Abstract
More precise estimation of the atherogenic lipid parameters could improve identification of residual risk beyond what is possible using traditional lipid risk factors. The aim of the present study was to explore the association between advanced analysis of lipoprotein subfractions and the prevalence of coronary artery calcium. Consecutive participants at intermediate cardiovascular risk who were undergoing computed tomographic assessment of coronary calcium (calcium score) were included. Using a validated ultracentrifugation method (the vertical autoprofile II test), cholesterol in eluting lipoprotein subfractions [i.e., low- (LDL), very-low-, intermediate-, and high-density lipoprotein subclasses, lipoprotein (a), and predominant LDL distribution] was directly quantified. A total of 410 patients were included (29% women, mean age 57 years), of whom 297 (72.4%) had coronary artery calcium. LDL pattern B (predominance of small dense particles) emerged as an independent predictor of coronary calcium after adjustment for traditional risk factors (odds ratio 4.46, 95% confidence interval 1.19 to 16.7). However, after additional stratification for dyslipidemia, as defined by conventional lipid profiling, a statistically significant prediction was only retained for high-density lipoprotein subfraction 2 (odds ratio 3.45, 95% confidence interval 2.03 to 50.1) and “real” LDL (odds ratio 6.10, 95% confidence interval 1.26 to 23.41) in the normolipidemia group and for lipoprotein (a) (odds ratio 7.81, 95% confidence interval 1.41 to 43.5) in the dyslipidemic group. In conclusion, advanced assessment of the lipoprotein subfractions [i.e., LDL pattern B, high-density lipoprotein subfraction 2, “real” LDL, and lipoprotein (a)] using the vertical autoprofile II test provided additional information to that of conventional risk factors on the prevalence of coronary artery calcium in patients at intermediate cardiovascular risk.
Evidence from large epidemiologic studies1–3 and clinical trials4,5 has established the importance of traditional lipid risk factors and their management. However, most cardiovascular events have not been prevented.6 More precise estimation of atherogenic lipid parameters could improve the identification of residual risk beyond that of traditional lipid risk factors.1,7 A variety of assays subfractionate lipoprotein particles according to size, density, or charge.8 Density gradient ultracentrifugation, such as in the vertical autoprofile II test (Atherotech, Birmingham, Alabama), measures the relative distribution of cholesterol within various lipoprotein subfractions, quantifying the cholesterol content of low-density (LDL), very-low-density, intermediate-density (IDL), and high-density (HDL) lipoprotein subclasses, and lipoprotein (a) [Lp(a)], real LDL [(LDL-R) calculated by subtracting Lp(a) and IDL from LDL]. Density gradient ultracentrifugation also determines the predominant LDL size distribution (e.g., A, large buoyant LDL particles; A/B, intermediate pattern; and B, small particles).9 The aim of the present study was to explore the association between these advanced lipid parameters and the prevalence of coronary artery calcium (CAC)—a widely accepted surrogate marker of prevalent coronary atherosclerosis and a very strong risk marker for incident cardiovascular events, including myocardial infarction, stroke, and cardiovascular death.10–13
Methods
Consecutive patient undergoing cardiovascular risk assessment at a preventive cardiology outpatient program of the Harbor-UCLA Medical Center (Los Angeles, California) were considered for inclusion. Participants without a history, or evidence, of cardiovascular disease at intermediate cardiovascular risk (10% to 20% 10-year risk of a major cardiovascular disease event) according to the Framingham risk score who were undergoing computed tomographic assessment of CAC score were included.11
Age and ethnicity were self-reported. Cardiovascular risk factors were measured or collected. These included hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or a history of physician-diagnosed hypertension and taking antihypertensive medication), diabetes (fasting glucose ≥126 mg/dl or taking antidiabetic medication), a family history of premature heart disease, tobacco consumption, and the lipid profile. The lipid profile was defined on the basis of high LDL cholesterol, low HDL cholesterol, and/or high triglyceride (TG) levels.7 Normolipidemia was defined as HDL >40 mg/dl in women and >50 mg/dl in men, LDL cholesterol <160 mg/dl, and TG <150 mg/dl. Dyslipidemia included the following categories: hypercholesterolemia—LDL cholesterol ≥160 mg/dl and TG <150 mg/dl; hypertriglyceridemia—HDL >40 mg/dl in women and >50 mg/dl in men, LDL cholesterol <160 mg/dl, and TG ≥150 mg/dl; isolated low HDL—HDL ≤40 mg/dl in women and ≤50 mg/dl in men, LDL cholesterol <160 mg/dl, and TG <150 mg/dl; metabolic dyslipidemia—HDL ≤40 mg/dl in women and ≤50 mg/dl in men, LDL cholesterol <160 mg/dl, and TG ≥150 mg/dl; and combined dyslipidemia—LDL cholesterol ≥160 mg/dl and TG ≥150 mg/dl. For analysis purposes, the study population was stratified into those with and without dyslipidemia.
CAC was assessed using electron beam tomographic imaging (GE, Milwaukee, Wisconsin). The typical acquisition parameters were used in a 40-slice study (3-mm slice thickness, 3-mm table increment, 100-ms acquisition times), as previously described.14,15 The CAC score of each lesion was calculated by multiplying the lesion area by a density factor derived from the maximum Hounsfield units within this area, as described by Agatston et al.14 The density factor was assigned as follows: 1 for lesions whose maximum density was 130 to 199 HU, 2 for lesions 200 to 299 HU, 3 for lesions 300 to 399 HU, and 4 for lesions >400 HU. A total CAC score was determined by summing the individual lesion scores at each anatomic site.15 The presence of CAC was defined by a score of >0 Agatston units.
The lipid and lipoprotein measurements were performed using fasting (12-hour) ethylenediaminetetraacetic acid plasma. The cholesterol associated with individual lipoprotein subfractions was quantified using the vertical autoprofile II method, a validated ultracentrifugation method that involves direct cholesterol measurements in eluting fractions, including very-low-density lipoprotein, IDL, 4 LDL subfractions (from the most buoyant LDL1 to the most dense LDL4), 3 LDL density/size subtype patterns (B, predominant, small, dense LDL particles; A, predominant, large, buoyant LDL particles, and A/B, an intermediate pattern), Lp(a), and HDL subfractions HDL2 and HDL3.9,16,17 A value for LDL, designated “real LDL” (LDL-R), was calculated from all fractions containing true LDL particles (LDL1–4) and excluded the contributions of IDL and Lp(a) included in the standard LDL measurements. All vertical autoprofile II lipid measurements were performed by Atherotech (Birmingham, Alabama).
Normally distributed continuous variables are described as the mean ± SD and were compared using the t test. Non-normally distributed continuous variables are described as the median and interquartile range and were compared using the Mann-Whitney U test. Categorical variables are described as numbers and/or percentages and were compared using the chi-square test. Multiple regression analysis was performed to determine the independent predictors for the presence of CAC (score >0 Agatston units) using the significant univariate predictors (i.e., age, gender, diabetes, hypertension, family history, smoking status, body mass index, and lipid parameters) as covariates. For the multivariate analysis, advanced lipid parameters were dichotomized according to cutoffs proposed by risk-identifying cohort studies and the current guidelines: LDL-R > 100 mg/dl, HDL2 >10 mg/dl in men and >15 mg/dl in women, HDL3 >20 mg/dl in men and >25 mg/dl in women, Lp(a) >50 mg/dl, very-low-density lipoprotein >30 mg/dl, and IDL >20 mg/dl.9,16–19 A p value of ≤0.05 was considered statistically significant. Statistical analyses were performed using PASW, version 18.0.0, for Windows.
Results
Of 508 eligible patients who had undergone cardiovascular screening, 410 had complete results from the computed tomographic calcium scan and demographic characteristics available and were included in the final analysis. The participants were 57 years old on average, almost 1/3 were women (28.8%), almost 60% were white (Table 1). Of the 410 participants, 198 (48%) had dyslipidemia, of whom, 162 (81.8%) had CAC compared to 135 participants (63.7%) with CAC in the normolipidemia group.
Table 1.
Baseline demographic and clinical characteristics, conventional lipid profile, and vertical autoprofile–derived lipid subfractions
| Variable | All (n = 410) | CAC
|
||
|---|---|---|---|---|
| Absent (n = 113) | Present (n = 297) | p Value | ||
| Women | 118 (28.8%) | 46 (40.7%) | 60 (20.2%) | <0.001 |
| Age (yrs) | 57.3 ± 11 | 50.3 ± 10 | 59.0 ± 10 | |
| White | 239 (58.3%) | 50 (44.3%) | 194 (65.3%) | <0.001 |
| Hypertension | 135 (32.9%) | 25 (22.1%) | 122 (41.1%) | 0.002 |
| Diabetes mellitus | 45 (11.0%) | 6 (5.3%) | 45 (15.2%) | 0.001 |
| Current smoking | 61 (14.9%) | 11 (9.7) | 54 (18.2%) | 0.103 |
| Body mass index (kg/m2) | 28 ± 4 | 27 ± 8 | 29 ± 6 | 0.092 |
| Total cholesterol (mg/dl) | 197 ± 45 | 214 ± 57 | 191 ± 40 | 0.216 |
| Low-density lipoprotein (mg/dl) | 128 ± 39 | 138 ± 39 | 124 ± 36 | 0.061 |
| High-density lipoprotein (mg/dl) | 0.016 | |||
| Median | 46 | 48 | 44 | |
| Interquartile range | 39–58 | 40–64 | 37–56 | |
| Triglycerides (mg/dl) | 0.102 | |||
| Median | 125 | 126 | 100 | |
| Interquartile range | 79–188 | 50–168 | 55–153 | |
| Low-density lipoprotein–real (mg/dl) | 108 ± 34 | 102 ± 32 | 112 ± 32 | 0.161 |
| High-density lipoprotein subtype 2 (mg/dl) | 12 ± 7 | 13 ± 7 | 11 ± 7 | 0.006 |
| High-density lipoprotein subtype 3 (mg/dl) | 26 ± 6 | 27 ± 6 | 25 ± 6 | 0.069 |
| Very-low-density lipoprotein (mg/dl) | 0.090 | |||
| Median | 16 | 19 | 15 | |
| Interquartile range | 10–25 | 7–24 | 8–22 | |
| Intermediate density lipoprotein (mg/dl) | 14 ± 8 | 17 ± 8 | 13 ± 7 | 0.508 |
| Lipoprotein (a) (mg/dl) | 7.5 ± 4.2 | 8.4 ± 3.6 | 7.1 ± 3.8 | 0.009 |
| Low-density lipoprotein pattern B | 205 (50%) | 27 (24%) | 178 (60%) | 0.011 |
Data are presented as mean ± SD, n (%), or median and interquartile range.
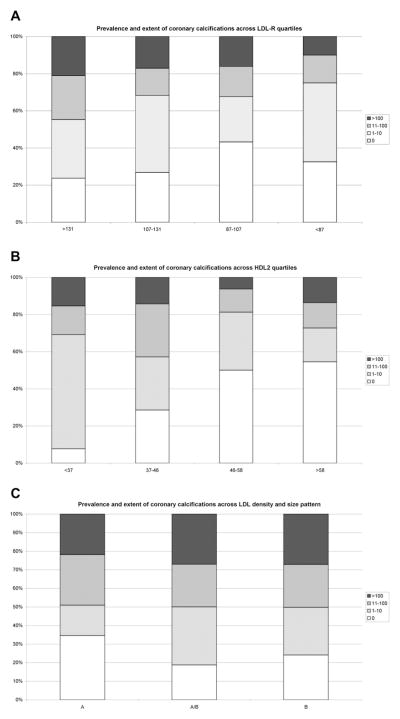
Overall, 297 participants (72.4%) had CAC. On average, the participants with CAC were older, more likely to be men and white, and had a greater prevalence of traditional risk factors (Table 1). In terms of lipid parameters, HDL (specifically, HDL2), Lp(a), and LDL pattern B were significantly different between patients with and without CAC. Across decreasing quartiles of HDL2 concentration, the prevalence and extent of CAC increased, with a reverse association observed across LDL density and size patterns from A (predominantly large, buoyant LDL) to B (predominantly small, dense LDL particles; Figure 1). We analyzed the cohort for both CAC >0 and CAC >100. The results were similar using either cutpoint (data not shown).
Figure 1.
Prevalence and extent of CAC deposits across (A) LDL aggregate subfractions 1, 2, 3, and 4 (LDL-R) quartiles, (B) HDL2, and (C) LDL particle size and density pattern (A, predominantly large, buoyant particles; B, predominantly small, dense LDL particles; A/B, intermediate pattern) according to typical CAC cutpoints.14,15
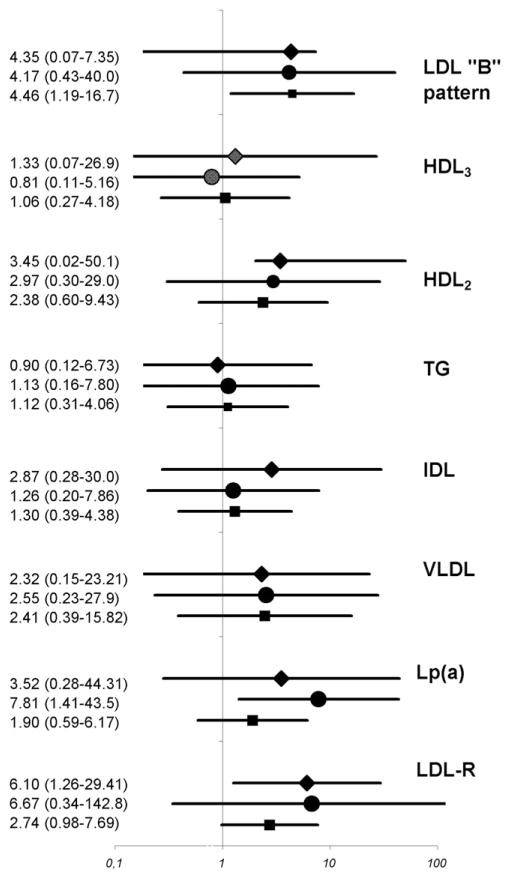
In a multivariate model adjusting for demographic characteristics (e.g., age, gender, ethnicity) and traditional risk factors (e.g., hypertension, diabetes, family history, body mass index, and smoking), none of the explored lipid parameters predicted the presence of CAC with reliable statistical significance, except for LDL pattern B. However, after stratification for dyslipidemia, LDL-R and HDL2 emerged as independent predictors of CAC in patients with normal lipid levels, and Lp(a) emerged as an independent predictor of CAC in patients with dyslipidemia (Figure 2).
Figure 2.
Odds ratios for prevalent CAC deposits adjusted for age, gender, ethnicity/race, arterial hypertension, diabetes mellitus, body mass index, and smoking in overall population (squares) and those with normal (diamonds) and abnormal (circles) conventional lipid profile. VLDL = very-low-density lipoprotein. Lines represent 95% confidence intervals.
Discussion
In the present study, we identified 3 distinctive lipid parameters that provided additional information on the prevalence of CAC beyond conventional lipid profiling. Low levels of HDL2 and high levels of LDL-R emerged as independent predictors of CAC in patients with normal lipid levels, and high levels of Lp(a) predicted CAC in patients with dyslipidemia (defined by the current cutoffs for lipo-protein and TG levels as proposed by the National Cholesterol Education Program guidelines). Additionally, the predominance of atherogenic, small, dense LDL particles (i.e., pattern B) was associated with CAC in the overall study population but failed to predict the CAC burden when patients were stratified according to the conventional lipid profile. Our results have confirmed that specific atherogenic derangements of the lipid profile are strongly associated with CAC and could therefore improve the risk assessment beyond the conventional lipoprotein and TG assessment.
Atherogenic LDL parameters, such as LDL-R, Lp(a), and B pattern (predominance of small, dense LDL particles) predicted CAC. However, the particle size and density pattern only predicted prevalent CAC in the overall study population. Although several reports have shown a strong univariate inverse relation between LDL particle size and coronary artery disease,20,21 such an association usually faded with statistical adjustments because of a significant covariance between LDL particle size and density and other lipid derangements (e.g., the metabolic syndrome lipid phenotype).20,21 In line with these findings, our results have confirmed that the strong association between an atherogenic LDL profile (predominantly composed of small, dense particles) and coronary atherosclerosis (as determined by the presence of CAC) vanishes after the study population has been stratified according to presence of National Cholesterol Education Program-defined lipid derangements.
In contrast, LDL-R and Lp(a) only predicted prevalent CAC after the study population was stratified according to the presence of dyslipidemia. LDL-R levels [a fraction that does not include Lp(a) and IDL in contrast to conventional LDL estimations] >100 mg/dl were associated with a sixfold increase in the prevalence of CAC in the subgroup of participants who were identified as having normolipidemia using conventional lipid profiling. This is particularly important in the face of recent evidence that conventionally determined LDL levels correlate poorly with the extent of CAC in subjects at low cardiovascular risk,22 implying a potential for improving risk stratification in this population.
In contrast to the LDL-R levels, Lp(a) only predicted CAC in those with dyslipidemia. Although many studies have reported a strong relation between Lp(a) and coronary artery disease,19 the evidence of an association between Lp(a) and coronary atherosclerosis is still controversial.22–24 Several studies have failed to confirm any significant association between Lp(a) and CAC or cardiovascular events,22,25 although most of these studies were undertaken in populations with normal lipid levels and explicitly suggested that Lp(a) was only predictive of coronary atherosclerosis in populations with dyslipidemia, consistent with our findings. In the present study, we did not measure the particle number. However, another study evaluated this measure and demonstrated that the total LDL particle number had a stronger association with CAC than the traditional lipoprotein measures. Patients in the highest tercile of total LDL particle number (1,935 to 3,560 nmol/L) were 3.7 times more likely to exhibit CAC as those in the lowest tercile (620 to 1,530 nmol/L).26
Regarding the HDL subfractions, both the prevalence and the extent of CAC increased across decreasing quartiles of HDL2 levels, although no significant association could be detected between the atherogenic HDL subtype 3 and CAC. This finding is consistent with a notable and well-recognized functional heterogeneity between different HDL subtypes.27 HDL2a, HDL2b, and HDL2c lipoproteins represent cardioprotective subfractions, and HDL3b is associated with increased cardiovascular risk.28 The inverse association between HDL2 and CAC was limited to those with normal lipid levels. Previous reports have already suggested that HDL fails to predict CAC in dyslipemic states such as diabetes,29 likely because lipid derangements, such as hypercholesterolemia30 and, especially, the dyslipidemia of the metabolic syndrome,31,32 tend to shift metabolic maturation of HDL from HDL2 toward HDL3. The HDL subtype might therefore be an indicator of a highly atherogenic metabolic turnover rather than an independent predictor of coronary atherosclerosis in its own right. Because several lipid-lowering agents have differential effects on the lipoprotein subfractions, these can be used to direct therapy for those at risk.
Despite providing novel insight into the association between CAC and lipid metabolism, our study had some limitations. First, it was a cross-sectional analysis that only allowed us to infer an association between lipid parameters and CAC, and causal and longitudinal relations could not be not addressed. Second, although CAC is a strong independent predictor of coronary heart disease, it only represents an estimate and not a direct measure of atherosclerosis and might have failed to detect some noncalcified plaques. More importantly, CAC represents a surrogate marker of cardiovascular risk; therefore, our study has only provided insight into the pathophysiology of the association between derangements of lipid metabolism and atherosclerosis. Clinically applicable conclusions remain speculative. In addition, we studied asymptomatic subjects who underwent cardiovascular risk assessment by computed tomography. Thus, our conclusions should only be applied to this intermediate-risk population. Therefore, large population-based clinical outcome-oriented studies should be undertaken.
Footnotes
Disclosures
The authors have disclosed no conflicts of interest.
References
- 1.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 2.Chirovsky DR, Fedirko V, Cui Y, Sazonov V, Barter P. Prospective studies on the relationship between high-density lipoprotein cholesterol and cardiovascular risk: a systematic review. J Cardiovascular Risk. 2009;16:404–423. doi: 10.1097/HJR.0b013e32832c8891. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376–b2384. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney P, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO., Jr Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735–1741. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35:159–168. [PubMed] [Google Scholar]
- 10.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2010;56:50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Nasir N, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Tseng PR, Flores FR, Raggi P, Berman DS, Blumenthal RS, Budoff MJ. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953–960. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Agatston A, Janowitz W, Hildner F, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography, a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni KR, Garber DW, Jones MK, Segrest JP. Identification and cholesterol quantify calcification of low density lipoprotein subclasses in young adults by VAP-II methodology. J Lipid Res. 1995;36:2291–2302. [PubMed] [Google Scholar]
- 17.Kulkarni KR, Marcovina SM, Krauss RM, Garber DW, Glasscock AM, Segrest JP. Quantification of HDL2 and HDL3 cholesterol by the vertical auto profile-II (VAP-II) methodology. J Lipid Res. 1997;38:2353–2364. [PubMed] [Google Scholar]
- 18.Zijaka P. Role of low-density lipoprotein apheresis. Am J Cardiol. 2005;96:67E–69E. doi: 10.1016/j.amjcard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, Sharp DS. Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am J Cardiol. 2000;86:412–416. doi: 10.1016/s0002-9149(00)00956-5. [DOI] [PubMed] [Google Scholar]
- 21.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 22.Nishino M, Malloy MJ, Naya-Vigne J, Russell J, Kane JP, Redberg RF. Lack of association of lipoprotein (a) levels with coronary calcium deposits in asymptomatic postmenopausal women. J Am Coll Cardiol. 2000;35:314–320. doi: 10.1016/s0735-1097(99)00555-0. [DOI] [PubMed] [Google Scholar]
- 23.Kullo IJ, Bailey KR, Bielak LF, Sheedy PF, Klee GG, Kardia SL, Peyser PA, Boerwinkle E, Turner ST. Lack of association between lipoprotein (a) and coronary artery calcification in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Mayo Clin Proc. 2004;79:1258–1263. doi: 10.4065/79.10.1258. [DOI] [PubMed] [Google Scholar]
- 24.Qasim AN, Martin SS, Mehta NN, Wolfe ML, Park J, Schwartz S, Schutta M, Iqbal N, Reilly MP. Lipoprotein(a) is strongly associated with coronary artery calcification in type-2 diabetic women. Int J Cardiol. 2011;150:17–21. doi: 10.1016/j.ijcard.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantin B, Gagnon F, Moorjani S, Despres J, Lamarche B, Lupien PJ, Dagenais GR. Is lipoprotein (a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular study. J Am Coll Cardiol. 1998;31:519–525. doi: 10.1016/s0735-1097(97)00528-7. [DOI] [PubMed] [Google Scholar]
- 26.Prado KB, Shugg S, Backstrand JR. Low-density lipoprotein particle number predicts coronary artery calcification in asymptomatic adults at intermediate risk of cardiovascular disease. J Clin Lipidol. 2011;5:408–413. doi: 10.1016/j.jacl.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw KT, Kastelein JJ. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 28.Lamarche B, Moorjani S, Cantin B, Degenais G, Lupien PJ, Després JP. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Atheroscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 29.Martin SS, Qasim AN, Wolfe M, St Clair C, Schwartz S, Iqbal N, Schutta M, Bagheri R, Mehta NN, Rader DJ, Reilly MP. Comparison of high-density lipoprotein cholesterol to apolipoprotein A-I and A-II to predict coronary calcium and the effect of insulin resistance. Am J Cardiol. 2010;107:393–398. doi: 10.1016/j.amjcard.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Yan B, Fu M, Xu Y, Tian Y. Relationship between plasma lipid concentrations and HDL subclasses. Clin Chem Acta. 2005;354:49–58. doi: 10.1016/j.cccn.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Jang Y, Kim K, Cho H, Jee SH, Park Y, Kim MK. Relationship between HDL3 subclasses and waist circumferences on the prevalence of metabolic syndrome: KMSRI-Seoul study. Atherosclerosis. 2010;213:288–293. doi: 10.1016/j.atherosclerosis.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 32.El Harchaoui K, Arsenault BJ, Franssen R, Despres J-P, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]