Abstract
Introduction
In Europe, vitamin D deficiency is highly prevalent varying between 40% and 60% in the healthy general adult population. The consequences of vitamin D deficiency for sepsis and outcome in critically ill patients remain controversial. We therefore systematically reviewed observational cohort studies on vitamin D deficiency in the intensive care unit.
Methods
Fourteen observational reports published from January 2000 to March 2014, retrieved from Pubmed and Embase, involving 9,715 critically ill patients and serum 25-hydroxyvitamin D3 (25 (OH)-D) concentrations, were meta-analysed.
Results
Levels of 25 (OH)-D less than 50 nmol/L were associated with increased rates of infection (risk ratio (RR) 1.49, 95% (confidence interval (CI) 1.12 to 1.99), P = 0.007), sepsis (RR 1.46, 95% (CI 1.27 to 1.68), P <0.001), 30-day mortality (RR 1.42, 95% (CI 1.00 to 2.02), P = 0.05), and in-hospital mortality (RR 1.79, 95% (CI 1.49 to 2.16), P <0.001). In a subgroup analysis of adjusted data including vitamin D deficiency as a risk factor for 30-day mortality the pooled RR was 1.76 (95% CI 1.37 to 2.26, P <0.001).
Conclusions
This meta-analysis suggests that vitamin D deficiency increases susceptibility for severe infections and mortality of the critically ill.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-014-0660-4) contains supplementary material, which is available to authorized users.
Introduction
Vitamin D deficiency, defined as serum 25-hydroxyvitamin D3 (25 (OH)-D) concentrations below 50 nmol/L, is highly prevalent in Dutch critically ill patients [1]. Several studies in critically ill patients report associations between vitamin D deficiency, a disturbed parathyroid hormone (PTH)-vitamin D axis and increased mortality [2-5]. A biological basis how hypovitaminosis D may cause mortality could be hypocalcaemia. Hypocalcaemia is a well-known abnormality in critically ill patients in the course of sepsis and rhabdomyolysis [6]. Second, vitamin D regulates both innate and adaptive immune systems. Vitamin D deficiency leads to immune dysregulation and has been proposed as an underlying pathogenic mechanism of infections [7]. Third, vitamin D deficiency is associated with increased markers of systemic inflammation associated with multi-organ failure [8]. Moreover hypovitaminosis D reduces, despite maximal upregulation of PTH levels, formation of 1,25-dihydroxyvitamin D3 (1,25 (OH)-D) at the tissue level. This may be critical in mediating the beneficial pleiotropic functions of vitamin D, involving innate immunity, mucosal barrier and endothelial function. Recently, a systematic review and meta-analysis including observational and interventional studies on vitamin D in non-critically ill patients, suggests an association of deficiency with cardiovascular diseases, diabetes, and all-cause mortality in the former but not in the latter studies [9]. In non-critically ill patients, of a prior meta-analysis of 18 randomised controlled trials, intake of supplementary doses of vitamin D was associated with a 7% decrease in mortality [10].
Therefore, we conducted a systematic review to pool the available data and to study the possible effect of vitamin D deficiency in critically ill patients on the incidence of infection, sepsis and association with mortality.
Materials and methods
Search strategy
The report of this protocol-driven systematic review and meta-analysis follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology guidelines (MOOSE) [11]. A Medline, Embase and Cochrane Library search was conducted with the help of biomedical information specialists, limited to publications from January 2000 until March 2014 in humans. The search consisted of two terms: vitamin D and intensive care. The controlled thesaurus terms we used for vitamin D were vitamin D and vitamin D deficiency. The concept intensive care was covered by the following keywords: intensive care unit (ICU), intensive care nursing, critical care and critically ill patients. For Medical Subject Heading (MeSH) terms and search strategy see S1 Table 1 in Additional file 1. References of included articles were cross-checked for other relevant studies. One author [12] was successfully contacted twice because not all required information could be retrieved from the publication.
Study selection
Two independent authors, a researcher (KdH) and an intensivist (AS) screened titles and abstracts for eligibility. In case of disagreement, agreement of a third author (ME) provided a final option. Studies eligible for inclusion in the systematic review were observational studies describing ICU patients, reporting on serum 25 (OH)-D concentrations, outcomes, and those written in English. Studies in which participants were younger than 18 years, sample size was less than 20 patients and participants suffered from parathyroid disease, human immunodeficiency virus infection and end-stage renal disease requiring chronic dialysis were excluded. Abstracts, letters, reviews and conference articles were excluded as well.
Definitions
In this meta-analysis vitamin D deficiency was a priori defined as a reported serum concentration <50 nmol/L, as advocated by the American Institute of Medicine [13]. However, the included studies unfortunately reported a wide range of cutoffs for vitamin D deficiency. Prior to analysing the data for each study, agreement was negotiated between two authors, KdH and AS, which vitamin D cutoffs to be used in the meta-analysis, see Table 1. Due to the designed search strategy, there was no clear definition of infection and sepsis between included studies. The reported occurrence of infections comprised any kind of infections such as pneumonia, urinary tract, bacteraemia and intra-abdominal infections. The included studies defined sepsis varying from having positive blood cultures or systemic inflammatory response syndrome (SIRS) criteria combined with a source of infection. We reported the complete overview of definitions used in S2 Table 2 in Additional file 1. Mortality rates were extracted as in-hospital and 30-day mortality. Thirty-day mortality rates are well described and defined in the included articles. All other definitions of mortality, like ICU mortality, in-hospital mortality, acute in-hospital mortality, were taken together as in-hospital mortality. When articles separately reported ICU and in-hospital mortality, we combined numbers together in the analysis as in-hospital mortality.
Table 1.
Overview of publications used in the meta-analysis
| Author | Year of publication | Study population | Study design | Number of patients | Endpoints | Comparison (in meta-analysis) | Study * quality |
|---|---|---|---|---|---|---|---|
| Amrein et al. [25] | 2014 | Medical, surgical ICU | Retrospective, cohort | 655 | Sepsis, in-hospital mortality | <50 nmol/l vs >75 nmol/l | 7 |
| Arnson et al. [21] | 2012 | Medical, surgical ICU | Prospective, cohort | 130 | Infections | ≤50 nmol/l vs >50 nmol/l | 6 |
| Aygencel et al. [24] | 2013 | Medical ICU | Prospective, cohort | 201 | Infections, sepsis, in-hospital mortality | <50 nmol/l vs ≥50 nmol/l | 4 |
| Braun et al. [5] | 2012 | Medical, surgical ICU | Two-centre, retrospective, cohort | 1,325 | Sepsis, 30-day, in-hospital mortality | ≤37 nmol/l vs ≥75 nmol/l | 8 |
| Braun et al. [3] | 2011 | Surgical ICU | Two-centre, retrospective, cohort | 2,399 | Infections, 30-day, in-hospital mortality | ≤37 nmol/l vs ≥75 nmol/l | 8 |
| Flynn et al. [22] | 2012 | Medical, surgical ICU | Prospective, cohort | 66 | Infections, sepsis, in-hospital mortality | ≤50 nmol/l vs >50 nmol/l | 2 |
| Higgins et al. [23] | 2012 | Medical, surgical ICU | Prospective, cohort | 196 | Infections, sepsis, 30-day mortality | ≤30 nmol/l vs ≥60 nmol/l | 7 |
| Lucidarme et al. [12] | 2012 | Medical, surgical ICU | Prospective, cohort | 134 | 30-day mortality | >15- ≤30 vs ≥60 nmol/l | 5 |
| Matthews et al. [30] | 2012 | Medical ICU | Prospective, cohort | 258 | In-hospital mortality | ≥10- ≤32 vs 67-97 nmol/l | 3 |
| Moromizato et al. [26] | 2014 | Medical, surgical ICU | Two-centre, retrospective, cohort | 3,386 | Sepsis | ≤37 nmol/l vs ≥75 nmol/l | 8 |
| Nair et al. [27] | 2012 | Medical ICU | Prospective, cohort | 100 | 30-day-, in-hospital mortality | <25 nmol/l vs ≥50 nmol/l | 6 |
| Remmelts et al. [28] | 2012 | Ward, medical ICU | Prospective, cohort | 272 | 30-day mortality | ≤50 nmol/l vs ≥75 nmol/l | 7 |
| Su et al. [29] | 2013 | Medical, surgical ICU | Prospective, cohort | 156 | 30-day mortality | ≤37 nmol/l vs ≥75 nmol/l | 6 |
| Venkatram et al. [4] | 2011 | Medical ICU | Retrospective, cohort | 437 | Sepsis, in-hospital mortality | ≤50 nmol/l vs ≥75 nmol/l | 4 |
*Study quality assessed by the Newcastle-Ottawa scale, see S8 in Additional file 1. ICU, intensive care unit.
Statistical analysis
Review Manager (version 5.2 for Windows, The Cochrane Collaboration, 2011) was used for the analysis. Occurrence of infections, sepsis and mortality, as defined in the studies, was calculated for each individual study and the estimated risk ratios (RRs) were pooled comparing the effect of deficient levels (sufficient levels as reference) of 25 (OH)-D with the use of the inverse variance (IV) method in a random-effects model, yielding RRs and 95% confidence intervals (CIs). The IV method was used because of the assumption that less variance in a study should contribute to its weight in significance. A random-effects model was used due to expected heterogeneity between studies. If raw data was not available to calculate the RRs, we used the reported odds ratios (ORs) and converted the reported ORs to RRs with corresponding 95% CIs. Otherwise, we manually calculated the RRs from the available data. Additionally, taking confounding into account, we decided to perform a subgroup analysis of adjusted data reported on 30-day mortality. Unfortunately, most studies do not report adjusted data on infections, sepsis and in-hospital mortality, so we were not able to make a subgroup analysis of those. Subgroup analyses were performed to examine the difference per outcome based on study design. To determine publication bias we used funnel plots (Figures S3-S7 in Additional file 1). Heterogeneity was assessed with the use of the Cochran Q statistics and the I2 test. We used the Newcastle-Ottawa scale to evaluate the quality of included studies. This scale uses a star system (with a maximum of nine stars) to evaluate a study in three domains: selection of participants, comparability of study groups, and the ascertainment of outcomes of interest. We judged studies that received a score of nine or eight stars to be at low risk of bias, studies that scored seven or six stars to be at medium risk, and scores below six to be at high risk of bias (S8a,b in Additional file 1).
Results
Search strategy
A total of 381 studies were screened; 358 were excluded for the following reasons: irrelevant (n = 229), review (n = 7), study design or too small in sample size (n = 26), only focused on vitamin D metabolism (n = 45), pediatric studies (n = 46) and animal studies (n = 5), after detailed evaluation, one additional study was excluded because of duplicates. All studies included in this analysis (n = 14) were prospective cohort studies (n = 9) or had retrospective designs (n = 5). We were not able to include all studies because they described different outcomes like effect on delirium, anti-microbial peptide levels and 90-day mortality [8,14,15] or their results [16] were not formatted to allow combining the results with the other studies. We also excluded four interventional trials because they were small in sample size and none reported on infections, sepsis and mortality [17-20], (S9 Figure 1 in Additional file 1).
Study characteristics
Fourteen observational reports published from January 2000 until March 2014 involving 9,715 critically ill patients were included. The average serum 25 (OH)-D level of the study population was 45 nmol/L. Mean age was 62 years and the majority of the patients, 53%, were male. The presence of infections was 31% and sepsis occurred in 28% of the patients. The average 30-day mortality in this study cohort was 17.5% and the in-hospital mortality rate 18.4%. Characteristics of the studies are presented in Table 1. For a complete overview of study characteristics of studies included in the meta-analysis see S2 Table 2 in Additional file 1.
25 (OH)-D levels and infections
Five [3,21-24] of the fourteen studies reported on infections. We pooled the manually calculated RRs for the effect of low levels of 25 (OH)-D on infections (Figure 1), including 1,967 patients. The pooled RR was 1.49 (95% CI 1.12 to 1.99).
Figure 1.
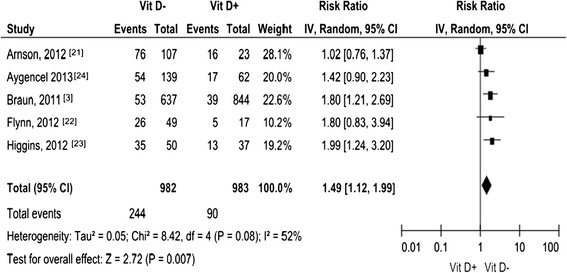
Forest plot of studies comparing deficient vitamin D levels with sufficient vitamin D levels on infection. CI, confidence interval; IV, inverse variance; Vit D-, deficient vitamin D level; Vit D+, sufficient vitamin D level.
25 (OH)-D levels and sepsis
The RRs in seven [4,5,22-26] of the fourteen studies were manually calculated, involving 3,844 patients (Figure 2). The pooled RR was 1.46 (95% CI 1.27 to 1.68).
Figure 2.
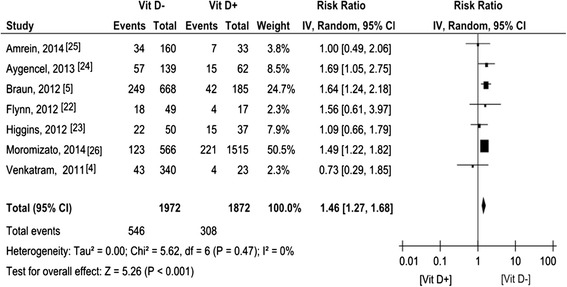
Forest plot of studies comparing deficient vitamin D levels with sufficient vitamin D levels on sepsis. CI, confidence interval; IV, inverse variance; Vit D-, deficient vitamin D level; Vit D+, sufficient vitamin D level.
25 (OH)-D levels and mortality
Seven of the fourteen studies reported on 30-day mortality, with ORs either available in the articles converted to RRs [3,5] (n = 2) or manually calculated RRs [12,23,27-29] (n = 5), involving 2,857 patients (Figure 3). The pooled RR was 1.42 (95% CI 1.00 to 2.02). Two studies [3,5] used a multivariate model adjusted for age, gender, race, disease severity, season and ICU type. Remmelts et al. [28] adjusted for age and heart failure in the multivariate analysis and Nair et al. [27] for age and disease severity. The pooled subgroup analysis of adjusted data involving 2,572 patients demonstrated an increased RR for 30-day mortality associated with vitamin D deficiency by 1.76 (95% CI 1.37 to 2.26) (Figure 4). Eight [3-5,22,24,25,27,30] of the fourteen studies reported in-hospital mortality of 3,606 patients (Figure 5). The pooled RR was 1.79 (95% CI 1.49 to 2.16).
Figure 3.
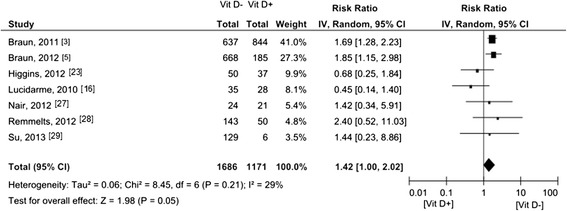
Forest plot of studies comparing deficient vitamin D levels with sufficient vitamin D levels on 30 day-mortality (univariate analysis). CI, confidence interval; IV, inverse variance; Vit D-, deficient vitamin D level; Vit D+, sufficient vitamin D level.
Figure 4.
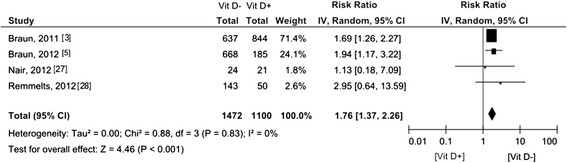
Forest plot of studies comparing deficient vitamin D levels with sufficient vitamin D levels on 30 day-mortality (multivariate analysis). CI, confidence interval; IV, inverse variance; Vit D-, deficient vitamin D level; Vit D+, sufficient vitamin D level.
Figure 5.
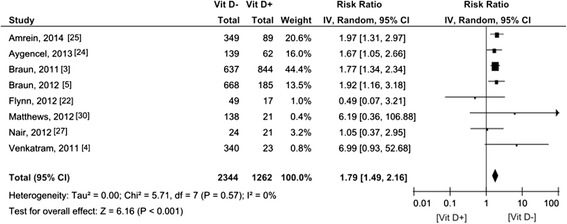
Forest plot of studies comparing deficient vitamin D levels with sufficient vitamin D levels on in-hospital mortality. CI, confidence interval; IV, inverse variance; Vit D-, deficient vitamin D level; Vit D+, sufficient vitamin D level.
Subgroup analyses per outcome based on study design
The effect on outcome in prospective study data is lower than in retrospective data see S10 in Additional file 1, but there were less prospective data.
Discussion
This study represents the first comprehensive systematic review and meta-analysis focused on studies in which the effects of vitamin D deficiency in critically ill patients on occurrence of infection, sepsis and mortality rates are described. These results show that vitamin D deficiency (<50 nmol/L) is associated with increase in infection rate, sepsis, 30-day mortality and in-hospital mortality in adult critically ill patients, worldwide.
The association between vitamin D status and immunity has been already supported by a number of studies [31-33]. However, results in healthy volunteers exposed to experimental human endotoxaemia suggest a lack of this association between vitamin D and inflammatory cytokine levels [34]. Therefore, it is suggested that the differences in the ability to produce vitamin D, may contribute to a difference in the susceptibility to microbial infection. Our study adds to the discussion on the association by an objectively derived pooled risk. The relation between 25 (OH)-D levels and sepsis has been described previously [8]. Vitamin D-deficient patients are at higher risk for blood culture positivity, which may contribute to higher sepsis rates [3]. However, Cecchi et al. found no clear relationship on outcome between lower vitamin D levels in septic patients when compared with a matched cohort [16]. The most recent study by Moromizato et al. included in our analysis specifically showed a threshold of 25 (OH)-D less than or equal to 40 nmol/L to be associated with sepsis [26]. Thus our results are in agreement with the hypothesis that vitamin D deficiency is a contributor to sepsis.
Some published studies [17,35] suggested an association between vitamin D deficiency and mortality in critically ill patients. In the study by Van den Berghe et al. both 25 (OH)-D and 1,25 (OH)-D levels were lower among non-survivors in critically ill patients [17]. Matthews et al. noted in their surgical ICU cohort that most deaths occurred at vitamin D levels less than 32 nmol/L and that no deaths occurred at levels higher than 65 nmol/L [30]. The CopD study done by Durup et al. reported a reversed J-shape relation between 25 (OH)-D and all-cause mortality, suggesting that too much and too little are deleterious. A serum 25 (OH)-D of 50 to 60 nmol/L was associated with the lowest mortality risk [36]. The results of this meta-analysis suggest that vitamin D levels below 50 nmol/L, increase 30-day mortality and in-hospital mortality with 76% and 79% respectively. To date, only four randomised trials in adult critically ill patients have been published, which were designed to study normalisation of vitamin D levels and its possible adverse effects such as hypercalcaemia and hypercalciuria [17-20]. These studies were not sufficiently powered to investigate the effects of vitamin D normalisation and potential benefits on hard outcomes such as incidence of severe infections and/or ICU mortality (S11 Table 3 in Additional file 1). The recently published Lancet review supports the relation between 25 (OH)-D deficiency and all-cause mortality in observational studies [9]. The discrepancy with the interventional studies could be due to underpowered numbers, low dosages or short duration of supplementation. The role for supplementation is unclear and appropriate dose-response studies with 1,25 and 25 (OH)-D must be done. Therefore the authors’ conclusion about low vitamin D as merely a marker of disease has to be confirmed in prospective interventional studies.
Data from biochemical and molecular studies indicate that vitamin D, in particular its active form 1,25 (OH)-D, has a much wider role than only the maintenance of calcium homeostasis and bone health. Sufficiency of vitamin D activity can thus also be defined by sufficient autocrine and paracrine production of 1,25 (OH)-D at serum 25 (OH)-D levels of at least around 75 nmol/L [37,38]. This active form is responsible for most, if not all, of the biological and pleiotropic effects including antimicrobial actions and immunomodulatory effects of vitamin D [7,39]. The study by Zittermann et al. demonstrated the superiority of predicting mortality by 1,25 (OH)-D as compared to 25 (OH)-D, supporting the assumption that adequately circulating 1,25 (OH)-D levels may play a role for survival [40]. Marshall et al. emphasised measuring 1,25 (OH)-D instead of total 25 (OH)-D as well; they postulated that the disease processes regulate vitamin D metabolism so that the low 25 (OH)-D levels observed in disease may be merely a biomarker of disease severity [39]. Unfortunately, the observational articles meta-analysed in this manuscript do not consider 1,25 (OH)-D on outcomes.
Two studies [3,5] had a time lag between admission into the ICU and vitamin D blood sampling. To illustrate the importance between 25 (OH)-D time of measurement and admission, the authors conducted a sensitivity analysis considering patients with 25 (OH)-D drawn before or after 90 days prior to hospital admission. This sensitivity analysis showed that the association that was found between vitamin D on outcomes was not modified by time lag.
Our study has several limitations. First, we included both prospective and retrospective studies in this meta-analysis, which is a matter of debate. In retrospective studies the control for confounders is difficult. We have addressed this by adding a subgroup analyses per outcome based on design, see S10 in Additional file 1. However, in the prospective study data the effect on mortality is lower, whereas the sample size in the prospective studies may have been insufficient to show an association. The five retrospective papers [3-5,25,26] contain large sample sizes enabling multivariate analysis for mortality ruling out confounders such as age, gender, race, glomerular filtration rate (GFR), C-reactive protein (CRP), season, disease severity and so on. Second, the studies included are observational so that a causative link between hypovitaminosis D and outcomes cannot be established. Additionally, the variability in measured 25 (OH)-D levels is probably multifactorial. It is possible that a random single 25 (OH)-D measurement in ICU patients does not appropriately reflect the vitamin D status [41]. Furthermore, alterations in vitamin D binding protein, fluid shifts [42] and assay variability with coefficients of variation ranging from 6% to 13% [43] may limit applicability of single measurements on outcome prediction used in most of the included studies. The different cutoff levels used by different studies are based on study endpoints (for example, fracture or osteoporosis) done in the general population. The applicability of these cutoff levels in the critically ill is unclear, especially because cutoff values between bone- specific and pleiotropic endpoints are different. There is heterogeneity in the definitions of infection and sepsis used in the included studies, sepsis was defined varying from positive blood cultures [3] to SIRS criteria together with a source of infection [23] but the I2 test did not show heterogeneity. We only found some heterogeneity (P = 0.08) in the forest plot combining studies with infection as outcome.
Conclusions
In conclusion, this is the first meta-analysis suggesting an association between vitamin D deficiency and infection and mortality in the critically ill. This information may help to design placebo-controlled randomised clinical trials on vitamin D supplementation in preventing severe infections and death in the ICU.
Key messages
25 (OH)-D deficiency is highly prevalent across intensive care population worldwide.
In critically ill patients, 25 (OH)-D deficiency is associated with mortality.
25 (OH)-D deficiency may be a risk factor for infections and sepsis.
We support the need for adequately powered prospective, dose-response trials to evaluate the effect of vitamin D substitution on infection rates, sepsis and mortality in the critically ill.
Abbreviations
- 1,25 (OH)-D
1,25-dihydroxyvitamin D3
- 25 (OH)-D
25-hydroxyvitamin D3
- CI
confidence interval
- CRP
C-reactive protein
- GFR
glomerular filtration rate
- ICU
intensive care unit
- IV
inverse variance
- MeSh
Medical Subject Heading
- MOOSE
Meta-Analysis Of Observational Studies in Epidemiology guidelines
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PTH
parathyroid hormone
- RR
risk ratio
- SIRS
systemic inflammatory response syndrome
Additional file
Results search strategy. S2 Table 2. Summary of characteristics of the included studies in the meta-analysis. S3. Funnel plot of studies comparing low vitamin D and normal vitamin D level on the occurrence of infections. S4. Funnel plot of studies comparing low vitamin D and normal vitamin D on the occurrence of sepsis. S5. Funnel plot of studies comparing low vitamin D and normal vitamin D on the occurrence of 30-day mortality (univariate). S6. Funnel plot of studies comparing low vitamin D and normal vitamin D on the occurrence of 30-day mortality (multivariate). S7. Funnel plot of studies comparing low vitamin D and normal vitamin D on the occurrence of in-hospital mortality. S8. Newcastle-Ottawa scale. S9 Figure 1. Flow diagram for the selection of studies evaluating the effect of vitamin D in critically ill patients. S10. Subgroup analyses per outcome based on study design. S11 Table 3. Summary of vitamin D interventional trials in the ICU.
Footnotes
Competing interests
The authors declare they have no competing interests. None of the authors received financial support for this study.
Authors’ contributions
KdH, ABJG and AS designed the study; KdH and ME performed the meta-analysis. KdH prepared the manuscript. ABJG supervised, and AS and HRHG edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kim de Haan, Email: k.dehaan@erasmusmc.nl.
AB Johan Groeneveld, Email: a.b.j.groeneveld@erasmusmc.nl.
Hilde RH de Geus, Email: h.degeus@erasmusmc.nl.
Mohamud Egal, Email: m.egal@erasmusmc.nl.
Ard Struijs, Email: a.struijs@erasmusmc.nl.
References
- 1.Weenink JJ, Oudemans-Van Straaten H, Yap HT, Slaats EH, Van Der Voort PH. High prevalence of severe vitamin D deficiency in intensive care patients. Crit Care. 2010;14:S198. doi: 10.1186/cc8820. [DOI] [Google Scholar]
- 2.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 2009;35:2028–2032. doi: 10.1007/s00134-009-1642-x. [DOI] [PubMed] [Google Scholar]
- 3.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaloga GP, Chernow B. Hypocalcemia in critical illness. JAMA. 1986;256(14):1924–1929. doi: 10.1001/jama.1986.03380140094029. [DOI] [PubMed] [Google Scholar]
- 7.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 10.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Lucidarme O, Messai E, Mazzoni T, Arcade M, Du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: Results from a prospective observational study. Intensive Care Med. 2010;36:1609–1611. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 13.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Magne ST, Rosen CJ, Shapes SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morandi A, Barnett N, Miller RR, Girard TD, Pandharipande PP, Ely EW, Ware LB. Vitamin D and delirium in critically ill patients: a preliminary investigation. J Crit Care. 2012;28:230–235. doi: 10.1016/j.jcrc.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quraishi SA, Bittner EA, Blum L, McCarthy CM, Bhan I, Camargo CA., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42:1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cecchi A, Bonizzoli M, Douar S, Mangini M, Paladini S, Gazzini B, Degl'innocenti S, Linden M, Zagli G, Peris A. Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol. 2011;77:1184–1189. [PubMed] [Google Scholar]
- 17.Van Den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 18.Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, de Castro MD L, Pavon RG, Quesada Gomez JM. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121:452–455. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 19.Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, Stojakovic T, Schnedl C, Dobnig H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vargas-Vasserot J, Mata-Granados JM, Luque De Castro M, Guerrero Pavon R, Quesada Gomez J. 25-hydroxyvitamin D3 treatment normalize the vitamin D status and the innate immune response mediated by cathelicidin in critically ill patients. Bone. 2011;48:S146–S147. doi: 10.1016/j.bone.2011.03.310. [DOI] [Google Scholar]
- 21.Arnson Y, Gringauz I, Itzhaky D, Amital H. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM. 2012;105:633–639. doi: 10.1093/qjmed/hcs014. [DOI] [PubMed] [Google Scholar]
- 22.Flynn L, Zimmerman LH, McNorton K, Dolman M, Tyburski J, Baylor A, Wilson R, Dolman H. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg. 2012;203:379–382. doi: 10.1016/j.amjsurg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Higgins DM, Wischmeyer PE, Queensland KM, Sillau SH, Sufit AJ, Heyland DK. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. J Parenter Enter Nutr. 2012;36:713–720. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 24.Aygencel G, Turkoglu M, Tuncel AF, Candir BA, Bildaci YD, Pasaoglu H. Is vitamin D insufficiency associated with mortality of critically ill patients? Crit Care Res Pract. 2013;2013:856747. doi: 10.1155/2013/856747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrein K, Zajic P, Schnedl C, Waltensdorfer A, Fruhwald S, Holl A, Urbanic Purkart T, Wunsch G, Valentin T, Grisold A, Stojakovic T, Amrein S, Pieber TR, Dobnig H. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;18:R47. doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42:97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 27.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, Center JR. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 28.Remmelts HHF, Van De Garde EMW, Meijvis SCA, Peelen ELGCA, Damoiseaux JGMC, Grutters JC, Biesma DH, Bos WJW, Rijkers GT. Addition of Vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. 2012;55:1488–1494. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- 29.Su LX, Jiang ZX, Cao LC, Xiao K, Song JP, Li H, Zhang X, Yan P, Feng D, Liu CT, Li X, Xie LX. Significance of low serum vitamin D for infection risk, disease severity and mortality in critically ill patients. Chin Med J (Engl) 2013;126:2725–2730. [PubMed] [Google Scholar]
- 30.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg O, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 32.Khoo AL, Chai LY, Koenen HJ, Kullberg BJ, Joosten I, van der Ven AJ, Netea MG. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: impact of seasonal variation of immune responses. J Infect Dis. 2011;203:122–130. doi: 10.1093/infdis/jiq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kox M, van den Berg MJ, van der Hoeven JG, Wielders JP, van der Ven AJ, Pickkers P. Vitamin D status is not associated with inflammatory cytokine levels during experimental human endotoxaemia. Clin Exp Immunol. 2013;171:231–236. doi: 10.1111/cei.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12:208–211. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97:2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 38.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willet WC, Zitterman A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 39.Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays. 2008;30:173–182. doi: 10.1002/bies.20708. [DOI] [PubMed] [Google Scholar]
- 40.Zittermann A, Schleithoff SS, Frisch S, Gotting C, Kuhn J, Koertke H, Kleesiek K, Tenderich G, Koerfer R. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55:1163–1170. doi: 10.1373/clinchem.2008.120006. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh B, Davidson B, Robinson K, Pascoe R, Appleton C, Jones M. Do random estimations of vitamin D3 and parathyroid hormone reflect the 24-h profile in the critically ill? Intensive Care Med. 2012;38:177–179. doi: 10.1007/s00134-011-2415-x. [DOI] [PubMed] [Google Scholar]
- 42.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:769–781. doi: 10.1016/j.beem.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]


