Abstract
Objective:
To report incidence and risk factors for motoric cognitive risk syndrome (MCR), a newly described predementia syndrome characterized by slow gait and cognitive complaints.
Methods:
We examined incidence rates of MCR in 3,128 adults aged 60 years and older, MCR- and dementia-free at baseline, participating in 4 US-based cohort studies. Hazard ratios (HRs) with 95% confidence intervals (CIs) for the association of modifiable risk factors with risk of MCR were computed using Cox models.
Results:
Over a median follow-up time of 3.2 years, 823 of the 3,128 participants met MCR criteria. The overall age- and sex-adjusted incidence of MCR was 65.2/1,000 person-years (95% CI: 53.3–77.1), and ranged from 50.8/1,000 person-years to 79.6/1,000 person-years in the individual cohorts. MCR incidence was higher with older age but there were no sex differences. In the pooled sample adjusted for age, sex, education, and cohort source, strokes (HR 1.42, 95% CI: 1.14–1.77), Parkinson disease (HR 2.52, 95% CI: 1.68–3.76), depressive symptoms (HR 1.65, 95% CI: 1.28–2.13), sedentariness (HR 1.76, 95% CI: 1.44–2.17), and obesity (HR 1.39, 95% CI: 1.17–1.65) predicted risk of incident MCR.
Conclusions:
The incidence of MCR is high in older adults. Identification of modifiable risk factors for MCR will improve identification of high-risk individuals and help develop interventions to prevent cognitive decline in aging.
There is increasing evidence that gait slowing occurs early in the course of dementia, precedes declines in cognitive tests,1–3 and is a strong predictor of dementia.4 Hence, incorporating gait performance into risk assessments may help improve dementia prediction. The motoric cognitive risk syndrome (MCR) is a predementia syndrome characterized by the presence of cognitive complaints and slow gait in older individuals without dementia or mobility disability.5,6 MCR can be detected without complex tests, enhancing accessibility in various clinical settings.5 The prevalence of MCR was 9.7% in a pooled analysis of 26,802 older adults from 17 countries.5 MCR predicted risk of developing major cognitive decline and dementia (Alzheimer disease and vascular dementia) in more than 5,000 older adults from 5 independent aging cohorts.5,6
We reported that individuals with MCR have more chronic illnesses than non-MCR elders at cross-section.5,6 Lifestyle variables such as physical activity levels are linked to dementia risk.7–9 Based on these observations, we hypothesized that disease burden at baseline and lifestyle characteristics will influence risk of MCR. To further delineate the epidemiology of MCR and gain insights into its causes, we examined incidence of MCR and associated risk factors in dementia-free older adults participating in 4 prospective cohort studies. Information on the incidence of MCR and modifiable risk factors can help identify individuals at high risk of cognitive decline and may lead to the development of preventive interventions for dementia.
METHODS
This MCR incidence study includes individual data from participants from 4 established aging studies in the United States. Baseline data were collected from 1994 to 2008 in the cohorts, and mean follow-up ranged from 1 to 10 years. Two studies recruited from single geographic sites,6,10 one regionally,11,12 and one nationally.13 All participating cohorts contained information at baseline and annual follow-up visits on cognitive complaints, cognitive test performance, gait speed, mobility disability, and dementia. From 4,659 individuals at baseline, we excluded those missing gait speed (n = 541) or cognitive complaints (n = 31). We excluded 395 participants who met MCR criteria at baseline. We also excluded 59 participants diagnosed with dementia at diagnostic case conferences at or before the baseline visit for this analysis. After exclusions, the final eligible sample included 3,128 individuals aged 60 years and older without MCR or dementia.
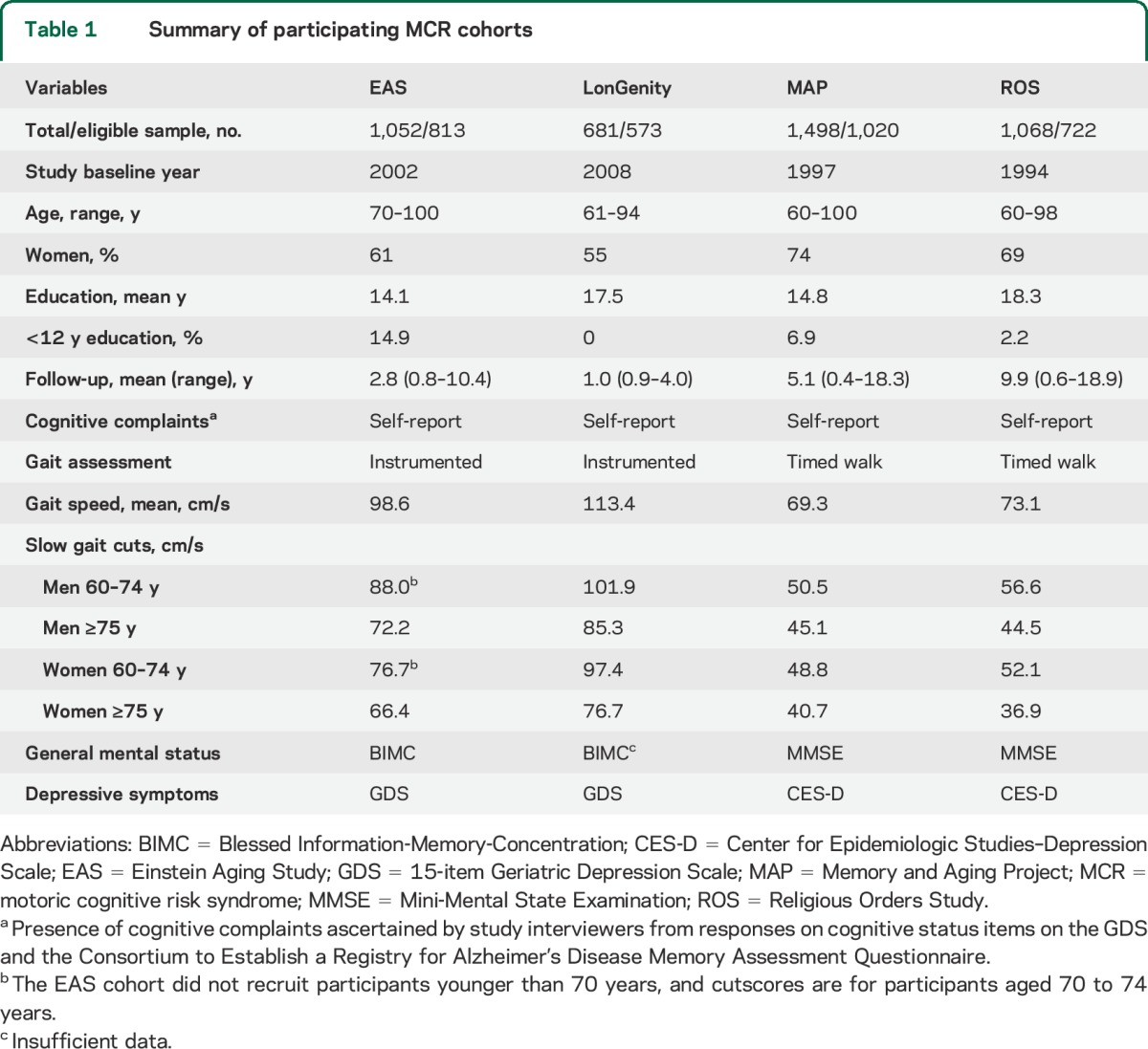
Individual study goals and design have been published. In brief, the Einstein Aging Study (EAS) is a longitudinal study of cognitive aging in a community-dwelling population sample from Bronx county (mean age 79.1 years, 61% women, 70% Caucasian, 24% African American).4,6 The LonGenity study recruited an Ashkenazi Jewish cohort from New York City and surrounding counties with the goal of identifying longevity-associated genotypes (mean age 76.0 years, 55% women, 100% Caucasian).12 The Chicago-based Memory and Aging Project (MAP) is a clinical-pathologic study of chronic conditions of aging (mean age 79.3 years, 74% women, 93% Caucasian, 6% African American).10 The Religious Orders Study (ROS) enrolled religious clergy from more than 40 groups across the United States (mean age 74.1 years, 69% women, 93% Caucasian, 6% African American).13
MCR diagnosis.
MCR diagnosis adapts operational criteria for the mild cognitive impairment (MCI) syndrome,5,14 and is defined as presence of cognitive complaints and slow gait in older individuals without dementia or mobility disability (inability to ambulate even with assistance or walking aids).6 Table 1 lists tests and procedures in each cohort. Cognitive complaints were recorded by interviewers based on responses to cognitive status items in standardized questionnaires.6 Unlike other predementia syndromes, cognitive tests were not used for MCR diagnosis. Informants were not available for all participants. Hence, informant reports were not used to define or confirm cognitive complaints for this analysis. Gait speed (cm/s) at normal pace was measured using an instrumented walkway (GAITRite; CIR Systems, Sparta, NJ) in EAS and LonGenity.12,15 Data collection started and stopped 3 feet from either end of the walkway edge to account for initial acceleration and terminal deceleration phases. Gait at normal pace was timed over a fixed distance from a standing start, and converted to speed in MAP and ROS.10,13 Slow gait was defined as walking speed 1 SD below age- and sex-specific means in each cohort to overcome variability in populations and procedures (table 1).6,16
Table 1.
Summary of participating MCR cohorts
Pooled multicountry prevalence rates of MCR defined using alternate criteria (global slow gait cutscores or gait speed measured using similar protocols) were similar to MCR prevalence estimates using cohort-specific slow gait cutscores.5 High reliability has been reported for gait speed measured using timed and instrumented methods.17 Furthermore, MCR prevalence rates in studies that used timed gait and those that used instrumented walkway to measure gait speed also showed excellent agreement.5
Other covariates.
Self-report of physician diagnosis of cardiovascular diseases (angina, myocardial infarction, and congestive heart failure), stroke, hypertension, diabetes, and Parkinson disease was noted. The EAS used the Blessed Information-Memory-Concentration (BIMC) Test to assess general mental status whereas MAP and ROS cohorts used the Mini-Mental State Examination (MMSE). We converted BIMC scores (range 0–32; lower scores better) to MMSE (range 0–30; higher better) using a validated conversion formula.18 Scores on tests of global mental status and depressive symptoms were standardized to facilitate comparisons.
We selected 3 potentially modifiable lifestyle variables that might influence MCR risk: cognitive reserve, obesity (body mass index ≥30 kg/m2), and sedentariness. Years of education is used as a proxy for cognitive reserve,19 and was linked to dementia risk in our cohorts.19,20 Sedentariness was defined as walking less than a quarter mile or difficulty negotiating stairs.
Analysis.
Participants adjudicated to not have dementia (at case conferences with access to all clinical and cognitive test information) and who did not meet MCR criteria at baseline were considered to be at risk of incident MCR. Results are reported for individual cohorts and pooling samples using meta-analysis. The onset of MCR was assigned at the follow-up visit at which criteria were met. Person-years of follow-up were calculated as the time between baseline visit and final follow-up examination, incident MCR, or death, whichever occurred first. We estimated age- and sex-adjusted incidence density rates (cases per 1,000 person-years) overall as well as stratified by age (60–69 years, 70–79 years, and ≥80 years) and sex (male vs female). Participants who developed incident dementia without an interim diagnosis of MCR at a follow-up visit (n = 298) were not counted as incident MCR because there may be dementia pathways without an MCR stage. Inclusion of person-years of follow-up preceding dementia diagnosis does not significantly alter MCR incidence rates. We examined stability of diagnosis in participants who had at least one follow-up visit after developing incident MCR.
Cox proportional-hazards models were used to compute hazard ratios (HRs) with 95% confidence intervals (CIs) associated with the selected risk factors for developing incident MCR. Model assumptions were examined graphically and analytically, and were adequately met.
Standard protocol approvals, registrations, and patient consents.
All participating studies obtained written informed consent and approval from local institutional review boards. The institutional review board of the Albert Einstein College of Medicine approved this analysis.
RESULTS
Cohort characteristics are presented in table 1. The ages of the 3,128 participants ranged from 60 to 100 years, with 66% women. Mean education level was 15.9 years.
Incidence.
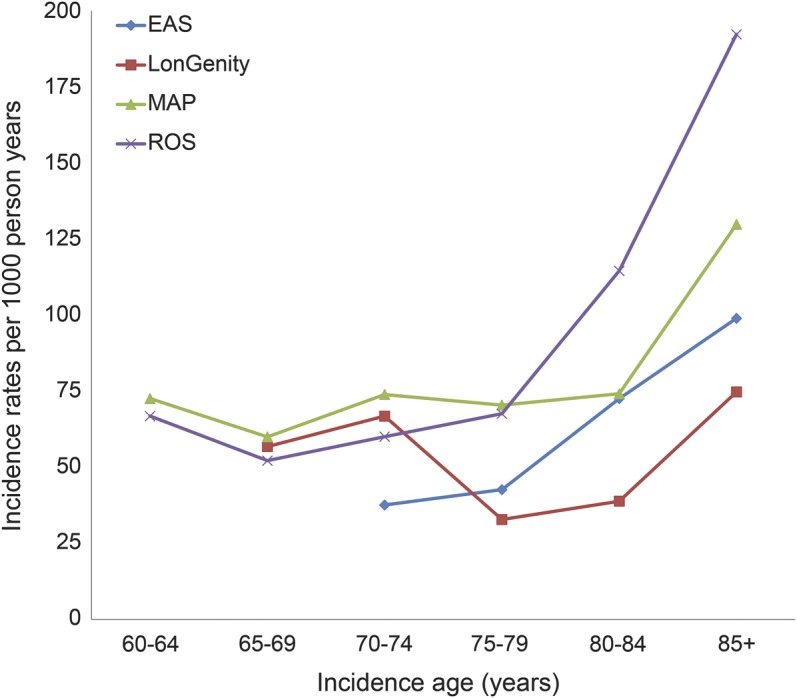
Over a median follow-up of 3.2 years, 823 of the 3,128 participants developed incident MCR. Table 2 presents incidence rates in individual cohorts and pooled: overall as well as by age and sex. The pooled age- and sex-adjusted incidence of MCR among individuals aged 60 years and older was 65.2/1,000 person-years. MCR incidence ranged from 50.8/1,000 person-years in the LonGenity cohort to 79.6/1,000 person-years in MAP. The figure shows that MCR incidence increases with increasing age. The pooled MCR incidence was higher in the 1,135 individuals aged 80 years and older at baseline (94.2/1,000 person-years) than the 1,508 individuals aged 70 to 79 years (56.6/1,000 person-years). Pooled incidence of MCR was lower in the 485 individuals aged 60 to 69 years (54.9/1,000 person-years); EAS did not include individuals younger than age 70. Pooled prevalence of MCR was similar in 1,060 men (63.4/1,000 person-years) and 2,068 women (68.0/1,000 person-years).
Table 2.
MCR incidence rates (per 1,000 person years) overall as well as by age and sex in study cohorts
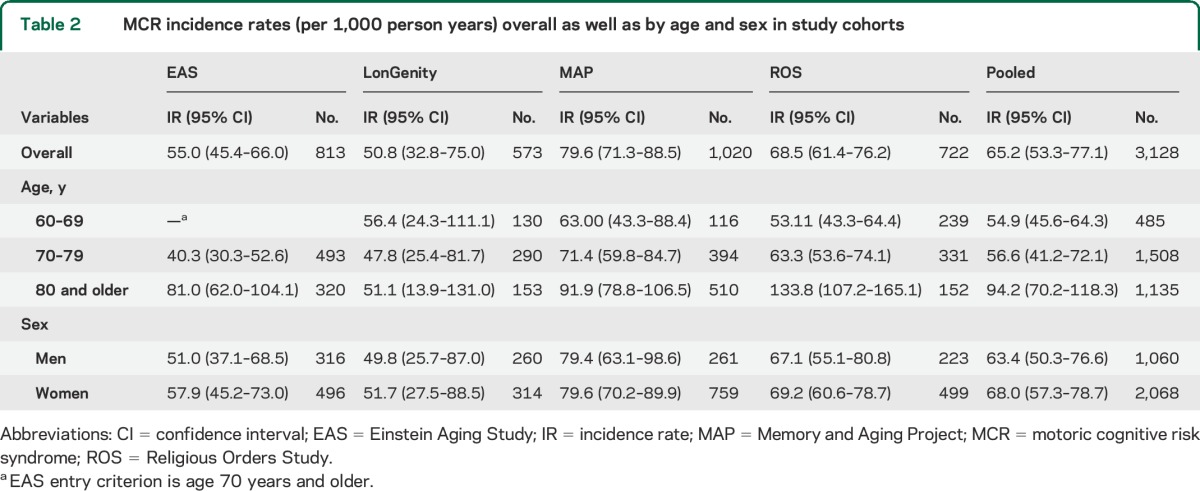
Figure. Incidence rates of motoric cognitive risk syndrome by age group and cohort.
EAS = Einstein Aging Study; MAP = Memory and Aging Project; ROS = Religious Orders Study.
Of the 823 participants who developed MCR, 615 had one or more subsequent annual follow-up visits: 354 (57.5%) remained MCR, 136 (22.1%) converted to dementia, and 125 (20.3%) reverted to non-MCR status. The participants who reverted were younger (75.4 vs 77.5 years nonreverters, p < 0.001) and more educated (16.7 vs 15.8 years, p = 0.004), but there were no sex differences (69% vs 66% women, p = 0.349).
MCR and risk factors.
Age was associated with increased risk of MCR (HR 1.05, 95% CI: 1.03–1.07). Sex did not predict MCR (HR 1.04, 95% CI: 0.94–1.15). The mean BIMC score was 1.8 ± 1.9 in EAS and mean MMSE scores were 28.3 ± 1.7 in MAP and 28.8 ± 1.3 in ROS. BIMC was not available for 23% of the LonGenity sample. General mental status predicted MCR (HR 0.82, 95% CI: 0.75–0.90). The mean gait speed was 85.9 ± 25.7 cm/s. Baseline gait speed (HR 0.95, 95% CI: 0.92–0.97) predicted incident MCR. The association remained (HR 0.95, 95% CI: 0.92–0.98) even when participants with slow gait (without cognitive complaints) were excluded.
Table 3 presents association of selected modifiable lifestyle and medical variables with incident MCR. While our main focus was to compare risk factors across cohorts, pooled estimates are presented to provide an epidemiologic perspective. The pooled estimates should be interpreted in the context of heterogeneity among studies, tested using the I2 statistic.21 Table 3 shows heterogeneity ranges from none (0%) for sex, cardiovascular diseases, Parkinson disease, and sedentariness; low (<25%) for depressive symptoms; moderate (25%–50%) for diabetes; and high (50%–75%) for education, obesity, stroke, and hypertension.21,22
Table 3.
Risk of motoric cognitive risk syndrome in individual cohorts and pooled sample
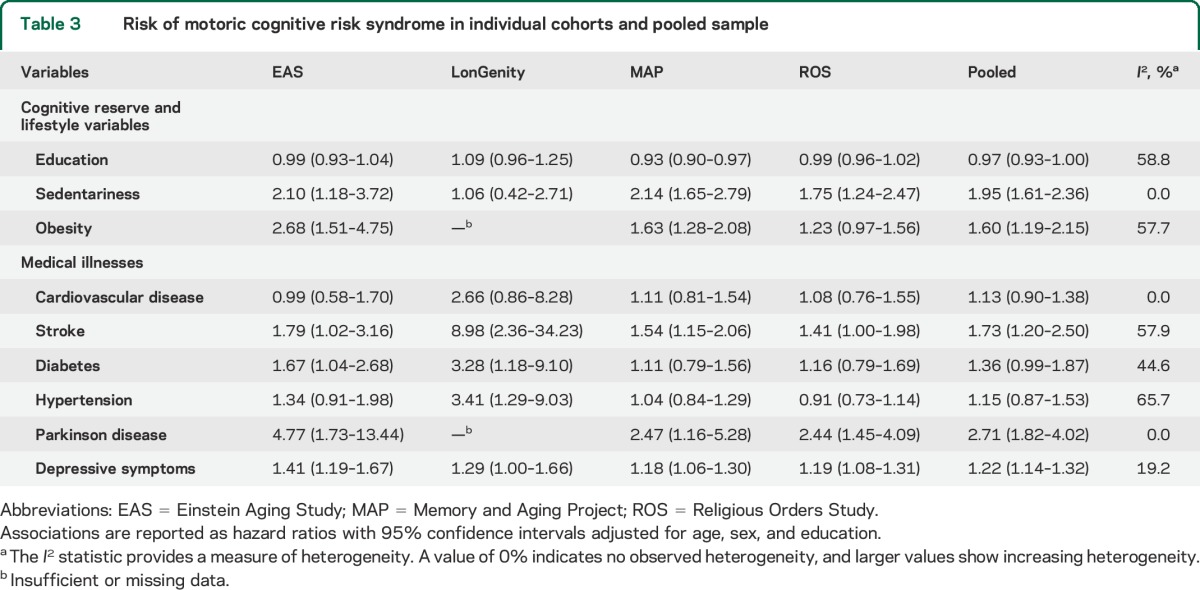
Education did not predict MCR overall, but was significant in MAP. Sedentariness (n = 475, 16.9%) predicted MCR in 3 cohorts. Obesity (n = 673, 26.4%) was significant in 2 of 3 cohorts in which it was measured. Cardiovascular diseases (n = 373, 11.9%) did not predict MCR. Individual cardiovascular diseases also did not predict MCR (data not shown). Strokes (n = 271, 9.1%) predicted MCR in all cohorts. Hypertension (n = 1,559, 50.3%) was associated with MCR only in LonGenity. Diabetes (n = 367, 11.7%) was associated with MCR in EAS and LonGenity. Parkinson disease had a low prevalence overall (n = 56, 2.2%) but predicted incident MCR in the 3 cohorts in which this information was available. Depressive symptoms predicted MCR in all cohorts.
In a summary model in the pooled sample including all risk factors and adjusted for cohort source, age (HR 1.04, 95% CI: 1.03–1.06), education (HR 0.97, 95% CI: 0.95–0.99), strokes (HR 1.42, 95% CI: 1.14–1.77), Parkinson disease (HR 2.52, 95% CI: 1.68–3.76), depressive symptoms (HR 1.65, 95% CI: 1.28–2.13), sedentariness (HR 1.76, 95% CI: 1.44–2.17), and obesity (HR 1.39, 95% CI: 1.17–1.65) predicted risk of incident MCR. Sex, cardiovascular disease, hypertension, and diabetes were not significant (data not shown).
DISCUSSION
In this multicenter study of 3,128 persons aged 60 years and older, age- and sex-adjusted pooled incidence of MCR was 65.2/1,000 person-years. A higher incidence of MCR was seen with advancing age, but there were no sex differences. The MCR concept is easily accessible in a wide variety of clinical settings. In a recent multicountry prevalence study,5 MCR criteria were applied using simple questions about cognitive complaints and timing gait over a fixed distance with a stopwatch in 11 studies worldwide (including MAP and ROS).
While no other incidence studies are available because MCR is newly proposed,6 comparison with other predementia syndromes is illustrative. MCR incidence rates were within a relatively narrow range in our cohorts (50.8–79.6 cases per 1,000 person years), similar to other predementia syndromes. Incidence rate for amnestic MCI was 3.7 per 100 person-years and for nonamnestic MCI 3.9 per 100 person-years in EAS.23 Of the 1,409 dementia-free participants at baseline in the MAP, 343 developed incident MCI (168 amnestic MCI) over 14 years of follow-up.10 In the ROS, 387 of 1,079 dementia-free participants evaluated between 1994 and 2011 developed incident MCI (132 amnestic).13 Predementia incidence rates have not been reported in LonGenity.
MCR was a robust predictor of cognitive decline in our multicountry study even after accounting for potential diagnostic misclassification of early dementia cases as MCR, overlap with MCI, and multiple confounders.5 MCR predicted vascular dementia in the EAS in our initial study.6 However, in the follow-up study,5 MCR predicted Alzheimer disease in more than 2,000 participants in the MAP and ROS cohorts. Parkinson disease predicted incident MCR in this analysis. Hence, MCR may be a marker for both vascular and neurodegenerative pathways to dementia. The association of baseline gait speed (even after excluding those with slow gait) and general mental status in dementia-free individuals with incident MCR suggests that subtle cognitive and motor dysfunction begins many years before diagnostic thresholds for predementia syndromes are met.1
Our investigation into modifiable risk factors provides insights into potential preventive strategies. Education, a proxy for cognitive reserve,19,24 was not associated with incident MCR, although the association was suggestive. The lack of significant findings may be explained by the high educational levels in our cohorts (93% ≥12 years). Cognitive leisure activity participation delays memory decline independent of education.24 Hence, continued intellectual stimulation may be necessary to maintain cognitive reserve later in life.24 We did not have information on other cognitive reserve markers such as cognitive activities or reading levels in all cohorts, and further study is required. Sedentariness and obesity increase risk of cognitive decline,7,9,25 and predicted MCR. Physical fitness is related to increased volumes and functional connectivity in the aging brain.7,8 Intervention studies can help establish whether participation in cognitive and physical activities as well as weight loss may help prevent or delay MCR. Cardiovascular diseases (overall or individual) did not predict MCR, whereas stroke was a significant predictor. Slow gait predicts strokes in older women,26 and gait impairment due to strokes predicts vascular dementia.27 Possible explanations for the association of depressive symptoms with MCR include reduced participation by depressed persons in healthy lifestyle behaviors, coexistence of common geriatric syndromes, shared pathologies,28 or depressive symptoms occurring as a prodromal dementia feature.29 Further examination of the role of depressive symptoms is warranted.
Over follow-up, 20% of participants who developed MCR reverted to normal. This rate is comparable to other clinical predementia syndromes. The reversion rates to normal for MCI range from 18% to 50% in previous studies,30–32 and variables associated with reversion have included younger age, shorter symptom duration, or lacking APOE ε4 allele.31,33 Younger age and higher education were associated with reversion of MCR.
A key strength is that our study was based on 4 large well-established aging cohorts with validated and reliable cognitive and motor assessment protocols.
Several limitations need to be discussed. The composition of our cohorts needs to be considered while interpreting incidence estimates and other findings. However, previous dementia-related findings from these 4 well-established cohorts were generalizable to other populations worldwide.4,10,11,13 Moreover, not many established dementia studies include annual gait speed measurements that are core to MCR diagnosis.5 Prospective studies in more diverse and population-based cohorts are needed to establish national and international MCR incidence rates.
The selected risk factors have established dementia links; however, this list is not meant to be exhaustive. Medical illnesses were based on self-report in this initial incidence study. Direct measures of pathology might have shown stronger associations, and should be examined to gain insights into MCR pathogenesis.
Because this was a retrospective analysis, all procedures and tests were not uniform. Each individual MCR criterion can be improved. Slow gait in older adults is multifactorial,34 but addition of cognitive complaints to the MCR criteria improves predictive validity.5 Informant reports of cognitive complaints may improve dementia identification but has lower sensitivity and will exclude many older adults who live alone, reducing the pool of at-risk older adults. Variability in any one MCR criterion is balanced by another criterion. In support, MCR had more incremental validity for predicting cognitive decline than either of its individual components of slow gait or cognitive complaints.5,6 That variability in individual criteria is reduced when combined is also suggested by the high consistency in MCR prevalence rates pooling studies using different cognitive and gait measurements and MCR prevalence from studies using the same cognitive and gait assessments.5 The agreement in MCR incidence rates across the 4 cohorts and associated risk factors is also reassuring. While MCR causes may vary regionally, risk factors for strokes and cardiovascular disease (2 major contributors to cognitive and motoric decline) were remarkably consistent worldwide.35,36 The heterogeneity between studies for most risk factors was relatively low and the direction of associations was in the same direction, an equally important consideration when interpreting meta-analyses.21,22
Gait variables other than speed may be considered for defining MCR4; however, the need for instrumented methods will restrict clinical utility but will be helpful in research settings.4,6 Use of insular slow gait cutscores for each cohort, the same approach used to define abnormal cognitive test performance for other predementia syndromes,5,14 helped address procedural and population differences. Use of a single slow gait cutscore for defining MCR is not optimal, because it does not account for age and population differences in gait performance.15,16
This multicenter study helps define the epidemiology of MCR in older adults. While not intended as a nationally representative sample, the inclusion of multiple sites enables comparison of incidence rates and risk factors for MCR in different regions and populations in the United States. The incidence rates and risk factor data derived from our study can serve as a foundation for future studies to improve dementia risk assessments, assist health care planning, and to develop novel interventions to prevent cognitive decline.
GLOSSARY
- BIMC
Blessed Information-Memory-Concentration
- CI
confidence interval
- EAS
Einstein Aging Study
- HR
hazard ratio
- MAP
Memory and Aging Project
- MCI
mild cognitive impairment
- MCR
motoric cognitive risk syndrome
- MMSE
Mini-Mental State Examination
- ROS
Religious Orders Study
AUTHOR CONTRIBUTIONS
Verghese was responsible for study concept, acquisition of data, initial draft, and analysis of data. Ayers and Wang were responsible revising draft for content and analysis of data. Barzilai, Bennett, Buchman, Holtzer, Katz, and Lipton were responsible for acquisition of data, interpretation of data, and revising draft for content.
STUDY FUNDING
No funding received for this analysis. Funding agencies for the participating cohorts are as follows. The Einstein Aging Study is funded by the NIH/National Institute on Aging (grant PO1 AG03949). The LonGenity study was funded by the NIH (R00AG037574, 1P01AG034906, R01AG046949, 1R01AG042188, P30AG038072, and NIH R37AG18381), CTSA KL2TR000088, Einstein Glenn Center, Paul Glenn Foundation, and the American Federation for Aging Research. The Memory and Aging Project and the Religious Orders Study are supported by the NIH/National Institute on Aging (grants P30AG10161, R01AG15819, R01AG17917, R01AG34374, and R01AG33678) and the Illinois Department of Public Health.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 2010;67:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2013;68:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology 1998;50:1496–1498. [DOI] [PubMed] [Google Scholar]
- 4.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007;78:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 2014;83:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013;68:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 2008;9:58–65. [DOI] [PubMed] [Google Scholar]
- 8.Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness, aging and neurocognitive function. Neurobiol Aging 2005;26(suppl 1):124–127. [DOI] [PubMed] [Google Scholar]
- 9.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–81. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA 2003;290:2030–2040. [DOI] [PubMed] [Google Scholar]
- 12.Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc 2011;59:1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med 2011;364:2227–2234. [DOI] [PubMed] [Google Scholar]
- 15.Oh-Park M, Holtzer R, Xue X, Verghese J. Conventional and robust quantitative gait norms in community-dwelling older adults. J Am Geriatr Soc 2010;58:1512–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capistrant BD, Glymour MM, Berkman LF. Assessing mobility difficulties for cross-national comparisons: results from the World Health Organization study on global ageing and adult health. J Am Geriatr Soc 2014;62:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thal LJ, Grundman M, Golden R. Alzheimer's disease: a correlational analysis of the Blessed Information-Memory-Concentration Test and the Mini-Mental State Exam. Neurology 1986;36:262–264. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007;69:1657–1664. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol 2014;127:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley; 2008. [Google Scholar]
- 23.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 2012;26:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 2009;73:356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064. [DOI] [PubMed] [Google Scholar]
- 26.McGinn AP, Kaplan RC, Verghese J, et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke 2008;39:1233–1239. [DOI] [PubMed] [Google Scholar]
- 27.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med 2002;347:1761–1768. [DOI] [PubMed] [Google Scholar]
- 28.Hajjar I, Yang F, Sorond F, et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci 2009;64:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry 2007;15:406–415. [DOI] [PubMed] [Google Scholar]
- 30.Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol 2011;68:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S, Unverzagt FW, Hall KS, et al. Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. Am J Geriatr Psychiatry 2014;22:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008;63:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology 2012;79:1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc 2006;54:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART Study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE Study): a case-control study. Lancet 2010;376:112–123. [DOI] [PubMed] [Google Scholar]


