Abstract
Objective:
To study activin signaling and its blockade in sporadic inclusion body myositis (sIBM) through translational studies and a randomized controlled trial.
Methods:
We measured transforming growth factor β signaling by SMAD2/3 phosphorylation in muscle biopsies of 50 patients with neuromuscular disease (17 with sIBM). We tested inhibition of activin receptors IIA and IIB (ActRII) in 14 patients with sIBM using one dose of bimagrumab (n = 11) or placebo (n = 3). The primary outcome was the change in right thigh muscle volume by MRI at 8 weeks. Lean body mass, strength, and function were secondary outcomes. Twelve of the patients (10 bimagrumab, 2 placebo) participated in a subsequent 16-week observation phase.
Results:
Muscle SMAD2/3 phosphorylation was higher in sIBM than in other muscle diseases studied (p = 0.003). Eight weeks after dosing, the bimagrumab-treated patients increased thigh muscle volume (right leg +6.5% compared with placebo, p = 0.024; left leg +7.6%, p = 0.009) and lean body mass (+5.7% compared with placebo, p = 0.014). Subsequently, bimagrumab-treated patients had improved 6-minute walking distance, which peaked at 16 weeks (+14.6%, p = 0.008) compared with placebo. There were no serious adverse events; the main adverse events with bimagrumab were mild acne and transient involuntary muscle contractions.
Conclusions:
Transforming growth factor β superfamily signaling, at least through ActRII, is implicated in the pathophysiology of sIBM. Inhibition of ActRII increased muscle mass and function in this pilot trial, offering a potential novel treatment of sIBM.
Classification of evidence:
This study provides Class I evidence that for patients with inclusion body myositis, bimagrumab increases thigh muscle volume at 8 weeks.
Sporadic inclusion body myositis (sIBM) is a slowly progressive degenerative and inflammatory skeletal muscle disease beginning in middle or later life.1 Its clinical features include a specific pattern of muscle involvement (preferential weakness of finger flexors and knee extensors) accompanied by progressive muscle atrophy, distinctive microscopic pathology including endomysial inflammation and rimmed vacuoles, and a recently identified serum autoantibody (against cytosolic 5′-nucleotidase 1A) biomarker.2–4 Despite a prominent adaptive immune response characterized by antigen-stimulated B- and T-cell maturation and prominent infiltration into muscle of immune system cells, sIBM is highly refractory to immunosuppressive therapies studied to date.2
Members of the transforming growth factor β (TGFβ) superfamily of ligands signal through a heterodimeric receptor system.5 They first bind a type II receptor, such as the TGFβRII, the activin receptors IIA and IIB, and the bone morphogenetic protein receptors, which then increases their affinity to a type I receptor, in the Alk family.5 The activin receptors IIA (ActRIIA) and IIB (ActRIIB), together abbreviated here as ActRII, mediate the signaling downstream of the TGFβ family member myostatin as well as other related ligands such as GDF11 and the activins, to inhibit the differentiation and growth of skeletal muscle.6 Increased signaling in this pathway causes muscle atrophy, while its blockade leads to muscle hypertrophy and increased strength and physical performance in animals and humans.7 ActRII receptors dimerize with Alk4/5 and signal intracellularly via the transcription factors Smad2 and Smad3.8,9 Phosphorylation of Smad2/3 results in downregulation of genes associated with muscle differentiation and inhibits Akt signaling,8,9 which is normally activated during muscle hypertrophy and often inhibited in settings of muscle atrophy.10
To understand the potential role of TGFβ family signaling in the pathogenesis of sIBM, we studied sIBM patient muscle biopsies and undertook a study of an ActRII inhibitory antibody, bimagrumab, in patients with sIBM.
METHODS
Translational studies of TGFβ family signaling.
Patients with muscle diseases provided informed consent under institutional review board–approved research studies of their muscle biopsy specimens. Western blot analyses of muscle SMAD2/3 and phosphorylated SMAD2/3 (pSMAD2/3) were performed on biopsy samples from 50 patients, 29 with myositis (sIBM n = 17, dermatomyositis n = 5, polymyositis n = 7), 16 with other muscle diseases (OtherMyo; toxic myopathy n = 4, mitochondrial myopathy n = 4, idiopathic degenerative myopathy n = 3, denervation atrophy n = 2, myotonic muscular dystrophy n = 1, sporadic nemaline myopathy n = 1, distal myopathy n = 1), and 5 without neuromuscular disease undergoing biopsy for pain or fatigue (called normal). The mean ages for each group were (in years): sIBM = 69, dermatomyositis = 36, polymyositis = 64, OtherMyo = 71, and normal = 45.
Cell and muscle lysates were prepared and studied in Western blots as described in e-Methods on the Neurology® Web site at Neurology.org. Briefly, 60 μg of muscle lysate underwent sodium dodecyl sulfate–polyacrylamide gel electrophoresis and gel transfer, and was then probed with rabbit monoclonal anti-pSMAD2 antibody (no. 3108; Cell Signaling Technology, Danvers, MA), rabbit polyclonal anti-SMAD2/3 primary antibody (no. 3102; Cell Signaling Technology), and rabbit polyclonal anti-actin primary antibody (no. sc1616; Santa Cruz Biotechnology, Santa Cruz, CA) and visualized after incubation with secondary horseradish peroxidase–tagged antibodies.
Clinical study design and participants.
We screened patients at 4 clinical centers in the United States, enrolling them in an 8-week randomized, placebo-controlled, double-blind, parallel-arm, proof-of-concept study. Before any primary analysis was conducted, the protocol was amended to add an additional 16-week extension observation phase to collect a total of 24 weeks of efficacy and safety data. Enrollment was planned at 12 patients, but 2 patients were screened after the enrollment target was reached, and these 2 were allowed to continue to enrollment. Patients were randomly assigned to active drug, a single IV dose of 30 mg/kg bimagrumab, or placebo in a 3:1 ratio. The primary outcome was change in muscle quantity, measured by thigh muscle volume (TMV) using MRI after 8 weeks. Additional outcomes were whole body composition, including lean body mass (LBM), assessed by dual-energy x-ray absorptiometry (DXA); isometric muscle strength measured by quantitative muscle testing (QMT); and functional performance measured by Timed Up and Go and 6-minute walking distance (6MWD).
The study population comprised men and women aged 40 to 80 years with a diagnosis of definite sIBM according to the European Neuromuscular Centre criteria.11 Patients had to be able to walk at least 3 m without assistance from another person, although use of assistive devices was allowed, and able to ingest adequate intake of energy and protein, defined as at least 20 kcal/kg/d and 0.6 g protein/kg/d measured by a Food Frequency Questionnaire.12
Standard protocol approvals, registrations, and patient consents.
The study was registered with ClinicalTrials.gov under identifier NCT01423110. Institutional review boards at each study site approved the study protocol, and all patients provided written informed consent. Novartis designed the study, conducted the statistical analyses, and interpreted the data in collaboration with the investigators.
Randomization and masking.
A randomization list was produced under the responsibility of Novartis Drug Supply Management using a validated system that automated the random assignment of treatment arms to randomization numbers in the specified ratio. The randomization scheme for patients was reviewed and approved by a member of the Novartis Biostatistics Quality Assurance Group. All patients and investigators were masked to treatment allocation.
Procedures.
Thigh muscle volume.
Patients were imaged using a similar MRI scanner in all sites (1.5T) and a Q-body coil. Patients remained horizontal for approximately 30 minutes before the scan to minimize variability caused by fluid shift. For TMV, after a rapid survey scan, lean muscle tissue volume was quantitatively assessed from proton density images of the thigh using a 2-dimensional multislice pulse sequence to cover the entire thigh (knee to hip). Scans were read at a central analysis site by experienced staff blinded to treatment assignment.
Total LBM.
DXA was used to assess total LBM changes. DXA instrument type and model, scan acquisition protocol, and analysis software remained consistent and their calibrations were monitored throughout the study using a calibration phantom. Scans were read at a central analysis site by experienced staff blinded to treatment assignment.
Muscle strength.
QMT (also called Maximum Voluntary Isometric Contraction Test) was performed using the QMA system (Computer Source, Atlanta, GA) or the Biodex system (Biodex Medical Systems, Shirley, NY). Six muscle groups were tested bilaterally (biceps brachii, triceps, quadriceps, hamstrings, ankle dorsiflexors, and hand grip). Each muscle group was tested twice while the patient was encouraged by the clinical evaluator to exert maximal effort. The maximum force generated by the patient from the 2 trials was recorded for each muscle group, and was recorded in pounds or newtons.
Muscle function.
Timed Up and Go, which is the time it takes a patient to stand up from a chair without armrests, walk 3 m, turn around, return to the chair, and sit down, was measured as previously described.13 The quicker of 2 trials (in seconds) was recorded. In addition, 6MWD was also measured as previously described.13
Statistical analysis.
All outcomes were analyzed by analysis of covariance with the pretreatment baseline as a covariate and treatment as a fixed effect. Two-sided p values and 95% confidence intervals for the difference between bimagrumab and placebo are presented. TMV and LBM were logarithmically transformed before analysis. Least mean squares estimates of treatment effects between the bimagrumab- and placebo-treated groups were reported on the original scale. The study was powered to detect a treatment difference in TMV from baseline to week 8, and this analysis was preplanned in the protocol. The remaining analyses reported here are exploratory in nature.
RESULTS
pSMAD2/3, a catabolic signal, is increased in sIBM muscle.
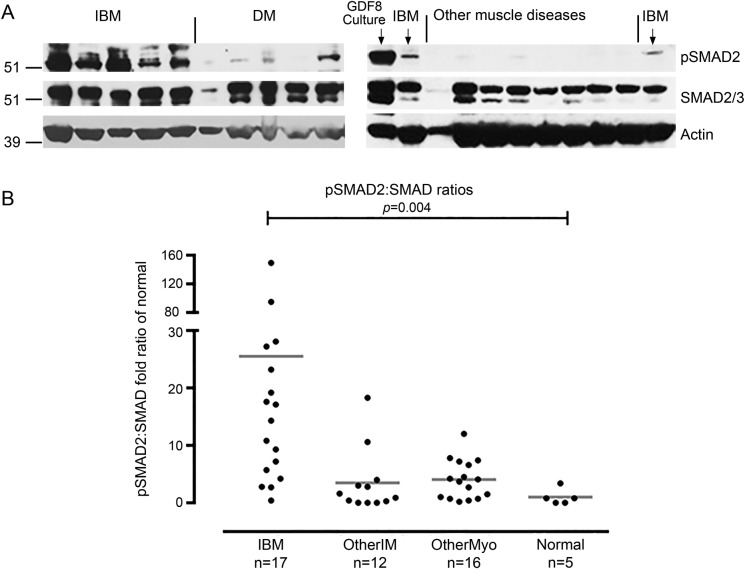
To understand the molecular events that could be driving muscle atrophy present in sIBM, we performed Western blots to quantitate pSMAD2/3 and total SMAD2/3 in 50 muscle biopsy samples (figure 1). pSMAD2/3, normalized to actin, was substantially increased in sIBM (27.4-fold increase; p = 0.003), but not in other forms of inflammatory muscle disease (2.5-fold; p = 0.3) or noninflammatory muscle diseases (1.7-fold; p = 0.1) (Mann-Whitney tests) compared with normal. Because SMAD phosphorylation reflects signaling through ligand-receptor pairs of the TGFβ superfamily, and because ActRII is known to be important in such signaling in muscle, we hypothesized that inhibition of ActRII could help improve muscle mass, strength, and function in patients with sIBM.
Figure 1. pSMAD2/3 is increased in sIBM muscle.
(A) Western blots of 7 sIBM samples show increased size and density of pSMAD2/3 bands, in comparison to 5 DM, and 8 noninflammatory other muscle disease samples. Myostatin (GDF8)-treated human skeletal muscle culture was a positive control. (B) Quantitation of pSMAD2 to SMAD Western blot band intensity ratios in 50 muscle samples show statistically significant increases in sIBM samples. DM = dermatomyositis; GDF8 = growth differentiation factor 8; IBM = inclusion body myositis; OtherIM = non-IBM inflammatory myopathy; OtherMyo = noninflammatory myopathy; pSMAD2/3 = phosphorylated SMAD2/3; sIBM = sporadic inclusion body myositis.
ActRII inhibition improves muscle mass in sIBM.
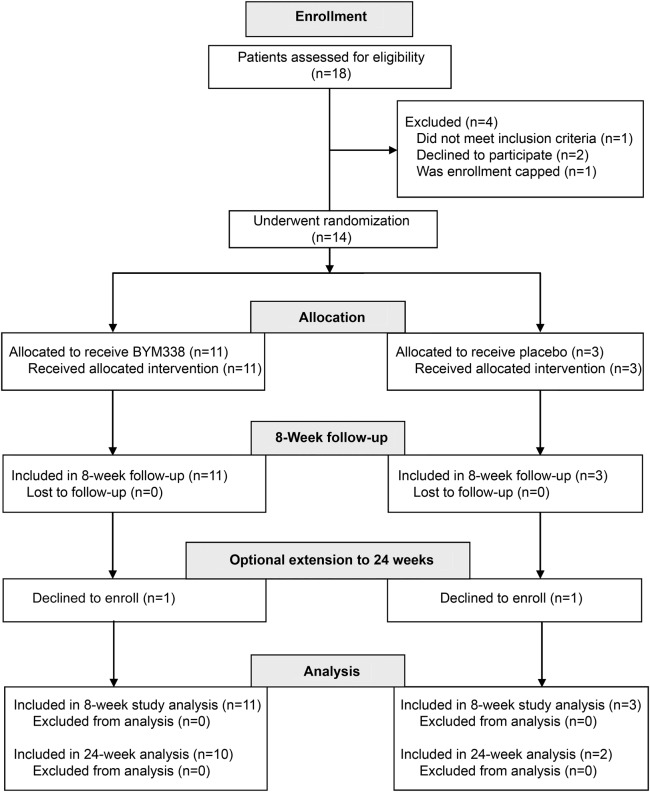
We screened 18 patients among 4 US sites for entry in the study (figure 2). Of these, 4 patients were deemed ineligible or declined further participation. Thus, 14 patients were enrolled from 3 sites and randomly assigned to treatment group in a 3:1 ratio (active to placebo), so that 11 patients received active study drug and 3 received placebo. All 14 patients completed the initial protocol 8-week study. Two participants declined to enroll in the optional 16-week extension to 24 weeks, leaving 10 patients in the bimagrumab arm and 2 in the placebo arm. Demographic characteristics of the study patients are summarized in table 1, and results are summarized in table 2.
Figure 2. Clinical trial design.
Table 1.
Demographics of study patients
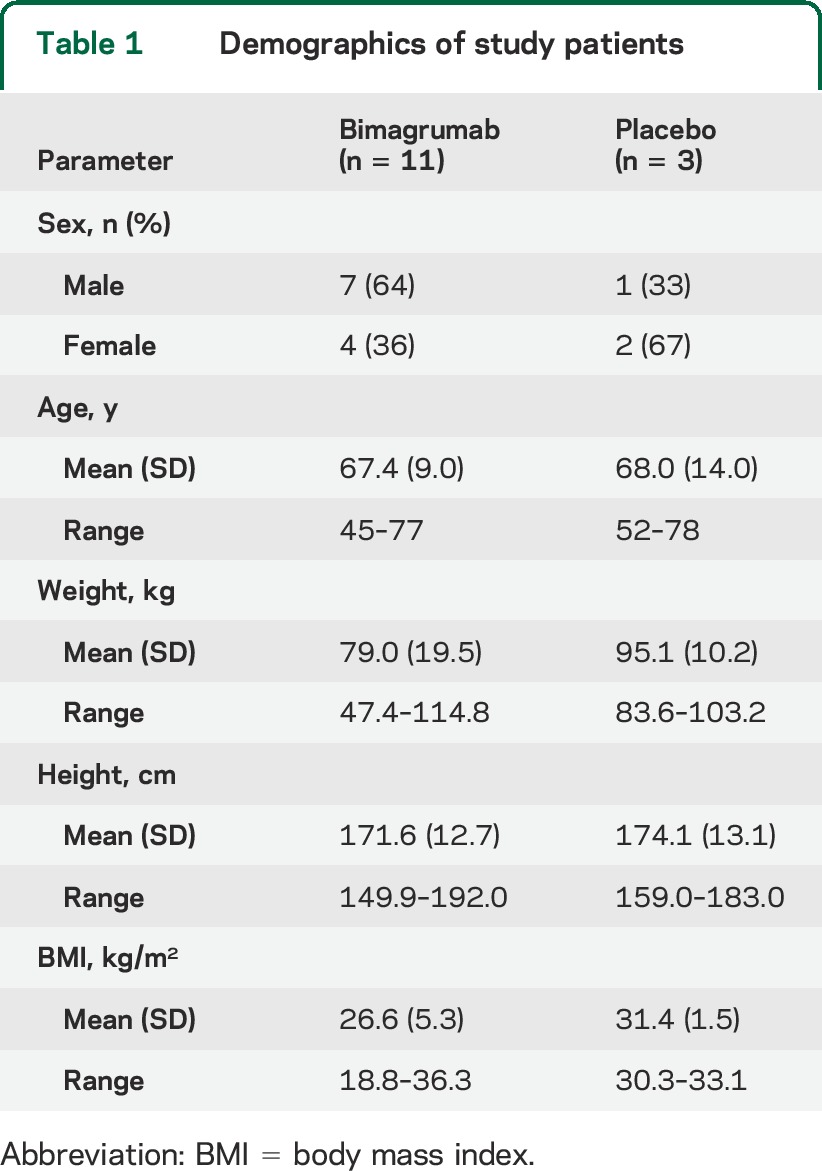
Table 2.
Outcomes measured over time in the clinical study by treatment group
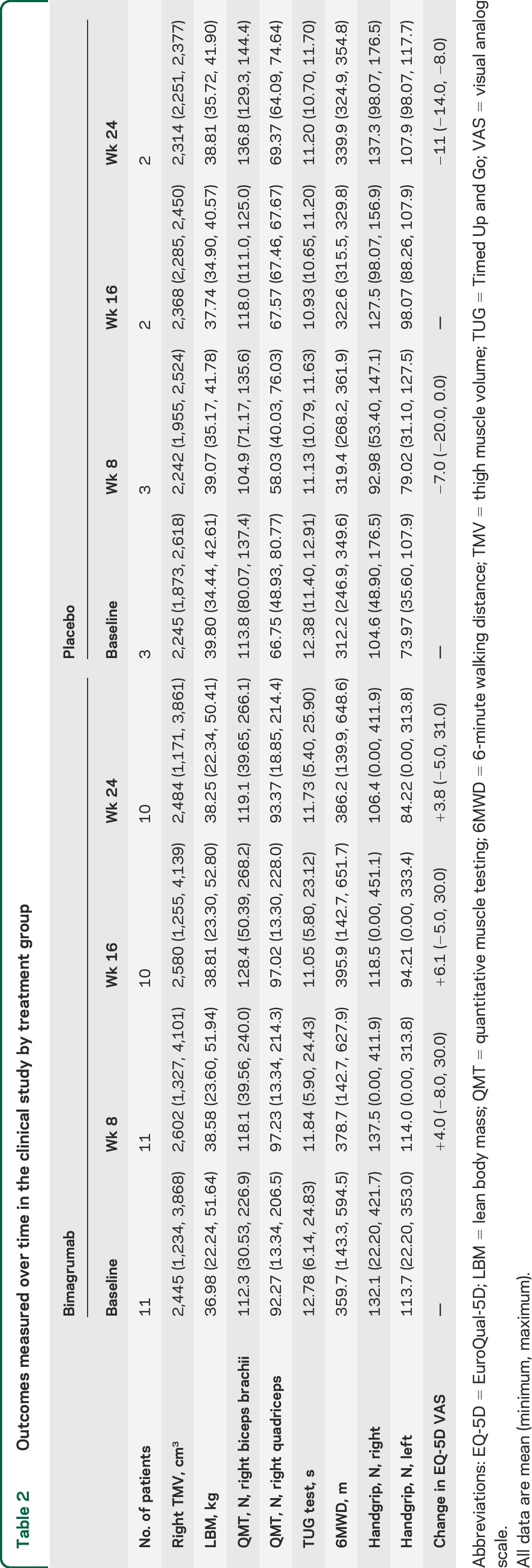
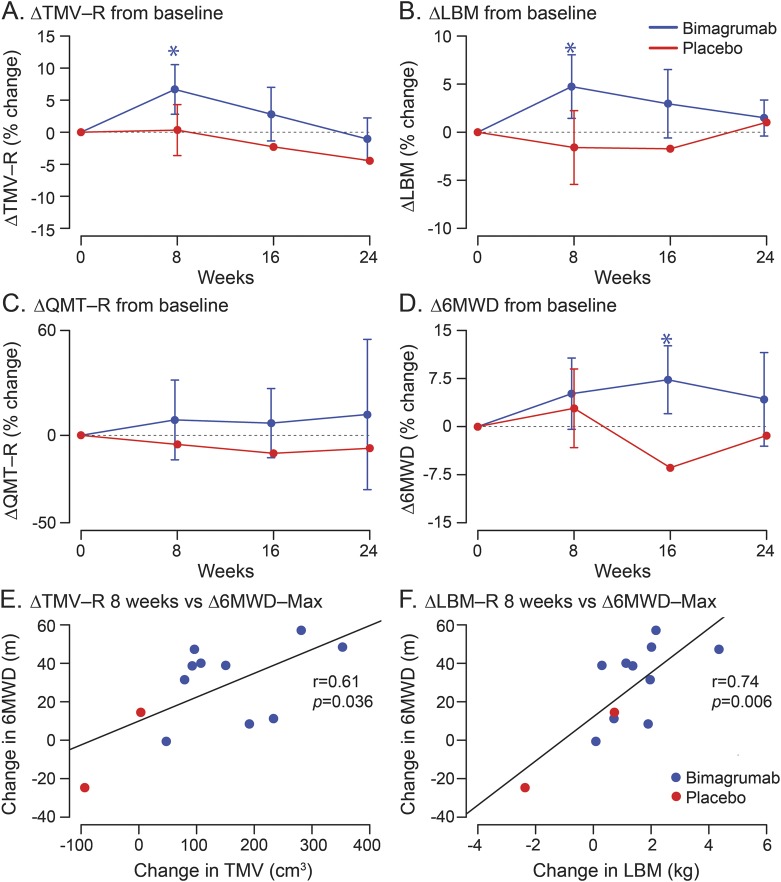
In the primary analysis preplanned in the study protocol, a single 30 mg/kg IV dose of bimagrumab increased the lean muscle volume primary outcome of right TMV compared with placebo at week 8 (least mean squares estimate of treatment effect 6.5%, p = 0.024; figure 3A). Left TMV also increased compared with placebo (least mean squares estimate 7.6%, p = 0.009). In secondary analyses, LBM increased 5.7% compared with placebo (p = 0.014) as well (figure 3B). The Timed Up and Go test showed no difference between bimagrumab and placebo at week 8 (mean [SD] of change from baseline were, respectively, −0.9 [0.9] second for bimagrumab and −1.2 [0.8] seconds for placebo). The 6MWD showed a trend favoring bimagrumab at 8 weeks: mean (SD) change from baseline was 19.0 (18.6) m for bimagrumab and 7.1 (17.3) m for placebo. There was also a trend favoring bimagrumab in right quadriceps QMT; mean (SD) of change from baseline of +5.0 (7.3) N in the bimagrumab group vs −1.7 (10.2) N in the placebo group. Nonsignificant trends in QMT favoring bimagrumab were present in all muscle groups except left biceps, left quadriceps, and right hamstrings. Measures of patient-reported outcomes (Inclusion Body Myositis Functional Rating Scale, 36-item Short Form Health Survey, or EuroQual-5D) showed no difference between groups at 8 weeks.
Figure 3. Effect of bimagrumab compared with placebo on primary and secondary study endpoints over 24 weeks.
(A–D) Changes in TMV-R, LBM, QMT-R, and 6MWD at 0, 8, 16, and 24 weeks. Sample sizes are 11 active and 3 placebo at 0 and 8 weeks, and 10 active and 2 placebo at 16 and 24 weeks. Mean and SDs of untransformed data are plotted, with SD bars provided for all means with more than 2 measurements. (E, F) Spearman correlation between change in muscle mass to week 8 (as measured by both TMV-R and LBM) and function (maximal change in 6MWD postbaseline). Sample sizes are 10 active and 2 placebo. LBM = lean body mass; QMT-R = right quadriceps quantitative muscle testing; 6MWD = 6-minute walking distance; TMV-R = right thigh muscle volume.
In the extension to 24 weeks (figure 3, A–D), TMV in the bimagrumab group remained elevated but not significantly. Right TMV was 5.2% above baseline compared with placebo at 16 weeks and 3.6% at 24 weeks. Left TMV was not measured in all patients in order to reduce the time patients were required to stay in the MRI scanner. LBM in the bimagrumab-treated group was 4.7% above baseline compared with placebo at 16 weeks and 0.4% at 24 weeks.
The 6MWD measure of muscle function in the bimagrumab-treated group improved 14.6% (p < 0.008; analysis of covariance) at 16 weeks and 5.7% at 24 weeks above baseline compared with placebo. Quadriceps muscle strength by QMT also showed a favorable trend toward bimagrumab: at 16 weeks the mean changes from baseline in the bimagrumab/placebo groups were +4.1 N/−8.1 N in the right leg and +3.9 N/−10.7 N in the left leg. At week 24, the same results were, respectively, −0.4 N/−6.3 N and 0.8 N/−8.3 N. Timed Up and Go did not show a difference between active treatment and placebo.
To investigate whether increased muscle mass leads to increased function, the increase in right TMV and LBM, respectively, were correlated with the maximal increase in 6MWD seen at any point past baseline (figure 3, E and F). Significant, positive Spearman rank correlation coefficients were found in both cases.
Drug exposure and safety of bimagrumab.
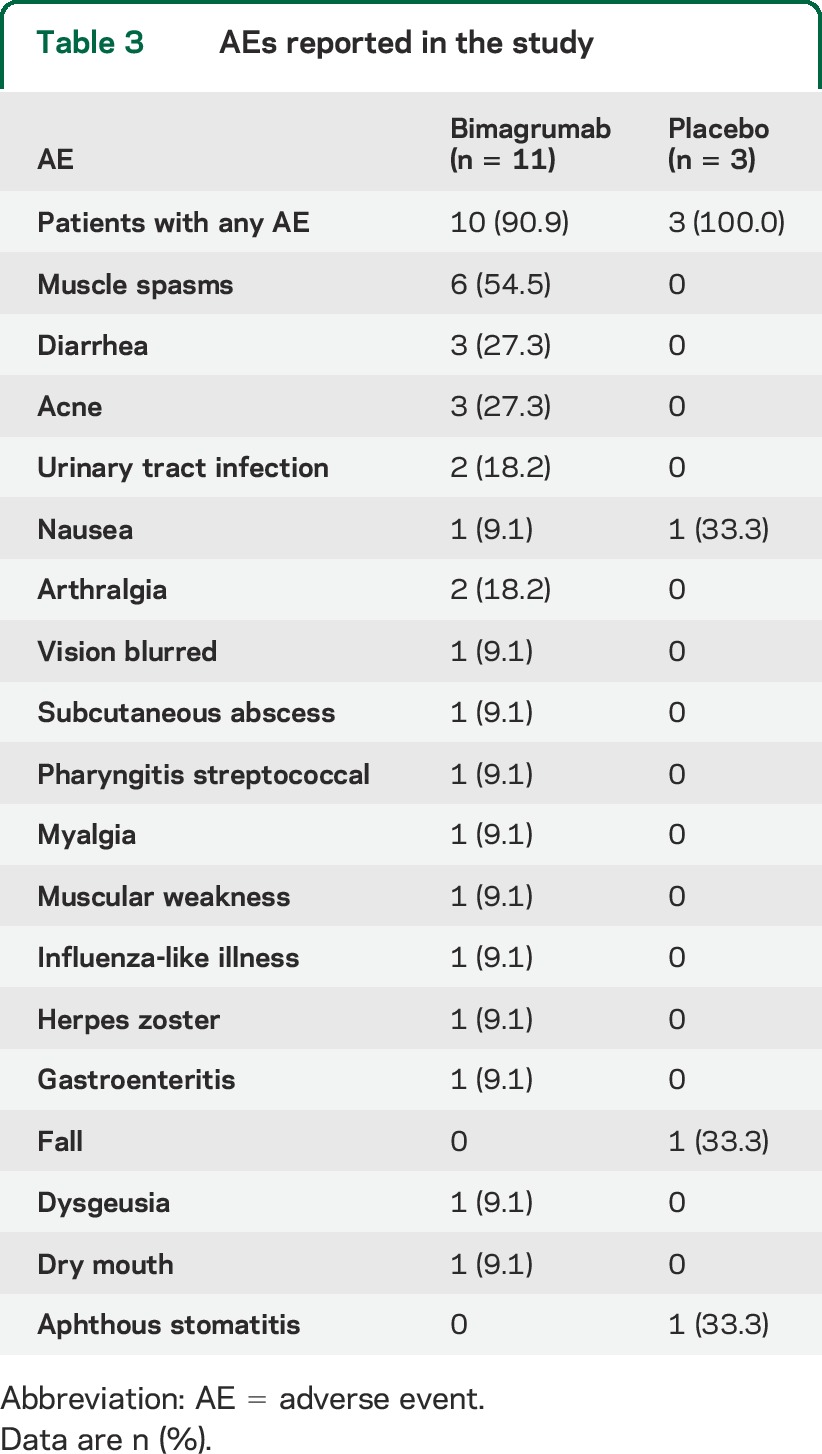
A single IV dose of 30 mg/kg bimagrumab gave exposure considered to be above saturation of the ActRIIB receptor for at least 56 days (data not shown). There was evidence of target-mediated drug disposition, with more rapid elimination of the drug once serum levels dropped below saturation. Bimagrumab was safe and tolerable: the most common adverse events were muscle spasms, which occurred in 6 of 11 bimagrumab-treated patients but no placebo-treated patients (table 3). These muscle contractions were fleeting, and considered mild in all cases. Three patients treated with bimagrumab reported mild diarrhea, compared with none receiving placebo. Three patients treated with bimagrumab developed mild acne, compared with none receiving placebo. There was one serious adverse event in a bimagrumab-treated patient, hospitalization for flu-like illness, which was considered to be unrelated to the study drug. There were no dropouts from adverse events over the 24 weeks of the study.
Table 3.
AEs reported in the study
DISCUSSION
sIBM is a poorly understood progressive muscle disease of middle and later life, with dual components of autoimmunity and degeneration. These 2 processes lead chronically to marked loss of muscle bulk. A key feature distinguishing sIBM from other forms of myositis, such as polymyositis or dermatomyositis, is that sIBM is refractory to corticosteroids and immunosuppressants tried to date.
To understand potential mechanisms underlying sIBM muscle atrophy, we examined the activation (by phosphorylation) of SMADs 2 and 3, which are transcription factors whose activation can result in skeletal muscle atrophy.8,9 SMAD2/3 are phosphorylated in response to certain TGFβ superfamily ligands including myostatin and related molecules such as GDF11 and the activins, signaling via ActRII.6 We found evidence of increased SMAD2/3 protein phosphorylation in sIBM muscle biopsy specimens. SMAD2/3 phosphorylation is sufficient to induce muscle atrophy by 2 mechanisms: inhibition of muscle-specific gene upregulation, and downregulation of Akt phosphorylation, and thus its signaling pathway (which is associated with muscle hypertrophy).14–16 The finding that SMAD2/3 phosphorylation is increased in sIBM suggested that targeting of ActRII through a neutralizing therapeutic might reverse sIBM muscle loss and improve disabling manifestations of sIBM. We therefore conducted a clinical trial of bimagrumab in sIBM focused on assessing changes in skeletal muscle mass.
A single dose of bimagrumab increased the primary outcome measure (muscle mass) at 8 weeks, and showed trends in improvement for strength and function during the subsequent 24 weeks of observation. In contrast, all previous sIBM randomized trials have not resulted in statistically significant improvements in their primary outcome measures.13,17–22 With a single dose of 30 mg/kg, bimagrumab exposure is thought to have been above the therapeutic target of saturating ActRII for approximately 2 months. During this time, muscle mass, measured by MRI of the thigh and by DXA of the whole body, increased substantially from baseline, by approximately 5% more than placebo (figure 3, A and B). That this muscle gain was functional is supported by the parallel increases in strength and 6MWD (figure 3, C and D). The development of muscle hypertrophy first, followed by increased function, has also been demonstrated after treatment with growth hormone and testosterone.23 This sequence seems to be true of hypertrophy-inducing pharmacologic agents, and is the opposite of what occurs with resistance exercise training, in which strength increases first.24
We have demonstrated that SMAD phosphorylation is frequently increased in the muscle of patients with sIBM, suggesting that at least part of the TGFβ superfamily may be implicated in the pathophysiology of sIBM. The finding that SMAD2/3 was elevated still left unresolved whether inhibiting this pathway in particular would be sufficient to perturb the clinical consequence of sIBM. This question was at least partly answered in a positive manner by the results of the clinical trial with bimagrumab, which demonstrated amelioration of sIBM using an inhibitor of a key TGFβ superfamily receptor, ActRII. Because the best-known ligands of ActRII, activin and myostatin, are both known to be associated with muscle wasting in animals,9 bimagrumab may offer a novel approach to treating muscle wasting in sIBM and other muscle diseases. Further studies exploring the optimal dose and duration of therapy, its safety and efficacy, and the spectrum of diseases that may benefit from bimagrumab, are now under way.
Supplementary Material
ACKNOWLEDGMENT
The authors thank MorphoSys for their contributions in isolation and characterization of the bimagrumab antibody.
GLOSSARY
- ActRII
activin receptors IIA and IIB
- DXA
dual-energy x-ray absorptiometry
- LBM
lean body mass
- pSMAD2/3
phosphorylated SMAD2/3
- QMT
quantitative muscle testing
- sIBM
sporadic inclusion body myositis
- 6MWD
6-minute walking distance
- TGFβ
transforming growth factor β
- TMV
thigh muscle volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Anthony A. Amato contributed to clinical study design and performance. Kumaraswamy Sivakumar contributed to clinical study performance. Namita Goyal contributed to clinical study performance. William S. David contributed to clinical study performance. Mohammad Salajegheh contributed to translational study design and performance. Jens Praestgaard contributed to clinical study design and data analysis. Estelle Lach-Trifilieff contributed to characterization and profiling of clinical antibody. Anne-Ulrike Trendelenburg contributed to signaling work and analyses. Didier Laurent contributed to clinical study design, imaging implementation, and data interpretation. David J. Glass contributed to signaling work and analyses, and translational design. Ronenn Roubenoff contributed to clinical study design and analysis, and obtaining study funding. Brian S. Tseng contributed to translational and clinical study design, data analysis, and interpretation. Steven A. Greenberg contributed to translational and clinical study designs, performance, and analyses.
STUDY FUNDING
Supported by Novartis Institutes for Biomedical Research, Boston Children's Hospital, and Brigham and Women's Hospital, Harvard Medical School. Role of the funding source: The study was designed and the data were analyzed by the sponsor, Novartis, in collaboration with the principal investigator (S.A.G.). Novartis participated in the interpretation of data, review, and approval of the report. An initial draft of the report was prepared by R.R. and S.A.G. All authors participated in interpretation of the data, contributed substantially to report drafts, and agreed to submit the report for publication. All authors had full access to the data and vouch for accuracy and completeness of the data and analyses.
DISCLOSURE
A. Amato, K. Sivakumar, N. Goyal, W. David, and M. Salajegheh report no disclosures relevant to the manuscript. J. Praestgaard is an employee of Novartis. E. Lach-Trifilieff is an employee of Novartis. A. Trendelenburg is an employee of Novartis. Didier Laurent is an employee of Novartis. D. Glass is an employee of Novartis. R. Roubenoff is an employee of Novartis. B. Tseng is an employee of Novartis. S. Greenberg reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol 2007;6:620–631. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SA. Pathogenesis and therapy of inclusion body myositis. Curr Opin Neurol 2012;25:630–639. [DOI] [PubMed] [Google Scholar]
- 3.Larman HB, Salajegheh M, Nazareno R, et al. Cytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 2013;73:408–418. [DOI] [PubMed] [Google Scholar]
- 4.Pluk H, van Hoeve BJ, van Dooren SH, et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann Neurol 2013;73:397–407. [DOI] [PubMed] [Google Scholar]
- 5.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 2012;11:790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sako D, Grinberg AV, Liu J, et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J Biol Chem 2010;285:21037–21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle 2011;2:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartori R, Milan G, Patron M, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 2009;296:C1248–C1257. [DOI] [PubMed] [Google Scholar]
- 9.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 2009;296:C1258–C1270. [DOI] [PubMed] [Google Scholar]
- 10.Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001;3:1014–1019. [DOI] [PubMed] [Google Scholar]
- 11.Badrising UA, Maat-Schieman M, van Duinen SG, et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology 2000;55:1385–1387. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1:58–64. [DOI] [PubMed] [Google Scholar]
- 13.Rutkove SB, Parker RA, Nardin RA, Connolly CE, Felice KJ, Raynor EM. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology 2002;58:1081–1087. [DOI] [PubMed] [Google Scholar]
- 14.Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 1999;286:1738–1741. [DOI] [PubMed] [Google Scholar]
- 15.Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 2001;3:1009–1013. [DOI] [PubMed] [Google Scholar]
- 16.Lai KM, Gonzalez M, Poueymirou WT, et al. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 2004;24:9295–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology 1997;48:712–716. [DOI] [PubMed] [Google Scholar]
- 18.Walter MC, Lochmuller H, Toepfer M, et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol 2000;247:22–28. [DOI] [PubMed] [Google Scholar]
- 19.Dalakas MC, Koffman B, Fujii M, Spector S, Sivakumar K, Cupler E. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology 2001;56:323–327. [DOI] [PubMed] [Google Scholar]
- 20.Muscle Study Group. Randomized pilot trial of betaINF1a (Avonex) in patients with inclusion body myositis. Neurology 2001;57:1566–1570. [DOI] [PubMed] [Google Scholar]
- 21.Badrising UA, Maat-Schieman ML, Ferrari MD, et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol 2002;51:369–372. [DOI] [PubMed] [Google Scholar]
- 22.Muscle Study Group. Randomized pilot trial of high-dose betaINF-1a in patients with inclusion body myositis. Neurology 2004;63:718–720. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder ET, He J, Yarasheski KE, et al. Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol 2012;112:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 2003;28:601–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.