002. Ministry of Food, Agriculture and Fisheries, Copenhagen, Denmark.
- 2.Baloda, S. B., L. Christensen, and S. Trajcevska. 2001. Persistence of a Salmonella enterica serovar Typhimurium serovar DT12 clone in a piggery and in agricultural soil amended with Salmonella-contaminated slurry. Appl. Environ. Microbiol. 67:2859-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berends, B. R., F. Van Knapen, J. M. Snijders, and D. A. Mossel. 1997. Identification and quantification of risk factors regarding Salmonella spp. on pork carcasses. Int. J. Food Microbiol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 4.Brunsgaard, G. 1998. Effects of cereal type and feed particle size on morphological characteristics, epithelial cell proliferation, and lectin binding patterns in the large intestine of pigs. J. Anim. Sci. 76:2787-2798. [DOI] [PubMed] [Google Scholar]
- 5.Budavari, S., and M. J. O'Neil (ed.). 1989. The Merck index: an encyclopedia of chemicals, drugs and biologicals, 11th ed., p. 41-308. Merck & Co., Inc., Rahway, N.J.
- 6.Eisemann, J. H., and R. A. Argenzio. 1999. Effects of diet and housing density on growth and stomach morphology in pigs. J. Anim. Sci. 77:2709-2714. [DOI] [PubMed] [Google Scholar]
- 7.Eisemann, J. H., and R. A. Argenzio. 1999. Effects of diets differing in propensity to promote gastric lesions on defense systems in gastric mucosae. J. Anim. Sci. 77:2715-2720. [DOI] [PubMed] [Google Scholar]
- 8.Guerin, S., Y. Ramonet, J. LeCloarec, M. C. Meunier-Salaun, P. Bourguet, and C. H. Malbert. 2001. Changes in intragastric meal distribution are better predictors of gastric emptying rate in conscious pigs than is meal viscosity of dietary fibre concentration. Br. J. Nutr. 85:343-350. [DOI] [PubMed] [Google Scholar]
- 9.Hills, B. P., C. Buff, K. M. Wright, L. H. Sutcliffe, and Y. Ridge. 2001. Microstructural effects on microbial survival: phase-separating dextran solutions. Int. J. Food Microbiol. 68:187-197. [DOI] [PubMed] [Google Scholar]
- 10.Hills, B. P., L. Arnould, C. Bossu, and Y. P. Ridge. 2001. Microstructural factors controlling the survival of food-borne pathogens in porous media. Int. J. Food Microbiol. 66:163-173. [DOI] [PubMed] [Google Scholar]
- 11.Holdeman, L. V., E. P. Cato, and E. C. Moore. 1977. Anaerobic laboratory manual. Virginia Polytechnic Institute and State University, Blacksburg.
- 12.Hurd, H. S., J. D. McKean, R. W. Griffith, I. V. Wesley, and M. H. Rostagno. 2002. Salmonella enterica infections in market swine with and without transport and holding. Appl. Environ. Microbiol. 68:2376-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen, B. B., and H. Jørgensen. 1994. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol. 60:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, M. T., R. P. Cox, and B. B. Jensen. 1995. Microbial production of skatole in the hind gut of pigs fed different diets and its relation to skatole deposition in backfat. Anim. Sci. 61:293-304. [Google Scholar]
- 15.Jørgensen, L., J. Dahl, B. B. Jensen, and H. D. Poulsen. 1999. Effects of expanding, pelleting, and grinding on Salmonella typhimurium infection, growth performance and gastrointestinal ecosystem in slaughter pigs. Publication no. 426. The National Committee for Pig Breeding Health and Production, Copenhagen, Denmark. (In Danish.)
- 16.Knarreborg, A., N. Miquel, T. Granli, and B. B. Jensen. 2002. Establishment and application of an in vitro methodology to study the effects of organic acids on coliform and lactic acid bacteria in the proximal part of the gastrointestinal tract of piglets. Anim. Feed Sci. Technol. 99:131-140. [Google Scholar]
- 17.Leontides, L. S., E. Grafanakis, and C. Genigeorgis. 2003. Factors associated with the serological prevalence of Salmonella enterica in Greek finishing swineherds. Epidemiol. Infect. 131:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littell, R. C., G. A. Milliken, W. W. Stroup, and R. D. Wolfinger. 1996. SAS system for mixed models. SAS Institute Inc., Cary, N.C.
- 19.Mavromichalis, I., J. D. Hancock, B. W. Senne, T. L. Gugle, G. A. Kennedy, R. H. Hines, and C. L. Wyatt. 2000. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs J. Anim. Sci. 78:3086-3095. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell, C. V., E. M. Reimann, W. G. Hoekstra, T. Kowalczyk, N. J. Benevenga, and R. H. Grummer. 1970. Effect of dietary particle size on lesion development and on the contents of various regions of the swine stomach. J. Anim. Sci. 30:911-922. [DOI] [PubMed] [Google Scholar]
- 21.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naughton, P. J., L. L. Mikkelsen, and B. B. Jensen. 2001. Effects of nondigestible oligosaccharides on Salmonella enterica serovar Typhimurium and nonpathogenic Escherichia coli in the pig small intestine in vitro. Appl. Environ. Microbiol. 67:3391-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen, B., L. Alban, H. Stege, L. L. Sørensen, V. Møgelmose, J. Bagger, J. Dahl, and D. L. Baggesen. 2001. A new Salmonella surveillance and control programme in Danish pig herds and slaughterhouses. Berl. Muench. Tierarztl. Wochenschr. 114:323-326. [PubMed] [Google Scholar]
- 24.Nielsen, E. K., and K. L. Ingvartsen. 2000. Effect of cereal type, disintegration method and pelleting on stomach content, weight and ulcers and performance in growing pigs. Livestock Prod. Sci. 66:271-282. [Google Scholar]
- 25.Regina, D. C., J. H. Eisemann, J. A. Lang, and R. A. Argenzio. 1999. Changes in gastric contents in pigs fed a finely ground and pelleted or coarsely ground meal diet. J. Anim. Sci. 77:2721-2729. [DOI] [PubMed] [Google Scholar]
- 26.Russel, J. B., and F. Diez-Gonzales. 1998. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 39:205-234. [DOI] [PubMed] [Google Scholar]
- 27.Scheppach, W. 1994. Effects of short chain fatty acids on gut morphology and function. Gut 35(Suppl. 1):S35-S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stege, H., J. Christensen, J. P. Nielsen, and P. Willeberg. 2001. Data-quality issues and alternative variable-screening methods in a questionnaire-based study on subclinical Salmonella enterica infection in Danish pig herds. Prev. Vet. Med. 48:35-54. [DOI] [PubMed] [Google Scholar]
- 29.Wegener, H. C., and D. L. Baggesen. 1996. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica ssp. enterica serovar Infantis by use of pulsed field gel electrophoresis. Int. J. Food Microbiol. 32:125-131. [DOI] [PubMed] [Google Scholar]
- 30.Wingstrand, A., J. Dahl, L. K. Thomsen, L. Jørgensen, and B. B. Jensen. 1997. Influence of dietary administration of organic acids and increased feed structure on Salmonella typhimurium infection in pigs, p. 170-172. In S. Bech-Nielsen and J. P. Nielsen (ed.), Proceedings of the 2nd International Symposium on Epidemiology and Control of Salmonella in Pork. Federation of Danish Pig Producers and Slaughterhouses, Copenhagen, Denmark.
- 31.Wondra, K. J., J. D. Hancock, K. C. Behnke, and C. R. Stark. 1995. Effects of mill type and particle size uniformity on growth performance, nutrient digestibility, and stomach morphology in finishing pigs. J. Anim. Sci. 73:2564-2573. [DOI] [PubMed] [Google Scholar]
- 32.Wondra, K. J., J. D. Hancock, K. C. Behnke, R. H. Hines, and C. R. Stark. 1995. Effects of particle size and pelleting on growth performance, nutrient digestibility, and stomach morphology in finishing pigs. J. Anim. Sci. 73:757-763. [DOI] [PubMed] [Google Scholar]