Abstract
The growth of the filamentous fungus Aspergillus niger, a common food spoilage organism, is inhibited by the weak acid preservative sorbic acid (trans-trans-2,4-hexadienoic acid). Conidia inoculated at 105/ml of medium showed a sorbic acid MIC of 4.5 mM at pH 4.0, whereas the MIC for the amount of mycelia at 24 h developed from the same spore inoculum was threefold lower. The MIC for conidia and, to a lesser extent, mycelia was shown to be dependent on the inoculum size. A. niger is capable of degrading sorbic acid, and this ability has consequences for food preservation strategies. The mechanism of action of sorbic acid was investigated using 31P nuclear magnetic resonance (NMR) spectroscopy. We show that a rapid decline in cytosolic pH (pHcyt) by more than 1 pH unit and a depression of vacuolar pH (pHvac) in A. niger occurs in the presence of sorbic acid. The pH gradient over the vacuole completely collapsed as a result of the decline in pHcyt. NMR spectra also revealed that sorbic acid (3.0 mM at pH 4.0) caused intracellular ATP pools and levels of sugar-phosphomonoesters and -phosphodiesters of A. niger mycelia to decrease dramatically, and they did not recover. The disruption of pH homeostasis by sorbic acid at concentrations below the MIC could account for the delay in spore germination and retardation of the onset of subsequent mycelial growth.
The filamentous fungus Aspergillus niger is a food spoilage organism that contaminates a variety of foods and beverages, including confectionery, dairy, and fruit juice products (12, 22, 37). A. niger is used to produce extracellular enzymes and organic acids for the food and pharmaceutical industries, and these products are generally regarded as safe (GRAS). However, some strains of this fungus have the capacity to produce the mycotoxin ochratoxin A (1, 5, 45), and this is of major concern to the food industry. Spoilage incidents occur due to the ability of the contaminating organism to overcome modern-day preservation technologies, such as low pH and water activity (aw) and high concentrations of chemical preservatives.
Naturally occurring weak organic acids, including sorbic acid (trans-trans-2,4-hexadienoic acid), benzoic acid, and acetic acid, are the most commonly used chemical preservatives of food and are GRAS, broad-spectrum antimicrobial agents (29). The antimicrobial activity of these acids in aqueous solution is pH dependent, with the maximum effect occurring at low pH, thus favoring the undissociated state of the acid (28). Because they are uncharged, undissociated acid molecules are lipophilic and will penetrate plasma membranes and thus enter cells. Theoretically, the higher-pH environment of the cell cytosol (ca. pH 7.8 in A. niger [13]) promotes the rapid dissociation of acid molecules into charged protons and anions, which cannot subsequently diffuse back across the plasma membrane. Intracellular acidification of the cell cytosol resulting from the accumulation of protons inhibits key metabolic activities involved in glycolysis (18) and hence inhibits ATP yields. A reduction in intracellular pH (pHint) and thus in the proton motive force (Δp) may also lead to reduced cellular uptake of amino acids (11).
Several attempts have been made to monitor changes pHint during weak-acid stress. Acetic acid decreases pHint in cultures of Saccharomyces cerevisiae (2). Furthermore, Krebs et al. (18) used [14C]benzoate (2 to 10 mM), coupled with mathematical modeling, to demonstrate a decrease in the intracellular pH of >1.0 unit in S. cerevisiae. In contrast, Bracey et al. (7), using a fluorescence-based probe, were unable to detect a decline in pHint when cells of S. cerevisiae were cultured with sorbic acid.
For all organisms, the maintenance of a stable intracellular pH is essential for normal cellular functions, including gene expression, protein synthesis, and enzyme activity (21). In this respect, A. niger can sustain pHcyt and pHvac at 7.6 and 6.2, respectively, when the extracellular pH (pHex) values are varied between 1.5 and 7.0 (13). The pHint homeostasis in fungal and plant cells is maintained by the removal of protons from the cytosol mediated by a P-type ATP-dependent plasma membrane H+-ATPase. This efflux pump also actively drives nutrient uptake and regulates ion homeostasis through the generation of an electrochemical potential or Δp across the plasma membrane (15, 30). In S. cerevisiae, the dissociation of weak acids in the cell cytosol activates the H+-ATPase in a response to counteract proton toxification (16, 31, 46).
Weak acids, such as sorbic acid, have been used extensively as food-preserving agents, but their mechanism of action in filamentous fungi remains unresolved. Consequently, the present investigation has studied the effects of sorbic acid on spore germination and mycelial growth in A. niger. 31P nuclear magnetic resonance (NMR) spectroscopy has been used for real-time monitoring of both cytosolic and vacuolar pH during weak acid stress in this organism.
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Sigma (Poole, United Kingdom) unless otherwise stated.
Strains and growth conditions.
Conidia of A. niger strains N402 (6) and NW131 (34) were propagated on potato dextrose agar (Oxoid Ltd., Basingstoke, United Kingdom) and complete medium (containing 1% [wt/vol] glucose, solidified with 1.5% agar) (32) slants at 28 and 30°C, respectively, before being stored at 4°C. Conidiospores were harvested with a solution containing 0.01% (wt/vol) Tween 80. Growth experiments were carried out using A. niger N402 in 250-ml Erlenmeyer flasks containing 100 ml of Aspergillus complete medium (ACM) (containing, per liter, 6 g of NaNO3 0.52 g of MgSO4 · 7H2O, 0.52 g of KCl, 1.52 g of KH2PO4, trace amounts of FeSO4 · 7H2O and ZnSO4 · 7H2O, 1.5 g of Casamino Acids [Difco, Detroit, Mich], 1.5 g of yeast extract, and 2.0 g of bacteriological peptone 2.0 g) supplemented with 0.02 μg of biotin per ml, 1.0 μg of p-aminobenzoic acid per ml, 5.0 μg of, pyridoxine per ml, 5.0 μg of thiamine per ml, 10.0 μg of nicotinic acid per ml, 10 μg of riboflavin per ml, and 2% (wt/vol) glucose at pH 4.0. The flasks were weighed before and after being autoclaved, and the weight difference was adjusted by adding sterile distilled water. The flasks were inoculated with 107 conidia (105/ml), unless otherwise stated, and incubated on an orbital shaker at 160 rpm for up to 96 h at 28°C. For mycelial inocula, the flasks were inoculated with 107 conidia and incubated as described above for 24 h before being washed with 50 ml of the same medium and then aseptically transferred to fresh ACM. All cultures were harvested through Mira cloth, washed twice with distilled water, and stored at −20°C before being freeze-dried. MICs of sorbic acid for spore inocula were determined on the basis of a lack of visible germ tubes (determined microscopically) and those for mycelial inocula were determined on the basis of biomass dry weights after 72 h.
Sorbic acid solutions.
A stock solution of sorbic acid was prepared by dissolving sorbic acid in pure ethanol to give a 10% (wt/vol) solution. The stock solution was subsequently diluted in ethanol before being adding to autoclaved growth medium. The final concentration of ethanol in each flask, apart from water controls, was always 1% (vol/vol), and the ethanol had no discernible effects on spore germination or mycelial growth in control studies. The pH of the medium following addition of the acid was adjusted, as necessary, to a value of 4.0 with NaOH.
Immobilization of conidia for NMR studies.
Immobilization of A. niger NW131 conidia in Ca2+ alginate beads (Manugel DJX; ISP Alginates, Tadworth, United Kingdom) and subsequent culturing were performed as described previously (14).
NMR perfusion conditions.
Immobilized biomass from the shake-flask cultures was washed with perfusion buffer (30°C at pH 4.0) as described previously (13) and perfused within the NMR tube for 2 h with 1 liter of the same buffer saturated with oxygen. Following a steady-state incubation period of 2 h, sorbic acid was added to the buffer reservoir. This addition did not alter the pH of the buffer. For all experiments, a 4-cm plug of immobilized biomass (with or without 12.5 ml of beads) was perfused at 15 ml/min.
31P NMR spectroscopy.
Experiments were carried out at 30°C as described previously (14). Briefly, 31P NMR spectra were recorded at 121.5 MHz on a AMX300 wide-bore spectrometer (Bruker, Bremen, Germany), using a 31P/31C probe tuned to the 31P nucleus. Spectra were collected over 20-min intervals (5,700 free induction decays) using acquisition parameters as described previously (14). Methylene diphosphonic acid (0.2 M, pH 8.9), contained in an in situ capillary, was used as an internal reference, resonating 16.92 ppm relative to 85% H3PO4 (0 ppm).
Cytoplasmic and vacuolar pH values of A. niger were determined by comparing the pH-sensitive chemical shifts of cytoplasmic and vacuolar inorganic phosphate resonance (Pcyt and Pvac, respectively), using a calibration curve for inorganic phosphate (Pi) (10), as utilized in previous work (24, 27, 38). The pH of the perfusion buffer was determined using a pH electrode.
Sorbic acid analysis.
Sorbic acid analysis on A. niger N402 culture filtrates was carried out by capillary electrophoresis on a Hewlett-Packard 3D capillary electropherograph with diode array detection and using HP Chemstations 3D software; dehydroacetic acid was the internal standard. The capillary used was an HPG1600-61332 capillary (64.5 cm by, 75 μm [internal diameter]) incorporating an extended light path. Positive control experiments with sorbic acid added to the culture medium showed a 94% recovery of the acid.
Binding/adsorption of sorbic acid.
An inoculum of 107 A. niger N402 conidia or young mycelia (∼24 h old) was inoculated into 100 ml of ACM (pH 4.0) containing 1.0 mM sorbic acid. Following a 20-min incubation period at 28°C, with shaking at 160 rpm, the concentration of sorbic acid in the culture filtrate was determined by capillary electrophoresis.
RESULTS
Effects of sorbic acid on spore germination and mycelial growth by A. niger.
The MIC of sorbic acid needed to completely inhibit spore germination (i.e., to cause a lack of germ tubes emerging from the spores) by A. niger for up to 72 h varied with the size of the inoculum (Table 1). As the inoculum size increased, there was a corresponding increase in the MIC needed to inhibit germination (Table 1). Samples from culture media were removed at regular intervals and examined microscopically for signs of germination if visual growth was not detectable. To ensure that an adequate incubation period had been allowed for the determination of the MIC of sorbic acid, the cultures were incubated for up to 7 days, but the MIC remained constant.
TABLE 1.
MIC of sorbic acid needed to completely inhibit spore germination or growth of young mycelia after 72 h of culturea
| Inoculum type | Inoculum size | MIC (mM) |
|---|---|---|
| Spore | 105/ml of medium | 4.5 |
| 104/ml of medium | 3.0 | |
| 103/ml of medium | 2.5 | |
| 102/ml of medium | 2.0 | |
| 10/ml of medium | <1.5 | |
| Mycelia | 1 × 24-h culture | 1.5 |
| 2 × 24-h culture | 2.0 | |
| 3 × 24-h culture | 2.0 |
For spore MIC determinations, different concentrations of conidia were inoculated in 100 ml of ACM (pH 4.0) containing sorbic acid. Mycelial inocula were propagated from 105 spores/ml in 100 ml of ACM (pH 4.0) grown for 24 h. Fresh ACM (pH 4.0) containing sorbic acid was inoculated with the combined biomass from one (i.e., 1 × 24-h culture), two, or three of the initial 24-h cultures. All culturing took place in 250-ml shake flasks incubated at 28°C with shaking at 160 rpm. Results presented are from independent cultures (n = 3 for each).
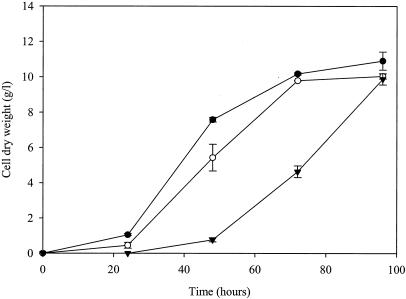
Figure 1 shows growth curves obtained using a spore inoculum (105 spores/ml of medium) when sorbic acid (1.0 or 3.0 mM) was added to the culture media. Sorbic acid delayed spore germination and significantly (P < 0.05) inhibited subsequent mycelial growth. In control cultures containing water or ethanol, spore germination occurred between 6 and 12 h, whereas in cultures containing 1.0 mM sorbic acid, spore germination occurred between 12 and 18 h, and in those containing 3.0 mM sorbic acid, spore germination was delayed for at least 24 h. Once spore germination had commenced, mean biomass yields after 24 and 48 h of culturing were inhibited by 57 and 28%, respectively, when 1.0 mM sorbic acid was present in the culture medium, compared to those for the corresponding controls (Fig. 1). When added to culture medium at 3.0 mM, sorbic acid inhibited biomass yields by 67 and 54% at 48 and 72 h, respectively, compared to the controls (Fig. 1). With 1.0 and 3.0 mM sorbic acid, the maximal biomass yields were reached by 72 and 96 h, respectively (Fig. 1). Transfer of the mycelia from the cultures containing 3.0 mM sorbic acid after 48 h of culture to fresh medium containing the same amount of the acid completely inhibited subsequent growth for up to 96 h (data not shown).
FIG. 1.
Mean cell dry weight yields of A. niger N402 in ACM (pH 4.0) (•, control) and with 1.0 mM (○) or 3.0 mM (▾) sorbic acid added to the medium. Shake flasks of 250-ml capacity, containing 100 ml of medium, were inoculated with 107 spores and incubated at 28°C with orbital shaking at 160 rpm. Vertical bars show standard errors of the mean (SEM) (n = 3) calculated from independent cultures. Results from ethanol controls were identical to those from water controls and are therefore not presented.
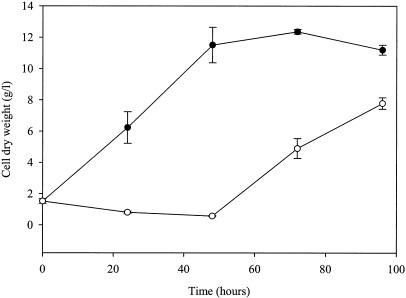
When young A. niger mycelia (24 h old, propagated from 105 spores/ml of medium) were transferred to fresh medium containing sorbic acid, the MIC needed to completely inhibit further growth was ∼66% lower than that required to inhibit spore germination (inoculum of 105 spores/ml of medium) (Table 1). The MIC increased slightly when the biomasses of two or three 24-h cultures were combined and used to inoculate medium containing sorbic acid. (Table 1). Figure 2 shows changes in cell dry weight over a 96-h culture period when young mycelia of A. niger were transferred to fresh medium containing 1.0 mM sorbic acid. Fungal growth was completely inhibited for up to 48 h following transfer, and when growth did eventually recommence, between 48 and 72 h, the biomass yields at 72 and 96 h were inhibited by 60 and 30%, respectively, compared to those of the controls.
FIG. 2.
Mean cell dry weight yields of A. niger N402 following transfer of mycelia grown for 24 h in ACM (pH 4.0) to fresh ACM (pH 4.0) (•, control) and with 1.0 mM sorbic acid (○). Shake flasks of 250-ml capacity, containing 100 ml of medium, were incubated at 28°C with orbital shaking at 160 rpm. Vertical bars show SEM (n = 3) calculated from independent cultures. Results from ethanol controls were identical to those from water controls and are therefore not presented.
Sorbic acid stability in the presence and absence of A. niger conidia and mycelia.
In the absence of A. niger cells, the concentration of sorbic acid (1.0 mM) in the medium remained constant for up to 72 h. However, when spores (105 spores/ml of medium) were cultured with sorbic acid (1.0 mM) for 24 h, the concentration of sorbic acid measured in the culture filtrate was below the minimum detection limit of ∼45 μM (Fig. 3). The concentration of sorbic acid in the medium was constant for the first 6 h of culture when conidia of A. niger were added as the inoculum, but it decreased by 27 and 95% by 12 and 18 h, respectively (Fig. 3). When a mycelial inoculum (propagated from 105 spores/ml of medium for 24 h) was used, the concentration of sorbic acid (1.0 mM) remained constant for the first 24 h of culture but the acid was undetectable at 72 h (data not shown). Analysis of capillary electrophoresis traces revealed that no obvious metabolites or degradation products of sorbic acid were present in the culture media when either a spore or a mycelial inoculum was used.
FIG. 3.
Mean sorbic acid concentrations in the culture filtrate during the growth of A. niger N402. Shake flasks of 250-ml capacity, containing 100 ml of medium (ACM, pH 4.0) supplemented with 1.0 mM sorbic acid, were inoculated with 107 spores and incubated at 28°C with orbital shaking at 160 rpm. Vertical bars show SEM (n = 3) calculated from independent cultures. Results from ethanol controls were identical to those from water controls and are therefore not presented.
Binding and adsorption of sorbic acid.
The above experiments showed that there was little if any removal of sorbic acid from the culture media by binding or adsorption of the acid to A. niger conidia or vegetative mycelia.
Sorbic acid causes intracellular acidification.
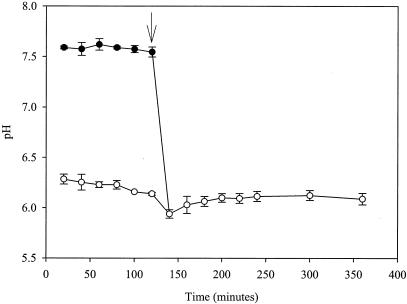
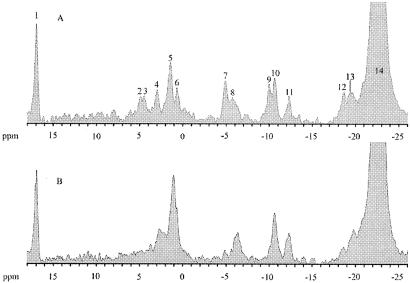
Immobilized mycelia (14) of A. niger in perfusion buffer were incubated for 2 h to reach a steady state before the addition of sorbic acid. During this time, pHcyt and pHvac were relatively constant at ca. pH 7.6 and 6.2, respectively (Fig. 4). The addition of sorbic acid (3.0 mM) resulted in a slight decrease in pHvac but caused pHcyt to rapidly decline by more than 1 pH unit within 20 min of addition (Fig. 4). This led to the collapse of the pH gradient over the vacuole (i.e., pHcyt with pHvac), making it impossible to distinguish between the Pcyt and Pvac peaks (peaks 4 and 5, respectively, Fig. 5A) which had merged to make one large phosphate resonance (Fig. 5B), corresponding to an intracellular pH (pHint) of ∼6.0 (Fig. 4). This large intracellular phosphate resonance did not return to its separate constituent Pcyt and Pvac resonances, and the pHint value remained at ∼6.0 for the rest of the experiment. Ethanol had no adverse effects on pHcyt and pHvac values, which were identical to those in water control experiments. In addition to the acidification of the cytosol and vacuole, a rapid and permanent loss of intracellular ATP (γ, α, and β isoforms [peaks 7, 9, and 12, respectively, in Fig. 5A; missing or reduced in Fig. 5B]), sugar-phosphomonoesters (peak 2, Fig. 5A), and sugar-phosphodiesters (peak 3, Fig. 5B) were also evident from the NMR spectra.
FIG. 4.
Cytosolic pH (pHcyt) (•) and vacuole pH (pHvac) (○) of A. niger NW131 in 25 mM citric-phosphate buffer (pH 4.0) at 30°C, containing 3.0 mM sorbic acid. The arrow indicates the time of addition of sorbic acid. Vertical bars show SEM (n = 2) calculated from independent cultures. Control experiments showed that the presence of ethanol in the perfusion buffer had no visible effects on pHcyt or pHvac and are therefore not presented.
FIG. 5.
31P NMR spectra of immobilized A. niger NW131 mycelium in the presence of 25 mM citrate-phosphate buffer (pH 4.0) before (A) and after (B) the addition of 3.0 mM sorbic acid. The peaks can be assigned to the following compounds: 1, inorganic phosphate standard; 2, sugar phosphomonoesters; 3, sugar phosphodiesters; 4, cytoplasmic inorganic phosphate; 5, vacuolar inorganic phosphate; 6, extracellular inorganic phosphate; 7, γ-ATP; 8, pyrophosphate; 9, α-ATP; 10, NAD(H) and uridine diphosphoglucose; 11, uridine diphosphoglucose (second peak); 12, β-ATP; 13, penultimate phosphates of polyphosphate; 14, polyphosphate.
DISCUSSION
This study represents the first systematic investigation of the mechanism of action of sorbic acid in A. niger. We show that in A. niger, sorbic acid delays spore germination and mycelial growth, causes intracellular acidification, and reduces intracellular ATP pools and levels of sugar-phosphomonoesters and -phosphodiesters. Although sorbic acid inhibited fungal growth in a concentration-dependent manner, A. niger was able to degrade the acid.
The ability of filamentous fungi to metabolize sorbic acid is well established, with early reports by Melnick et al. (23) suggesting that degradation of the acid occurs via β-oxidation, producing CO2 and H2O. Several authors have reported that some fungi can metabolize sorbic acid and thereby detoxify it. For example, certain Penicillium and yeast (notably Zygosaccharomyces rouxii and Debaryomyces hansenii) species can degrade sorbic acid to the volatile compound 1,3-pentadiene through a decarboxylation reaction (9, 17). The metabolism of sorbic acid to 4-hexanol by Mucor species has also been reported (19, 20, 42). However, the metabolic pathway(s) and implicated enzyme(s) that convert sorbic acid into its metabolite products are still elusive. Sorbic acid is thought to undergo auto-oxidative degradation in aqueous systems, forming malonaldehyde and other carbonyls (3, 4, 44). However, in the present study, in the absence of A. niger cells, the concentration of the acid remained constant for up to 72 h. When inocula of spores or vegetative mycelia were used, sorbic acid was undetectable in the culture media after 24 and 72 h, respectively. No metabolites or degradation products of sorbic acid were detected in the culture filtrate, possibly due to the production of volatile metabolites, as discussed below.
In the present study, there was no evidence to suggest that the bioavailability of sorbic acid was reduced by the possible binding or adsorption of the acid by cell walls or lipids of spores or mycelia. Steels et al. (39) similarly found no evidence that sorbic acid binds or adsorbs to yeast cells, although Stratford et al. (41) have provided proof that sulfite, another weak acid preservative, reacts with acetaldehyde produced by active metabolism in yeast.
In our study, sorbic acid was effective at delaying spore germination and reducing biomass yields of A. niger. This growth inhibition was concentration dependent, with the higher concentrations of the acid being most effective. The disappearance of sorbic acid (1.0 mM) from the culture media with time (Fig. 3) coincided with the onset of spore germination and subsequent mycelial growth (Fig. 1) and the recovery of mycelial growth in the case of a mycelial inoculum (Fig. 2).
When the size of the spore inoculum and, to a lesser extent, the mycelial inoculum was increased, the MIC correspondingly increased. A similar inoculum effect has been shown for a number of organisms exposed to weak acids (25, 39, 43). Steels et al. (39) suggested that a large inoculum size increases the phenotypic diversity of a culture, thereby enhancing the likelihood that resistant cells are present.
In this study, conidia inoculated at 105/ml of medium showed a sorbic acid MIC of 4.5 mM whereas the amount of mycelia at 24 h developed from the same spore inoculum, grown in the absence of sorbic acid, showed a threefold-lower sorbic acid MIC. The conidia appear to retain some resistance to sorbic acid during germination and, at the same time, develop a capacity to degrade sorbic acid.
At the cellular level, our studies support the classic weak acid theory that, at low pH, weak acids cause intracellular acidification following their dissociation within the cell cytosol. As mentioned above, A. niger secretes large quantities of organic acids, such as citric acid and gluconic acid, and acidifies its medium to pH values below 2.0. Despite this, the fungus maintains its intracellular pH within a very narrow range (13). The present investigation shows that sorbic acid rapidly disrupts such pH homeostasis. This is supported by the finding that pHcyt rapidly decreased by more than 1.0 pH unit to a value below that of pHvac, which also declined slightly, shortly after addition of the acid. This, in turn, completely collapsed the pH gradient across the vacuolar membrane, leading to the measurement of one large intracellular phosphate resonance (pHint).
Bracey et al. (8) showed that sorbic acid, at low pH, causes intracellular acidification in S. cerevisiae. However, the same authors subsequently used a technique that allowed direct measurement of the pHint of growing cells in vivo and were unable to detect the previously noted large reduction in pHint during sorbic acid stress (7). They proposed that sorbic acid was indeed lowering pHint but suggested that actively growing cells were able to induce an energy-dependent response to counteract this detrimental effect. This energy-dependent response was proposed to be the proton-pumping H+-ATPase, which is known to aid weak acid adaptation in yeast by actively extruding protons from the cell cytosol (16, 31, 46). The active efflux of protons from the cell is a major energy investment for the organism since the H+-ATPase can consume up to 60% of cellular ATP (35, 36). In addition to the loss of the pH gradient over the vacuole, such changes could account for the long-term acidification of the cytosol observed in the present investigation.
Interestingly, NMR spectra in our studies showed that sorbic acid also caused the rapid depletion of intracellular ATP and that these yields were not replaced for the duration of the experiment. Thus, it is possible that the activity of the H+-ATPase could cease due to a lack of substrate (ATP); this may be one reason why pHcyt was unable to recover. Przybylski and Bullerman (33) similarly reported that ATP pools were decreased during sorbic acid stress in conidia of A. parasiticus. These authors also suggested, similar to the subsequent claims of Bracey et al. (7), that the rapid decline in ATP level was linked to the active efflux of protons from the cell by a proton pump. Energy (ATP) depletion through the strong inhibitory effects on glycolytic enzymes, e.g., phosphofructokinase, has also been reported to occur during weak acid stress in S. cerevisiae (18, 26).
Acetic acid has the same pKa value as sorbic acid (4.76), and therefore both acids in theory should release the same concentration of protons on dissociation in the cell cytosol. Interestingly, the MIC of acetic acid needed to completely inhibit spore germination and mycelial growth is 20-fold higher than that of sorbic acid (our unpublished data). This suggests that the inhibitory action of sorbic acid is not due solely to intracellular acidification. Stratford and Anslow (40) also reported that MICs of sorbic acid and acetic acid in S. cerevisiae differ despite their equal pKa values.
In conclusion, our studies show that sorbic acid is an effective antifungal agent that delays spore germination and retards mycelial growth. Consistent with some previous findings, sorbic acid causes rapid and long-term acidification of the cytosol and subsequently collapses the pH gradient over the vacuole. Such growth-inhibitory properties are limited since A. niger appears to have the ability to metabolize or degrade the acid, but the precise mechanisms involved and the nature of the breakdown products are still to be determined.
Acknowledgments
This work was funded by a BBSRC CASE studentship supported by Unilever R&D, awarded to A.P.
We are grateful to Chris Tier (Unilever R&D, Sharnbrook, Bedford, United Kingdom) for assistance with capillary electrophoresis analyses.
REFERENCES
- 1.Abarca, M. L., M. R. Bragulat, G. Castella, and F. J. Cabanes. 1994. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 60:2650-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arneborg, N., L. Jespersen, and M. Jakobsen. 2000. Individual cells of Saccharomyces cerevisiae and Zygosaccharomyces bailii exhibit different short-term intracellular pH responses to acetic acid. Arch. Microbiol. 174:125-128. [DOI] [PubMed] [Google Scholar]
- 3.Arya, S. S. 1980. Stability of sorbic acid in aqueous solutions. J. Agric. Food Chem. 28:1246-1249. [Google Scholar]
- 4.Arya, S. S., and B. R. Thakur. 1988. Degradation products of sorbic acid in aqueous solutions. Food Chem. 29:41-49. [Google Scholar]
- 5.Battilani, P., A. Pietri, T. Bertuzzi, L. Languasco, P. Giorni, and Z. Kozakiewicz. 2003. Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. J. Food Prot. 66:633-636. [DOI] [PubMed] [Google Scholar]
- 6.Bos, C. J., A. J. M. Debets, K. Swart, A. Huybers, G. Kobus, and S. M. Slakhorst 1988. Genetic analysis and the construction of master strains for assignment of genes to six linkage groups in Aspergillus niger. Curr. Genet. 14:437-443. [DOI] [PubMed] [Google Scholar]
- 7.Bracey, D., C. D. Holyoak, and P. J. Coote. 1998. Comparison of the inhibitory effect of sorbic acid and amphotericin B on Saccharomyces cerevisiae: is growth inhibition dependent on reduced intracellular pH? J. Appl. Microbiol. 85:1056-1066. [DOI] [PubMed] [Google Scholar]
- 8.Bracey, D., C. D. Holyoak, G. Nebe-von Caron, and P. J. Coote. 1998. Determination of the intracellular pH (pHi) of growing cells of Saccharomyces cerevisiae: the effect of reduced-expression of the membrane H+-ATPase. J. Microbiol. Methods 31:113-125. [Google Scholar]
- 9.Casas, E., M. J. Valderrama, and J. M. Peinado. 1999. Sorbate detoxification by spoilage yeasts isolated from marzipan products. Food Technol. Biotechnol. 37:87-91. [Google Scholar]
- 10.den Hollander, J. A., K. Ugurbil, T. R. Brown, and R. G. Shulman. 1981. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry 20:5871-5880. [DOI] [PubMed] [Google Scholar]
- 11.Freese, E., C. W. Sheu, and E. Galliers. 1973. Function of lipophilic acids as antimicrobial food additives. Nature 241:321-325. [DOI] [PubMed] [Google Scholar]
- 12.Fustier, P., A. Lafond, C. P. Champagne, and F. Lamarche. 1998. Effect of inoculation techniques and relative humidity on the growth of molds on the surfaces of yellow layer cakes. Appl. Environ. Microbiol. 64:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse, S. J. A., G. J. G. Ruijter, C. Dijkema, and J. Visser. 2002. Intracellular pH homeostasis in the filamentous fungus Aspergillus niger. Eur. J. Biochem. 269:3485-3494. [DOI] [PubMed] [Google Scholar]
- 14.Hesse, S. J. A., G. J. G. Ruijter, C. Dijkema, and J. Visser. 2000. Measurement of intracellular (compartmental) pH by 31P NMR in Aspergillus niger. J. Biotechnol. 77:5-15. [DOI] [PubMed] [Google Scholar]
- 15.Holyoak, C. D., D. Bracey, P. W. Piper, K. Kuchler, and P. J. Coote. 1999. The Saccharomyces cerevisiae weak-acid-inducible ABC transporter pdr12 transports fluorescein and preservative anions from the cytosol by an energy- dependent mechanism. J. Bacteriol. 181:4644-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holyoak, C. D., M. Stratford, Z. McMullin, M. B. Cole, K. Crimmins, A. J. P. Brown, and P. J. Coote. 1996. Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak acid preservative sorbic acid. Appl. Environ. Microbiol. 62:3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinderlerer, J. L., and P. V. Hatton. 1990. Fungal metabolites of sorbic acid. Food Addit. Contam. 7:657-669. [DOI] [PubMed] [Google Scholar]
- 18.Krebs, H. A., D. Wiggins, M. Stubbs, A. Sols, and F. Bedoya. 1983. Studies on the mechanism of the anti-fungal action of benzoate. Biochem. J. 214:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurogochi, S., S. Tahara, and J. Mizutani. 1974. Fungal metabolites of sorbic acid. Agric. Biol. Chem. 38:893-895. [Google Scholar]
- 20.Kurogochi, S., S. Tahara, and J. Mizutani. 1975. Fungal reduction of C6 α,β-unsaturated carboxylic acids. Agric. Biol. Chem. 39:825-831. [Google Scholar]
- 21.Madshus, I. H. 1988. Regulation of intracellular pH in eukaryotic cells. Biochem. J. 250:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin, S., M. E. Guynot, P. Neira, M. Bernado, V. Sanchis, and A. J. Ramos. 2002. Risk assessment of the use of sub-optimal levels of weak acid preservatives in the control of mould growth on bakery products. Int. J. Food Microbiol. 79:203-211. [DOI] [PubMed] [Google Scholar]
- 23.Melnick, D., F. H. Luckmann, and C. M. Gooding. 1954. Sorbic acid as a fungistatic agent for foods. VI. Metabolic degradation of sorbic acid in cheese by molds and the mechanism of mold inhibition. Food Res. 19:44-58. [Google Scholar]
- 24.Nicolay, K., W. A. Scheffers, P. M. Bruinenberg, and R. Kaptein. 1982. Phosphorus-31 nuclear magnetic resonance studies of intracellular pH, phosphate compartmentation and phosphate transport in yeasts. Arch. Microbiol. 133:83-89. [Google Scholar]
- 25.Nielsen, P. V. 1991. Preservative and temperature effect on growth of three varieties of the heat-resistant mould, Neosartorya fischeri, as measured by an impedimetric method. J. Food Sci. 56:1735-1740. [Google Scholar]
- 26.Pearce, A. K., I. R. Booth, and A. J. P. Brown. 2001. Genetic manipulation of 6-phosphofructo-1-kinase and fructose 2,6-bisphosphate levels affects the extent to which benzoic acid inhibits the growth of Saccharomyces cerevisiae. Microbiology 147:403-410. [DOI] [PubMed] [Google Scholar]
- 27.Pilatus, U., and D. Techel. 1991. 31P-NMR-studies on intracellular pH and metabolite concentrations in relation to the circadian rhythm, temperature and nutrition in Neurospora crassa. Biochim. Biophys. Acta 1091:349-355. [DOI] [PubMed] [Google Scholar]
- 28.Piper, P., C. Ortiz Calderon, K. Hatzixanthis, and M. Mollapour. 2001. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635-2642. [DOI] [PubMed] [Google Scholar]
- 29.Piper, P., Y. Mahe, S. Thompson, R. Pandjaitan, C. Holyoak, R. Egner, M. Muhlbauer, P. Coote, and K. Kuchler. 1998. The Pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 17:4257-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piper, P. W. 1999. Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radic. Biol. Med. 27:1219-1227. [DOI] [PubMed] [Google Scholar]
- 31.Piper, P. W., C. Ortiz Calderon, C. Holyoak, P. Coote, and M. Cole. 1997. Hsp30, the integral plasma membrane heat shock protein of Saccharomyces cerevisiae, is a stress-inducible regulator of plasma membrane H+-ATPase. Cell Stress Chaperones 2:12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontecorvo, G., J. A. Roper, L. J. Hemmons, K. J. MacDonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 33.Przybylski, K. S., and L. B. Bullerman. 1980. Influences of sorbic acid on viability and ATP content of conidia of Aspergillus parasiticus. J. Food Sci. 45:375-376. [Google Scholar]
- 34.Ruijter, G. J. G., H. Panneman, and J. Visser. 1997. Overexpression of phosphofructokinase and pyruvate kinase in citric acid-producing Aspergillus niger. Biochim. Biophys. Acta 1334:317-326. [DOI] [PubMed] [Google Scholar]
- 35.Serrano, R. 1988. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 157:533-544. [DOI] [PubMed] [Google Scholar]
- 36.Serrano, R., C. Montesinos, M. Roldan, G. Garrido, C. Ferguson, K. Leonard, B. C. Monk, D. S. Perlin, and E. W. Weiler. 1991. Domains of yeast plasma membrane and ATPase-associated glycoprotein. Biochim. Biophys. Acta 1062:157-164. [DOI] [PubMed] [Google Scholar]
- 37.Shearer, A. E. H., A. S. Mazzotta, R. Chuyate, and D. E. Gombas. 2002. Heat resistance of juice spoilage microorganisms. J. Food Prot. 65:1271-1275. [DOI] [PubMed] [Google Scholar]
- 38.Shipanova, I., Y. Bartoshevich, L. Sibeldina, P. Zaslavskaya, and A. Michtchenko. 1995. Relationship between intracellular pH and antibiotic biosynthesis in fusidium coccineum. Appl. Microbiol. Biotechnol. 43:514-517. [Google Scholar]
- 39.Steels, H., S. A. James, I. N. Roberts, and M. Stratford. 2000. Sorbic acid resistance: the inoculum effect. Yeast 16:1173-1183. [DOI] [PubMed] [Google Scholar]
- 40.Stratford, M., and P. A. Anslow. 1998. Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett. Appl. Microbiol. 27:203-206. [DOI] [PubMed] [Google Scholar]
- 41.Stratford, M., P. Morgan, and A. H. Rose. 1987. Sulfur dioxide resistance in Saccharomyces cerevisiae and Saccharomycodes ludwigii. J. Gen. Microbiol. 133:2173-2179. [Google Scholar]
- 42.Tahara, S., S. Kurogochi, M. Kudo, and J. Mizutani. 1977. Fungal metabolism of sorbic acid. Agric. Biol. Chem. 41:1635-1642. [Google Scholar]
- 43.Terrell, F. R., J. R. Morris, M. G. Johnson, E. E. Gbur, and D. J. Makus. 1993. Yeast inhibition in grape juice containing sulphur dioxide, sorbic acid and dimethyldicarbonate. J. Food Sci. 58:1132-1134. [Google Scholar]
- 44.Thakur, B. R., R. K. Singh, and S. S. Arya 1994. Chemistry of sorbates—a basic perspective. Food Rev. Int. 10:71-91. [Google Scholar]
- 45.Urbano, G. R., M. H. Taniwaki, M. F. D. Leitao, and M. C. Vicentini. 2001. Occurrence of ochratoxin A-producing fungi in raw Brazilian coffee. J. Food Prot. 64:1226-1230. [DOI] [PubMed] [Google Scholar]
- 46.Viegas, C. A., and I. Sacorreia 1991. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J. Gen. Microbiol. 137:645-651. [DOI] [PubMed] [Google Scholar]