Abstract
Purpose of Review
This review discusses management of status epilepticus in children including both anticonvulsant medications and overall management approaches.
Recent Findings
Rapid management of status epilepticus is associated with a greater likelihood of seizure termination and better outcomes, yet data indicate there are often management delays. This review discusses an overall management approach aiming to simultaneously identify and manage underlying precipitant etiologies, administer anticonvulsants in rapid succession until seizures have terminated, and identify and manage systemic complications. An example management pathway is provided.
Summary
Status epilepticus is a common neurologic emergency in children and requires rapid intervention. Having a predetermined status epilepticus management pathway can expedite management.
Keywords: Status epilepticus, Seizure, Pediatric, Management, EEG
Introduction
Status epilepticus (SE) refers to a prolonged seizure or recurrent seizures without a return to baseline and is the most common neurological emergency in childhood. The incidence of SE is 18–23 per 100,000 children per year.[1] Management involves three simultaneous components: (1) identification and management of underlying precipitant etiologies, (2) administration of anticonvulsants to terminate the seizure(s), and (3) identification and management of systemic complications that could result in secondary brain injury.
Status Epilepticus Timing
Historically, SE was defined as a seizure lasting longer than 30 minutes or a series of seizures in a period of 30 minutes without return to baseline level of alertness between seizures.[2] The temporal definition has gradually shortened due to increasing recognition that most seizures are brief (3–4 minutes)[3] and anticonvulsant administration delays are associated with more refractory seizures. The most recent Neurocritical Care Society guideline for SE management in children and adults defines SE as “5 minutes or more of (i) continuous clinical and/or electrographic seizure activity or (ii) recurrent seizure activity without recovery (returning to baseline) between seizures.”[4] Immediate aggressive management may be particularly important in post-operative neurosurgical and cardiac surgical patients, patients with (or at risk of) elevated intracranial pressure (e.g. traumatic brain injury, brain tumor, central nervous system infections), and children with multi-system organ failure.[5]
The terminology used to describe SE timing has evolved over time. During the prodromal or incipient stage (<5 minutes) it is unknown whether the seizure will self-terminate or evolve into SE. Persisting SE has been divided into early SE (5–30 minutes), established SE (>30 minutes), or refractory SE (RSE) (seizures that persist despite treatment with adequate doses of two or three anticonvulsants). The recent guideline states that “definitive control of SE should be established within 60 minutes of onset.”[4] In contrast to some earlier timing terminology which considered medications as first, second, and third line agents, the new guideline uses the terms “emergent”, “urgent”, and “refractory” to help convey a sense of time urgency and that medications should be administered sequentially if seizures persist. RSE is defined as clinical or electrographic seizures which persist after an adequate dose of an initial benzodiazepine and a second appropriate anti-seizure medication; no specific time must elapse before initiation of RSE management.
Importance of Pre-Determined Management Pathways
Several studies have described associations between SE management delays and more prolonged seizures as well as lower anticonvulsant responsiveness. One study of children with convulsive SE found that for every minute delay between SE onset and Emergency Room arrival there was a 5% cumulative increase in the risk of having SE that lasted more than 60 minutes.[6] Further, studies have demonstrated that when anticonvulsants were administered quickly, they were more effective in terminating SE. A study that included 71 children who continued to seize despite first and second line anticonvulsants, reported that seizures were terminated by a third anticonvulsant in 100% or 22% of children when it was administered within one hour or more than one hour after the first anticonvulsant, respectively.[7] A study of 27 children documented that the first and second line anticonvulsants were effective in terminating SE in 86% or 15% when administered in less than 15 minutes or greater than 30 minutes, respectively.[8] An observational study of 157 children with seizures lasting longer than 5 minutes reported that treatment delays exceeding 30 minutes were associated with delays in achieving seizure control.[9] A study of 358 children found that midazolam efficacy was significantly lower when treatment was initiated more than 3 hours after seizure onset, and there was a trend toward reduced efficacy even at one hour.[10] These findings may be explained by data indicating that prolonged SE leads to internalization of inhibitory gamma-aminobutyric acid (GABA) receptors, thereby making benzodiazepines less effective.[11–13]
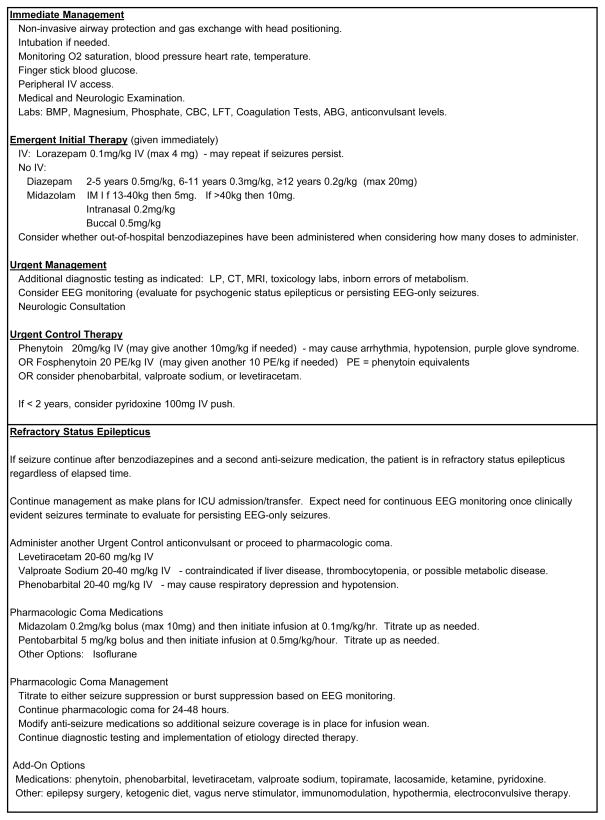
Unfortunately, variability in management and treatment delays are common, potentially contributing to worse outcomes. A study evaluating the management of children with SE reported that laboratory parameters were often not checked and long delays often occurred in obtaining glucose results.[14] A retrospective multi-center study reported that even once in the Emergency Department, the median time to administer a second-line anticonvulsant to a seizing child was 24 minutes.[15] Benzodiazepine dosing has been reported to be outside usual dosing guidelines in 23% of children with SE.[14] Excess benzodiazepine dosing, which often occurs when prehospital doses have been administered, contributes to respiratory insufficiency and need for intensive care unit admission.[14, 16, 17] To expedite therapeutic decisions, a recent consensus document recommended that all units have a written management pathway with a clear structured time frame.[18] An example SE management pathway is provided in Figure 1.
Figure 1.
Example status epilepticus evaluation and management pathway.
Evaluating for Precipitating and Etiological Conditions
A separate paper in this journal addresses the evaluation of status epilepticus in detail.[19] Multiple studies have characterized the various potential etiologies for SE.[1, 20–22] The most common cause of pediatric SE is febrile SE. SE may be unprovoked and occur in a patient with epilepsy, but this is rare.[23] Acute symptomatic etiologies are identified in 15–20% of children presenting with SE[1, 21, 24] and are especially likely in children under two years of age.[25] The American Academy of Neurology practice parameter addressing the diagnostic assessment of a child with convulsive SE reported that abnormal results among children who underwent testing included low anticonvulsant levels (32%), neuroimaging abnormalities (8%), electrolytes (6%), inborn errors of metabolism (4.2%), ingestion (3.6%), central nervous system infections (2.8%), and positive blood cultures (2.5%).[26] A prospective study reported that the most common acute symptomatic etiologies were central nervous system infection (55%), vascular insult (21%), toxin (8%), electrolyte imbalance (8%), and trauma (8%).[21]
The recent Neurocritical Care Society guideline indicates that initial etiologic testing should include bedside finger stick blood glucose (0–2 minutes), and lab testing including blood glucose, complete blood count, basic metabolic panel, calcium, magnesium, and anti-seizure medication levels (5 minutes). Depending on the clinical scenario, other diagnostic testing may be required such as neuroimaging or lumbar puncture (0–60 minutes), other laboratory testing (including liver function tests, coagulation studies, arterial blood gas, toxicology screen, and inborn errors of metabolism screening), and continuous electroencephalographic (EEG) monitoring if the patient is not waking up after clinical seizures cease (15–60 minutes).[4] These most recent recommendations are similar to a prior American Academy of Neurology practice parameter.[26] Rarer infectious, metabolic, autoimmune and paraneoplastic etiologies may be considered if no etiology is identified.[27]
Neuroimaging abnormalities have been reported in 30% of children with SE and described to alter acute management in 24%.[21] Computerized tomography (CT) is more widely available, rapid, and does not require sedation, but it may not detect some smaller lesions which can be identified by magnetic resonance imaging (MRI). Among 44 children who underwent head CT and MRI, 14 had a normal head CT but an abnormal MRI, leading to the conclusion that MRI had a superior yield and should be considered whenever available if head CT is non-diagnostic.[21]
Central nervous system infections are a common cause for acute symptomatic SE.[21] Lumbar puncture should be performed whenever there is a clinical suspicion for infection. Children who are less than two years old, immune suppressed, or have received recent antibiotics may present with SE prior to other clear signs of central nervous system infection. Hence, in these patients there should be a low threshold for performing a lumbar puncture.
There are two main urgent EEG indications. First, psychogenic SE diagnosis by EEG monitoring may avoid continued exposure to anticonvulsants and pharmacologic coma induction with potential adverse effects.[28, 29] Second, there is increasing data that after convulsive SE terminates some children have persisting EEG-only (non-convulsive) seizures.[30, 31] A multi-center retrospective study of 122 subjects who underwent EEG monitoring concluded that the likelihood of experiencing an electrographic seizure was five times higher among children who presented with convulsive SE.[32] A multi-center study of children who underwent EEG monitoring while in the ICU reported that 33% of 98 children who presented with convulsive SE had electrographic seizures identified. Among those with seizures, 34% had exclusively EEG-only seizures which would not have been identified without EEG monitoring. Further, the seizure burden was often high; 47% had electrographic status epilepticus. Risk factors for identifying seizures on EEG monitoring included a prior diagnosis of epilepsy and the presence of inter-ictal epileptiform discharges.[33] Observational studies have reported that in multivariable analyses aiming to account for encephalopathy etiology and severity, high electrographic seizure burdens in critically ill children are associated with worse outcomes.[31, 34–37] Based on these data, the recent guideline states that in order to identify electrographic seizures, “continuous electroencephalographic monitoring should be initiated within one hour of status epilepticus onset if ongoing seizures are suspected” and also recommends 48 hours of EEG monitoring to identify possible non-convulsive seizures in children with recent SE without a return to baseline mental status in 10 minutes. It recommends that the management goal be termination of convulsive and electrographic seizures.[4] However, further study is needed to determine whether efforts to identify and manage these electrographic seizures improve patient outcomes.
Medical Management
Systemic changes may cause secondary brain injury. In early SE, brain glucose and oxygen requirements increase. If cerebral autoregulation is preserved then substrate delivery also increases. However, in later SE hypotension and respiratory compromise may occur as a result of SE itself and anticonvulsant administration, leading to subsequent brain hypoxia, hypoglycemia, and acidosis.[38] Hyperthermia and rhabdomyolysis (and secondary renal failure) may develop with prolonged convulsions,[39], and rarely SE is associated with ictal bradycardia, stress cardiomyopathy, neurogenic pulmonary edema, and bone fractures.
The Neurocritical Care Society guideline provides a timed treatment outline. Steps include non-invasive airway protection and gas exchange with head positioning (0–2 minutes), intubation if airway or gas exchange is compromised or intracranial pressure is elevated (0–10 minutes), vital signs assessment (0–2 minutes), vasopressor support if needed (5–15 minutes), neurologic examination (0–5 minutes), and placement of peripheral intravenous access for administration of emergent anti-seizure medication therapy and fluid resuscitation (0–5 minutes).[4]
Seizure Management
Medication management aims to terminate clinical and electrographic seizures. The guideline states that “definitive control of SE should be established within 60 minutes of onset.”[4] In contrast to some earlier SE management algorithms which considered medications as first, second, and third line agents, the new guideline uses the terms “emergent”, “urgent”, and “refractory” to help convey a sense of time urgency.
Benzodiazepines are the “emergent” medications of choice; lorazepam for intravenous administration, midazolam for intramuscular or intranasal administration, and diazepam for rectal administration.[4] Repeat dosing may be provided in 5–10 minutes if needed. Since publication of the guidelines the results of a double-blind randomized trial of 273 children that compared lorazepam (0.1mg/kg) and diazepam (0.2mg/kg) for convulsive status epilepticus in the emergency department setting became available. A half-dose of either medication could be administered at 5 minutes if seizures persisted. The primary outcome was status epilepticus cessation by 10 minutes without recurrence in 30 minutes, and was not significantly different in the two groups (72.1% with diazepam and 72.9% with lorazepam). Patients receiving lorazepam were more likely to be sedated (67% with lorazepam, 50% with diazepam) but there was no difference in requirement for assisted ventilation (18% with lorazepam, 16% with diazepam). The study concluded that the data did “not support the preferential use of lorazepam” over diazepam for pediatric status epilepticus.[40] Care should be taken to assess whether any pre-hospital benzodiazepines were administered in which case progressing to an urgent medication may be indicated. Since benzodiazepines are relatively short acting, unless the SE etiology has been identified and definitively corrected, all children should also receive an “urgent” category anticonvulsant in addition to a benzodiazepine.[4]
Nearly half of children will have persisting SE after receiving benzodiazepines,[7, 15] yet there are few comparative data. A survey of pediatric emergency medicine physicians reported that phenytoin was chosen as the second line agent by 88%,[41] and this is consistent with surveys of neurologists.[42] Fosphenytoin is a pro-drug of phenytoin, and it is prescribed in phenytoin equivalents (PE). Fosphenytoin may be administered more rapidly than phenytoin but is then converted to phenytoin internally, which may take about fifteen minutes. Thus, fosphenytoin and phenytoin likely reach therapeutic concentrations in the brain in about the same time. The recent guideline considers phenytoin/fosphenytoin to be emergent treatment options, urgent treatment options, and refractory treatment options.[4] Cardiac arrhythmias are rare, especially with fosphenytoin, but may occur with both.[43] Fosphenytoin is associated with less tissue injury if infiltration occurs. Phenytoin and fosphenytoin are considered focal anticonvulsants, and they may be ineffective in treating SE related to generalized epilepsy. Phenytoin is a strong hepatic enzyme inducer and highly protein bound, leading to drug interactions.
Phenobarbital is commonly used as a first line agent to treat neonatal seizures and SE,[44] and it is often considered a third or fourth line drug in pediatric SE algorithms. The recent guideline considers phenobarbital to be an emergent treatment option and an urgent control treatment option.[4] Phenobarbital may cause sedation, respiratory depression and hypotension. It is a hepatic enzyme inducer leading to drug interactions.
Valproate sodium is a broad spectrum anticonvulsant and has been reported to be safe and highly effective in terminating SE and RSE without adverse effects. The recent SE guideline considers valproic acid to be an emergent treatment option, an urgent control treatment option and a refractory treatment option.[4] Black box warnings include hepatotoxicity (highest risk in children less than two years of age, those receiving anticonvulsant polytherapy, and children with mitochondrial disorders), pancreatitis, and teratogenicity. Other adverse effects include pancytopenia, thrombocytopenia, platelet dysfunction, hypersensitivity reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis), and encephalopathy (with or without elevated ammonia). Valproate is a hepatic enzyme inhibitor leading to drug interactions.
Levetiracetam is a broad spectrum anticonvulsant and there is increasing evidence that levetiracetam may be safe and effective for treating SE. The recent status epilepticus guideline considers levetiracetam to be an urgent therapy option.[4] Levetiracetam has no hepatic metabolism, which may be beneficial in complex patients with liver dysfunction, metabolic disorders, or in those at risk for major drug interactions. In comparison to other intravenous anticonvulsants, levetiracetam has a low risk of sedation, cardio-respiratory depression, or coagulopathy. Since levetiracetam clearance is dependent on renal function, maintenance dosage reduction is required in patients with renal impairment.
RSE management has been reviewed recently.[45–47] Management involves use of medications that terminate SE by substantially reducing overall brain activity (e.g. midazolam and pentobarbital) to induce “pharmacologic coma” while simultaneously identifying and managing underlying conditions and administering anticonvulsants to provide seizure control once the high-dose suppressive medications are weaned. Several medications may be used to terminate RSE including midazolam and pentobarbital, and continuous infusions of anesthetics and anticonvulsants are reviewed in a separate paper within this journal. [48] Midazolam is a fast acting benzodiazepine that rapidly penetrates the blood brain barrier and has a short duration of action. Pentobarbital is a barbiturate with a reported seizure termination efficacy of 74–100%. Both midazolam and pentobarbital may produce respiratory depression and hypotension. Anesthetics such as isoflurane are effective in inducing a burst suppression pattern and terminating seizures, but hypotension requiring vasopressors is common and seizures may recur after weaning the anesthetic.[49] Propofol may also be used to terminate seizures, but propofol infusion syndrome limits pediatric use. Case reports and series have described several add-on medications and other techniques have been reported useful in reducing seizure recurrence as pharmacologic coma is weaned including topiramate, ketamine, lidocaine, the ketogenic diet, epilepsy surgery, immunomodulation, and electroconvulsive therapy.
Outcome
Outcome is reviewed in detal in another paper within this journal.[50] Morbidity and mortality are largely related to SE etiology.[1, 7, 51–54] Children with febrile SE or epilepsy related SE have a 0–2% mortality while children with acute symptomatic SE have a 12–16% mortality.[52] Mortality may also be higher in younger children, although this may relate to the high occurrence of central nervous system infections causing SE in this group.[52] In a prospective study of children with SE, 9% of survivors were found to have new neurologic deficits, and almost all occurred in children with acute or progressive neurologic insults. Additionally, younger children were more likely to have new neurologic deficits (29% younger than one year and 6% older than three years), although this related to the higher occurrence of acute symptomatic etiologies in younger children. Of children without prior epilepsy, 30% had subsequent seizures.[54]
Outcome may be at least partially dependent on SE duration. RSE is associated with higher mortality and morbidity (both new neurological deficits and subsequent epilepsy) as compared to SE episodes aborted by the initial two anti-convulsants.[7]
SE recurrence occurs in 3–67% of children, and is rare with febrile or idiopathic etiologies but common with acute symptomatic or progressive etiologies.[55] Since most episodes of SE begin outside the hospital, it is important that children with epilepsy who have had an SE episode have out-of-hospital management plans in place and rescue medication available.[56]
Conclusions
Status epilepticus is a common neurologic emergency requiring rapid and simultaneous efforts to identify and manage acute precipitants, manage systemic complications, and terminate seizures. Having a pre-determined plan may streamline management and avoid delays.
Key Points.
Status epilepticus is a common neurologic emergency.
Management involves three simultaneous components: (1) identification and management of underlying precipitant etiologies, (2) administration of anticonvulsants to terminate the seizure(s), and (3) identification and management of systemic complications that could result in secondary brain injury.
Having a predetermined status epilepticus management pathway can expedite management.
Acknowledgments
Funding:
Nicholas Abend is funded by NIH K23NS076550.
Tobias Loddenkemper receives support from the National Institutes of Health/NINDS, a Career Development Fellowship Award from Harvard Medical School and Boston Children’s Hospital, the Program for Quality and Safety at Boston Children’s Hospital, the Payer Provider Quality Initiative, The American Epilepsy Society, The Epilepsy Foundation of America, the Center for Integration of Medicine and Innovative Technology, the Epilepsy Therapy Project, the Pediatric Epilepsy Research Foundation, the Danny Did Foundation, the HHV6 Foundation, and from investigator initiated research grants from Lundbeck and Eisai.
Footnotes
Conflicts of Interest:
Dr. Abend has given expert testimony in medico-legal cases and receives royalties from Demos Medical Publishing for Pediatric Neurocritical Care.
Dr. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an Associate Editor for Seizure, and performs video electroencephalogram long-term monitoring, electroencephalograms, and other electrophysiological studies at Boston Children’s Hospital and bills for these procedures.
Contributor Information
Nicholas S Abend, Email: abend@email.chop.edu, Departments of Neurology and Pediatrics, The Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania (Philadelphia, PA).
Tobias Loddenkemper, Email: Tobias.Loddenkemper@childrens.harvard.edu, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children’s Hospital and Harvard Medical School (Boston, MA).
References
- 1.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368(9531):222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Epidemiology and Prognosis, International League Against Epilepsy: Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34(4):592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Shinnar S, Berg AT, Moshe SL, et al. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659–64. [PubMed] [Google Scholar]
- 4**.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. This guideline from the Neurocritical Care Society provides direction regarding evaluation and management of children and adults with status epilepticus. [DOI] [PubMed] [Google Scholar]
- 5.Riviello JJ., Jr Seizures in the context of acute illness. Curr Opin Pediatr. 2009;21(6):731–736. doi: 10.1097/MOP.0b013e328332c77d. [DOI] [PubMed] [Google Scholar]
- 6.Chin RF, Neville BG, Peckham C, et al. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7(8):696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49(4):615–25. doi: 10.1111/j.1528-1167.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewena S, Young S. When benzodiazepines fail: how effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006;18(1):45–50. doi: 10.1111/j.1742-6723.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson K, Metsaranta P, Huhtala H, et al. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65(8):1316–8. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K, Osawa M, Aihara M, et al. Efficacy of Intravenous Midazolam for Status Epilepticus in Childhood. Pediatr Neurol. 2007;36(6):366–372. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246–56. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 12.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28(10):2527–38. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobias JD, Berkenbosch JW. Management of status epilepticus in infants and children prior to pediatric ICU admission: deviations from the current guidelines. South Med J. 2008;101(3):268–72. doi: 10.1097/SMJ.0b013e318164e3f0. [DOI] [PubMed] [Google Scholar]
- 15.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care. 2009;25(2):83–7. doi: 10.1097/PEC.0b013e318196ea6e. [DOI] [PubMed] [Google Scholar]
- 16.Chin RF, Verhulst L, Neville BG, et al. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75(11):1584–8. doi: 10.1136/jnnp.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirupathi S, McMenamin JB, Webb DW. Analysis of factors influencing admission to intensive care following convulsive status epilepticus in children. Seizure. 2009;18(9):630–3. doi: 10.1016/j.seizure.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Shorvon S, Baulac M, Cross H, et al. The drug treatment of status epilepticus in Europe: Consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia. 2008;49(7):1277–1286. doi: 10.1111/j.1528-1167.2008.01706_3.x. [DOI] [PubMed] [Google Scholar]
- 19.Freilich ER, Schreiber JM, Zelleke T, et al. Pediatric Status Epilepticus: Identification and Evaluation. Curr Opin Pediatr. 2014;26(6) doi: 10.1097/MOP.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 20.Hussain N, Appleton R, Thorburn K. Aetiology, course and outcome of children admitted to paediatric intensive care with convulsive status epilepticus: a retrospective 5-year review. Seizure. 2007;16(4):305–12. doi: 10.1016/j.seizure.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Singh RK, Stephens S, Berl MM, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology. 2010;74(8):636–42. doi: 10.1212/WNL.0b013e3181d0cca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama I, Ohtsuka Y, Tsuda T, et al. An epidemiological study of children with status epilepticus in Okayama, Japan. Epilepsia. 2007;48(6):1133–7. doi: 10.1111/j.1528-1167.2007.01106.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Shinnar S, Testa FM, et al. Status epilepticus after the initial diagnosis of epilepsy in children. Neurology. 2004;63(6):1027–34. doi: 10.1212/01.wnl.0000138425.54223.dc. [DOI] [PubMed] [Google Scholar]
- 24.Berg AT, Shinnar S, Levy SR, et al. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol. 1999;45(5):618–23. doi: 10.1002/1531-8249(199905)45:5<618::aid-ana10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Shinnar S, Pellock JM, Moshe SL, et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38(8):907–14. doi: 10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 26.Riviello JJ, Ashwal S, Hirtz D, et al. Practice Parameter: Diagnostic assessment of the child with status epilepticus (an evidence-based review) Neurology. 2006;67:1542–1550. doi: 10.1212/01.wnl.0000243197.05519.3d. [DOI] [PubMed] [Google Scholar]
- 27.Watemberg N, Segal G. A suggested approach to the etiologic evaluation of status epilepticus in children: what to seek after the usual causes have been ruled out. J Child Neurol. 2010;25(2):203–11. doi: 10.1177/0883073809337032. [DOI] [PubMed] [Google Scholar]
- 28.Pakalnis A, Paolicchi J, Gilles E. Psychogenic status epilepticus in children: psychiatric and other risk factors. Neurology. 2000;54(4):969–70. doi: 10.1212/wnl.54.4.969. [DOI] [PubMed] [Google Scholar]
- 29.Papavasiliou A, Vassilaki N, Paraskevoulakos E, et al. Psychogenic status epilepticus in children. Epilepsy Behav. 2004;5(4):539–46. doi: 10.1016/j.yebeh.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–7. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology. 2013;81(4):383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52(6):1130–6. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- 33*.Sanchez Fernandez I, Abend NS, Arndt DH, et al. Electrographic seizures after convulsive status epilepticus in children and young adults. A retrospective multicenter study. Journal of Pediatrics. 2014;164(2):339–346. doi: 10.1016/j.jpeds.2013.09.032. This multi-center retrospective observational studies describs the occurrence of electrographic seizures after termination of convulsive status epilepticus in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38(5):853–62. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014 doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(Pt 5):1429–38. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5 Suppl 2):13–23. [PubMed] [Google Scholar]
- 39.Bleck TP. Intensive care unit management of patients with status epilepticus. Epilepsia. 2007;48(Suppl 8):59–60. doi: 10.1111/j.1528-1167.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 40**.Chamberlain JM, Okada P, Holsti M, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–60. doi: 10.1001/jama.2014.2625. This large randomized trial compared lorazepam and diazepam in children with convulsive status epilepticus in the emergency department setting. [DOI] [PubMed] [Google Scholar]
- 41.Babl FE, Sheriff N, Borland M, et al. Emergency management of paediatric status epilepticus in Australia and New Zealand: practice patterns in the context of clinical practice guidelines. J Paediatr Child Health. 2009;45(9):541–6. doi: 10.1111/j.1440-1754.2009.01536.x. [DOI] [PubMed] [Google Scholar]
- 42.Claassen J, Hirsch LJ, Mayer SA. Treatment of status epilepticus: a survey of neurologists. J Neurol Sci. 2003;211(1–2):37–41. doi: 10.1016/s0022-510x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 43.Adams BD, Buckley NH, Kim JY, et al. Fosphenytoin may cause hemodynamically unstable bradydysrhythmias. J Emerg Med. 2006;30(1):75–9. doi: 10.1016/j.jemermed.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37(2):85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Abend NS, Dlugos DJ. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38(6):377–90. doi: 10.1016/j.pediatrneurol.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Owens J. Medical Management of Refractory Status Epilepticus. Semin Pediatr Neurol. 2010;17(3):176–181. doi: 10.1016/j.spen.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Wheless JW. Treatment of Refractory Convulsive Status Epilepticus in Children: Other Therapies. Semin Pediatr Neurol. 2010;17(3):190–194. doi: 10.1016/j.spen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Tasker R. Continuous infusions of anticonvulsants and anesthetics used in status epilepticus. Curr Opin Pediatr. 2014;26(6) [Google Scholar]
- 49.Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61(8):1254–9. doi: 10.1001/archneur.61.8.1254. [DOI] [PubMed] [Google Scholar]
- 50.Scott RC. Consequences of febrile seizures in childhood. Curr Opin Pediatr. 2014;26(6) doi: 10.1097/MOP.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 51.Arzimanoglou A. Outcome of status epilepticus in children. Epilepsia. 2007;48(Suppl 8):91–3. doi: 10.1111/j.1528-1167.2007.01361.x. [DOI] [PubMed] [Google Scholar]
- 52.Raspall-Chaure M, Chin RF, Neville BG, et al. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006;5(9):769–79. doi: 10.1016/S1474-4422(06)70546-4. [DOI] [PubMed] [Google Scholar]
- 53.Sadarangani M, Seaton C, Scott JA, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol. 2008;7(2):145–50. doi: 10.1016/S1474-4422(07)70331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maytal J, Shinnar S, Moshe SL, et al. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83(3):323–31. [PubMed] [Google Scholar]
- 55.Shinnar S, Maytal J, Krasnoff L, et al. Recurrent status epilepticus in children. Ann Neurol. 1992;31(6):598–604. doi: 10.1002/ana.410310606. [DOI] [PubMed] [Google Scholar]
- 56.Glauser TA. Designing practical evidence-based treatment plans for children with prolonged seizures and status epilepticus. J Child Neurol. 2007;22(5 Suppl):38S–46S. doi: 10.1177/0883073807303068. [DOI] [PubMed] [Google Scholar]