Abstract
Dinoflagellates represent a cosmopolitan group of phytoplankton with the ability to form harmful algal blooms. Featuring a Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) with very low CO2 affinities, photosynthesis of this group may be particularly prone to carbon limitation and thus benefit from rising atmospheric CO2 partial pressure (pCO2) under ocean acidification (OA). Here, we investigated the consequences of OA on two bloom-forming dinoflagellate species, the calcareous Scrippsiella trochoidea and the toxic Alexandrium tamarense. Using dilute batch incubations, we assessed growth characteristics over a range of pCO2 (i.e. 180–1200 µatm). To understand the underlying physiology, several aspects of inorganic carbon acquisition were investigated by membrane-inlet mass spectrometry. Our results show that both species kept growth rates constant over the tested pCO2 range, but we observed a number of species-specific responses. For instance, biomass production and cell size decreased in S. trochoidea, while A. tamarense was not responsive to OA in these measures. In terms of oxygen fluxes, rates of photosynthesis and respiration remained unaltered in S. trochoidea whereas respiration increased in A. tamarense under OA. Both species featured efficient carbon concentrating mechanisms (CCMs) with a CO2-dependent contribution of HCO3− uptake. In S. trochoidea, the CCM was further facilitated by exceptionally high and CO2-independent carbonic anhydrase activity. Comparing both species, a general trade-off between maximum rates of photosynthesis and respective affinities is indicated. In conclusion, our results demonstrate effective CCMs in both species, yet very different strategies to adjust their carbon acquisition. This regulation in CCMs enables both species to maintain growth over a wide range of ecologically relevant pCO2.
Introduction
Since the Industrial Revolution, alterations in fossil fuel combustion and land-use have caused atmospheric CO2 partial pressure (pCO2) to increase from approximately 280 toward approximately 395 µatm at present-day, and is predicted to reach values of approximately 900 µatm by the end of the 21st century (IPCC 2007). Regarding the oceans, elevated pCO2 causes an increase in CO2 and bicarbonate (HCO3−) concentrations, while carbonate ion concentrations (CO32−) decrease. These changes in the speciation of dissolved inorganic carbon (DIC) result in lowered pH values, a phenomenon also known as ocean acidification (OA; Wolf-Gladrow et al. 1999, Caldeira and Wickett 2003). OA and associated changes in the carbonate chemistry have been shown to impact marine organisms in many ways (Fabry et al. 2008). Especially for phytoplankton, being the basis of the marine food web and the driver of the biological carbon pumps, such changes may have far reaching consequences (Falkowski et al. 1998, Doney et al. 2009).
Phytoplankton take up inorganic carbon and fix CO2 into organic compounds by Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO). This enzyme generally features low affinities for CO2, and a competing reaction with O2 further reduces its overall efficiency (Badger et al. 1998). To overcome these catalytic limitations imposed by RubisCO, phytoplankton developed so-called carbon concentrating mechanisms (CCMs). Common features of a CCM include active uptake of CO2 and HCO3− as well as means to reduce the CO2 leakage (Giordano et al 2005, Rost et al. 2006). CCMs may further involve carbonic anhydrase (CA), an enzyme which accelerates the otherwise slow interconversion between CO2 and HCO3−. The mode and cost of CCMs will to a great extent determine the sensitivity of phytoplankton toward OA (Rost et al. 2008, Reinfelder 2011).
The functioning of CCMs has been intensively studied in various phytoplankton species. In diatoms, CCMs were generally downregulated with increasing pCO2 as reflected by lowered photosynthetic affinities for CO2 and DIC (Burkhardt et al. 2001, Trimborn et al. 2008). Often, these changes were also accompanied by lowered contribution of HCO3− uptake or decreased activities of extracellular CA (eCA). In other taxa such as the coccolithophore Emiliania huxleyi or the cyanobacterium Trichodesmium, affinities for CO2 and DIC were also downregulated under OA, yet eCA activity did not seem to play a role in the functioning of their CCMs (Rost et al. 2003, Kranz et al. 2009). These different modes of CCMs and their regulation with pCO2 have increased our understanding about species-specific responses toward OA in diatoms, coccolithophores and cyanobacteria. Little is yet known about other taxa, such as dinoflagellates.
Earlier work suggested severe CO2 limitation in photosynthesis of dinoflagellates (Colman et al. 2002, Dason et al. 2004). This was attributed to their type II RubisCO, which has the lowest affinity for CO2 of all eukaryotic phytoplankton (Morse et al. 1995, Badger et al. 1998), as well as limited ability to use HCO3−. Recent studies have, however, demonstrated high HCO3− uptake rates in the dinoflagellates species Ceratium lineatum, Heterocapsa triquetra, Prorocentrum minimum (Rost et al. 2006, Fu et al. 2008) and Protoceratium reticulatum (Ratti and Giordano 2007), indicating rather efficient modes of CCMs that may make them relatively independent from changes in CO2 availability. In some dinoflagellates, CCMs have been shown to respond to changes in carbonate chemistry, e.g. by lowered photosynthetic affinities for CO2 and DIC (Rost et al. 2006, Ratti and Giordano 2007), or by downregulation of CA transcripts (Van de Waal et al. 2013) with increasing pCO2. Such apparent differences in the regulation of CCMs may explain the observed variability in responses to OA in growth and primary production of different dinoflagellate species (Fu et al. 2007, 2010) or strains (Brading et al. 2011, 2013).
To improve our understanding about growth responses and the functioning of CCMs in dinoflagellates under OA, this study investigated the eco-physiology of two distinct dinoflagellate species, the calcareous Scrippsiella trochoidea and the toxic Alexandrium tamarense over a range of pCO2. Both are bloom-forming species that co-occur in the North Sea (Fistarol et al 2004, McCollin et al. 2011). As one has the potential to calcify and the other is a potent toxin producer, different ecological strategies can be expected, which may also be reflected in the functioning of their CCM. Hence, measurements on growth and biomass production were accompanied by measurements on inorganic carbon fluxes and CA activities using membrane-inlet mass spectrometry (MIMS).
Material and methods
Species and growth conditions
Scrippsiella trochoidea GeoB267 (culture collection of the University of Bremen) and Alexandrium tamarense Alex5 (Tillmann et al. 2009), both isolates from the North Sea, were cultured at 15°C in 0.2 µm filtered North Sea water (salinity 34). Vitamins and trace metals were added according to f/2 medium (Guillard and Ryther 1962), except for FeCl3 (1.9 µmol l−1), H2SeO3 (10 nmol l−1) and NiCl2 (6.3 nmol l−1). Nitrate and phosphate were added to final concentrations of 100 and 6.25 µmol l−1, respectively. Culture medium was pre-aerated with air containing pCO2 of 180 µatm (Last Glacial Maximum), 380 µatm (present-day), 800 µatm and 1200 µatm (scenarios of the year 2100 and beyond). These concentrations were obtained by mixing CO2-free air (<0.1 µatm pCO2; Domnick Hunter, Willich, Germany) with pure CO2 (Air Liquide Deutschland, Düsseldorf, Germany) using mass flow controllers (CGM 2000 MCZ Umwelttechnik, Bad Nauheim, Germany). CO2 concentrations were regularly verified by a non-dispersive infrared analyzer system (LI6252, LI-COR Biosciences, Bad Homburg, Germany).
Cultures were grown in 2.4 l borosilicate bottles and placed on a roller table to allow homogenous mixing. Light was provided by OSRAM daylight tubes (18 W/965 Biolux) at a light: dark cycle of 16:8 h. Light was adjusted to an incident photon flux density (PFD) of 250 ± 25 µmol photons m−2 s−1 using a spherical micro quantum sensor (Walz, Effeltrich, Germany). Prior to the onset of the experiments, cells were acclimated to the respective CO2 concentrations for at least 14 days. To ensure dilute batch conditions with minor changes in carbonate chemistry, cultures were diluted about once a week and population densities were kept <400 cells ml−1. Experiments were run in triplicates (n = 3) over at least 5 days.
Sampling and analyses
Samples were always taken 5–7 h after the start of the light period. Every other day, pH was measured with a 2-point calibrated WTW pH meter 3110 (Wissenschaftlich-Technische Werkstätten GmbH, Weilheim, Germany). Samples for total alkalinity (TA) were analyzed by a fully automated titration system (SI Analytics, Mainz, Germany) with a mean accuracy of 13 µmol l−1. DIC samples were analyzed in a QuAAtro high performance microflow analyzer (Seal, Mequon, WI) with a mean accuracy of 8 µmol l−1. Changes in TA and DIC over the course of the incubations were <2 and <3.4%, respectively. Owing to the decreasing buffer capacity with increasing pCO2 (Egleston et al. 2010), DIC consumption caused higher variability in pH and pCO2 in the high CO2 treatments (Table1). Carbonate chemistry was calculated with CO2sys (Pierrot et al. 2006) using pHNBS (National Bureau of Standards) and TA of each incubation. Equilibrium constants of Mehrbach et al. (1973), refitted by Dickson and Millero (1987) were chosen.
Table 1.
Carbonate chemistry for the different CO2 treatments. Values for TA, DIC and pH indicate the mean of triplicate incubations (n = 3; ± sd). pCO2 was calculated based on pH and TA of each incubation, using equilibrium constants by Mehrbach et al. (1973), refitted by Dickson and Millero (1987)
| CO2 treatment | TA (µmol l−1) | DIC (µmol l−1) | pHNBS | pCO2 (µatm) |
|---|---|---|---|---|
| S. trochoidea | ||||
| 180 | 2386 ± 1 | 1972 ± 16 | 8.45 ± 0.01 | 180 ± 6 |
| 380 | 2388 ± 2 | 2096 ± 10 | 8.21 ± 0.02 | 358 ± 15 |
| 800 | 2385 ± 1 | 2223 ± 11 | 7.91 ± 0.03 | 785 ± 55 |
| 1200 | 2386 ± 4 | 2268 ± 18 | 7.77 ± 0.04 | 1133 ± 97 |
| A. tamarense | ||||
| 180 | 2434 ± 3 | 1992 ± 33 | 8.50 ± 0.06 | 162 ± 24 |
| 380 | 2439 ± 1 | 2117 ± 41 | 8.27 ± 0.07 | 315 ± 57 |
| 800 | 2434 ± 2 | 2245 ± 37 | 7.97 ± 0.10 | 706 ± 154 |
| 1200 | 2418 ± 1 | 2283 ± 34 | 7.83 ± 0.12 | 995 ± 248 |
To determine population densities, 20–60 ml culture suspension was and fixed with Lugol's solution (2% final concentration). Each day, triplicate cell counts were performed with an Axiovert 40C inverted microscope (Carl Zeiss MicroImaging GmbH, Hamburg, Germany). Specific growth rates (μ) were calculated by an exponential fit through cell counts over at least 4 days for each biological replicate (n = 3).
At the end of each experiment, samples were taken to assess particulate organic carbon and nitrogen (POC and PON), particulate inorganic carbon (PIC, as difference between total particulate carbon and POC), as well as chlorophyll a (Chl a). For analyses of POC and PON, 300–400 ml culture suspension was filtered in duplicate on pre-combusted GF/F filters (500°C, 6 h). Prior to POC measurements, 200 ml of HCl (0.1 mol l−1) was added to the filters to remove all PIC, and filters were dried overnight. Filters were wrapped in tin foil cups and analyzed by an ANCA-SL 20–20 mass spectrometer (SerCon Ltd., Crewe, UK). To determine Chl a, 100–200 ml culture suspension was filtered in duplicate on cellulose-nitrate filters (Whatman, Maidstone, UK), rapidly frozen in liquid nitrogen and subsequently stored at −80°C. Extraction and fluorometric determination of Chl a were done according to Knap et al. (1996), using a TD-700 Fluorometer (Turner Designs, Sunnyvale, CA).
Oxygen and inorganic carbon flux measurements
O2 and CO2 fluxes were measured by means of MIMS (Isoprime, GV Instruments, Manchester, UK) to determine photosynthetic O2 evolution and respiratory O2 uptake, as well as CO2 and HCO3− fluxes. Net O2 fluxes were converted to inorganic carbon (Ci) fluxes by applying a photosynthetic quotient of 1.4 (as nitrate was the only nitrogen source in the growth medium) and a respiratory quotient of 1.0 (Williams and Robertson 1991). The applied approach by Badger et al. (1994) depends on a chemical disequilibrium between CO2 and HCO3−, which is induced by photosynthetic Ci uptake in the absence of eCA activity. O2 and CO2 fluxes were measured simultaneously during steady-state photosynthesis in consecutive light–dark intervals with increasing amounts of DIC. Maximum rates (Vmax) and half-saturation concentrations (K1/2) for respective Ci species (CO2 and HCO3−) and DIC were determined by applying a Michaelis–Menten fit. Negative estimates of HCO3− concentrations, which were occasionally calculated for the lowest DIC concentrations, were omitted from the Michaelis–Menten fit. Measurements were performed in a 4-(2-hydroxylethyl)-1-piperazine-ethanesulfonic acid (HEPES, 50 mmol l−1) buffer in f/2 medium with a pH of 8.0 ± 0.1 at 15 ± 0.3°C. The applied pH in the MIMS assay represents an intermediate value of the pH values of the acclimations. Provided that these differences in pH have minor effects on Ci uptake kinetics, rates in the assays are also representative for the acclimation. For more details on the method see Badger et al. (1994) and Rost et al. (2007).
Prior to the experimental series, the shape and speed of the stirrer in the MIMS-cuvette were tested on both species to eliminate biases from mechanical and physiological stress for the dinoflagellate species. In test runs, photosynthetic O2 evolution was measured in intervals for about 1 h confirming that rates remained unaffected over the duration of the assay. Light and dark intervals were adjusted to 4.5 and 3.5 min, respectively, to allow the CO2 and O2 traces to reach steady-state conditions (i.e. a linear slope; see Rost et al. 2006). The light intensity in the cuvette was set to the light intensity of the experiments (tested with the same light meter) with very comparable light spectra as similar daylight tubes have been used. Prior to the measurements, acclimated dinoflagellate cells were concentrated by gentle vacuum filtration (<200 mbar) over a 10 µm membrane filter (Millipore, Billerica, MA). Culture medium was exchanged with DIC-free assay medium and 8 ml of this concentrated cell suspension was transferred into the MIMS cuvette. During the first dark phase, membrane-impermeable dextran-bound sulphonamide (DBS; Synthelec AB, Lund, Sweden) was added to a final concentration of 50 µmol to inhibit any potential eCA activity. In order to normalize rates, duplicate Chl a samples were taken after each measurement.
Extracellular carbonic anhydrase activities
The determination of eCA activity was monitored by the 18O depletion rate of doubly labeled 13C18O2 in sea water via alternating hydration and dehydration steps (Silverman 1982). As CA catalyzes the interconversion between HCO3− and CO2, it concomitantly enhances the exchange of 18O in 13C18O18O (m/z = 49) with 16O from water molecules, forming 13C18O16O (m/z = 47) and subsequently 13C16O16O (m/z = 45). In the dark, NaH13C18O3 label was injected into the cuvette containing 8 ml HEPES-buffered culture medium with a pH of 8.0 ± 0.1 at 15 ± 0.3°C. After recording the steady-state depletion in 18O enrichment for approximately 8 min (S1), 400 µl of the concentrated cell suspension was injected, and the 18O depletion was followed for another 10 min (S2). Units of eCA activity (U) were calculated using the catalyzed and non-catalyzed rates S2 and S1, respectively, and subsequently normalized to Chl a (Badger and Price 1989). As a consequence, U corresponds to the enhancement in the interconversion between CO2 and HCO3−, expressed as % µg−1 Chl a. For more details on the method see Palmqvist et al. ()1994 and Rost et al. (2007).
Statistics
Normality of data was confirmed using the Shapiro–Wilk. Variables were log-transformed if this improved the homogeneity of variances, as tested by Levene's test. Significant differences between treatments were tested using one way anova, followed by post hoc comparison of the means using Tukey's HSD (α = 0.05; Quinn and Keough 2002); significant differences between species, i.e. comparing the respective treatments, were tested using t-test; significance of relationships between HCO3− to net C fixation and CO2 concentrations were tested by means of linear regression.
Results
Growth characteristics
In both species, growth remained largely unaffected by changes in pCO2 (Table2), but S. trochoidea grew significantly faster than A. tamarense (P < 0.001) with average growth rates of 0.60 ± 0.05 day−1 compared to 0.47 ± 0.02 day−1, respectively. Scrippsiella trochoidea displayed a decrease in POC quota in response to elevated pCO2 (Table2), which was in line with a reduction in cell size (data not shown). In A. tamarense, POC quota remained unaltered over the applied pCO2 range (Table2), and were about twofold higher compared to S. trochoidea. Chl a quota in S. trochoidea showed maximum values in the 380 and 800 µatm CO2 treatments, whereas in A. tamarense, they remained largely constant over the pCO2 range (Table2). Average Chl a quota were about sixfold higher in A. tamarense as compared to S. trochoidea. As a consequence of the differences in growth and POC quota, POC production rates of S. trochoidea decreased from 180 to 1200 µatm pCO2 (P = 0.005; Table2). Alexandrium tamarense displayed no changes in POC production rates toward elevated pCO2 (Table2), which were on average twice as high as in S. trochoidea.
Table 2.
Growth characteristics of Scrippsiella trochoidea and Alexandrium tamarense in the different CO2 treatments. A significant difference between treatments is denoted by different letters. Values represent the mean ± sd of triplicate incubations (n = 3)
| pCO2 | Growth rate | POC production | POC production | Chl a | POC | POC:PON | POC:Chl a |
|---|---|---|---|---|---|---|---|
| (µatm) | (day−1) | (ng cell−1 day−1) | (pg pg−1 Chl a day−1) | (pg cell−1) | (ng cell−1) | (atomic) | (mass) |
| S. trochoidea | |||||||
| 180 | 0.61 ± 0.03 | 1.21 ± 0.04a | 283 ± 38a | 4.3 ± 0.71a | 1.99 ± 0.04a | 7.6 ± 0.2ac | 469 ± 81a |
| 380 | 0.61 ± 0.05 | 1.08 ± 0.08ab | 143 ± 13b | 7.6 ± 1.19ab | 1.76 ± 0.02ab | 8.1 ± 0.3ab | 236 ± 42b |
| 800 | 0.61 ± 0.04 | 1.10 ± 1.14a | 127 ± 20b | 8.7 ± 0.52b | 1.79 ± 0.22ab | 8.4 ± 0.3b | 206 ± 28b |
| 1200 | 0.58 ± 0.02 | 0.87 ± 0.02b | 188 ± 52b | 4.9 ± 1.25a | 1.50 ± 0.09b | 7.4 ± 0.1c | 321 ± 77ab |
| A. tamarense | |||||||
| 180 | 0.46 ± 0.02ab | 1.47 ± 0.08 | 40.5 ± 3.9 | 36.3 ± 1.52 | 3.17 ± 0.25 | 5.8 ± 0.1 | 88 ± 11 |
| 380 | 0.46 ± 0.02ab | 1.68 ± 0.12 | 42.0 ± 4.6 | 40.1 ± 2.75 | 3.62 ± 0.31 | 5.8 ± 0.3 | 91 ± 9 |
| 800 | 0.48 ± 0.01a | 1.67 ± 0.06 | 42.4 ± 3.1 | 39.5 ± 3.34 | 3.46 ± 0.15 | 5.7 ± 0.1 | 88 ± 6 |
| 1200 | 0.45 ± 0.01b | 1.55 ± 0.06 | 43.2 ± 7.7 | 36.4 ± 5.82 | 3.46 ± 0.17 | 5.6 ± 0.1 | 97 ± 15 |
The POC:PON ratio (molar) of S. trochoidea ranged between 7.4 and 8.4 with highest values in the 380 and 800 µatm CO2 treatments, whereas the POC:Chl a ratio (mass) were highest in the 180 and 1200 µatm CO2 treatments with 469 ± 81 and 321 ± 77, respectively (Table2). In A. tamarense, POC:PON and POC:Chl a ratios remained unaffected by elevated pCO2 with average values of 5.7 ± 0.2 and 90 ± 10, respectively. Calcification in S. trochoidea was very low with PIC:POC ratios <0.1 in all CO2 treatments (data not shown), suggesting that calcite cyst formation in exponentially growing cells remains low (Wang et al. 2007).
Oxygen and carbon fluxes
In S. trochoidea, net photosynthesis (Vmax) and dark respiration remained largely unaltered between the different CO2 treatments (Fig. 1). With mean values of 323 ± 68 and 173 ± 21 µmol O2 mg−1 Chl a h−1, respectively, net photosynthetic rates were about twofold higher than dark respiration rates. In A. tamarense, net photosynthetic rates decreased from 142 ± 4 to 86 ± 11 µmol O2 mg−1 Chl a h−1 (P = 0.034), while dark respiration rates increased from 88 ± 5 to 116 ± 9 µmol O2 mg−1 Chl a h−1 from the lowest to the highest CO2 treatment (P = 0.009).
Figure 1.
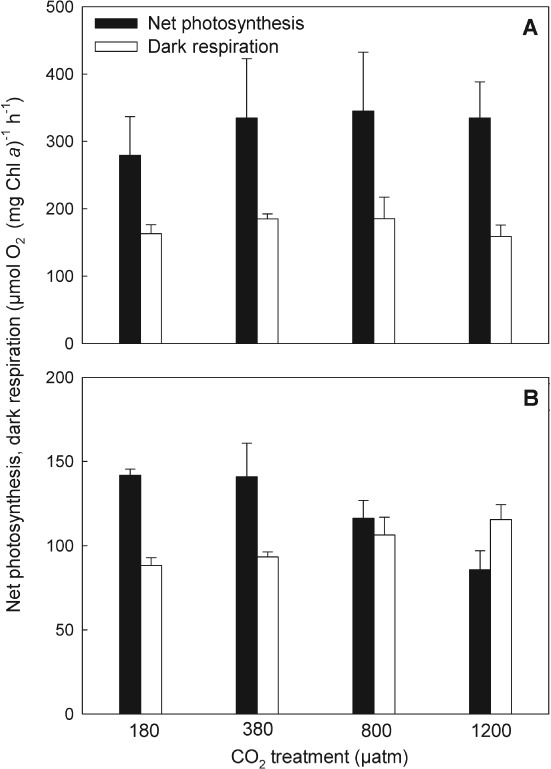
Chl a-specific rates of net photosynthesis and dark respiration of Scrippsiella trochoidea (A) and Alexandrium tamarense (B) acclimated to different CO2 concentrations. Bars represent mean ± sd (n = 3).
Scrippsiella trochoidea preferentially took up HCO3− with high affinities [i.e. low K1/2 (HCO3−); Table3]. In contrast, A. tamarense exhibited a high CO2 uptake with high affinities [i.e. low K1/2 (CO2); Table3]. Hence, the relative contribution of CO2 and HCO3− to net C fixation differed between the investigated species and furthermore changed under elevated pCO2. Scrippsiella trochoidea showed a decrease in the HCO3− to net C fixation ratio from 0.99 ± 0.17 at the lowest to 0.70 ± 0.14 at the highest CO2 treatment (f = 1.065 − 0.0009x; R2 = 0.48; P = 0.0128; Fig. 2). Alexandrium tamarense used both HCO3− and CO2 as carbon source, with an increase in the HCO3− to net C fixation ratio from 0.36 ± 0.06 at the lowest to 0.64 ± 0.27 at the highest CO2 treatment (f = 0.29 + 0.0003x; R2 = 0.40; P = 0.0280; Fig. 2).
Table 3.
Net C fixation, net CO2 uptake, HCO3− uptake, eCA activity and leakage of Scrippsiella trochoidea and Alexandrium tamarense in the different CO2 treatments. Values for Vmax and K1/2 are given in µmol mg−1 Chl a h−1 and µmol l−1, respectively. A dash indicates that values could not be determined. If not stated otherwise, values represent the mean ± sd of triplicate incubations (n = 3). A significant difference between treatments is denoted by different letters
| pCO2 (µatm) | Net C fixation | Net CO2 uptake | HCO3− uptake | eCA activity U (µg−1 Chl a) | Leakage CO2 efflux: total Ci uptake | ||||
|---|---|---|---|---|---|---|---|---|---|
| Vmax | K1/2 (CO2) | K1/2 (DIC) | Vmax | K1/2 (CO2) | Vmax | K1/2 (HCO3−) | |||
| S. trochoidea | |||||||||
| 180 | 199 ± 41 | 3.8 ± 0.5 | 94 ± 50 | −13 ± 37a | – | 194 ± 5 | 7.2 ± 12.4 | 1573 ± 108 | 0.56 ± 0.06 |
| 380 | 239 ± 62 | 4.7 ± 1.1 | 160 ± 40 | −7 ± 29a | – | 225 ± 40 | 17 ± 1.4 | 1416 ± 22 | 0.53 ± 0.06 |
| 800 | 246 ± 62 | 5.5 ± 0.9 | 263 ± 51 | −3 ± 40ab | – | 202 ± 32 | 3.7 ± 5.5 | 1232 ± 144 | 0.54 ± 0.01 |
| 1200 | 239 ± 38 | 5.1 ± 0.4 | 269 ± 78 | 89 ± 22b | 20.1 ± 0.6 | 165 ± 26 | 10.3 ± 17 | 1301 ± 99 | 0.48 ± 0.04 |
| A. tamarense | |||||||||
| 180 | 101 ± 2a | 2.4 ± 0.1 | 267 ± 43 | 66 ± 9a | 3.3 ± 0.2 | 36 ± 5 | 105 ± 45 | 19 ± 48 (n = 2) | 0.44 ± 0.01a |
| 380 | 101 ± 14ab | 2.8 ± 0.2 | 309 ± 67 | 60 ± 16ab | 3.3 ± 0.3 | 38 ± 7 | 220 ± 96 | 86 ± 2 (n = 2) | 0.46 ± 0.02a |
| 800 | 83 ± 7ab | 2.0 ± 0.9 | 206 ± 65 | 42 ± 8ab | 3.1 ± 1.0 | 42 ± 10 | 158 ± 143 | 156 ± 12 (n = 2) | 0.53 ± 0.02b |
| 1200 | 61 ± 8c | 2.5 ± 0.2 | 173 ± 15 | 23 ± 26b | 4.5 ± 0.8 | 38 ± 13 | 148 ± 28 | 124 (n = 1) | 0.63 ± 0.05c |
Figure 2.
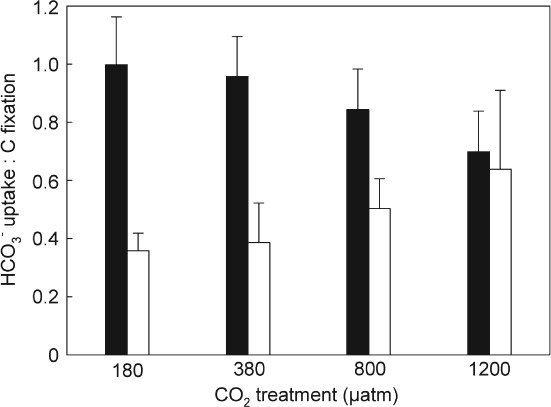
Contribution of HCO3− uptake relative to net C fixation of Scrippsiella trochoidea (black bars) and Alexandrium tamarense (white bars) acclimated to different CO2 concentrations. Ratios were calculated using the Michaelis–Menten kinetics (Table3) and the corresponding carbonate chemistry of the respective CO2 treatments (Table1). Bars represent mean ± sd (n = 3).
Carbonic anhydrase activity
Scrippsiella trochoidea displayed exceptionally high eCA activities with up to 1600 U µg−1 Chl a irrespective of the CO2 treatments (Table3). In contrast, A. tamarense contained relatively low eCA activities with mean values of 95 U µg−1 Chl a.
Discussion
In this study, two bloom-forming dinoflagellate species with different traits, the calcifying S. trochoidea and the toxic A. tamarense, were exposed to a range of pCO2 to investigate the effects of OA. While growth rates remained largely unaltered, elemental composition and production rates were responsive to OA. Both species also strongly regulated their underlying physiology with surprisingly different strategies to deal with changes in CO2 supply.
Growth and biomass production
Both species showed relatively small effects in terms of growth rates, yet we observed CO2-dependent differences in POC production rates between species. In S. trochoidea, POC production rates decreased by almost 30%, which is reflected by a reduced cell size (data not shown) as well as lowered POC quota under elevated pCO2 (Table2). On the contrary, in A. tamarense, POC production rates and POC quotas remained largely unaltered (Table2), the latter being similar to Leong et al. (2010). Average Chl a quota in S. trochoidea was largely comparable with earlier findings (Haardt and Maske 1987), whereas for A. tamarense, the average Chl a quota was about twice as high as earlier reported values (Carreto et al. 2001, Hu et al. 2006). Note that in none of the mentioned studies carbonate chemistry was controlled.
Regarding elemental composition, S. trochoidea showed highest POC:PON ratios under intermediate CO2 concentrations (Table2), with average ratios being lower than previously observed (approximately 9.3 in Burkhardt et al. 1999). These changes in POC:PON ratios are the result of disproportionally decreasing POC and PON quota under elevated pCO2. In A. tamarense, POC:PON ratios were unaltered by the applied CO2 treatments, and values were comparable with results of Leong et al. (2010). The significantly lower POC:PON ratio of A. tamarense, compared to S. trochoidea, may partly be attributed to the fact that it produces nitrogen-rich paralytic shellfish poisoning toxins (PST; Bates et al. 1978). However, the overall contribution of PST to total cellular nitrogen for this strain of A. tamarense accounts for less than 4% (Van de Waal et al. submitted), and thus cannot alone explain the observed differences in POC:PON between both species.
In contrast to our expectations, processes like growth and elemental ratios were not strongly affected by OA. With respect to POC production, however, species differed in their responses, which could be attributed to CO2-dependent changes in photosynthesis, in particular in their mode of Ci acquisition. We therefore performed MIMS measurements targeting those underlying processes.
Photosynthesis and respiration
In S. trochoidea, rates of O2 evolution (i.e. net photosynthesis) were more than twofold higher than in A. tamarense, which is in line with higher growth rates as well as higher POC:Chl a ratios (Table2). Both species exhibited high dark respiration rates compared to net photosynthetic rates (Fig. 1). Provided that measured respiration during darkness is representative also for the light phase, respiration was approximately 50% of net photosynthesis in S. trochoidea, whereas in A. tamarense both rates were equally high. Comparable high dark respiration rates have since long also been shown for other dinoflagellate species, including zooxanthellae (e.g. Burris 1977). In A. tamarense, net photosynthesis and respiration furthermore showed opposing trends in response to elevated pCO2. The decrease in net photosynthesis in A. tamarense may be largely caused by the increased dark respiration under elevated pCO2. Other processes affecting O2 uptake in the light, such as Mehler Reaction and photorespiration, can however not be excluded here and may potentially alter the trends. Brading et al. (2013), for example, observed significant light depended O2 uptake in four Symbiodinium strains, which remained unaltered under OA. Other studies showed that OA effects can be modulated under different light levels and may enhance mitochondrial respiration, photorespiration and ultimately reduce growth and biomass production under high light (Gao et al. 2012, Rokitta and Rost 2012, Li and Campbell 2013). Interestingly, the increase in respiration with pCO2 observed in A. tamarense was found to have no effect on growth or POC production rates. Overall, it can be concluded that the sum of net photosynthesis and respiration, i.e. gross photosynthesis, remained largely unaffected in both tested species.
Previous studies on Protoceratium reticulatum and four strains of Symbiodinium showed basically no CO2 effect on photosynthesis and respiration (Ratti and Giordano 2007, Brading et al. 2011), with the exception of one Symbiodinium strain that showed higher rates of net photosynthesis under OA (Brading et al. 2011). Interestingly, in Protoceratium reticulatum and another Symbiodinium strain, growth nonetheless increased with elevated pCO2 (Ratti and Giordano 2007, Brading et al. 2011). These findings, together with our current results, demonstrate that responses in growth and biomass production toward OA cannot always be explained by changes in O2 fluxes, but instead may be attributed to the mode of Ci acquisition. High sensitivities in growth and biomass production toward OA, for instance, have often been associated with a strong dependency on CO2 as a Ci source for photosynthesis (Colman et al. 2002, Fu et al. 2008), whereas when HCO3− is the dominant Ci source, much less sensitivity toward changes in CO2 is expected (Burkhardt et al. 1999, Rost et al. 2008). Therefore, we assessed various key components of the CCM and their potential CO2-dependent regulation to understand the responses of S. trochoidea and A. tamarense toward OA.
Carbon source and carbonic anhydrase
Among the various studies on carbon acquisition in dinoflagellates, either CO2 (Colman et al. 2002, Dason et al. 2004, Fu et al. 2008, Lapointe et al. 2008, Brading et al. 2013) or HCO3− (Rost et al. 2006, Ratti and Giordano 2007, Fu et al. 2008) was estimated to be the dominant Ci source. Here we show that S. trochoidea and A. tamarense used CO2 as well as HCO3− for photosynthesis, though their contribution to net C fixation and response to elevated pCO2 were very different. As one would expect, S. trochoidea displayed an increase in relative CO2 uptake, or in other words, a decrease in relative HCO3− uptake to net fixation under elevated pCO2 (Fig. 2). Such a trend has also been observed in other functional groups, e.g. diatoms (Burkhardt et al. 2001, Trimborn et al. 2009, 2013), coccolithophores (Rost et al. 2003) or cyanobacteria (Kranz et al. 2010). The response in A. tamarense, however, was surprising as it showed the reverse trend, i.e. an increase of HCO3− uptake in response to elevated pCO2 (Fig. 2). This could be associated with the generally high and increasing rates of respiration and CO2 efflux observed in this species (Fig. 1, Table3). HCO3− uptake may therefore be simply upregulated to compensate for the increasing CO2 efflux. Even though respiration can partly cause the high loss of Ci from the cell, it could be speculated that the increase in respiration may also provide the required ATP to fuel the higher HCO3− uptake. Why mitochondrial activity, in the first place, is stimulated under OA scenarios remains elusive, but it could be associated to altered proton gradients across the mitochondrial membrane or to pH-dependent changes in the functioning of respiratory enzymes (Amthor 1991).
According to the common notion, eCA functions to replenish the CO2 pool in the CO2 depleted boundary layer of a cell, thereby fuelling the CO2 uptake systems (Badger and Price 1989, Sültemeyer 1998, Elzenga et al. 2000). Such mechanism would obviously be most effective when a cell predominantly uses CO2 as its Ci source. For dinoflagellates, a major role of eCA activity in CCM functioning was only indicated for the CO2 user Lingulodinium polyedrum and Symbiodinium A20 (Lapointe et al. 2008, Brading et al. 2013). Activities of eCA in most other tested dinoflagellates, including A. tamarense in this study, were close to detection limits and therefore likely play only a minor role, if any, in Ci acquisition (Table3; Colman et al. 2002, Rost et al. 2006, Ratti and Giordano 2007). In S. trochoidea, however, we observed exceptionally high eCA activities of up to 1600 U µg−1 Chl a over the entire pCO2 range (Table3). Why would a predominant HCO3− user have such high eCA activities? Comparable high eCA activities in concert with high HCO3− contribution have been observed previously (Martin and Tortell 2008, Trimborn et al. 2008, 2013), and our observation that eCA and HCO3− uptake are both upregulated at high pH casts further doubts on an universal role of eCA.
Trimborn et al. (2008) proposed that in HCO3− users, eCA may convert effluxing CO2 to HCO3−, which is subsequently taken up again by the cell. Such a ‘CO2 recycling mechanism’ would be particularly advantageous for species with high respiration rates, which was indeed the case for S. trochoidea (Fig. 1). For Thalassiosira spp., however, the effectiveness of such a mechanism was recently questioned as it would increase the Ci uptake rate by less than 1% only (Hopkinson et al. 2013). This situation may, however, strongly differ between species as model estimates depend on the net CO2 uptake, which is large for Thalassiosira spp. (Hopkinson et al. 2013) but not for S. trochoidea (Table3). In fact, net CO2 uptake in S. trochoidea was close to zero or even negative and there was a high leakage, i.e. about 50% of the Ci taken up by the cell was leaking out as CO2 (Table3), which is not accounted for in the model calculations (Hopkinson et al. 2013). Particular high leakage has also been measured in other dinoflagellates (Rost et al. 2006). It should be noted, however, that Ci fluxes are typically determined using disequilibrium approaches and thus require the inhibition of potential eCA activity (Badger et al. 1994). If eCA activity would indeed be involved in minimizing the CO2 efflux, this approach may overestimate leakage for S. trochoidea, while estimates in A. tamarense, which lacks eCA activity, would not be biased by the approach. In any case, although eCA presumably contributes to the CCM, its role and correlation with high HCO3− uptake remains puzzling and requires further investigations.
CCMs and trade-offs within
With respect to net C fixation, both S. trochoidea and A. tamarense displayed half-saturation concentrations (K1/2) of <6 µmol CO2 l−1 at all applied CO2 levels (Table3). These results were consistent with previously published K1/2 values of other dinoflagellates (Rost et al. 2006, Ratti and Giordano 2007) and fall in the same range as those measured for temperate diatoms (Burkhardt et al. 2001, Trimborn et al. 2008, 2009, Yang and Gao 2012), which are known to feature very effective CCMs (Reinfelder 2011 for review). Interestingly, the Km value of the type II RubisCO employed in dinoflagellates (80–250 µmol CO2 l−1) is much higher than the Km of type I in diatoms (31–41 µmol CO2 l−1; Badger et al. 1998). In other words, the CCM in these dinoflagellates increased not only their CO2 affinities by more than one order of magnitude relative to their RubisCO kinetics, but also demonstrates that the activity of the CCM in dinoflagellates must be up to sixfold higher than that of diatoms. Additionally, dinoflagellate cells are typically bigger than those of diatoms, which automatically reduces the surface to volume ratio and hence the specific reaction diffusion-supply rate of CO2 to the cell surface (Reinfelder 2011). The correspondingly higher energy expenditure for running their CCM could thus, to a large degree, explain why dinoflagellates grow generally much slower than diatoms and thrive under different environmental conditions (Smayda 1997).
Next to the K1/2 value, also the maximum rate (Vmax) plays an important role in determining the competitive success of a species (Healey 1980). Interestingly, our data indicate a trade-off between Vmax and K1/2 values between both species. Scrippsiella trochoidea displayed relatively high Vmax and high K1/2 values, while A. tamarense showed the inverse pattern, i.e. relatively low Vmax and low K1/2 (Fig. 3). The observed trade-off within the kinetic properties of Ci acquisition is also present between the different CO2 treatments, especially for S. trochoidea showing a relative decrease in affinities with increasing maximum rates. This correlation may reflect fundamental characteristics of nutrient uptake in microalgae (Raven 1980, Aksnes and Egges 1991, Lichtman et al. 2007). Given the limited area of the cell's surface available for nutrient uptake, the number of transporters with small active area per transporter (leading to higher Vmax and higher K1/2) ‘compete’ with the number of uptake sites with relatively large active areas (leading to lower Vmax and lower K1/2). The fact that cells do not come up with transporters being characterized by high Vmax as well as low K1/2 is probably dictated by biochemical constraints, i.e. transporters can be faster only at the expense of lower affinities or vice versa (Fersht 1974). Our findings on the trade-off between Vmax and K1/2 in Ci acquisition are in line with previously observed characteristics on N acquisition in the major eukaryotic phytoplankton groups (Lichtman et al. 2007) as well as different strains of two N2 fixing cyanobacteria species (Hutchins et al. 2013). However, whether this trade-off in Ci acquisition is a general feature holding true also for other species and strains (Brading et al. 2013 shows Vmax and K1/2 values of two Symbiodinium strains being similar to S. trochoidea) and even taxa needs to be further investigated.
Figure 3.
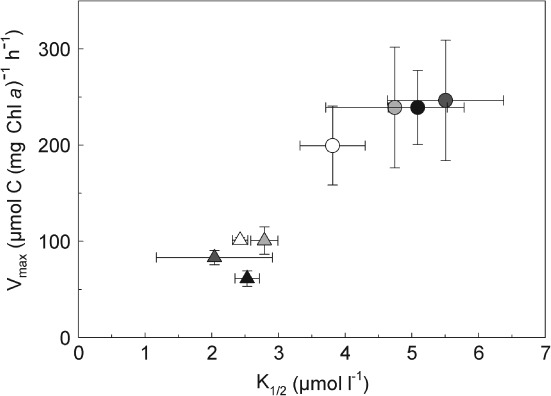
Vmax vs K1/2 of photosynthetic carbon fixation of Scrippsiella trochoidea (circles), Alexandrium tamarense (triangles) acclimated to different CO2 concentrations. Color of symbols indicates CO2 treatments from low (white) to high (black). Symbols represent mean ± sd (n = 3).
Ecological implications
To compensate potential limitations in carboxylation reaction of RubisCO, S. trochoidea and A. tamarense operate effective CCMs, allowing both species to grow unaltered over the applied range of pCO2. More specifically, both species substantially increased their overall affinities for photosynthesis, relative to what would be predicted by RubisCO, and were also able to use HCO3− as Ci source. However, the high levels of Ci accumulation required for the low affine RubisCO, the predominant HCO3− uptake, as well as the high CO2 leakage cause Ci acquisition to be very costly, which may have profound ecological consequences. For instance, it could partly explain why dinoflagellates display generally lower growth rates compared to other major groups of marine phytoplankton, which employ a more affine type I RubisCO (Smayda 1997). Reasons why dinoflagellates yet thrive well in many environments can partly be attributed to their mixotrophic behavior (Jeong et al. 2005), the potential of some species to produce allelopathic compounds (Cembella 2003) and the ability to migrate within the water column to circumvent nutrient and light limitation (MacIntyre et al. 1997). Active swimming may as well lower diffusion limitation (Pahlow et al. 1997), in particular for nutrients like nitrate or trace elements, but it could also enhance the CO2 supply to the cell surface and thereby possibly reduce the costs of CCMs in dinoflagellates.
The observed trade-off between maximum uptake rates and affinities for CO2 may also play a role in optimizing the competitive success of both species at different CO2 levels. More specifically, having a higher Vmax and higher growth rate, S. trochoidea exhibits the ‘velocity’ strategy (Sommer 1984), which will be favored under high and dynamic Ci availabilities. With a lower K1/2, on the other hand, A. tamarense exhibits an ‘affinity’ strategy (Sommer 1984) that will have a competitive advantage under low Ci concentrations (Fig. 4, Table2). During phytoplankton blooms, carbonate chemistry may substantially change and drift toward high pH and low CO2 concentrations (Hansen 2002). As a consequence, species with a low K1/2 for CO2, such as A. tamarense, may be favored. At the same time, however, carbonate chemistry may also exhibit strong daily fluctuations as results of day-time photosynthesis and night-time respiration. Under these conditions, species with a high Vmax and growth rate, like S. trochoidea, are likely to be favored. On top of that, the high preference of S. trochoidea for HCO3− may further support its growth during blooms. It thus seems that A. tamarense and S. trochoidea exhibit different strategies allowing them to cope with dense bloom conditions. Such differences in competitive strategies, induced by physiological characteristics, may furthermore allow coexistence of multiple species.
Species being able to regulate their CCM in response toward high pCO2 and low pH conditions will have advantages in a future ocean. Even though both tested species were regulating their CCM, S. trochoidea showed strongest changes in response to OA. Modes of CCMs and thus CO2 sensitivities in growth and biomass production may, however, change strongly under resource limitation, i.e. nutrient depleted or low light conditions, and therefore alter the outcome of competition under OA. For bloom-forming species like S. trochoidea and A. tamarense, which tend to flourish late in the succession, investigations on the interactive effects of nutrient limitation and OA as well as dynamic changes therein are crucial to improve our understanding of the response of this important group of phytoplankton in a future, high CO2 world.
Acknowledgments
Grant support was provided by European Community's Seventh Framework Programme (FP7/2007-2013)/ERC No. 205150, EPOCA No. 211384 and BIOACID programme, financed by the German Ministry of Education and Research. We thank Karin Zonneveld (University of Bremen, Germany) for providing Scrippsiella trochoidea strain 267 and Urban Tillmann (Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, Germany) for providing Alexandrium tamarense strain Alex5. We thank Klaus-Uwe Richter, Ulrike Richter and Yvette Bublitz for assistance during the work and Sebastian Rokitta for having a critical view on the manuscript.
Abbreviations
- CA
carbonic anhydrase
- CCM
carbon concentrating mechanism
- Chl a
chlorophyll a
- Ci
inorganic carbon
- CO32-
carbonate ion
- DBS
dextran-bound sulphonamide
- DIC
dissolved inorganic carbon
- eCA
extracellular carbonic anhydrase
- HCO3
bicarbonate
- HEPES
4-(2-hydroxylethyl)-1-piperazine-ethanesulfonic acid
- K1/2
half-saturation concentration
- MIMS
membrane-inlet mass spectrometry
- OA
ocean acidification
- pCO2
atmospheric CO2 partial pressure
- CO2
partial pressure
- PFD
photon flux density
- PIC
particulate inorganic carbon
- POC
particulate organic carbon
- PON
particulate organic nitrogen
- PST
paralytic shellfish poisoning toxins
- RubisCO
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- TA
total alkalinity
References
- Aksnes DL, Egge JK. A theoretical model for nutrient uptake in phytoplankton. Mar Ecol Prog Ser. 1991;70:65–72. [Google Scholar]
- Amthor JS. Respiration in a future, higher-CO2 world. Plant Cell Environ. 1991;14:13–20. [Google Scholar]
- Badger MR, Price GD. Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989;89:51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Palmqvist K, Jian-Wei Y. Measurement of CO2 and HCO3– fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant. 1994;90:529–536. [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot. 1998;76:1052–1071. [Google Scholar]
- Bates HA, Kostriken R, Rapoport H. The occurrence of saxitoxin and other toxins in various dinoflagellates. Toxicon. 1978;16:595–601. doi: 10.1016/0041-0101(78)90187-3. [DOI] [PubMed] [Google Scholar]
- Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP, Suggett DJ. Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae) Limnol Oceanogr. 2011;56:927–938. [Google Scholar]
- Brading P, Warner ME, Smith DJ, Suggett DJ. Contrasting modes of inorganic carbon acquisition amongst Symbiodinium (Dinophyceae) phylotypes. New Phytol. 2013;200:432–442. doi: 10.1111/nph.12379. [DOI] [PubMed] [Google Scholar]
- Burkhardt S, Zondervan I, Riebesell U. Effect of CO2 concentration on C:N:P ratio in marine phytoplankton: a species comparison. Limnol Oceanogr. 1999;44:683–690. [Google Scholar]
- Burkhardt S, Amoroso S, Riebesell U, Sültemeyer D. CO2 and HCO3– uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr. 2001;46:1378–1391. [Google Scholar]
- Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Carreto JI, Carignan MO, Montoya NG. Comparative studies on mycosporine-like amino acids, paralytic shellfish toxins and pigment profiles of the toxic dinoflagellates Alexandrium tamarenseA. catenella and A. minutum. Mar Ecol Prog Ser. 2001;223:49–60. [Google Scholar]
- Cembella AD. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia. 2003;42:420–447. [Google Scholar]
- Colman B, Huertas IE, Bhatti S, Dason JS. The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol. 2002;29:261–270. doi: 10.1071/PP01184. [DOI] [PubMed] [Google Scholar]
- Dason JS, Huertas IE, Colman B. Source of inorganic carbon for photosynthesis in two marine dinoflagellates. J Phycol. 2004;40:229–434. [Google Scholar]
- Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Ann Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Egleston ES, Sabine CL, Morel FMM. Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Global Biogeochem Cycles. 2010;24:GB1002. [Google Scholar]
- Elzenga JTM, Prins HBA, Stefels J. The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Prymnesiophyceae): a comparison with other marine algae using the isotope disequilibrium technique. Limnol Oceanogr. 2000;45:372–380. [Google Scholar]
- Fabry JV, Seibel AB, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
- Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- Fersht AR. Catalysis, binding and enzyme-substrate complementarity. Proc R Soc Lond B Biol Sci. 1974;187:397–407. doi: 10.1098/rspb.1974.0084. [DOI] [PubMed] [Google Scholar]
- Fistarol GO, Legrand C, Rengefors K, Granéli E. Temporary cyst formation in phytoplankton: a response to allelopathic competitors? Environ Microbiol. 2004;6:791–798. doi: 10.1111/j.1462-2920.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- Fu FX, Warner ME, Zhang Y, Feng Y, Hutchins DA. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorcoccus (Cyanobacteria) J Phycol. 2007;43:485–496. [Google Scholar]
- Fu FX, Zhang Y, Warner ME, Feng Y, Sun J, Hutchins DA. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae. 2008;7:76–90. [Google Scholar]
- Fu FX, Place AR, Garcia NS, Hutchins DA. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat Microb Ecol. 2010;59:55–56. [Google Scholar]
- Gao K, Xu J, Gao G, Li Y, Hutchins DA, Huang B, Wang L, Zheng Y, Jin P, Cai X, Häder D-P, Li W, Xu K, Liu N, Riebesell U. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat Clim Change. 2012;2:519–523. [Google Scholar]
- Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Ryther JH. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea Cleve. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Haardt H, Maske H. Specific in vivo absorption coefficient of chlorophyll a at 675 nm. Limnol Oceanogr. 1987;32:608–619. [Google Scholar]
- Hansen PJ. Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat Microb Ecol. 2002;28:279–288. [Google Scholar]
- Healey FP. Slope of the Monod equation as an indicator of advantage in nutrient competition. Microb Ecol. 1980;5:281–286. doi: 10.1007/BF02020335. [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Meile C, Chen S. Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role. Plant Physiol. 2013;162:1142–1152. doi: 10.1104/pp.113.217737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shi Y, Cong W. Improvement in growth and toxin production of Alexandrium tamarense by two-step culture method. J Appl Phycol. 2006;18:119–126. [Google Scholar]
- Hutchins DA, Fei-Xue F, Webb EA, Walworth N, Tagliabue A. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nat Geosci. 2013;6:790–795. [Google Scholar]
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge; New York: Cambridge University Press; 2007. [Google Scholar]
- Jeong HJ, Yoo YD, Park JY, Song JY, Kim ST, Lee SH, Kim KY, Yih WH. Feeding by phototrophic red-tide dinoflagellates: fine species newly revealed and six species previously known to be mixotrophic. Aquat Microb Ecol. 2005;40:133–150. [Google Scholar]
- Kranz SA, Sültemeyer D, Richter K-U, Rost B. Carbon acquisition by Trichodesmium: the effect of pCO2 and diurnal changes. Limnol Oceanogr. 2009;54:548–559. [Google Scholar]
- Lapointe M, MacKenzie TBD, Morse D. An external δ-carbonic anhydrase in a free-living marine dinoflagellate may circumvent diffusion-limited carbon acquisition. Plant Physiol. 2008;147:1427–1436. doi: 10.1104/pp.108.117077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SCY, Maekawa M, Taguchi S. Carbon and nitrogen acquisition by the toxic dinoflagellate Alexandrium tamarense in response to different nitrogen sources and supply modes. Harmful Algae. 2010;9:48–58. [Google Scholar]
- Li G, Campbell DA. Rising CO2 interacts with growth light and growth rate to alter photosystem ii photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS ONE. 2013;8:e55562. doi: 10.1371/journal.pone.0055562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman E, Klausmeier CA, Schofield OM, Falkowski PG. The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol Lett. 2007;10:1170–1181. doi: 10.1111/j.1461-0248.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre JG, Cullen JJ, Cembella AD. Vertical migration, nutrition and toxicity in the dinoflagellate Alexandrium tamarense. Mar Ecol Prog Ser. 1997;148:201–216. [Google Scholar]
- Martin CL, Tortell PD. Bicarbonate transport and extracellular carbonic anhydrase in marine diatoms. Physiol Plant. 2008;133:106–116. doi: 10.1111/j.1399-3054.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- McCollin T, Lichtman D, Bresnan E, Berx B. A study of phytoplankton communities along a hydrographic transect on the north east coast of Scotland. 2011. . Marine Scotland Science Report 04/11.
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- Morse D, Salois P, Markovic P, Hastings JW. A nuclear-encoded form II RuBisCO in dinoflagellates. Science. 1995;268:1622–1624. doi: 10.1126/science.7777861. [DOI] [PubMed] [Google Scholar]
- Pahlow M, Riebesell U, Wolf-Gladrow DA. Impact of cell shape and chain formation on nutrient acquisition by marine diatoms. Limnol Oceanogr. 1997;42:1660–1672. [Google Scholar]
- Pierrot DE, Lewis E, Wallace DWR. Program Developed for CO2 System Calculations. 2006. . Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory. Available at: http://cdiac.ornl.gov/oceans/co2rprt.html.
- Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Ratti S, Giordano M. CO2-concentrating mechanisms of the potentially toxic dinoflagellate Protoceratium reticulatum (Dinophyceae, Gonyaulacales) J Phycol. 2007;43:693–701. [Google Scholar]
- Raven JA. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- Raven JA, Johnston AM. Mechanisms of inorganic-carbon aquisition in marine phytoplankton and their implications for the use of other resources. Limnol Oceanogr. 1991;36:1701–1714. [Google Scholar]
- Reinfelder JR. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Ann Rev Mar Sci. 2011;3:291–315. doi: 10.1146/annurev-marine-120709-142720. [DOI] [PubMed] [Google Scholar]
- Rokitta SD, Rost B. Effects of CO2 and their modulation by light in the life-cycle stages of the coccolithophore Emiliania huxleyi. Limnol Oceanogr. 2012;57:607–618. [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D. Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr. 2003;48:55–67. [Google Scholar]
- Rost B, Richter K-U, Riebesell U, Hansen PJ. Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ. 2006;29:810–822. doi: 10.1111/j.1365-3040.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- Rost B, Kranz S, Richter K-U, Tortell P. Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnol Oceanogr Methods. 2007;5:328–337. [Google Scholar]
- Rost B, Zondervan I, Wolf-Gladrow DA. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser. 2008;373:227–237. [Google Scholar]
- Silverman DN. Carbonic anhydrase. Oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- Smayda TJ. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr. 1997;42:1137–1153. [Google Scholar]
- Sommer U. The paradox of the plankton: fluctuations of phosphorus availability maintain diversity of phytoplankton in flow-through cultures. Limnol Oceanogr. 1984;29:633–636. [Google Scholar]
- Sültemeyer D. Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can J Bot. 1998;76:962–972. [Google Scholar]
- Tillmann U, Alpermann TL, da Purificação RC, Krock B, Cembella A. Intra-population clonal variability in allelochemical potency of the toxigenic dinoflagellate Alexandrium tamarense. Harmful Algae. 2009;8:759–769. [Google Scholar]
- Trimborn S, Lundholm N, Thoms S, Richter K-U, Krock B, Hansen PJ, Rost B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant. 2008;133:92–105. doi: 10.1111/j.1399-3054.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- Trimborn S, Wolf-Gladrow DA, Richter K-U, Rost B. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J Exp Mar Bio Ecol. 2009;376:26–36. [Google Scholar]
- Trimborn S, Brenneis T, Sweet E, Rost B. Sensitivity of Antarctic phytoplankton species to ocean acidification: growth, carbon acquisition, and species interaction. Limnol Oceanogr. 2013;58:997–1007. [Google Scholar]
- Van de Waal DB, John U, Ziveri P, Reichart G-J, Hoins M, Sluijs A, Rost B. Ocean acidification reduces growth and calcification in a marine dinoflagellate. PLoS ONE. 2013;8:e65987. doi: 10.1371/journal.pone.0065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Qi YZ, Yang YF. Cyst formation: an important mechanism for the termination of Scrippsiella trochoidea (Dinophyceae) bloom. J Plankton Res. 2007;29:209–218. [Google Scholar]
- Williams PJL, Robertson JE. Overall planktonic oxygen and carbon dioxide metabolisms: the problem of reconciling observations and calculations of photosynthetic quotients. J Plankton Res. 1991;13:153–169. [Google Scholar]
- Wolf-Gladrow DA, Riebesell U, Burkhardt S, Bijma J. Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus. 1999;B51:461–476. [Google Scholar]
- Yang G, Gao K. Physiological responses of the marine diatom Thalassiosira pseudonana to increased pCO2 and seawater acidity. Mar Environ Res. 2012;79:142–151. doi: 10.1016/j.marenvres.2012.06.002. [DOI] [PubMed] [Google Scholar]


