Abstract
Objective:
The purpose of this study was to investigate functional connectivity (FC) changes in epileptogenic networks in intractable partial epilepsy obtained from resting-state fMRI by using intrinsic connectivity contrast (ICC), a voxel-based network measure of degree that reflects the number of connections to each voxel.
Methods:
We measured differences between intrahemispheric- and interhemispheric-ICC (ICCintra−inter) that could reveal localized connectivity abnormalities in epileptogenic zones while more global network changes would be eliminated when subtracting these values. The ICCintra−inter map was compared with the seizure onset zone (SOZ) based on intracranial EEG (icEEG) recordings in 29 patients with at least 1 year of postsurgical follow-up. Two independent reviewers blindly interpreted the icEEG and fMRI data, and the concordance rates were compared for various clinical factors.
Results:
Concordance between the icEEG SOZ and ICCintra−inter map was observed in 72.4% (21/29) of the patients, which was higher in patients with good surgical outcome, especially in those patients with temporal lobe epilepsy (TLE) or lateral temporal seizure localization. Concordance was also better in the extratemporal lobe epilepsy than the TLE group. In 85.7% (18/21) of the cases, the ICCintra−inter values were negative in the SOZ, indicating decreased FC within the epileptic hemisphere relative to between hemispheres.
Conclusions:
Assessing alterations in FC using fMRI-ICC map can help localize the SOZ, which has potential as a noninvasive presurgical diagnostic tool to improve surgical outcome. In addition, the method reveals that, in focal epilepsy, both intrahemispheric- and interhemispheric-FC may be altered, in the presence of both regional as well as global network abnormalities.
Epilepsy can be conceptualized as a network disorder that may involve not only epileptogenic regions but also remote cortical areas.1,2 Examination of epileptogenic networks may provide a better understanding of seizure generation and propagation to other brain regions. Furthermore, understanding the network properties may help guide surgical intervention for better postoperative outcomes in refractory epilepsy. Various measures of connectivity have been used to better understand epileptogenic networks.2–6
Functional connectivity (FC) measurements from resting-state fMRI (rs-fMRI) data can provide new insights into epileptogenic networks.7 Previous studies in focal epilepsy, focusing mostly on medial temporal lobe epilepsy (TLE), reported variable results: FC usually decreased but sometimes increased in the seizure focus, and also changed in the contralateral temporal and other cortical areas.8–16 Examining FC changes might help localize the epileptogenic zone (EZ).17 Nevertheless, few rs-fMRI studies have compared this directly with postsurgical outcome.
To apply fMRI FC analysis to localize the EZ regardless of the seizure focus requires a voxel-based analysis method that does not rely on a priori assumptions as to specific regions of interest. Epileptogenic networks may involve nodes ipsilateral or contralateral to the seizure focus, thus network properties could lead to both intrahemispheric- and interhemispheric-FC alterations.7 To distinguish the seizure focus from other changes in remote brain areas, a contrast between intrahemispheric- and interhemispheric-FC was introduced. Differences between intrahemispheric- and interhemispheric-FC maps were compared with the seizure onset zone (SOZ) identified based on intracranial EEG (icEEG) recordings in epilepsy surgery patients. We also compared the concordance rates in subgroups of various clinical factors.
METHODS
Subjects.
We analyzed data retrospectively from 29 patients with intractable partial epilepsy. The inclusion criteria were patients who (1) were referred for anatomical MRI and rs-fMRI between October 2004 and February 2011, (2) subsequently underwent epilepsy surgery after icEEG seizure recordings, and (3) had at least 1 year of postsurgical follow-up. The patients' data were compared with those of 85 normal healthy volunteers (male/female = 44:41, mean age 34.7 ± 11.3 years).
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants. This study was approved by the Human Investigational Committee of the Yale University School of Medicine.
Data acquisition.
All patients had 2 to 8 runs of rs-fMRI using a 3T MR scanner (Siemens Trio, Siemens Medical, Erlangen, Germany) or a 1.5T MR scanner (Siemens Sonata) with short breaks between runs (less than a minute) to provide high-quality data free of motion artifacts. Images were acquired with an echoplanar imaging sequence with a 2-second repetition time (TR), 30-millisecond echo time (TE), 256-mm field of view (FoV), 90° flip angle, 64 × 64 matrix size, 4 × 4 mm in-plane resolution, and 4-mm slice thickness without gap, generating 220 image volumes. Each control subject had 8 runs of rs-fMRI using the 3T MR scanner with the same imaging parameters.
Anatomical images were acquired with 2-dimensional (300-millisecond TR, 2.43-millisecond TE, 60° flip angle) and 3-dimensional T1-weighted sagittal magnetization-prepared rapid acquisition with a gradient echo (MPRAGE) sequence (2,530-millisecond TR, 3.34-millisecond TE, 7° flip angle) with 256-mm FoV, 256 × 256 matrix size, 1-mm thickness, and 176 contiguous slices.
FC analysis using intrinsic connectivity contrast.
To calculate the intrinsic connectivity contrast–degree (ICC-dth) measure, the correlation between the rs-fMRI BOLD time course of a reference voxel and all other gray matter voxels was determined, and the number of such correlations above a correlation threshold (>0.25) was used as a measure of the degree reflecting network connectivity.18
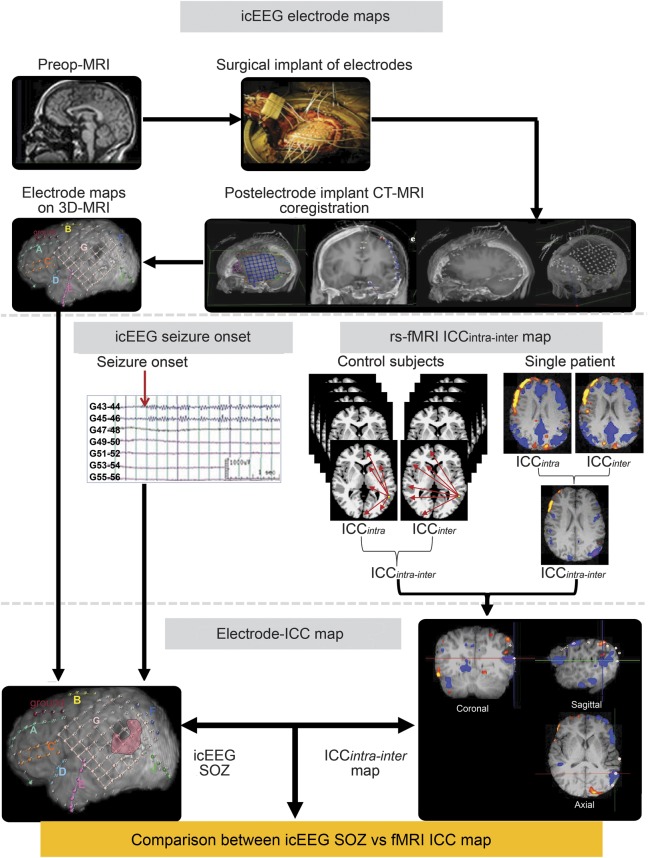
First, 2 ICC-dth maps were computed for each voxel to capture hemispheric differences in FC after preprocessing. The first map was of a voxel's connectivity within the ipsilateral hemisphere (ICCintra). The second map was of voxel's connectivity to the contralateral hemisphere (ICCinter). Both maps were generated for each voxel in the gray matter. Finally, the difference map was created by subtracting ICCinter from ICCintra (ICCintra−inter) to cancel out global network alterations (figure 1). ICC values were normalized to fit a gaussian distribution with zero mean and unitary variance, and smoothed with a 6-mm gaussian kernel. To compute the group statistics, single subject results were normalized to the Montreal Neurological Institute (MNI) standard template using the intensity-only component as implemented in BioImage Suite software (www.bioimagesuite.org).19,20
Figure 1. Diagram summarizing the preprocessing steps and the creation of the ICC map to compare with the icEEG SOZ.
The ICC was measured for each voxel, reflecting the number of connections to each voxel from all other voxels in the gray matter either within the same hemisphere (intrahemispheric) or between hemispheres (interhemispheric). After normalization of the ICC maps with those from control subjects, the within-subject difference of intrahemispheric- and interhemispheric-ICC (ICCintra−inter) was compared with the icEEG SOZ in individual patients. ICC = intrinsic connectivity contrast; icEEG = intracranial EEG; rs-fMRI = resting-state fMRI; SOZ = seizure onset zone.
Differences between each individual patient and the 85 control subjects were identified using a group t test (p < 0.05 with family-wise error correction, 20-voxel cluster threshold). The ICCintra−inter map was initially created with a threshold t value of 2.0 in each patient, then adjusted stepwise by a t value of 0.05 to a higher threshold if the map showed diffuse and widespread changes, or to a lower threshold if it revealed too subtle changes. Regions with reliable ICC differences were identified anatomically by using the Talairach Daemon Atlas21 after transforming MNI coordinates into Talairach coordinates corrected using a nonlinear transformation.22
Anatomical localization of 3-dimensional electrode positions and coregistration to ICC maps.
Intracranial electrodes (Ad-Tech Medical Instruments Corp., Racine, WI) were implanted as part of surgery procedures. All patients underwent pre- and postoperative MRI and CT scans with subdural electrodes. MRI was performed as described above, and Lightspeed CT (Siemens Medical) was done with parameters of 120 kVp, 600 milliseconds/220 mA, helical/64 mode, 1.5-mm thickness without gap, and 260 × 220 mm FoV. Electrode locations were identified using the postoperative CT and postoperative MRI, and registered to the preoperative MRI,23 then coregistered to the MPRAGE image of individual patients using BioImage Suite.19 This sequence of registrations allows the electrode locations and ICC maps to be coregistered and displayed on the individual's MPRAGE image (figure 1).
Identification of the icEEG SOZ.
All patients underwent icEEG recording using 128-channel video-EEG monitoring equipment (Bio-Logic Systems Corp., Mundelein, IL) with 16-bit A/D conversion, 256-Hz sampling, 90-dB common mode rejection ratio, and 0.1- to ∼70-Hz bandpass filter. First, the icEEG was reviewed separately, blind to other clinical information, by 2 expert neurologists (H.W.L. and P.F.) to identify the SOZ using traditional icEEG visual analysis. If the results disagreed, the 2 reviewers discussed the case to reach a final consensus or declare the case nonlocalizing if they could not localize the SOZ or failed to reach an agreement.
Concordance between the icEEG SOZ and fMRI-ICC maps.
The concordance rate was evaluated between the fMRI-ICC map and icEEG SOZ, not resected cortical area in postoperative imaging. This was because not all of these patients had postoperative MRI (some of them had postoperative CT instead) and several patients had multiple subpial transections with or without partial surgical resection since the cortical area included a functionally eloquent region. The results of ICC maps were interpreted separately by 2 reviewers (H.W.L. and R.T.C.) who were blinded to all other clinical data. The fMRI-ICC maps were presented to the 2 reviewers separately, in random patient order. In cases with multiple cortical regions with mixed increased or decreased ICC changes, only the cortical area showing the largest cluster with the peak intensity difference was taken into account. In the same manner as for the icEEG, if the 2 reviewers disagreed, they discussed the case to reach a consensus or categorize it as nonlocalizing.
For both the icEEG SOZ and fMRI-ICC maps, the reviewers interpreted the cortical localization of abnormality as to the side, lobar, or sublobar. The concordance between these modalities was determined on the coregistered 3-dimensional brain surface rendering of electrode locations and ICC maps in each patient. Concordance was determined only when there was spatial overlap between the SOZ and ICC change regions at the same sublobar location. Surgical outcomes were determined based on the Engel classification24 and International League Against Epilepsy outcome criteria.25
Statistical analysis.
To assess the reliability of each test modality, agreement rates were calculated between the reviewers based on their initial independent interpretations using Cohen κ coefficient. The reliability of concordance between the icEEG SOZ and ICC maps was determined based on the final consensus using generalized κ-type statistics.26 The χ2 test for proportions was used to compare concordance rates in subgroups of patients with various clinical factors including surgical outcome and final localization. Statistical results were calculated using Stata 10.0 (StataCorp, College Station, TX) for κ coefficients and SPSS 16.0.0 (SPSS Inc., Chicago, IL) for other tests, with p < 0.05 considered significant.
RESULTS
The patients were 14 males and 15 females, with the age range of 7 to 55 years (mean 29.4 ± 11.3). There were 13 patients with TLE and 16 with extratemporal lobe epilepsy (ETLE), with a mean seizure onset age of 12.4 ± 8.1 years and a mean epilepsy duration of 13.9 ± 7.4 years (table 1). The interrater agreement rate for ICCintra−inter interpretation was 89.7% (κ = 0.66, 95% confidence interval [CI] = 0.31–1.00), which was comparable to that of the icEEG SOZ (agreement rate 93.1%, κ = 0.79, 95% CI 0.51–1.00). ICCintra−inter maps showed localized changes in 23 of 29 patients. Among them, 21 patients showed concordance of their ICCintra−inter maps with their icEEG SOZ (91.3%, κ = 0.76, 95% CI 0.44–1.00).
Table 1.
Clinical information of all patients and concordance between intracranial EEG seizure onset and fMRI-ICC maps
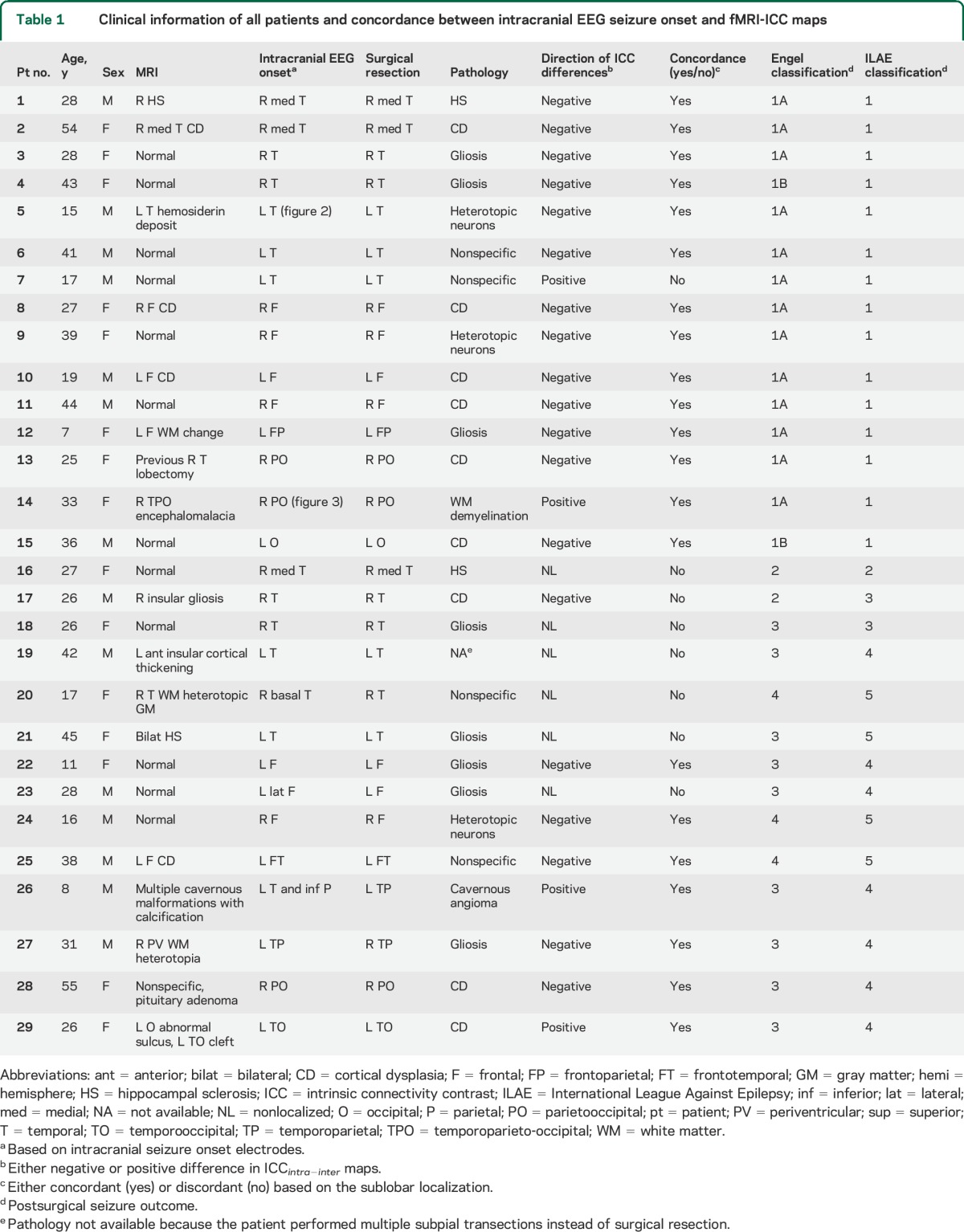
In 18 of the cases with concordance (18/21, 85.7%), ICCintra−inter was negative in the SOZ, indicating decreased FC within the epileptic hemisphere relative to between hemispheres. The remaining 3 patients with concordance (3/21, 14.3%) had positive values of ICCintra−inter in the SOZ, indicating lower interhemispheric FC than intrahemispheric FC.
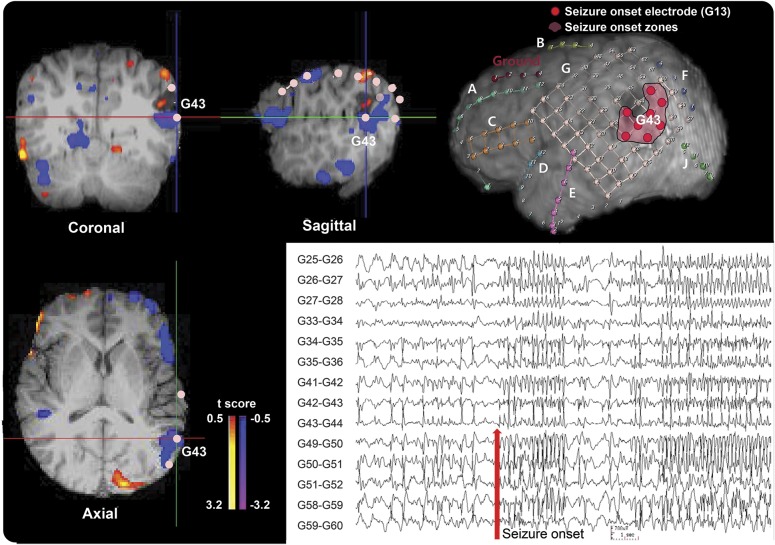
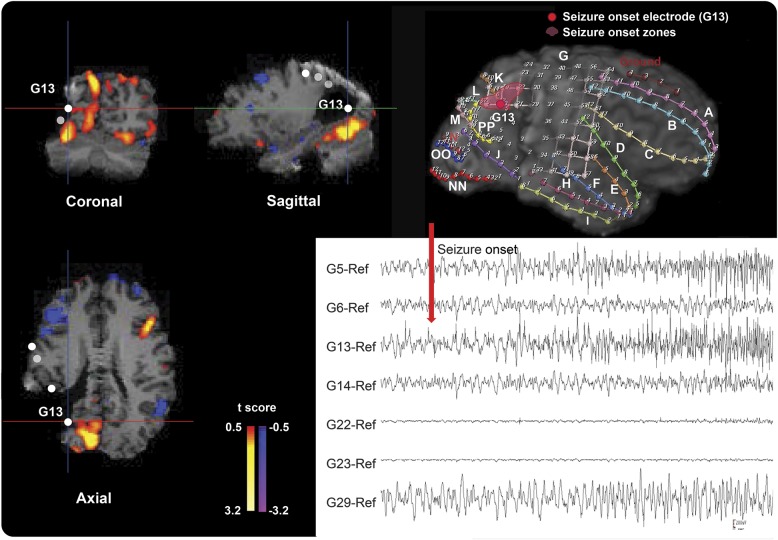
Representative cases are shown in figures 2 and 3. Patient 5 in table 1 with lesional TLE had negative values on the ICCintra−inter map in the left lateral temporal cortical region that matched well with the icEEG SOZ at the posteroinferior border of the structural lesion (figure 2), indicating lower connectivity within the epileptic hemisphere relative to between hemispheres in this area. In contrast, patient 14 with lesional ETLE had positive values on the ICCintra−inter map in the right parietooccipital SOZ (figure 3), indicating lower interhemispheric relative to intrahemispheric FC in this area. These 2 patients were seizure free after surgery.
Figure 2. Example of the icEEG SOZ/ICCintra−inter map comparison in a 15-year-old male with lesional temporal lobe epilepsy.
Patient 5 (table 1) with cavernous hemangioma shows negative values on the ICCintra−inter map at the posteroinferior border of a structural lesion in the left lateral temporal area in the top and bottom images on the left and the top image in the middle. This is concordant with the icEEG SOZ as shown in the 3-dimensional MRI surface rendering at the top right (marked as pink) and the bottom right icEEG finding (the red arrow marks a seizure onset). The color bar to the right of the ICC map indicates the t score for difference between intrahemispheric- and interhemispheric-ICC values. Both the results of the ICC map and the icEEG SOZ suggest that the epileptogenic zone is in the left lateral temporal lobe, and this patient has been seizure-free since resection of this region. ICC = intrinsic connectivity contrast; icEEG = intracranial EEG; SOZ = seizure onset zone.
Figure 3. Example of the icEEG SOZ/ICCintra−inter map comparison in a 33-year-old woman with lesional extratemporal lobe epilepsy.
Patient 14 (table 1) with right parietooccipital lobe epilepsy shows positive values on the ICCintra−inter map in the right parietooccipital region in the top and bottom images on the left and the top image in the middle. This matches well with the icEEG SOZ shown in the 3-dimensional MRI surface rendering at the top right (marked as pink) and the bottom right icEEG finding (the red arrow marks a seizure onset). The color bar to the right of the ICC map indicates the t score for difference between intrahemispheric- and interhemispheric-ICC values. Both the results of the ICC map and the icEEG SOZ suggest that the epileptogenic zone is in the right parietooccipital lobe, and this patient has been seizure-free since resection of this region. ICC = intrinsic connectivity contrast; icEEG = intracranial EEG; SOZ = seizure onset zone.
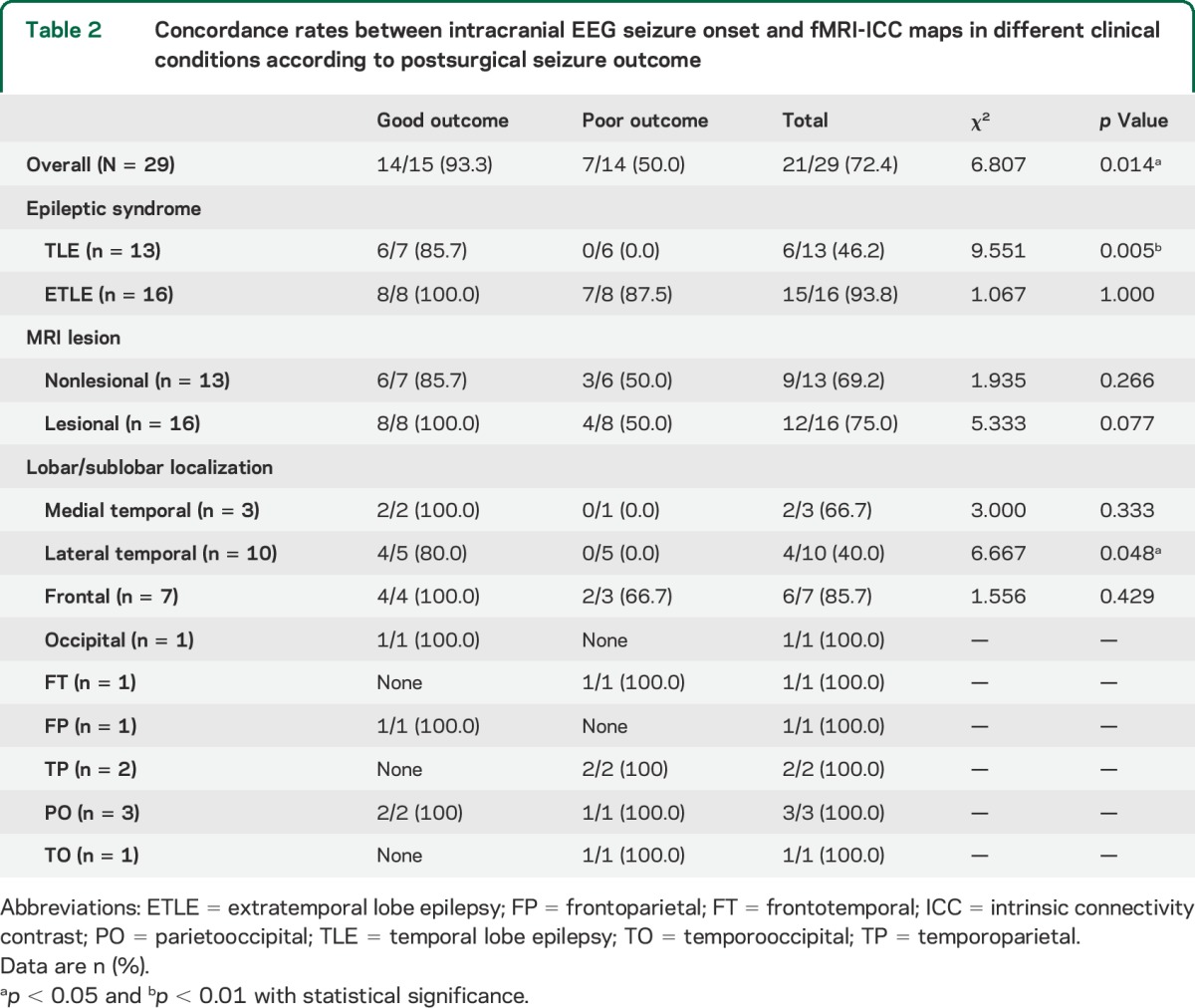
The overall concordance between the icEEG SOZ and ICCintra−inter map was higher in patients with good surgical outcome than those with poor outcome (93.3% vs 50.0%). This was particularly the case in patients with TLE (85.7% vs 50.0%) and lateral temporal seizure localization (80.0% vs 0%). Concordance was also better in the ETLE than TLE group (93.8% vs 46.2%, χ2 = 8.134, p = 0.010). Of note, concordance was good in ETLE regardless of seizure outcome (100% vs 87.5%), while in TLE, it was better with good outcome than poor outcome (85.7% vs 0%) (table 2). No specific trends were observed in other patient subgroups (table 2, table e-1 on the Neurology® Web site at Neurology.org).
Table 2.
Concordance rates between intracranial EEG seizure onset and fMRI-ICC maps in different clinical conditions according to postsurgical seizure outcome
DISCUSSION
This study demonstrates that FC mapping at the voxel level, based on rs-fMRI data, may provide additional information for the identification of the EZ, potentially improving surgical outcome. Differences between intrahemispheric- and interhemispheric-ICC values (ICCintra−inter) were mapped and compared with the icEEG SOZ in 29 epilepsy surgery patients. The main findings of this study were as follows: (1) concordance between the icEEG SOZ and ICCintra−inter map was observed in 72.4% (21/29), (2) ICCintra−inter maps in the SOZ showed lower FC within the epileptic hemisphere relative to between hemispheres in 85.7% (18/21), and (3) concordance was higher in patients with good surgical outcome (especially in those with TLE and lateral temporal seizure localization) and also better in the ETLE than TLE group. Our findings suggest the possibility that this approach could add another information dimension in the workup of patients who are candidates for epilepsy surgery.
Most rs-fMRI studies in partial epilepsy, mainly targeting medial TLE, have reported decreased FC,6,8,9,14,16 but a few studies have shown increases in connectivity.11,17 In addition to changes in the seizure focus, contralateral temporal or remote cortical changes were reported as well.6,12,13,15,16 The variability of these findings might be related to patient heterogeneity or methodologic differences. Only a few patients with neocortical epilepsy (included mainly in the TLE cohorts) have been studied with rs-fMRI so far.4,17 Changes in areas remote to the seizure focus or in the contralateral hemisphere have also been reported in other human studies using different neuroimaging modalities such as PET.27,28 The mechanisms for changes outside the seizure foci are not completely understood, but frequent seizure spread with comorbid neurobehavioral abnormalities and possible compensatory mechanisms have been suggested.29 A study using a tetanus toxin rat model for focal neocortical epilepsy, where the etiology and the seizure focus were controlled to be identical in all animals, showed decreased interhemispheric but increased intrahemispheric FC in the epileptic and nonepileptic hemispheres, but these changes altered with time.30 In this focal epilepsy model, the functional alterations observed were not restricted to the epileptic focus but were in multiple brain regions, supporting the possibility that both regional and global changes occur in neocortical epilepsy.
It is somewhat counterintuitive that the location of the SOZ stands out when subtracting the interhemispheric-ICC from intrahemispheric-ICC in the majority of our patients. This finding suggests that FC changes in the EZ have characteristic features that are distinct within the epileptic hemisphere or between hemispheres, while FC outside the epileptic foci is relatively stable within the epileptic and the contralateral hemispheres. FC could be influenced in different ways when comparing local vs remote connectivity (e.g., the former is more related to the epileptogenesis itself while the latter is associated with more global changes in network properties).
Recent evidence has suggested that a laterality index based on FC could be helpful to predict surgical outcomes but this does not provide information on the EZ.4 In the current study, we examined whether FC changes could have localizing value in epilepsy surgery patients. The concordance between the icEEG SOZ and fMRI FC changes was analyzed to investigate whether these FC changes truly represent the EZ. We observed that the concordance rate between the 2 modalities was higher in patients with good surgical outcome. Of note, concordance was good in ETLE regardless of surgical outcome, while in TLE it was higher in patients with good outcome. These findings suggest that this kind of FC mapping approach may be helpful in improving seizure localization for subgroups of patients with otherwise poor outcome.
Various methods of network analysis based on graph theory have been used to assess FC in epileptic networks.3,6–17,31,32 A recent study revealed increased FC in the icEEG SOZ when using local FC measures within close cortical areas.17 In the current analysis, we used the ICCintra−inter map, since this highlighted localized changes while the individual ICCintra and ICCinter maps showed diffuse and bilateral changes that were insufficient for localization in most cases. Healthy normal brain tissue typically demonstrates more within hemisphere connectivity and less contralateral hemisphere connectivity.7 In contrast, the negative values of ICCintra−inter in the EZ in most of our patients may indicate less FC in that region. Traditionally, the interictal epileptiform discharges observed on EEG are characterized as increased synchrony in the EZ.33,34 Previous studies using scalp or icEEG reported increased connectivity within the seizure focus, but decreased connectivity outside of the focus in TLE11,35 and ETLE.36 Several other studies have challenged this classic view showing that the synchrony and/or correlation of EEG activity are dynamically changing over time, or even decreased at seizure onset.37 Our study suggests that the epileptic hemisphere exhibits lower connectivity than the contralateral side, although that does not indicate absolute changes. To assess that, future studies in larger and more homogeneous groups of patients would be needed.
One of the difficulties in localizing the ICC changes was that the ICC maps often showed multifocal changes in cortical areas in addition to those areas that matched the SOZ. It is unclear at this time whether the multiple focal regions (1) result from an epileptogenic network rather than a single seizure focus, (2) reflect other aspects of functional changes such as comorbid neurobehavioral abnormalities,31,38 (3) relate to artifacts that require further technical improvement in analysis methods, or (4) result from a combination of all of the above. The next logical step to fully consider all the possibilities would be to perform network analyses (using the same rs-fMRI data) on these multiple nodes to determine the central node and other network properties and to correlate these with detailed clinical data. However, the overall validity of this method is still under investigation and warrants further studies to develop an automated and quantifiable method to select significant connectivity clusters of interest.
FC measurement using rs-fMRI has the potential to provide a novel noninvasive method to localize the SOZ as a part of presurgical evaluation. In our study, the concordance rate between the icEEG SOZ and fMRI-ICC map was better in patients with good outcome, suggesting that this kind of approach can be useful as a biomarker that reflects epileptogenesis and also predicts surgical outcome in advance when planning epilepsy surgery. For instance, patients in whom brain areas showing ICC changes are resected completely would be expected to have better surgical outcome. Although it is still in the early stage of development, with further validation in patients with intractable partial epilepsy, this kind of approach may ultimately be useful for both clinical and scientific purposes, especially as part of presurgical evaluations. We hope that our study represents a step forward in developing clinically useful analysis paradigms using fMRI-based intrinsic connectivity measurements.
Supplementary Material
GLOSSARY
- CI
confidence interval
- ETLE
extratemporal lobe epilepsy
- EZ
epileptogenic zone
- FC
functional connectivity
- FoV
field of view
- ICC
intrinsic connectivity contrast
- icEEG
intracranial EEG
- MNI
Montreal Neurological Institute
- MPRAGE
magnetization-prepared rapid acquisition with a gradient echo
- rs-fMRI
resting-state fMRI
- SOZ
seizure onset zone
- TE
echo time
- TLE
temporal lobe epilepsy
- TR
repetition time
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Hyang Woon Lee: design and conceptualization of the study design, subject selection, analysis and interpretation of the data, statistical analysis, drafting and revising the manuscript. Jagriti Arora: data collection, analysis of the fMRI data, statistical analysis, and revising the manuscript. Xenophon Papademetris: analysis of the fMRI data and revising the manuscript. Fuyuze Tokoglu: data collection and analysis of the fMRI data. Michiro Negishi: data collection and analysis of the fMRI data. Dustin Scheinost: analysis of the fMRI data and revising the manuscript. Pue Farooque: analysis, interpretation of the icEEG data, and revising the manuscript. Hal Blumenfeld: subject selection, data collection, interpretation of the icEEG data, and revising the manuscript. Dennis D. Spencer: data collection, analysis of the icEEG data, and revising the manuscript. R. Todd Constable: study design, interpretation of the fMRI data, and revising the manuscript.
STUDY FUNDING
Dr. Lee is funded by the Basic Science Research Program through the National Research Foundation (NRF) of Korea by the Ministry of Science, ICT, and future planning (NRF-2011-0015788 and 2014-R1A2A1A11052103), and by the Ewha Global Top 5 Grant 2011 of Ewha Womans University of Korea. Dr. Blumenfeld is funded by the NIH grant NS055829. Dr. Constable is funded by the NIH grant EB009666-02.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist 2012;18:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemieux L, Daunizeau J, Walker MC. Concepts of connectivity and human epileptic activity. Front Syst Neurosci 2011;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One 2011;6:e20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia 2011;52:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilke C, Worrell GA, He B. Analysis of epileptogenic network properties during ictal activity. Conf Proc IEEE Eng Med Biol Soc 2009;2009:2220–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Tokoglu F, Negishi M, et al. Social network theory applied to resting-state fMRI connectivity data in the identification of epilepsy networks with iterative feature selection. J Neurosci Methods 2011;199:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constable RT, Scheinost D, Finn ES, et al. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Front Neurol 2013;4:39. 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettus G, Guedj E, Joyeux F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 2009;30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettus G, Bartolomei F, Confort-Gouny S, et al. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2010;81:1147–1154. [DOI] [PubMed] [Google Scholar]
- 10.Frings L, Schulze-Bonhage A, Spreer J, Wagner K. Reduced interhemispheric hippocampal BOLD signal coupling related to early epilepsy onset. Seizure 2009;18:153–157. [DOI] [PubMed] [Google Scholar]
- 11.Liao W, Zhang Z, Pan Z, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One 2010;5:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan VL, Gore JC, Abou-Khalil B. Cluster analysis detection of functional MRI activity in temporal lobe epilepsy. Epilepsy Res 2007;76:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res 2010;88:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira FR, Alessio A, Sercheli MS, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 2010;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Lu G, Zhong Y, et al. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol 2009;256:1705–1713. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Lu G, Zhong Y, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp 2010;31:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stufflebeam SM, Liu H, Sepulcre J, Tanaka N, Buckner RL, Madsen JR. Localization of focal epileptic discharges using functional connectivity magnetic resonance imaging. J Neurosurg 2011;114:1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martuzzi R, Ramani R, Qiu M, Shen X, Papademetris X, Constable RT. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage 2011;58:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi A, Scheinost D, Okuda H, et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics 2011;9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Integrated intensity, point-feature nonrigid registration. Med Image Comput Comput Assist Interv 2001;3216:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000;10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage 2008;42:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer JA, Zaveri HP, Goncharova II, et al. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex 2008;18:1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel J, Jr, van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J, Jr, editor. Surgical Treatment of the Epilepsies, 2nd ed New York: Raven Press; 1993:609–622. [Google Scholar]
- 25.Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report: proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001;42:282–286. [PubMed] [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 27.Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol 2002;51:202–208. [DOI] [PubMed] [Google Scholar]
- 28.Chassoux F, Semah F, Bouilleret V, et al. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain 2004;127:164–174. [DOI] [PubMed] [Google Scholar]
- 29.Koepp MJ, Woermann FG. Imaging structure and function in refractory focal epilepsy. Lancet Neurol 2005;4:42–53. [DOI] [PubMed] [Google Scholar]
- 30.Otte WM, Dijkhuizen RM, van Meer MP, et al. Characterization of functional and structural integrity in experimental focal epilepsy: reduced network efficiency coincides with white matter changes. PLoS One 2012;7:e39078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes M, Folley BS, Sonmezturk HH, et al. Resting state functional connectivity of the hippocampus associated with neurocognitive function in left temporal lobe epilepsy. Hum Brain Mapp 2014;35:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes MJ, Yang X, Landman BA, et al. Functional networks in temporal-lobe epilepsy: a voxel-wise study of resting-state functional connectivity and gray-matter concentration. Brain Connect 2013;3:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dichter MA, Ayala GF. Cellular mechanism of epilepsy: a status report. Science 1987;237:157–164. [DOI] [PubMed] [Google Scholar]
- 34.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed New York: McGraw-Hill; 2000. [Google Scholar]
- 35.Bettus G, Wendling F, Guye M, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res 2008;81:58–68. [DOI] [PubMed] [Google Scholar]
- 36.Shevon CA, Cappell J, Emerson R, et al. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage 2007;35:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindler K, Leung H, Elger CE, Lehnertz K. Assessing seizure dynamics by analyzing the correlation structure of multichannel intracranial EEG. Brain 2007;130:65–77. [DOI] [PubMed] [Google Scholar]
- 38.Vlooswijk MC, Vaessen MJ, Jansen JF, et al. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology 2011;77:938–944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.