Abstract
Industrial forestry typically leads to a simplified forest structure and altered species composition. Retention of trees at harvest was introduced about 25 years ago to mitigate negative impacts on biodiversity, mainly from clearcutting, and is now widely practiced in boreal and temperate regions. Despite numerous studies on response of flora and fauna to retention, no comprehensive review has summarized its effects on biodiversity in comparison to clearcuts as well as un-harvested forests.
Using a systematic review protocol, we completed a meta-analysis of 78 studies including 944 comparisons of biodiversity between retention cuts and either clearcuts or un-harvested forests, with the main objective of assessing whether retention forestry helps, at least in the short term, to moderate the negative effects of clearcutting on flora and fauna.
Retention cuts supported higher richness and a greater abundance of forest species than clearcuts as well as higher richness and abundance of open-habitat species than un-harvested forests. For all species taken together (i.e. forest species, open-habitat species, generalist species and unclassified species), richness was higher in retention cuts than in clearcuts.
Retention cuts had negative impacts on some species compared to un-harvested forest, indicating that certain forest-interior species may not survive in retention cuts. Similarly, retention cuts were less suitable for some open-habitat species compared with clearcuts.
Positive effects of retention cuts on richness of forest species increased with proportion of retained trees and time since harvest, but there were not enough data to analyse possible threshold effects, that is, levels at which effects on biodiversity diminish. Spatial arrangement of the trees (aggregated vs. dispersed) had no effect on either forest species or open-habitat species, although limited data may have hindered our capacity to identify responses. Results for different comparisons were largely consistent among taxonomic groups for forest and open-habitat species, respectively.
Synthesis and applications. Our meta-analysis provides support for wider use of retention forestry since it moderates negative harvesting impacts on biodiversity. Hence, it is a promising approach for integrating biodiversity conservation and production forestry, although identifying optimal solutions between these two goals may need further attention. Nevertheless, retention forestry will not substitute for conservation actions targeting certain highly specialized species associated with forest-interior or open-habitat conditions.
Our meta-analysis provides support for wider use of retention forestry since it moderates negative harvesting impacts on biodiversity. Hence, it is a promising approach for integrating biodiversity conservation and production forestry, although identifying optimal solutions between these two goals may need further attention. Nevertheless, retention forestry will not substitute for conservation actions targeting certain highly specialized species associated with forest-interior or open-habitat conditions.
Keywords: biodiversity, boreal forest, clearcut, disturbance, forestry, structural retention, temperate forest, variable retention
Introduction
Forests are used to produce pulp, timber and bioenergy in large parts of the global forest estate (FAO 2010). Intensive, industrial forestry has modified forests world-wide, resulting in the simplification of managed stands and forest landscapes with negative implications for biodiversity and ecosystem services (Secretariat of the Convention on Biological Diversity 2010). The frequency, extent and intensity of disturbances that result from industrial forestry differ dramatically from those associated with natural disturbance regimes (Lindenmayer & Franklin 2002).
A forest management model – ‘retention forestry’ – was introduced about 25 years ago as a response to the rapid ongoing transformation and homogenization of forests, and the need to better balance the goals of wood prod-uction and biodiversity conservation (Franklin 1989; Gustafsson et al. 2012). Retention forestry aims to reduce structural and functional contrasts between production forests and natural forests, mainly by increasing the abundance in logged stands of key structures important for many elements of biodiversity, such as old and dead trees (Lindenmayer, Laurance & Franklin 2012; Stokland, Siitonen & Jonsson 2012). The fundamental practice of retention forestry is that single trees and/or intact forest patches are retained at the time of harvest, with the overall aim of achieving a level of continuity in forest structure and complexity that more closely resembles the outcomes of natural disturbance, thereby conserving forest biodiversity and sustaining ecological functions (Gustafsson et al. 2012). The amount of trees retained usually ranges between a few per cent and about 30% but vary greatly among regions and forest-owner categories (Gustafsson et al. 2012). The main difference between retention forestry and traditional industrial forestry is that the trees are deliberately selected and retained over the long term to sustain biodiversity. Retention forestry is promoted as a way to integrate biodiversity concerns into industrial forestry and thus differs from traditional conservation approaches that have mainly focused on setting aside reserves.
Heavy logging can result in substantial changes in environmental conditions, such as altered light, humidity and wind speed, thereby constraining forest species adapted to closed forest conditions (e.g. Heithecker & Halpern 2007). Species interactions may also be altered in logged areas, such as increased predation rates resulting from reduced shelter and increased detectability of prey (e.g. Robertson & Hutto 2007). Conversely, a more open post-logging environment presents a window of opportunity for many species that require recently disturbed habitat, as it might result in an increase in resources and regeneration niches (Swanson et al. 2011). Species characteristic of open habitat often have good dispersal capacities and high reproduction rates that are life-history attributes often associated with common species. Yet, numerous uncommon species also depend on early-seral stages, such as those associated with dead wood and old remnant trees in open, disturbed habitat (e.g. Kouki et al. 2001). Retention forestry aims to assist the long-term viability of both forest species and open-habitat species (Gustafsson et al. 2012).
A better understanding of biodiversity responses to retention forestry is badly needed since this forest harvest model is currently practiced on more than 150 million ha of boreal and temperate forests (Gustafsson et al. 2012), and application is increasing (Kraus & Krumm 2013). Numerous studies assessing responses of flora and fauna to retention forestry have been conducted (Lindenmayer et al. 2012), including several large experiments (listed in Gustafsson et al. 2012). To date, however, the few meta-analyses published have examined one species group (birds; Vanderwel, Malcolm & Mills 2007) or made comparisons only between areas harvested with retention and clearcuts (Rosenvald & Lõhmus 2008). Thus, no thorough meta-analysis has been conducted on how biodiversity responds to retention forestry in relation to clearcuts as well as un-harvested forest. We address the following questions:
What are the effects of retention forestry on forest species and open-habitat species, in comparison with clearcuts and un-harvested forests? We expect that forest species and open-habitat species will benefit from the retention compared to clearcut and closed forests, respectively (Fig.1). One implication of both species groups being promoted will be a higher species number in retention cuts than in either clearcuts or un-harvested forests.
Do taxonomic groups differ in their response to retention forestry? We predict that responses will be similar for different taxonomic groups within forest and open-habitat species groups, respectively, since the division into these ecological groups is likely to be a better predictor of response than taxonomic affiliation.
Does the richness and abundance of forest species and open-habitat species vary with the proportion of retained trees, the size of retention cuts, the spatial arrangement of trees (dispersed or aggregated) and years after harvest? We predict that forest species will increase and open-habitat species will decrease with an increasing proportion of retention. The identification of possible thresholds – where any further increase in retention elements does not bring about additional significant effects on biodiversity – is of special interest since ‘how much should be left?’ is a key question from practitioners. Large retention cuts could possibly disadvantage forest species since extensive open areas represent harsher conditions for these species than small open areas, but on the other hand, open-habitat species should be promoted. The spatial arrangement will impact on species that require a special microclimate, since humidity and temperature will vary between retention cuts with dispersed or aggregated trees. Additionally, we hypothesize that forest species will increase with time since harvest, due to ongoing forest recovery and dispersal.
Do species responses to retention differ between temperate and boreal biomes? We predict that there will be no differences because regardless of biome, species within a given seral group will respond similarly to the logging disturbance, that is, early-seral species assemblages will respond in a similar way, as will late-seral species assemblages.
Figure 1.
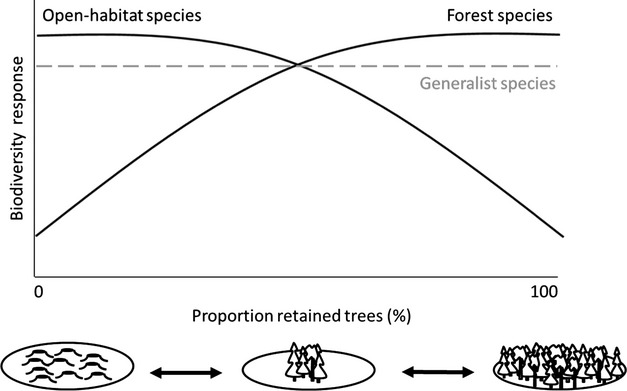
Expected biodiversity responses (species richness and abundance) for different ecological groups of taxa (forest species, open-habitat species, generalists) to tree retention in the gradient from clearcut to forest. Response curves are schematically drawn; different shapes are possible. In the meta-analysis, retention was compared to two kinds of external controls: clearcuts and forest (arrows). Forest species and open-habitat species were the main target groups, and generalists were less emphasized.
The overall aim of our meta-analysis is to establish whether retention forestry is an effective way to integrate biodiversity conservation into production forestry. Thus, our intention is to summarize knowledge in a way that will provide advice regarding future application of this comparatively new practice.
Materials and methods
Terminology
We use the term ‘forest’ (or sometimes ‘un-harvested forest’) for forests that have reached maturity from a production point of view, that is, they are old enough to be logged. In some cases, these forests have been previously harvested and regenerated, and in others, they have never been previously logged. The term ‘clearcut’ corresponds to forests that have been harvested with <2% retention. ‘Treatment unit’ is used for stands in which retention forestry has been applied (‘retention cuts’) or for controls (clearcuts or forests). ‘Observation’ is used for a comparison between a retention cut and a clearcut or between a retention cut and a forest.
Literature Search
We conducted our meta-analysis based on a peer-reviewed systematic review protocol according to the guidelines of the Collaboration for Environmental Evidence (CEE; www.environmentalevidence.org) (Fedrowitz & Gustafsson 2012). We searched the electronic data bases, Web of Science and Scopus on 10 May 2012, using search terms associated with retention forestry and biodiversity outcomes. In addition, we searched Google Scholar and The Directory of Open Access Journals (DOAJ) on 19 December 2012, using the simplified search strings [retention and forest and biodiversity] or [retention and biodiversity], respectively, and examined only the first fifty hits, sorted on relevance. We added further papers from reference lists in published studies or that were suggested by colleagues. We obtained additional grey literature by contacting researchers within the field.
From an initial number of >5000 articles that were downloaded, we kept 603 articles after reading titles and abstracts. Of these, 116 articles were summaries of studies or narrative studies, 22 articles could not be retrieved, and 213 articles were determined to be not relevant. We scrutinized the remaining 252 articles further for data extraction. See Table S1 (Supporting Information) for study exclusion criteria. We contacted several authors to provide data or to clarify their study design.
Our final data base comprised 78 articles (including one unpublished study) (see Appendix S1, Supporting Information) from which mean values on abundance or species richness, sample size (n) and either standard error (SE) or standard deviation (SD) were extracted. From a small proportion of the articles where means and variation of means were not presented in tables, we extracted such data by digitizing graphs using plot digitizer 2.6.2. (http://plotdigitizer.sourceforge.net/). In addition, we recorded information on the proportion of trees retained, spatial arrangement of trees (aggregated vs. dispersed), years after harvest, size of the treatment unit and biome (temperate or boreal) (Olson et al. 2001).
We analysed data only from those studies which reported quantities of retained living trees, because the number of articles with empirical data on dead wood retention suitable for our meta-analysis was too small (three studies). Furthermore, we included only those articles that referred to the whole treatment unit, that is, comprising the cleared, open areas and areas with retained trees. Thus, comparisons were made on a stand basis, that is, over the whole harvested areas, since almost all studies had this study design.
We used reported mean values for species richness or abundance of individual species and also different taxonomic groups. Abundance measures included mean values of vegetation cover (e.g. percentage cover, crown volume index, summed frequency, mean density estimates), biomass, number of individuals or records, or activity (e.g. for bats: mean passes per site).
Where possible, we assigned species to one of the following ecological groups based on descriptions in the article: forest, open-habitat or generalist species. Species that could not be assigned to a category were treated as ‘unclassified’ (see Appendix S2, Supporting Information). The category ‘forest species’ included all species that were classified as associated with closed canopy (mature or old-growth forest), as well as indicator species for late-successional forest conditions, late-seral species, species sensitive to clearcutting or species that prefer a more shaded and cool habitat. The category ‘open-habitat species’ included species associated with early-successional habitats, such as species that prefer areas with low-canopy tree cover or species that were classified as open-habitat species. Generalist species included species that can be found in several different habitat types or those that were classified as edge generalists or generalist species. Specifically for mammal species from North America, we further used information on habitat classification from the Smithsonian National Museum of Natural History (http://www.nmh.si.edu; accessed 18 June 2013). When calculating a combined effect size, we pooled taxa based on ecological group (forest, open-habitat, generalist, unclassified), treatment and study location.
We classified species into the following taxonomic groups: amphibians, reptiles, birds, mammals, beetles, spiders, other invertebrates, lichens, bryophytes, fungi and plants. Understorey vegetation was often reported as a single value and not separated into specific taxonomic groups and was consequently classified into the broader group of plants. In a few cases, this included trees or shrubs which were otherwise excluded from the analysis as well as bryophytes that were usually reported separately. The group ‘mammals’ included small forest-floor mammals, bats and squirrels.
Treatments included various designs, that is, trees retained as patches or as dispersed individuals, or a combination of the two. The proportion of trees/forest retained was measured by basal area, number of trees, merchantable volume, area or canopy cover, either in comparison with the original amount of trees or to forest controls. We compared retention cuts against two kinds of controls: clearcuts with <2% retention that were harvested at approximately the same time as the treatment units (in two articles, there was a 3–6 or 7-year difference) or un-harvested forests. Only post-treatment species data were used. To be included in the meta-analysis, studies needed to have at least two independent replicates per treatment and control, respectively.
Meta-Analysis
We conducted the meta-analysis using the package ‘metafor’ (Viechtbauer 2010) in r 2.15.2 (R Core Team 2012). We chose the standardized mean difference Hedges’ d (Hedges & Olkin 1985) as the effect size metric for comparing means between the treatment (retention) and control (clearcut or forest). Positive values of Hedges’ d indicate higher species richness or abundance of organisms in retention cuts relative to the control (clearcut or un-harvested forest), that is, positive effects of retention forestry. While the magnitude of the mean effect size d is difficult to interpret, Cohen's benchmark gives a rough estimation with mean effect sizes of d = 0·2 indicating a small effect, d = 0·5 a moderate effect and d = 0·8 a large effect (Koricheva, Gurevitch & Mengersen 2013).
Hedges’ d was chosen as effect size measure because it adjusts for differences in sampling effort among studies and corrects for small sample size. In addition, it was deemed more appropriate to use Hedges’ d than the response ratio because several studies reported zero values for either treatment or control groups, and 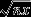 /SD was <3 in a large number of observations, when using either clearcut or forest as control (Koricheva, Gurevitch & Mengersen 2013).
/SD was <3 in a large number of observations, when using either clearcut or forest as control (Koricheva, Gurevitch & Mengersen 2013).
We estimated the mean effect sizes for species richness and abundance by using random-effects models, which include two components of variance around the mean, a within-study variance (sampling error) and a between-study variance (τ2). The restricted maximum likelihood estimator (REML) was used for estimating the between-study variance component of the effect size. Effect size from each study was weighted by the inverse of study variance calculated as a sum of within- and between-study variance, and the weighted mean effect was calculated as the sum of the products (study-specific effect sizes multiplied by weights) divided by the sum of weights. The variance of the summary effect was estimated as the reciprocal of the sum of the weights, and the SE of the mean effect was calculated as a square root of the variance adjusted by the sample size. The SE was used to calculate 95% confidence intervals around the mean effect, and mean effect sizes were considered significantly different from 0 if their confidence intervals did not include 0.
Most articles contributed more than one observation to our analysis, for example several taxonomic groups and retention levels were included in the same study. When publications reported separate values for two or more study locations or tree species with at least two replicates for both treatment and control, we regarded each as an independent observation. If an article reported single mean values for a number of different species within the same taxonomic group (e.g. within mammals) or for several observations within the same year, we calculated the combined effect size for the group or the year (Borenstein et al. 2009).
We used mixed-effects meta-regression models to examine the influence of the following moderator variables on the mean effect size: proportion of retained trees (%), time since harvest (years), treatment unit size (ha), the spatial arrangement of trees (dispersed vs. aggregated) and forest biome (temperate vs. boreal). We also tested the interaction between treatment size and proportion of retention because the effect of retention amount may differ between large and small areas. Total heterogeneity (QT) in these structural models is partitioned into heterogeneity explained by the model structure (QM) and unexplained heterogeneity (QE). We used the QM test implemented in ‘metafor’ to test for a significant difference in the mean effect size between different levels in the following moderator variables: ecological groups (forest species, open-habitat species, generalists), taxonomic groups, the spatial arrangement of trees (dispersed vs. aggregated) and forest biome (temperate vs. boreal).
We also tested for publication bias, that is, the probability that significant results are more likely to be published than non-significant results, using the trim and fill method (Duval & Tweedie 2000a,b; Viechtbauer 2010). This is a sensitivity analysis that adjusts for funnel plot asymmetry, that is, it adds values for missing studies to create a symmetric funnel plot from which a new mean effect size can be estimated (Koricheva, Gurevitch & Mengersen 2013).
Results
Data Description
Most studies were conducted in North America with 40% from the USA and 36% from Canada, while 21% were from Europe. A total of 68% of studies were from the temperate zone and the remainder from the boreal zone. Retention levels in the included studies ranged between 2% and 88% (mean 36·4% ± 24·8 SD) including some studies that used shelterwood cutting or partial harvest to describe the retention, and treatment unit sizes ranged between 0·6 and 78 ha. The 78 studies produced a data set comprising 944 observations, that is, comparisons between treatment (retention cuts) and control (clearcut or forest). Comparisons of mammals were most common (36% of observations), followed by birds (18%), vascular plants (12%), beetles (9%), spiders (8%), bryophytes (4%), amphibians (4%), other invertebrates (3%), lichens (3%), reptiles (1%) and fungi (1%).
In most studies (61), species were sampled directly after harvest or soon after harvest (0–5 years). In 23 studies, sampling was conducted additionally or solely >5 years after harvest; of these, 14 were sampled 10–31 years after harvest (some studies made observations on more than one occasion). See Appendices S1 and S2 (Supporting Information) for more details on included studies.
Forest Species, Open-Habitat Species and Generalists
The effect of retention cuts on species richness and abundance differed among forest species, open-habitat species and generalists, both when the studies included clearcuts as the control (species richness – QM =12·38, d.f. = 2, P < 0·002; abundance – QM = 38·18, d.f. = 2, P < 0·0001) and when un-harvested forest was the control (species richness – QM = 27·42, d.f. = 2, P < 0·0001; abundance – QM = 98·91, d.f. = 2, P < 0·0001).
Retention cuts supported higher forest species richness and abundance compared to clearcuts, but lower richness and abundance than un-harvested forest (Fig.2). In contrast, species richness and abundance of open-habitat species in retention cuts were lower than in clearcuts, but higher than in un-harvested forests (Fig.2). Species richness and abundance of generalists did not differ between retention cuts and clearcuts (Fig.2a). Their abundance but not richness was higher in retention cuts than in un-harvested forests (Fig.2b). Similar results were obtained when the trim and fill analysis was conducted, indicating that our results were robust to possible publication bias.
Figure 2.
Effects of retention cuts (mean effect size ± 95% CI) on species richness and abundance of forest, generalist and open-habitat species when using (a) clearcut or (b) un-harvested forest as the control. Numbers of observations are stated in brackets. Effects are not significantly different from 0 when 95% CIs include 0. For significant effects, P-values are shown as *P<0·05, **P<0·01 or ***P<0·001.
Taxonomic Groups
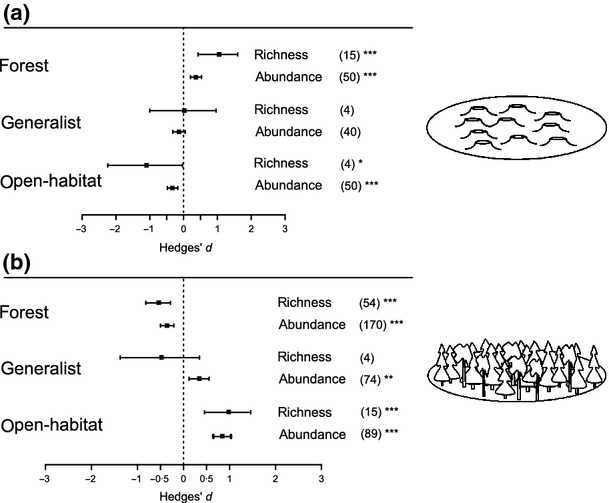
Within forest species, there were differences among taxonomic groups in richness-response to retention cuts compared with un-harvested forest (QM = 15·24, d.f. = 7, P = 0·033). The number of observations was not large enough to test for richness difference between retention cuts and clearcuts for individual taxonomic groups. For analyses of the abundance of forest species, there was a significant difference among the taxonomic groups when retention cuts were compared with clearcuts (QM = 13·75, d.f. = 5, P < 0·017) as well as with forest (QM = 79·15, d.f. = 9, P < 0·0001).
For forest species, retention cuts supported lower levels of richness of birds and bryophytes as well as lower abundances of amphibians and reptiles, mammals, birds, beetles, lichens and fungi than un-harvested forest (Figs3 and 4b). The abundance of forest birds, bryophytes and lichens was higher in retention cuts than in clearcuts, while spiders had higher abundance in retention cuts than in unlogged forest (Fig.4a,b).
Figure 3.
Effects of retention cuts (mean effect size ± 95% CI) on species richness of forest species with forest as control. Numbers of observations are stated in brackets. Effects are not significantly different from 0 when 95% CIs include 0. For significant effects, P-values are shown as *P<0·05, **P<0·01 or ***P<0·001.
Figure 4.
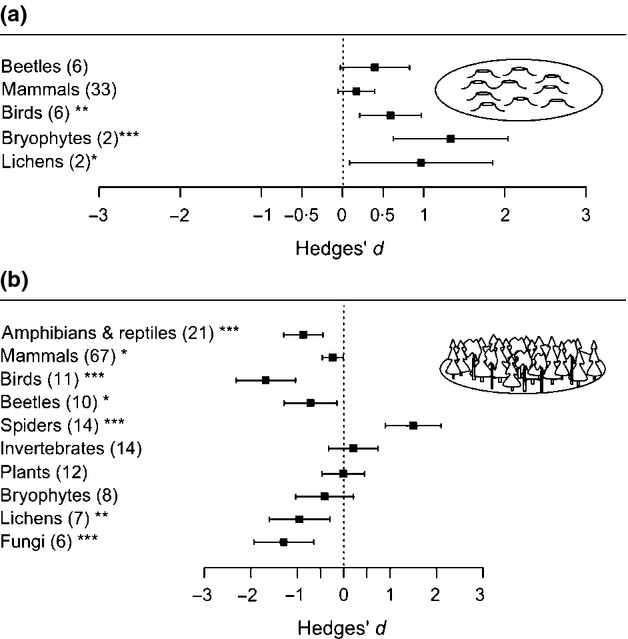
Effects of retention cuts (mean effect size ± 95% CI) on forest species abundance with clearcut (a) or forest (b) as control. Numbers of observations are stated in brackets. Effects are not significantly different from 0 when 95% CIs include 0. For significant effects, P-values are shown as *P<0·05, **P<0·01 or ***P<0·001.
Plants and birds were the only taxonomic groups associated with open habitats that could be tested regarding richness, and both had higher richness in retention cuts than in un-harvested forest [plants: mean effect = 0·85; 95% CI (0·49–1·22); n = 12; P < 0·0001; birds: mean effect = 1·26; 95% CI (0·87–1·64); n = 3; P < 0·0001]. There were not enough data to make comparisons with clearcuts. The abundance of open-habitat birds, mammals, beetles and spiders was lower in retention cuts compared to clearcuts (Fig.5). In contrast, when comparing to un-harvested forest, birds, mammals, beetles, spiders and plants had higher abundance in retention cuts (Fig.5).
Figure 5.
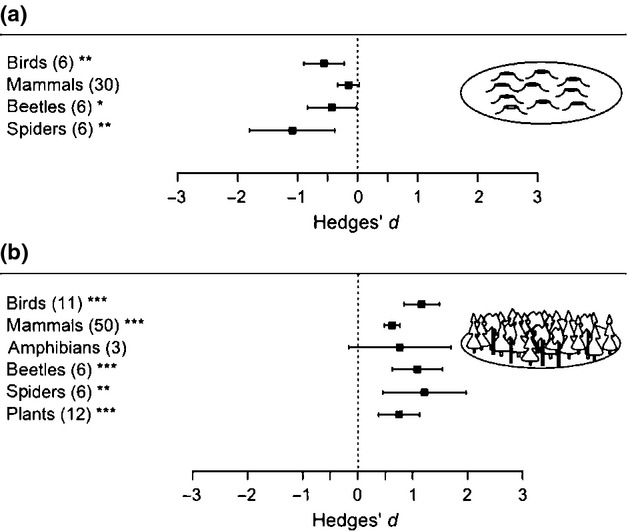
Effects of retention cuts (mean effect size ± 95% CI) on open-habitat species abundance with clearcut (a) or forest (b) as control. Numbers of observations are stated in brackets. Effects are not significantly different from 0 when 95% CIs include 0. For significant effects, P-values are shown as *P<0·05, **P<0·01 or ***P<0·001.
Proportion of Retained Trees, Size of Treatment Unit, Years after Harvest, Spatial Arrangement and Biome
The difference in forest species richness between retention cuts and clearcuts increased with the proportion of retained trees [slope 0·03; 95% CI (0·02–0·04); n = 15; P < 0·0001], while there was no such effect for forest species abundance compared to clearcuts, or for forest species richness or abundance compared to forests. The effect of retention (as compared to clearcuts) on the abundance of open-habitat species decreased with the proportion of retained trees [slope −0·01; 95% CI (−0·02 to −0·00); n = 45; P = 0·008], but there was no such effect when compared to forests. There were not enough data to test open-habitat species richness compared to clearcuts, and there was no significant effect for open-habitat species richness compared to forests (Tables S2 and S3, Supporting Information).
There was no effect of treatment unit size on the effect of retention cuts on richness or abundance of either forest species or open-habitat species (Tables S2 and S3, Supporting Information). The effect of retention cuts on the number of forest species increased with years after harvest, using clearcuts as a control [slope 0·36; 95% CI (0·11–0·61); n = 15; P = 0·0042]. The effect on abundance of open-habitat species also increased with time since harvest [slope 0·04; 95% CI (0·01–0·07); n = 45; P = 0·0187] compared to clearcuts. Interaction terms between treatment size and the proportion of retention were non-significant. Spatial arrangement of the trees (aggregated vs. dispersed) had no effect on richness or abundance of either forest species or open-habitat species (Tables S2 and S3, Supporting Information).
There were differences between biomes in the abundance responses of forest species and open-habitat species to retention harvest, both in comparison with clearcuts (forest species: QM = 10·44, d.f. = 1, P = 0·001, open-habitat species: QM = 7·12, d.f. = 1, P = 0·008) and forests (forest species: QM = 17·39, d.f. = 1, P = 0·001, open-habitat species: QM = 6·37, d.f. = 1, P = 0·012). In a single moderator analysis, the direction of the effect was the same in both biomes, but the magnitude of the retention cut effect was larger in the boreal biome, for all comparisons (Table S4, Supporting Information).
Response of Overall Species Richness and Abundance
For all species together (forest species, open-habitat species, generalists and unclassified species), retention cuts supported higher total species richness and greater levels of abundance when compared with clearcuts (Fig.6a) (only significant when using the trim and fill analysis). There also was a positive effect of retention cuts on both total species richness and total species abundance compared to forests (Fig.6b). However, when using the trim and fill analysis, the effect was negative and significant for abundance but not for richness.
Figure 6.
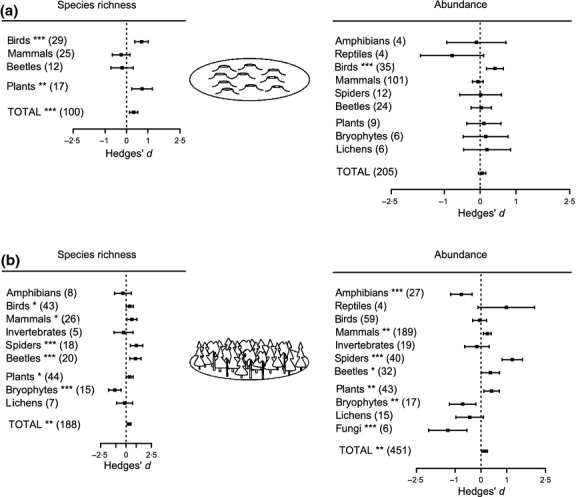
Effects of retention cuts (mean effect size ± 95% CI) on richness and abundance for all species combined (forest species, open-habitat species, generalist species, unclassified species) for comparisons with (a) clearcuts and (b) un-harvested forests. Number of observations is stated in brackets. Effects are not significantly different from 0 when 95% CIs include 0. For significant effects, P-values are shown as *P<0·05, **P<0·01 or ***P<0·001.
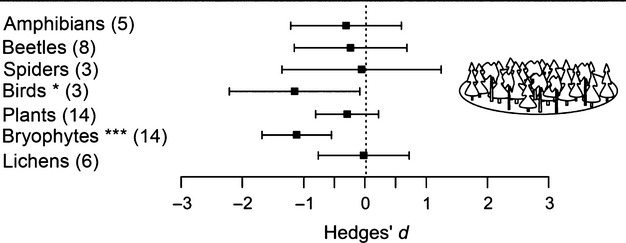
Taxonomic groups differed for richness of all species taken together when using clearcuts (QM = 29·06, d.f. = 10, P = 0·001) or forests as the control (QM = 46·61, d.f. = 10, P < 0·0001), and for abundance when using forests (QM = 88·23, d.f. = 10, P < 0·0001) but not clearcuts as the control (QM = 15·36, d.f. = 10 P = 0·12).
Compared to clearcuts, retention cuts supported higher richness of birds and plants, and a greater abundance of birds (Fig.6a). Compared to un-harvested forest, retention cuts supported higher richness of birds, mammals, spiders, beetles and plants but lower bryophyte richness (Fig.6b). Compared to un-harvested forest, retention cuts had lower abundance of amphibians, bryophytes and fungi but higher abundance of mammals, spiders, beetles and plants (Fig.6b).
Discussion
Understanding the effectiveness of retention forestry in sustaining biodiversity is one of the critical steps in promoting the integration of wood production and conservation in production forests (Lindenmayer et al. 2012), particularly as the use of this approach is widespread and increasing around the world. Our meta-analysis yielded clear results for many questions that we addressed and thus offers valuable new insights into biodiversity responses to retention forestry. Moreover, our investigation examined a large number of taxonomic groups, and a novel aspect of our work was that we explored the impacts of a suite of potential explanatory variables such as the amount of retention, geographical location (temperate vs. boreal forest), spatial arrangement of the retention trees and time since logging. We found evidence-based support for a wider use of retention harvesting practices in that any declines in forest species and open-habitat species were reduced in retention cuts as compared to un-harvested and clearcut forests, respectively. Other important findings include increased effect on forest species richness with the proportion of trees retained and a positive effect on forest species richness with time since harvest.
Many Forest Species are Promoted by Retention Forestry
Retention cuts supported higher total species richness and a greater abundance of forest species than clearcuts. Also as anticipated, forest species richness and abundance on retention cuts were lower compared with forests, indicating that retention cuts may not effectively conserve all forest species. Thus, for the long-term viability of species requiring forest-interior conditions, large reserves may be critical. One example of such a species is the pendulous lichen Usnea longissima Ach. which is largely confined to old trees in northern hemisphere forest with a high humidity (Esseen et al. 1981; Keon & Muir 2002). It is also noteworthy that the studies we analysed only rarely if ever included observations of red-listed and very rare forest species. For example, in Fennoscandia alone, there are at least 500 red-listed species (categories RE, EN, VU, NT) that are currently assumed to depend on old-growth forests (Gärdenfors 2010; Rassi et al. 2010). Our findings of the positive effects of retention cuts to general biodiversity cannot easily be extended to cover these species and their conservation because of the limitations in our data.
Many Open-Habitat Species Also Benefit from Retention Cuts
We found that retention cuts supported more open-habitat species than did un-harvested forest, but open-habitat species richness and abundance were lower in retention cuts than in clearcuts. Our findings thus suggest that retention cuts do not provide optimal habitat for all species that depend on early-successional post-disturbance conditions. In some regions, such species are probably commonly also found in other types of habitats, like many vascular plants rapidly colonizing after disturbance, and thus are not a target for conservation. However, in other regions, industrial forestry practices often greatly shorten the open post-disturbance phase of forest development, so providing high-quality habitat for early-seral species is important (Swanson et al. 2011). The endemic Florida Scrub Jay Aphelocoma coerulescens (Bosc, 1795) is an example of a species probably disadvantaged by retained trees. This bird species depends on open, fire-dominated oak scrub habitat, and in a production forest landscape, it would instead be promoted by open clearcuts (Greenberg, Harris & Neary 1995).
Overall, care needs to be taken to secure conditions after harvest that are suitable for rare species with specialized associations with early-successional environments (e.g. Runnel, Rosenvald & Lõhmus 2013). Many rare species in early-successional habitats are associated with dead wood, but such organisms were uncommon in our meta-analysis. There are also rare species associated with live trees in open environments (e.g. Hunter & Bond 2001). Thus, provision of dead trees and single remnant living trees in open conditions are essential components of retention forestry, although the relative importance of such substrates may depend on landscape properties and forest history (Sverdrup-Thygeson, Gustafsson & Kouki 2014).
Taxonomic Groups
Taxonomic groups of forest or open-habitat species differed in their strength of response but showed the same general direction in their effect size, for example forest taxa generally had lower richness and abundance in retention cuts than in un-harvested forests. Forest spiders had an idiosyncratic pattern with a higher abundance in retention cuts than in forests. The mechanisms to explain this need further investigation, but spiders are known to respond favourably to structural heterogeneity, including variation in the forest-floor layer and presence of gaps, qualities that increase after retention harvesting (Oxbrough & Ziesche 2013). Despite the overall similar responses within taxonomic groups, it is quite likely that there are important differences among species within a taxa, but this was not possible to explore in our meta-analysis.
Total Species Richness is Higher on Retention Cuts Than on Clearcuts
Our results show that for all species taken together (i.e. forest species, open-habitat species, generalist species and unclassified species), richness was higher in retention cuts than in clearcuts. The analysis also indicates higher species abundance in retention cuts compared to clearcuts, while no clear results could be drawn for the comparison of retention cuts with un-harvested forests. We were unable to distinguish between species that survived within harvest areas because of retained forest elements or buffered microclimatic conditions and those that were able to quickly re-establish, either because of proximity to source populations or other factors (reviewed in Baker et al. 2013). However, both processes are likely to be important in explaining high species richness in retention cuts. For species-oriented conservation, high species richness is not an aim per se; rather, rare and declining species are often of primary conservation concern. But for ecosystem functioning, it may be essential to maintain the full array of species that would occur in intact landscapes, and therefore, retention harvesting practices may have an important role in this regard.
Proportion Retention and Time since Harvest
An important question for practitioners is how much retention is enough to benefit biodiversity. We found a positive but weak relationship between the proportion of retained trees and forest species richness. The sample size for this comparison was low (n = 15), and we found no significant relationship with species abundance, indicating that more data are needed to draw firm conclusions. Studies from individual experiments in the USA and Canada have suggested retention amount thresholds between 10% and 20% for late-seral abundance and diversity (Craig & Macdonald 2009; Halpern et al. 2012), and expert recommendations point to a strict minimum retention volume of 5–10% (Gustafsson et al. 2012), with even higher retention being preferable. With more data, it may be possible to analyse whether there are thresholds for habitat amounts. Such analyses preferably should be made for groups of species, since responses are likely species specific (Lindenmayer & Luck 2005; Ranius & Fahrig 2006). Nevertheless, it may be that beneficial effects of retention on biodiversity increase continuously and that no clear threshold can be found that will satisfy all groups of species.
We found some evidence of faster recovery of biodiversity in retention cuts than on clearcuts because the effect size for forest species richness increased with time since harvest. However, our meta-analysis included few investigations of retention cuts >20 years old, with >70% of investigations completed ≤5 years after harvest. Nevertheless, it is known that some sensitive species use retained trees only after very long time after harvest (Phillips & Hall 2000; Blakesley, Noon & Anderson 2005).
Spatial Configuration of Trees
Contrary to our expectations, we did not detect differences in biodiversity response between dispersed and aggregated retention patterns. Our assumption was that the spatial arrangement of trees will affect local climate, which in turn is important for many species. There are several reasons to not draw strong conclusions from our results, but instead await more research that specifically targets the spatial design of retained trees. One reason is inconsistent classification of retention pattern in different studies included in our meta-analysis. A second is that the size of aggregates, a key variable impacting effectiveness of tree groups (e.g. Hautala, Laaka-Lindberg & Vanha-Majamaa 2011), was not included as a factor as data were often not available. A third reason is that, due to limited data, we analysed all taxonomic groups together. There is already some existing literature showing that some taxa benefit from particular retention patterning, for example ectomycorrhizal fungi prefer dispersed trees (Hagerman et al. 1999) and bryophytes tree groups (Aubry, Halpern & Peterson 2009). In the future, it will be important to focus more research on understanding likely complex interactions between retention level, retention pattern and aggregate size (e.g. Halpern et al. 2012). Also, certain individual species are likely to benefit from either dispersed or aggregated patterns (Lindenmayer & Franklin 2002).
Different Strength in Response between Biomes
An unexpected result was that the impact of retention was more pronounced in boreal than in temperate regions. Mean effect sizes for total species abundance for both forest and open-habitat species were larger for the boreal than the temperate biome. Boreal studies were from Canada and northern Europe where fire is a strong disturbance factor (Kneeshaw, Bergeron & Kuuluvainen 2011). For the temperate biome, >90% of the studies were from the Pacific NW in the USA and Canada, a region with a warmer and more humid climate than boreal forests. These temperate forests are also less influenced by fire than boreal forests, although fire is important in some temperate forest ecosystems (Franklin et al. 2002). It may be that species survive better and also colonize more rapidly after logging in the boreal biome because they are adapted to larger and more frequent disturbances than species in the temperate biome. An additional explanation may be that regrowth after harvest is more rapid in temperate regions due to a warmer climate, thus the contrast between retention cuts and forests decreases more rapidly.
Better Reporting would Increase Sample Size
Our sample size of 944 observations was large but could have been considerably larger had more data in the original papers been presented in a way that could be used in the meta-analysis. For over 150 relevant studies, it was not possible to extract sample size, mean and variation around the mean, that is, data that are necessary for meta-analysis, even after consulting with the authors. Thus, to enable large high-quality knowledge compilations in the future, better reporting of primary results is necessary. We also found it hard in many cases to determine how the sampling had been conducted, because the description of the study design was often limited. Furthermore, data from several studies could not be used because controls were not replicated.
Ways Ahead for Research
Retention forestry is a way to combine two goals: biodiversity conservation and wood production, often with likely trade-offs between the two. There is a potential to fine-tune retention models, for example regarding selection of site types and tree species, to increase biodiversity benefits while simultaneously minimizing loss of revenue. Studies to identify cost-efficient solutions are few (but see Perhans, Haight & Gustafsson 2014), and more studies targeting this are urgently needed. Such studies preferably should recognize regional or even local variations in site conditions and forestry practices, to increase applicability of results. Modelling of biodiversity response over time including effects on wood production is also essential, to assess the role of retention forestry in future production forest landscapes. The value of retained trees to biodiversity will likely increase with time since they will become increasingly older compared to the production forest trees. Post-harvest treatments like soil scarifi-cation, prescribed burning and use of herbicides also warrant further study since they may affect biodiversity response.
Conclusions
Retention forestry is a comparatively new but widely applied forest management practice. It differs from traditional forestry in that it includes the deliberate, long-term retention of single trees and tree patches to allow integration of biodiversity conservation and wood production in managed forest stands. One of the advantages of retention forestry is that it can easily be implemented, for example, in comparison with more complex multicohort and selection cutting harvesting systems. Our meta-analysis of a relatively large set of published studies shows that retention approaches at harvest represent a way to moderate harvest impacts on forest species while at the same time promoting species requiring disturbance. Thus, the answer to the question posed in the title ‘Can retention forestry help preserve biodiversity?’ is yes; retention forestry is usually more beneficial to biodiversity than traditional harvest systems, in particular clearcutting, and consequently a wider use of this forestry practice is warranted.
Acknowledgments
We are very grateful to respondents to questions and those who contributed data (see Table S5, Supporting Information for a list of the contributors). This project was funded by The Swedish Research Council Formas (Grant nr. 215-2009-569) to L. Gustafsson.
Supporting Information
Appendix S1. Studies included in the meta-analysis.
Appendix S2. Meta-data.
Table S1. Study exclusion criteria.
Table S2. Results of the mixed-effects meta-regression models, comparison with clearcut.
Table S3. Results of the mixed-effects meta-regression models, comparison with un-harvested forests.
Table S4. Abundance responses of forest species and open-habitat species to biome.
Table S5. List of contributors.
References
- Aubry KB, Halpern CB. Peterson CE. Variable-retention harvests in the Pacific Northwest: a review of short-term findings from the DEMO study. Forest Ecology and Management. 2009;258:398–408. [Google Scholar]
- Baker SC, Spies TA, Wardlaw TJ, Balmer J, Franklin JF. Jordan GJ. The harvested side of edges: effect of retained forests on the re-establishment of biodiversity in adjacent harvested areas. Forest Ecology and Management. 2013;302:107–121. [Google Scholar]
- Blakesley JA, Noon BR. Anderson DR. Site occupancy, apparent survival, and reproduction of California spotted owls in relation to forest stand characteristics. Journal of Wildlife Management. 2005;69:1554–1564. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT. Rothstein HR. Introduction to Meta-Analysis. New York, NY: John Wiley and Sons; 2009. [Google Scholar]
- Craig A. Macdonald SE. Threshold effects of variable retention harvesting on understory plant communities in the boreal mixedwood forest. Forest Ecology and Management. 2009;258:2619–2627. [Google Scholar]
- Duval SJ. Tweedie R. A nonparametric ‘trim and fill’ method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association. 2000a;95:89–98. [Google Scholar]
- Duval SJ. Tweedie RL. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000b;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Esseen PA, Ericson L, Lindström H. Zackrisson O. Occurrence and ecology of Usnea longissima in central Sweden. Lichenologist. 1981;13:177–190. [Google Scholar]
- FAO. Global Forest Resources Assessment 2010. Rome: Food and Agricultural Organization of the United Nations; 2010. pp. 1–378. , FAO Forestry Paper 163. [Google Scholar]
- Fedrowitz K. Gustafsson L. Does the amount of trees retained at clear felling of temperate and boreal forests influence biodiversity response? Environmental Evidence. 2012;1:5. [Google Scholar]
- Franklin JF. Towards a new forestry. American Forests. 1989;95:37–44. [Google Scholar]
- Franklin JF, Spies TA, Van Pelt R, Carey AB, Thornburgh DA, Berg DR. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. Forest Ecology and Management. 2002;155:399–423. [Google Scholar]
- Gärdenfors U. Rödlistade arter i Sverige 2010 – The 2010 Red List of Swedish Species. Uppsala: ArtDatabanken, SLU; 2010. p. 496. [Google Scholar]
- Greenberg CH, Harris LD. Neary DG. A comparison of bird communities in burned and salvage-logged, clear-cut, and forested Florida sand pine scrub. Wilson Bulletin. 1995;107:40–54. [Google Scholar]
- Gustafsson L, Baker SC, Bauhus J, Beese WJ, Brodie A, Kouki J. Retention forestry to maintain multifunctional forests: a world perspective. BioScience. 2012;62:633–645. [Google Scholar]
- Hagerman SM, Jones MD, Bradfield GE. Sakakibara SM. Ectomycorrhizal colonization of Picea engelmannii × Picea glauca seedlings planted across cut blocks of different sizes. Canadian Journal of Forest Research. 1999;29:1856–1870. [Google Scholar]
- Halpern CB, Halaj J, Evans SA. Dovčiak M. Level and pattern of overstory retention interact to shape long-term responses of understories to timber harvest. Ecological Applications. 2012;22:2049–2064. doi: 10.1890/12-0299.1. [DOI] [PubMed] [Google Scholar]
- Hautala H, Laaka-Lindberg S. Vanha-Majamaa I. Effects of retention felling on epixylic species in boreal spruce forests in southern Finland. Restoration Ecology. 2011;19:418–429. [Google Scholar]
- Hedges LV. Olkin I. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Heithecker TD. Halpern CB. Edge-related gradients in microclimate in forest aggregates following structural retention harvests in western Washington. Forest Ecology and Management. 2007;248:163–173. [Google Scholar]
- Hunter JE. Bond ML. Residual trees: wildlife associations and recommendations. Wildlife Society Bulletin. 2001;29:995–999. [Google Scholar]
- Keon DB. Muir PS. Growth of Usnea longissima across a variety of habitats in the Oregon Coast Range. Bryologist. 2002;105:233–242. [Google Scholar]
- Kneeshaw D, Bergeron Y. Kuuluvainen T. Forest ecosystem structure and disturbance dynamics across the circumboreal forest. In: Schickhoff U, editor; Millington AC, Blumler MB, editors. The SAGE Handbook of Biogeography. Los Angeles, CA: SAGE; 2011. pp. 263–280. [Google Scholar]
- Koricheva J, Gurevitch J. Mengersen K. Handbook of Meta-analysis in Ecology and Evolution. Oxford: Princeton University Press; 2013. [Google Scholar]
- Kouki J, Löfman S, Martikainen P, Rouvinen S. Uotila A. Forest fragmentation in Fennoscandia: linking habitat requirements of wood-associated threatened species to landscape and habitat changes. Scandinavian Journal of Forest Research. 2001;3(Suppl):27–37. [Google Scholar]
- Kraus D. Krumm F. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity. Freiburg: European Forest Institute; 2013. p. 284. [Google Scholar]
- Lindenmayer DB. Franklin JF. Conserving Forest Biodiversity. A Comprehensive Multiscaled Approach. Washington, DC: Island Press; 2002. [Google Scholar]
- Lindenmayer DB, Laurance WF. Franklin JF. Global decline in large old trees. Science. 2012;338:1305–1306. doi: 10.1126/science.1231070. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB. Luck G. Synthesis: thresholds in conservation and management. Biological Conservation. 2005;124:351–354. [Google Scholar]
- Lindenmayer DB, Franklin JF, Lõhmus A, Baker SC, Bauhus J, Beese W. A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conservation Letters. 2012;5:421–431. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC. Terrestrial ecoregions of the worlds: a new map of life on earth. BioScience. 2001;51:933–938. [Google Scholar]
- Oxbrough A. Ziesche T. Spiders in forest ecosystems. In: Krumm F, editor; Kraus D, editor. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity. Freiburg: European Forest Institute; 2013. pp. 186–193. [Google Scholar]
- Perhans K, Haight RG. Gustafsson L. The value of information in conservation planning: selecting retention trees for lichen conservation. Forest Ecology and Management. 2014;318:175–182. [Google Scholar]
- Phillips LC. Hall BS. A historical view of Red-cockaded Woodpecker habitat on Fort Polk, Louisiana. Journal of Field Ornithology. 2000;71:585–596. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/ [Google Scholar]
- Ranius T. Fahrig L. Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds. Scandinavian Journal of Forest Research. 2006;21:201–208. [Google Scholar]
- Rassi P, Hyvärinen E, Juslén A. Mannerkoski I. The 2010 Red List of Finnish Species. Helsinki: Ympäristöministeriö & Suomen ympäristökeskus; 2010. p. 685. [Google Scholar]
- Robertson BA. Hutto RL. Is selectively harvested forest an ecological trap for Olive-sided Flycatchers? Condor. 2007;109:109–121. [Google Scholar]
- Rosenvald R. Lõhmus A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. Forest Ecology and Management. 2008;255:1–15. [Google Scholar]
- Runnel K, Rosenvald R. Lõhmus A. The dying legacy of green-tree retention: different habitat values for polypores and wood-inhabiting lichens. Biological Conservation. 2013;159:187–196. [Google Scholar]
- Secretariat of the Convention on Biological Diversity. Global Biodiversity Outlook 3. Montreal, QC: Secretariat of the Convention on Biological Diversity; 2010. p. 94. [Google Scholar]
- Stokland JN, Siitonen J. Jonsson BG. Biodiversity in Dead Wood. Cambridge: Cambridge University Press; 2012. [Google Scholar]
- Sverdrup-Thygeson A, Gustafsson L. Kouki J. Spatial and temporal scales relevant for conservation of dead-wood associated species – current status and perspectives. Biodiversity & Conservation. 2014;23:513–535. [Google Scholar]
- Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmayer DB. Swanson FJ. The forgotten stage of forest succession: early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment. 2011;9:117–125. [Google Scholar]
- Vanderwel MC, Malcolm JR. Mills SC. A meta-analysis of bird responses to uniform partial harvesting across North America. Conservation Biology. 2007;21:1230–1240. doi: 10.1111/j.1523-1739.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. http://www.jstatsoft.org/v36/i03/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Studies included in the meta-analysis.
Appendix S2. Meta-data.
Table S1. Study exclusion criteria.
Table S2. Results of the mixed-effects meta-regression models, comparison with clearcut.
Table S3. Results of the mixed-effects meta-regression models, comparison with un-harvested forests.
Table S4. Abundance responses of forest species and open-habitat species to biome.
Table S5. List of contributors.


