Summary
Activity-dependent gene transcription and protein synthesis underlie many forms of learning-related synaptic plasticity. At excitatory glutamatergic synapses, the immediate early gene product Arc/Arg3.1 couples synaptic activity to postsynaptic endocytosis of AMPA-type glutamate receptors. Although the mechanisms for Arc induction have been described, little is known regarding the molecular machinery that terminates Arc function. Here we demonstrate that the RING domain ubiquitin ligase Triad3A/RNF216 ubiquitinates Arc, resulting in its rapid proteasomal degradation. Triad3A associates with Arc, localizes to clathrin-coated pits, and is associated with endocytic sites in dendrites and spines. In the absence of Triad3A, Arc accumulates, leading to the loss of surface AMPA receptors. Furthermore, loss of Triad3A mimics and occludes Arc-dependent forms of synaptic plasticity. Thus, degradation of Arc by clathrin-localized Triad3A regulates the availability of synaptic AMPA receptors and temporally tunes Arc-mediated plasticity at glutamatergic synapses.
Introduction
Both long-term synaptic plasticity and behavioral learning require de novo RNA and protein synthesis (Costa-Mattioli et al., 2009). Several immediate early genes (IEGs) are rapidly induced in response to neuronal activity (Flavell and Greenberg, 2008). Among these IEG products, the activity-regulated cytoskeleton-associated protein Arc/Arg3.1 is particularly notable since its mRNA is rapidly trafficked following neuronal stimulation, where it is locally translated (Lyford et al., 1995; Moga et al., 2004; Steward et al., 1998). Arc regulates synaptic strength (Guzowski et al., 2000; Rial Verde et al., 2006; Shepherd et al., 2006; Waung et al., 2008) and promotes the endocytosis of AMPA receptors at glutamatergic synapses (Rial Verde et al., 2006; Shepherd et al., 2006; Waung et al., 2008). Indeed, Arc directly binds dynamin-2 and endophilin-3, which are important components of the endocytic machinery (Chowdhury et al., 2006). Recent findings have shown that Arc participates in multiple forms of synaptic plasticity including homeostatic scaling (Gao et al., 2010; Korb et al., 2013; Shepherd et al., 2006), metabotropic glutamate receptor-dependent long-term depression (mGluR-LTD) (Jakkamsetti et al., 2013; Park et al., 2008; Waung et al., 2008), and inverse synaptic tagging where it mediates endocytosis of AMPA receptors at inactive synapses that recently experienced strong stimulation (Okuno et al., 2012). A large body of work has shown that activity-dependent endocytosis and AMPA receptor recycling mediate diverse forms of learning-related synaptic plasticity (Kessels and Malinow, 2009; Newpher and Ehlers, 2008). Thus, the transient induction and tight regulation of Arc levels has been proposed to tune synaptic strength by adjusting postsynaptic trafficking of AMPA receptors. Notably, once induced, Arc undergoes rapid protein turnover (Rao et al., 2006), ensuring a discrete temporal window for Arc-dependent plasticity.
Across phylogeny, protein degradation by the ubiquitin-proteasome system (UPS) regulates many aspects of synapse function (DiAntonio and Hicke, 2004; Mabb and Ehlers, 2010). At mammalian hippocampal synapses, long-term alterations in synaptic activity cause global changes in the composition of postsynaptic proteins via the UPS (Ehlers, 2003). Furthermore, long-term potentiation (LTP) at CA1 synapses in the hippocampus requires a balance between protein synthesis and proteasomal degradation (Fonseca et al., 2006), suggesting that newly synthesized plasticity proteins are subject to ubiquitin-dependent turnover for reliable synapse function. Additionally, a variety of activity-induced proteins, including Arc, are degraded by the UPS (Greer et al., 2010; Rao et al., 2006). However, the mechanisms by which Arc is targeted for UPS degradation and how Arc turnover is coupled to endocytic function remain poorly defined.
In the present study, we demonstrate that the RING domain E3 ubiquitin ligase, Triad3A/RNF216 ubiquitinates Arc and promotes its proteasomal degradation. Using live-cell imaging and biochemical analysis, we show that Triad3A localizes to clathrin-coated pits and controls Arc turnover. Overexpression of Triad3A reduces levels of Arc, resulting in an increased abundance of synaptic AMPA receptors. Conversely, loss of Triad3A leads to elevated Arc levels and downregulation of AMPA receptors. Furthermore, overexpression of Triad3A prevents homeostatic synaptic scaling and mGluR-dependent synaptic depression, whereas in the absence of Triad3A, these Arc-dependent forms of synaptic plasticity are mimicked and occluded. Thus, degradation of Arc by clathrin-localized Triad3A regulates synaptic strength by limiting the endocytic trafficking of AMPA receptors. Such spatial control of protein degradation at synapses provides a novel mechanism for limiting the duration of plasticity protein action in response to bouts of activity.
Results
Proteasomal Degradation Regulates Arc Turnover in Neurons
The translation of Arc mRNA is rapidly induced by synaptic activity in an NMDA receptor-dependent manner in vivo (Lyford et al., 1995; Steward and Worley, 2001). We recapitulated this manipulation in vitro by treating cultured hippocampal neurons with 4-aminopyridine (4AP), a blocker of Kv1 family K+ channels, together with the γ-aminobutyric acid (GABA) receptor antagonist bicuculline (4AP/Bic), to enhance synaptic and network activity (Kawashima et al., 2009). Using this protocol, Arc protein expression is robustly induced (Figure S1A) (Kawashima et al., 2009). The 4AP/Bic-induced increase in Arc protein was prevented by the Na+ channel blocker tetrodotoxin (TTX, 2 μM) (Figure S1A). Following its induction by 4AP/Bic, Arc protein rapidly decays in the presence of protein synthesis inhibitors (Figure S1A), indicating robust degradation of newly synthesized Arc. To measure the half-life of newly synthesized Arc following increased neuronal activity, we performed pulse-chase metabolic labeling experiments in cortical neurons. Newly synthesized Arc was radiolabeled with 35S-cysteine/methionine (35S-Cys/Met) under conditions of elevated activity (4AP/Bic). Neurons were then chased by incubation with unlabeled amino acids over time (Figure 1A). With elevated synaptic activity, we observed robust induction followed by a rapid decay of labeled Arc protein. The half-life of newly synthesized Arc protein was 45 ± 10 min (Figure 1A), which is consistent with previous observations (Rao et al., 2006).
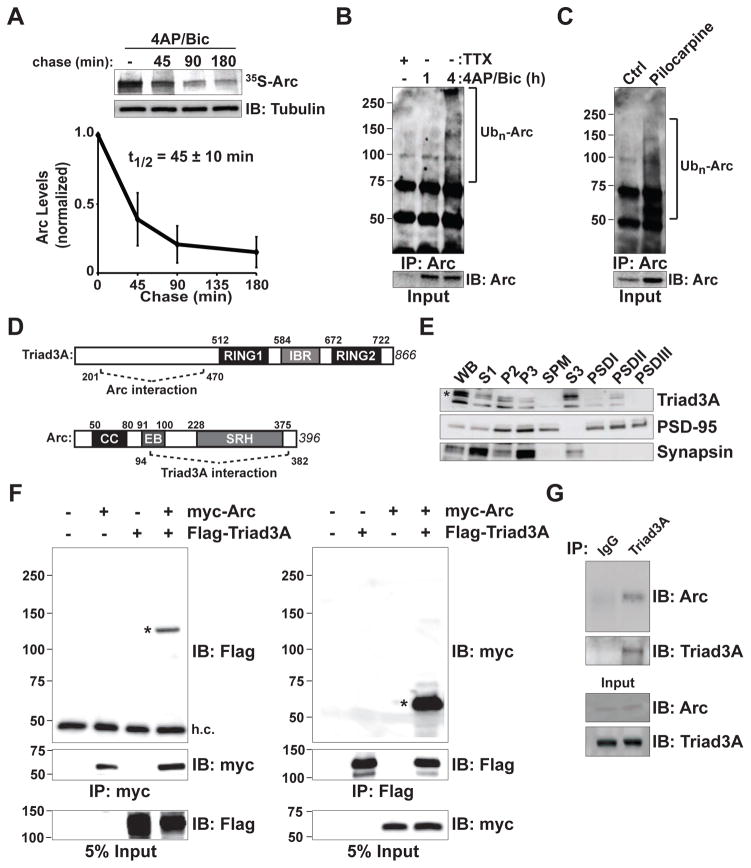
Figure 1. Rapid Turnover of Arc and Interaction with the RING E3 Ligase Triad3A.
(A) Pulse-chase analysis of endogenous Arc. Cortical neurons at 14–18 days in vitro (DIV14–18) were treated with 40 μM 4-aminopyridine (4AP) and 50 μM bicuculline (Bic) for 4 h in the presence of 35S-Cys/Met. Labeled neurons were chased with unlabeled amino acids for various times in the continued presence of 4AP/Bic. Cell lysates were immunoprecipitated (IP) with an anti-Arc antibody, resolved by SDS-PAGE, and visualized by autoradiography. The remaining labeled Arc was plotted over time. Data indicate means ± SEM and half-life (t1/2) is shown. n = 3.
(B) Endogenous Arc is upregulated and ubiquitinated upon 4AP/Bic treatment. DIV14 cortical neurons were incubated with 4AP/Bic for 1 and 4 h. Endogenous Arc was immunoprecipitated (IP) under denaturing conditions, resolved by SDS-PAGE and immunoblotted (IB) with an antiubiquitin (Ub) antibody. Brackets in (B) and (C) indicate polyubiquitinated Arc species.
(C) Arc is ubiquitinated in vivo. Hippocampal extracts were isolated from mice following pilocarpine-induced seizures to elevate Arc levels. Endogenous ubiquitinated Arc was immunoprecipitated and detected as in (B).
(D) Identification of Triad3A as a binding partner of Arc by yeast two-hybrid screening. Schematic diagram indicates domain structures of Arc and Triad3A and their identified binding regions. Numbers indicate amino acid residues. CC, coiled-coil domain; EB, endophilin-3 binding domain; SRH, spectrin repeat homology domain; RING (Really Interesting New Gene) domain; IBR, In-Between-RING domain.
(E) Subcellular distribution of Triad3A in brain. Subcellular fractions from adult rat forebrain were immunoblotted for Triad3A, PSD-95, and synapsin I. For each lane, 25 μg of S1, P2, and P3 fractions, or 8 μg each of total synaptosomal plasma membrane (SPM) and S3 fractions, or 3 μg of postsynaptic density (PSD) fractions (PSDI, PSDII, and PSDIII) were loaded for immunoblot analysis. Note that Triad3A is present in both S3 and PSDII fractions.
(F) Triad3A and Arc form a complex in heterologous cells. FLAG-Triad3A and myc-Arc were expressed in HEK293 cells and immunoprecipitated (IP) with antibodies to their corresponding epitope tags (myc, left; Flag, right), followed by immunoblotting (IB) with anti-Flag (left) or anti-myc antibodies (right). Asterisks represent the corresponding co-immunoprecipitation of Triad3A or Arc. h.c., IgG heavy chain band. Molecular mass markers are shown on the left.
(G) In vivo interaction of endogenous Arc and Triad3A in mouse hippocampus. Following seizure induction with pilocarpine, lysates from mouse hippocampus were immunoprecipitated (IP) with an anti-Triad3A antibody and precipitated proteins were subjected to immunoblot (IB) analysis using an anti-Arc antibody.
To test whether the rapid degradation of Arc is mediated by the ubiquitin-proteasome system (UPS), we incubated cortical neurons with the proteasome inhibitor MG132 (30 μM, 1 h), following treatment with 4AP/Bic. In addition to induction of Arc protein by synaptic activity (3-fold induction compared to TTX, p < 0.05), Arc levels were further augmented upon treatment with MG132 (Figures S1B and S1C; 1.5-fold relative to 4AP/Bic alone, p < 0.05). We also observed increased Arc levels upon MG132 treatment by immunocytochemical staining of hippocampal neurons (Figures S1D and S1E). Endogenous Arc protein was robustly induced and ubiquitinated in cortical neurons following treatment with 4AP/Bic (Figure 1B). A similar increase in total and ubiquitinated Arc occurred in mouse hippocampus in vivo following seizure induction using pilocarpine (Figure 1C). Thus, upon increased activity, Arc protein is rapidly synthesized, but then quickly degraded by the UPS both in vitro and in vivo.
Triad3A/RNF216 Interacts with Arc and Promotes Its Ubiquitination
To identify binding partners that regulate Arc degradation, we performed a yeast two-hybrid screen using full-length Arc as bait against a mouse brain cDNA library. We identified a strong interaction between Arc and the RING domain E3 ubiquitin ligase Triad3A/RNF216 (Figure S1F). Analysis of isolated clones mapped the interaction to the N-terminal domain of Triad3A upstream of the RING-IBR-RING domain (amino acids 201–470) and the C-terminal domain of Arc encompassing the spectrin-like homology repeats (amino acids 94–382) (Figure 1D). Triad3A was initially identified as a RING domain ubiquitin E3 ligase involved in the ubiquitination of toll-like receptors (TLRs) (Chuang and Ulevitch, 2004). Triad3A is the major splice variant of Triad3, which encodes five splice variants, Triad3A–E (Chuang and Ulevitch, 2004). Among these splice variants, Triad3A is most highly expressed in brain (Chuang and Ulevitch, 2004), including hippocampus, neocortex, olfactory bulb, and cerebellum (Figures S1G and S1H). Biochemical fractionation indicated the presence of Triad3A protein in both presynaptic vesicle (S3) and postsynaptic density (PSD) fractions, although abundance in the PSD fraction was lower (Figure 1E). When co-expressed in HEK293T cells, myc-tagged Arc co-immunoprecipitated with Flag-tagged Triad3A and vice versa (Figure 1F). In addition, endogenous Arc protein co-immunoprecipitated with Triad3A from hippocampal extracts of mice upon induction of Arc by pilocarpine-induced seizures (Figure 1G).
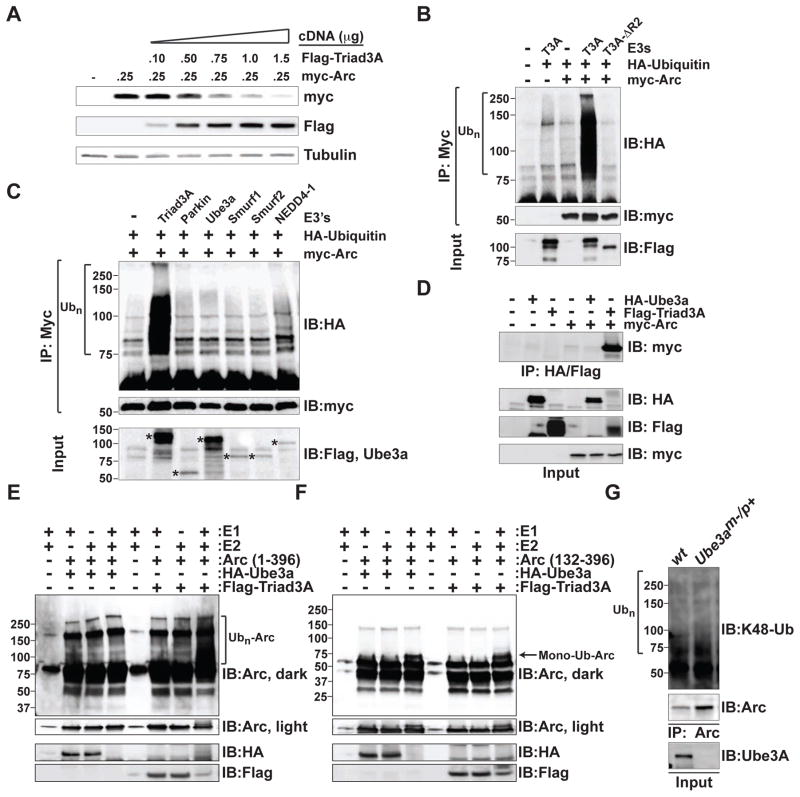
Upon transfecting HEK293 cells with Arc and increasing amounts of Triad3A cDNA, immunoblot analysis indicated a progressive loss of Arc protein with increased Triad3A (Figure 2A). In addition, co-expression of myc-Arc with HA-ubiquitin (HA-Ub) revealed robust Arc ubiquitination in the presence of Triad3A (Figure 2B). In contrast to wildtype Triad3A, expression of mutant Triad3A lacking the second RING domain (Triad3A-ΔR2) did not promote Arc ubiquitination (Figure 2B). The predominant Triad3 isoforms in the brain are Triad3A, 3D, and 3E (Chuang and Ulevitch, 2004), splice variants that differ only in their N-terminal domain (Figure S2A, top panel). Although variably spliced, Triad3C, Triad3D, and Triad3E encode for the same protein (Figure S2A, top panel). Both Triad3A and Triad3B, but not Triad3C, promoted Arc ubiquitination (Figure S2A). Whereas Triad3A and Triad3B strongly induced Arc ubiquitination, the related RING-IBR-RING E3 ligase parkin did not (Figure S2A). Moreover, we did not detect ubiquitination of Arc upon co-expression with other known E3s that are expressed in the brain including Ube3a, Smurf1, Smurf2, and NEDD4-1 (Figure 2C).
Figure 2. Triad3A Ubiquitinates Arc.
(A) HEK293T cells expressing myc-Arc were transfected with increasing amounts of Flag-Triad3A cDNA. Cell lysates were resolved by SDS-PAGE and immunoblotted with indicated antibodies.
(B) Lysates from HEK293 cells expressing myc-Arc and HA-ubiquitin (Ub) along with wildtype Flag-Triad3A or inactive FLAG-Triad3A-ΔR2 lacking the RING2 domain were subjected to immunoprecipitation (IP) with an anti-myc antibody followed by immunoblot (IB) analysis using an anti-HA antibody. Ubiquitinated Arc appears as a high molecular mass smear. In (B–C) and (E–G), molecular mass markers in kilodaltons are shown on the left.
(C) Examination of Arc ubiquitination with multiple ubiquitin E3 ligases. Lysates from HEK293 cells expressing myc-Arc and HA-ubiquitin (Ub) along with various ubiquitin E3 ligases were subjected to immunoprecipitation (IP) with an anti-myc antibody followed by immunoblot (IB) analysis using an anti-HA antibody to detect ubiquitinated Arc. Asterisks in the bottom panel identify input control bands.
(D) Comparison of co-immunoprecipitation of Arc with Ube3a and Triad3A. HEK293T cells were transfected with HA-Ube3a, Flag-Triad3A, or myc-Arc. Cell extracts were immunoprecipitated with an anti-HA or anti-Flag antibody. Immunoprecipitates (IP) were separated by SDS-PAGE and immunoblotted (IB) with anti-myc, anti-HA, or anti-FLAG antibodies. Arc robustly co-precipitated with Flag-Triad3A.
(E–F) HA-Ube3a and Flag-Triad3A were expressed in 293T cells and immunopurified from cell extracts. Immunopurified E3s were added to an in vitro ubiquitination reaction containing 0.15 μg of GST-tagged full-length Arc(1–396) (E) or a GST-tagged truncated Arc(132–396) fragment (F), 0.1 μg E1, 0.5 μg of UbcH7, and 10 μg of recombinant ubiquitin. Samples were incubated for 2 h at 30°C, resolved by SDS-PAGE, and subjected to immunoblot (IB) analysis with anti-Arc antibody to detect ubiquitin conjugates or anti-HA and anti-Flag antibodies to detect immunopurified E3s. Immunopurified HA-Ube3a and FLAG-Triad3A are both active as they are capable of self-ubiquitination. Whereas high molecular mass ubiquitin conjugates of Arc(1–396) were observed with Triad3A (E), a single apparent mono-ubiquitinated species of Arc(132–396) was observed with both Triad3A and Ube3a (F) (mono-Ub).
(G) Arc levels are increased and Arc ubiquitin conjugates are present in neurons lacking Ube3a. DIV14 cortical neurons from Ube3am−/p+ mice were treated with 2 μM TTX for 24 h to normalize activity levels prior to solubilization. Cell lysates were immunoprecipitated (IP) with an anti-Arc antibody, and immunoblotted (IB) using anti-ubiquitin (Ub), anti-K48-linked ubiquitin, or anti-Arc antibodies. Ubiquitinated Arc appears as a high molecular mass smear.
Previously, Ube3a was identified as a ubiquitin E3 ligase for Arc (Greer et al., 2010). In contrast to Triad3A, we were unable to detect Ube3a-dependent Arc ubiquitination (Figure 2C), similar to recent findings (Kuhnle et al., 2013). However, we found wildtype Ube3a, but not the catalytically inactive mutant Ube3a (C883A) to reduce Arc protein levels (Figure S2B), as previously reported (Greer et al., 2010). Compared to Triad3A, which efficiently coimmunoprecipitated with Arc, we were unable to detect an interaction between Ube3a and Arc in HEK 293 cells (Figure 2D). However, we did observe a weak interaction between endogenous Ube3a and Arc in hippocampal neurons (Figure S2C). Knockdown of Triad3A in hippocampal neurons did not increase the interaction between Arc and Ube3a, suggesting that Triad3A does not compete with Ube3a for Arc binding (Figure S2C). Using in vitro ubiquitination assays, we found that Triad3A efficiently ubiquitinated full-length recombinant Arc, whereas Ube3a did not (Figure 2E, compare lanes 4 and 8). We then purified a fragment of Arc (amino acids 132–396) that lacks the N-terminal coiled coil and endophilin-3 binding domains and was previously demonstrated to be a Ube3a substrate in vitro (Greer et al., 2010). Both Triad3A and Ube3a could ubiquitinate this Arc fragment (Figure 2F). However, unlike the high molecular mass poly-ubiquitinated smear observed with full-length Arc (Figure 2E), we observed a small ~7 kDa shift in molecular weight to an apparent single species (Figure 2F). Finally, cortical neurons from Ube3am−/p+ mice were examined for Arc K48-linked poly-ubiquitination using a K48 linkage-specific antibody that recognizes the predominant ubiquitin chain associated with proteasome-dependent degradation (Newton et al., 2008). Immunoprecipitation and immunoblot analysis revealed an increase in Arc protein levels in Ube3am−/p+ cortical neurons compared to wild-type neurons as previously described (Greer et al., 2010) (Figure 2G). However, we also detected an increase in K48-linked Arc ubiquitin conjugates, indicating that Arc is still capable of being ubiquitinated even in the absence of Ube3a (Figure 2G).
Triad3A Ubiquitinates Arc at Lysines 268 and 269
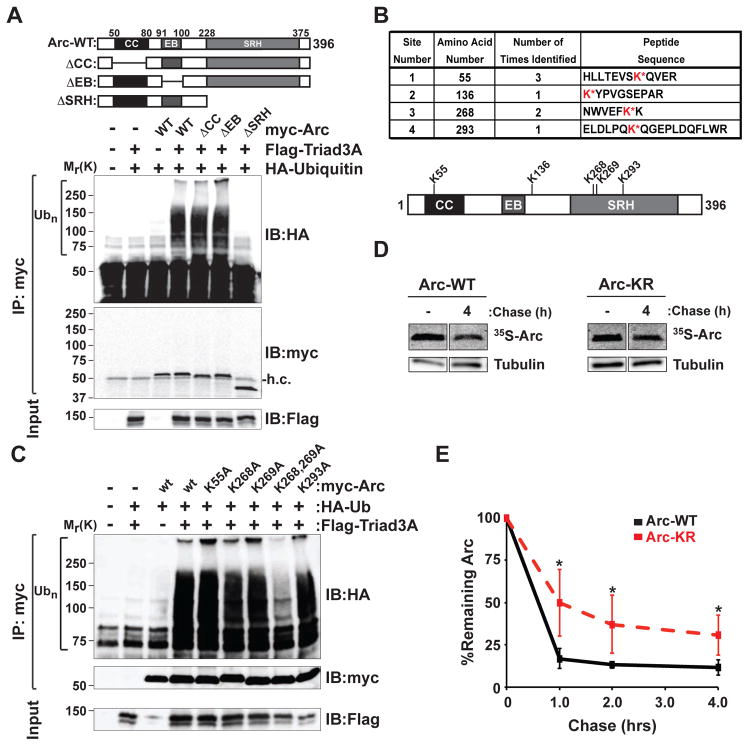
To determine which region of Arc serves as the target for Triad3A-dependent ubiquitination, we co-expressed Flag-Triad3A and HA-Ub with various Arc deletion mutants lacking the coiled-coil domain (ΔCC), endophilin-3 binding domain (ΔEB), and spectrin-repeat homology domains (ΔSRH) (Figure 3A). Deletion of the spectrin repeat homology domain of Arc abolished ubiquitination by Triad3A (Figure 3A). To identify the lysine residue(s) of Arc that are ubiquitinated by Triad3A, we performed mass spectrometry on purified Arc following in vitro ubiquitination with Triad3A. His-tagged Arc was purified from insect cells and Flag-tagged Triad3A was isolated from HEK293 cells (Figure S3A). When screening various E2 proteins, we found that both UbcH5 and UbcH7 efficiently supported Triad3A-dependent ubiquitination of Arc in vitro (Figure S3B). We then performed in vitro Arc ubiquitination assays in the presence of E1, E2 (UbcH7), recombinant His-Arc, Flag-Triad3A, and purified ubiquitin lacking internal lysines (Figure S3C). Arc was purified under denaturing conditions, resolved by SDS-PAGE, stained with Coomassie Blue (Figure S3C), band isolated, and subjected to in-gel trypsin digestion for liquid chromatography and tandem mass spectrometry (LC-MS/MS) analysis. Our analysis identified four lysines (K55, K136, K268, and K293) as sites of ubiquitination (Figure 3B). Of these sites, lysines 55 and 268 were ubiquitinated by Triad3A in more than one experiment (Figure 3B).
Figure 3. Triad3A Ubiquitinates Arc at Lysines 268 and 269.
(A) The spectrin-repeat homology (SRH) domain of Arc is required for Triad3A-dependent ubiquitination. Lysates from HEK293 cells expressing myc-Arc mutants and HA-ubiquitin (Ub) along with wildtype Flag-Triad3A were subjected to immunoprecipitation (IP) with an anti-myc antibody followed by immunoblot (IB) analysis using an anti-HA antibody. Ubiquitinated Arc appears as a high molecular mass smear. Deletion of the SRH domain abolishes Arc ubiquitination. h.c., IgG heavy chain. Top panel shows Arc deletion mutants. CC, coiled-coil domain; EB, endophilin-3 binding domain; SRH, spectrin repeat homology domain.
(B) Ubiquitin conjugated lysine residues following in vitro ubiquitination of Arc by Triad3A. Four ubiquitination sites were identified in three independent experiments by mass spectrometry. Top, table of isolated Arc tryptic peptides containing ubiquitinated lysines (red). Bottom, schematic of Arc showing ubiquitinated lysines (K55, K136, K268, K293) and the adjacent lysine K269. Protein domain labels as in (A).
(C) Triad3A-mediated ubiquitination requires lysines 268 and 269 of Arc. HEK293 cells expressing FLAG-Triad3A and HA-ubiquitin (Ub) along with either wildtype (wt) myc-Arc or myc-Arc mutants were subjected to immunoprecipitation (IP) with an anti-myc antibody, followed by immunoblot (IB) analysis with an anti-HA antibody to detect ubiquitinated Arc. Arc ubiquitination by Triad3A was nearly abolished by mutation of both K268 and K269. Molecular mass markers in kDa are shown.
(D) Arc protein stability increases upon mutation of both K268 and K269 residues. Examples of 35S-Cys/Met pulse-chase data of myc-Arc (WT, left) and myc-Arc K268R/K269R (KR, right) co-expressed with Triad3A in HEK cells are shown.
(E) Quantitative analysis of 35S-Cys/Met pulse-chase data. Data represent means ± SEM of the percent remaining labeled Arc following various times of chase. *p < 0.05, n = 3.
We next mutated candidate lysine residues and co-expressed Arc mutants with Triad3A in HEK293 cells. Single-site mutation of each of the four lysines (K55A, K136A, K268A, and K293A) had no effect on Triad3A-induced ubiquitination (Figure 3C). However, we noted the presence of a conserved lysine residue adjacent to K268, which was not identified in the mass spectrometric analysis. Indeed, whereas Arc-K268A and Arc-K269A were each robustly ubiquitinated by Triad3A, mutation of both residues to alanine almost completely abolished Triad3A-induced ubiquitination (Figure 3C). Similar results were obtained with a lysine to arginine double mutant Arc-K268R/K269R (data not shown). We then co-expressed wildtype Arc or Arc-K268R/K269R (hereafter referred to as Arc-KR) in HEK293 cells and performed 35S-Cys/Met metabolic labeling and pulse-chase analysis. Introduction of K268R and K269R mutations significantly slowed Arc degradation (Figures 3D and 3E). Since Arc decay was still observed in the Arc-KR mutant, we mutated all lysines of Arc (K55R/K136R/K268R/K269R//K293R) that were identified as being ubiquitinated (referred to as Arc-ALL) and monitored decay of protein levels following cyclohexamide treatment (Figure S3D). As expected, Arc-KR levels decayed slower than Arc-WT (Figure S3D). However, we did not observe a difference in decay between Arc-KR and Arc-ALL suggesting that K268 and K269 are the primary sites of Arc ubiquitination (Figures S3D and S3E). The continued degradation of Arc-KR and Arc-ALL could be due to the presence of a strong predicted PEST motif that is located at the C-terminus of Arc (Rao et al., 2006).
Triad3A Regulates Arc Turnover in Neurons
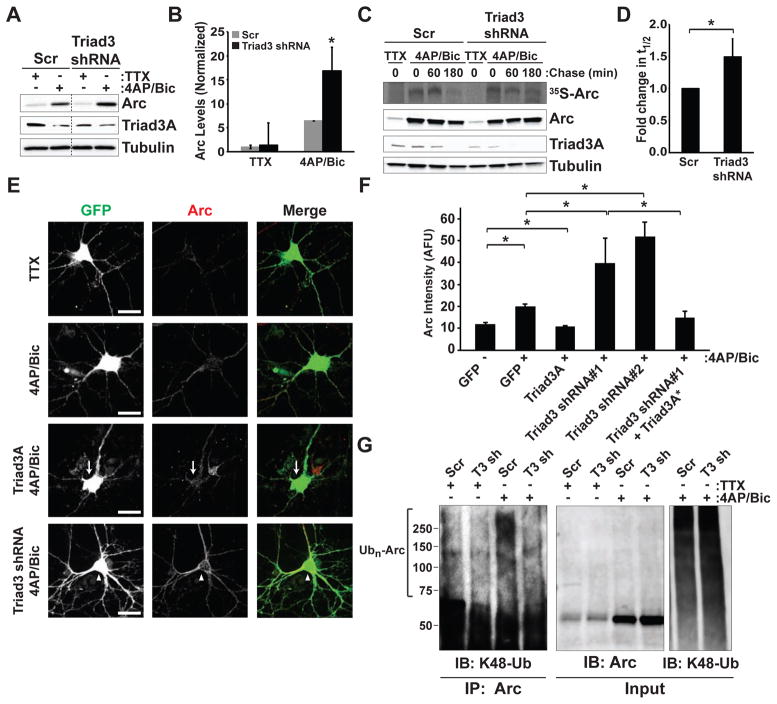
To examine the role of endogenous Triad3A in regulating Arc stability, we utilized RNA interference (RNAi) for loss-of-function analysis. We identified two short hairpin (shRNA) sequences that efficiently reduced Triad3A protein levels (Figure S4). Expression of these shRNAs in hippocampal neurons decreased Triad3A immunostaining by approximately 75% (Figures S4C and S4D). Lentiviral transduction of Triad3 shRNA resulted in a significant reduction of endogenous Triad3A relative to a scrambled shRNA control (Figure S4E). We next examined Arc expression elicited by 4AP/Bic in hippocampal neurons transduced with Triad3 shRNA (Figure 4A) and observed an increase in Arc accumulation compared to scrambled control (2.6 ± 0.4 fold increase, p < 0.05; Figures 4A and 4B). Along with an increase in Arc, Triad3A levels were slightly decreased with 4AP/Bic, potentially due to Triad3A autoubiquitination. Following induction by 4AP/Bic, degradation of 35S-labeled Arc was significantly slower in neurons expressing Triad3 shRNA, compared to neurons expressing a scrambled control shRNA (Figures 4C and 4D). Immunoblot analysis of total Arc from radiolabeled lysates confirmed the persistence of Arc following 4AP/Bic in neurons lacking Triad3A (Figure 4C).
Figure 4. Triad3A Regulates Arc Stability and Turnover.
(A) Enhanced Arc induction upon depletion of Triad3A in hippocampal neurons. Hippocampal neurons were transduced with control or Triad3 lentiviral shRNAs and treated with either TTX (2 μM) or 4AP/Bic. Cell lysates were resolved by SDS-PAGE and immunoblotted with Arc or Triad3A specific antibodies. Note that Triad3A knockdown results in higher Arc levels after 4AP/Bic induction.
(B) Means ± SEM of Arc levels under the indicated conditions displayed as a fold change relative to the Scr control. *p < 0.05, n = 3.
(C) Loss of Triad3A increases Arc half-life in neurons. Hippocampal neurons were incubated with control lentiviral shRNA or Triad3-shRNA and treated with either TTX (2 μM) or 4AP/Bic. Neurons were then incubated in 35S-Cys/Met for metabolic labeling and pulse-chase analysis, upper panel. Immunoblots of radiolabeled extracts using anti-Arc, anti-Triad3A, and anti-tubulin antibodies are shown in the lower blots. Note the reduced turnover of Arc (35S-Arc) as well as the increased remaining total Arc in neurons lacking Triad3A.
(D) Quantification of Arc half-life 3 h after 4-AP/Bic treatment displayed as a fold change relative to Scr control. Data represent means ± SEM. *p < 0.05, n = 3.
(E) Immunostaining for Arc upon Triad3A overexpression or downregulation. DIV14 hippocampal neurons were transfected with cDNA encoding GFP along with either myc-Triad3A or Triad3-shRNA for 72 h. Cells were treated with TTX or 4-AP/Bic for 4 h prior to fixation and immunostaining for Arc. Arrows indicate reduced Arc levels in a Triad3A overexpressing neuron. Arrowheads indicate elevated Arc in neurons expressing Triad3 shRNA. Scale bars, 25 μm.
(F) Data represent means ± SEM of Arc staining in neurons expressing GFP-Triad3A, Triad3-shRNA, or shRNA-resistant Triad3A (Triad3A*) from (E). AFU, arbitrary fluorescence units. *p < 0.01, n = 6–22.
(G) Triad3A is required for activity-induced Arc ubiquitination in neurons. Cortical neurons transduced with Scr or Triad3 (T3) shRNA lentivirus and incubated with TTX or 4AP/Bic. Arc was immunoprecipitated from cell extracts under denaturing conditions. Immunoprecipitates were resolved by SDS-PAGE and probed with an anti-K48-linkage specific ubiquitin antibody or anti-Arc antibody. 4AP/Bic induced Arc polyubiquitination (lane 3) that was prevented by Triad3-shRNA (lane 4).
In further experiments, we monitored endogenous Arc in hippocampal neurons upon Triad3A overexpression or knockdown in the presence or absence of elevated synaptic activity (Figure 4E). As expected, 4AP/Bic treatment increased Arc levels within 4 h (Figures 4E and 4F). In neurons with reduced levels of Triad3A, activity-induced Arc levels were increased by two-fold (Figures 4E and 4F). Co-expression of RNAi-resistant Triad3A reversed the increase in Arc (Figure 4F). In addition, overexpression of Triad3A blocked the activity-induced increase in Arc, resulting in Arc levels that were similar to those observed without activity (Figures 4E and 4F). Furthermore, knockdown of Triad3A abolished 4AP/Bic-induced Arc ubiquitination in cortical neurons (Figure 4G).
Triad3A Associates with Arc at Sites of Endocytosis
Recent studies have found that Arc facilitates the endocytosis of AMPA receptors through interactions with dynamin-2 and endophilin-3 (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006). We investigated whether Triad3A-mediated Arc ubiquitination affects the kinetics and localization of Arc within the endocytic pathway. In COS7 cells, GFP-tagged Triad3A puncta colocalized with clathrin-dsRed, which marks sites of endocytosis (Figures 5A and 5B). Further, mCherry-Triad3A puncta colocalized with the clathrin endocytic adaptor, GFP-Eps15 (Figure 5A, bottom panel) and GFP-dynamin 2 (data not shown). We next co-expressed GFP-Triad3A with DsRed-tagged clathrin light chain (LC) in COS7 cells and examined surface colocalization using total internal reflection fluorescence microscopy (TIRFM) (Merrifield et al., 2005). TIRFM revealed punctate spots of GFP-Triad3A that colocalized with clathrin (Figures 5C, S5A, and S5B). We also observed colocalization of Arc and Triad3A in COS7 cells, albeit to a lesser degree (Figures 5B and S5C).
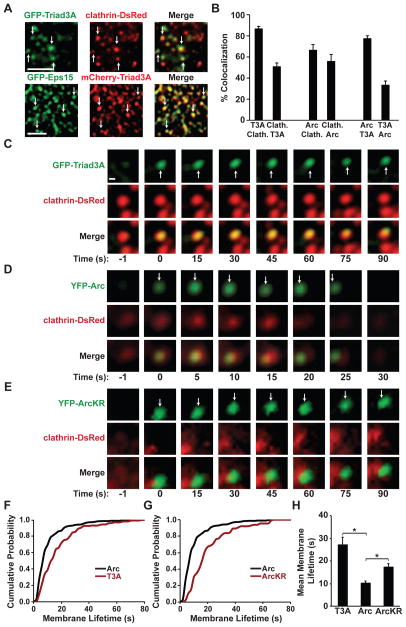
Figure 5. Ubiquitination by Clathrin-Localized Triad3A Regulates Arc Dynamics.
(A) Confocal image of GFP-Triad3A and clathrin-DsRed (top panels) or mCherry-Triad3A and GFP-Eps15 (bottom panels) in COS7 cells. Arrows indicate Triad3A localized to clathrin-coated pits (yellow). Scale bar, 5 μm.
(B) TIRF data representing means ± SEM of the percent colocalization between the two indicated proteins at presumptive endocytic pits. For example, the first bar indicates the percentage of Triad3A puncta (T3A) that colocalize with clathrin (Clath.).
(C) Time-lapse images demonstrating recruitment dynamics of GFP-Triad3A to clathrin-DsRed puncta.
(D) Time-lapse images demonstrating recruitment dynamics of YFP-Arc to clathrin-DsRed puncta.
(E) Time-lapse images demonstrating persistent association of YFP-ArcKR at or near clathrin-DsRed puncta.
(F) Cumulative probability plot of YFP-Arc and GFP-Triad3A lifetimes at the membrane as measured by TIRF microscopy.
(G) Cumulative probability plot of wildtype Arc and ArcKR lifetimes at the membrane as measured by TIRF microscopy.
(H) Mean lifetimes of GFP-Triad3A (T3A), YFP-Arc, and YFP-ArcKR puncta at the cell membrane ± SEM *p < 0.001
Using live cell TIRFM imaging, compared to Arc, Triad3A persisted for longer periods at membrane puncta (Triad3A, 27.1 ± 2.3 s; Arc-WT, 10.2 ± 1.0 s; Figures 5C–5F, 5H and S5D; Movies S1 and S2). The transient association of Arc with clathrin-coated pits (CCPs) is similar to the reported dynamics of its interacting partners, endophilin-3 and dynamin-2 (Perrais and Merrifield, 2005). Mutation of the K268 and K269 ubiquitination sites of Arc (Arc-KR) resulted in a more stable association at the membrane (Arc-KR, 17.3 ± 1.6 s) (Figures 5E, 5G, 5H and S5E; Movie S3). Intriguingly, Arc-KR puncta were near but seldom directly overlapped with CCPs, suggesting that Triad3A-dependent ubiquitination might couple Arc to sites of endocytosis or prevent persistent association of Arc with endocytic intermediates following clathrin uncoating (Chowdhury et al., 2006; Wu et al., 2011). In neurons, GFP-Triad3A colocalized with clathrin-DsRed in dendritic shafts and spines of hippocampal neurons (Figure 6A), but not with the postsynaptic density marker Homer-DsRed (Figure 6B). To test whether Triad3A localizes to the endocytic zone (EZ) within spines, we co-expressed GFP and clathrin-DsRed in hippocampal neurons and immunostained for endogenous Triad3A (Figure 6C). Endogenous Triad3A in dendritic spines tightly overlapped with clathrin-DsRed within a subdomain of the spine (Figure 6C). Consistent with the lateral localization of the EZ, Triad3A staining was localized adjacent to the PSD (Figure 6D). The percentage of PSDs containing adjacent Triad3A puncta was 55 ± 5%, which is slightly lower than previous findings for EZ-positive containing spines (Blanpied et al., 2002; Lu et al., 2007). Furthermore, Triad3A did not tightly overlap with syntaxin-4, a marker of exocytic domains within dendritic spines (Figure S5F) (Kennedy et al., 2010). To examine the synaptic distribution of Triad3A in vivo, we performed immunogold electron microscopy (EM) of rat CA1 hippocampus. Triad3A immunogold labeling was present near the membrane and on vesicle-like structures in both presynaptic terminals and postsynaptic spines (Figure 6E), similar to our biochemical observations (Figure 1E). Quantitative analysis indicated that a significant fraction (>30%) of gold particles were positioned along the plasma membrane near the PSD (black arrows), with a two-fold decrease in Triad3A labeling at spine membrane locations further away from the PSD (Figure 6F).
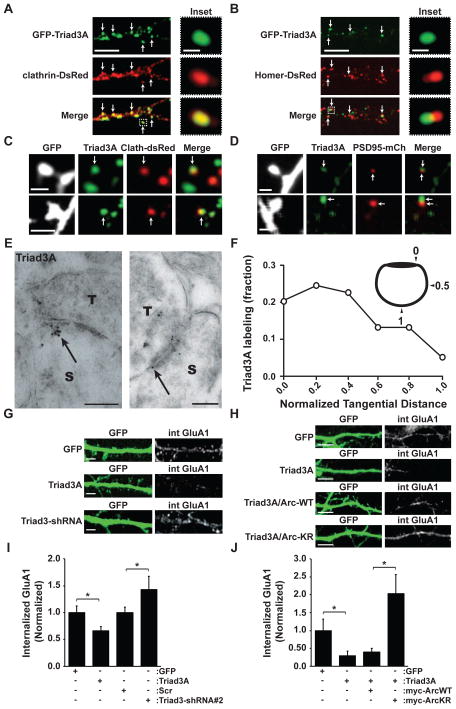
Figure 6. Clathrin-Localized Triad3A controls GluA1 AMPAR Endocytosis.
(A) Co-localization of GFP-Triad3A and clathrin-DsRed in hippocampal neurons. Arrows indicate co-localized puncta of GFP-Triad3A and clathrin-DsRed. The inset (right) shows an example of colocalized GFP-Triad3A and clathrin-DsRed. Scale bars, 10 μm and 2 μm, respectively.
(B) GFP-Triad3A localizes lateral to the PSD. Arrows indicate adjacent puncta of GFP-Triad3A (green) and the PSD-marker Homer-DsRed (red). Scale bars, 10 μm and 2 μm, respectively.
(C) Colocalization of endogenous Triad3A with clathrin-DsRed in dendritic spines. Hippocampal neurons expressing GFP and clathrin-DsRed were fixed and immunostained with an anti-Triad3A antibody. Two examples are shown. Scale bars, 2 μm.
(D) Hippocampal neurons (DIV19) expressing GFP and PSD95-mCh were fixed and stained for Triad3A. Triad3A puncta partially overlapped with PSD95-mCh, but were primarily positioned lateral to the PSD. Two examples are shown. Scale bars, 2 μm.
(E) Immunogold EM of Triad3A at asymmetric synapses in CA1 hippocampus in vivo. Triad3A labeling was present at membrane sites and vesicular structures in both presynaptic terminals (T) and postsynaptic spines (S). Within spines, Triad3A labeling was at sites adjacent to the PSD (arrows). Scale bars, 200 nm.
(F) Quantitative analysis of the tangential distribution of Triad3A labeling at the spine membrane as a function of distance from the PSD. Triad3A labeling is most abundant just lateral to the PSD. n = 69 particles.
(G) Representative images of DIV21 hippocampal neurons expressing GFP, GFP-Triad3A, or Triad3-shRNA. Internalized GluA1 AMPA receptors (int GluA1) were visualized by liveantibody feeding using an antibody directed against an N-terminal extracellular epitope of GluA1. Scale bars, 10 μm.
(H) Representative images of DIV21 hippocampal neurons expressing GFP, GFP-Triad3A, myc-Arc (Arc-WT), and myc-Arc K268R/K269R (Arc-KR). Internalized GluA1 AMPA receptors (int GluA1) were visualized by live-antibody feeding using an antibody directed against an Nterminal extracellular epitope of GluA1. Scale bars, 10 μm.
(I) Data represent means ± SEM of internalized GluA1 labeling in hippocampal neurons from G. *p < 0.05, n = 10–12. Scr, scrambled shRNA control.
(J) Data represent means ± SEM of internalized GluA1 labeling in hippocampal neurons from H. *p < 0.05, n = 7–10.
Triad3A is Required for Arc-Dependent Synaptic Plasticity
Postsynaptic endocytosis regulates synaptic AMPA receptor levels (Luscher et al., 1999; Newpher and Ehlers, 2008), and Arc is known to facilitate AMPA receptor endocytosis (Chowdhury et al., 2006; Rial Verde et al., 2006; Waung et al., 2008). Regulation of Arc levels results in reciprocal changes in the expression of surface AMPA receptors that can occur under baseline conditions and is most robust upon chronic changes in synaptic activity (Beique et al., 2011; Craig et al., 2012; Gao et al., 2010; Shepherd et al., 2006; Waung et al., 2008). We reasoned that a reduction of Triad3A should phenocopy Arc overexpression, whereas overexpression of Triad3A should mimic effects of Arc knockdown or knockout (KO). Consistent with this notion, shRNA knockdown of Triad3A led to increased endocytosis of the AMPA receptor subunit, GluA1 (Figures 6G and 6I). Conversely, overexpression of Triad3A decreased endocytosis of GluA1 AMPA receptors (Figures 6G and 6I), similar to that observed in Arc KO neurons (Chowdhury et al., 2006). Notably, shRNA knockdown of Triad3A decreased GluA1 receptor levels at the cell surface (Figures S6A–B) and this effect could be rescued by co-expressing an RNAi-resistant form of Triad3A (Figure S6B). Conversely, overexpression of Triad3A produced an increase in surface AMPA receptors (Figures S6A and S6B), similar to that observed in Arc knockout (KO) neurons. Thus, Triad3A and Arc exert reciprocal effects on AMPA receptor surface trafficking. To determine if Triad3A functions in the same endocytic pathway as Arc to mediate GluA1 endocytosis, we co-expressed Endophilin 3 (Endo3-WT) and a dominant negative version of Endophilin 3 (172–347) (Endo3-CT) that interacts with Arc and interferes with Arc-induced AMPAR endocytosis (Chowdhury et al., 2006). As above (Figures 6G and 6I), expression of Triad3-shRNA increased GluA1 endocytosis compared to Scr control (Figures S6C–S6D). However, co-expression of Triad3-shRNA with Endo3-CT prevented this increase in GluA1 endocytosis mediated by Triad3-shRNA (Figures S6C–S6D). Triad3A dependent Arc ubiquitination occurs on K268 and K269. To examine if ubiquitination of Arc by Triad3A mediates GluA1 endocytosis, we co-expressed Arc-WT or Arc-KR with Triad3A. Overexpression of Triad3A reduced GluA1 endocytosis and this was not affected by coexpression of Arc-WT (Figures 6H and 6J). However, expression of Arc-KR stimulated GluA1 endocytosis despite co-expression of Triad3A (Figures 6H and 6J), indicating that Triad3A negatively regulates GluA1 endocytosis by ubiquitination of Arc at K268/269.
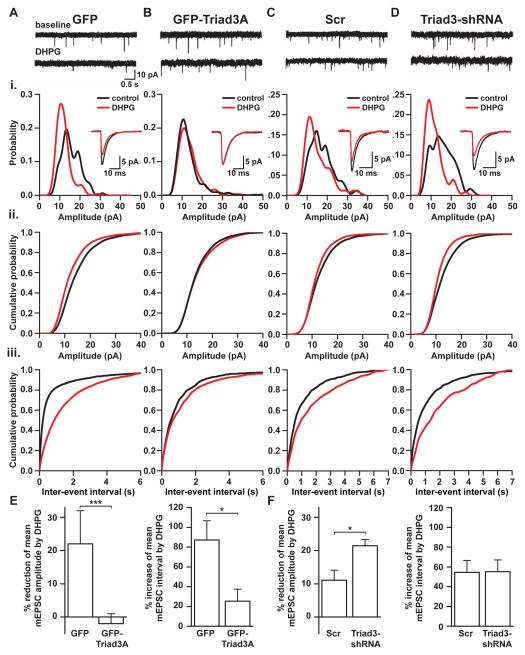
Arc is required for multiple forms of activity-dependent synaptic plasticity, including homeostatic scaling, hippocampal LTP, and protein synthesis-dependent LTD (Gao et al., 2010; Guzowski et al., 2000; Jakkamsetti et al., 2013; Rial Verde et al., 2006; Shepherd and Bear, 2011; Shepherd et al., 2006; Waung et al., 2008). In hippocampal neurons, overexpression of Triad3A mimicked and occluded TTX-induced upregulation as measured by surface expression of synaptic GluA1 (Figures 7A and 7B). In contrast, Triad3A knockdown blocked the scaling effects of TTX (Figures 7A and 7C). To confirm our findings, we tested the effect of Triad3A on miniature excitatory postsynaptic currents (mEPSCs) following chronic activity manipulations. Whereas Triad3A overexpression mimicked and occluded the increase of mEPSC amplitudes normally induced by TTX treatment (48 h, 1 μM), Triad3A knockdown prevented any TTX-induced increase in mEPSC amplitudes (Figures 7D–7H). Given the inverse relationship between Arc levels and its ubiquitinating enzyme Triad3A, these data support a model whereby negative regulation of Arc by Triad3A mediates TTX-induced upscaling (Shepherd et al., 2006).
Figure 7. Triad3A is Required for Activity-Induced Upscaling of Synaptic Strength.
(A) Representative images of DIV21–24 hippocampal neurons expressing GFP, GFP-Triad3A, Scr shRNA, or Triad3-shRNA treated in the presence of 1 μM TTX for 48 h. Surface AMPA receptors (sGluA1) were visualized by live GluA1 antibody labeling and subsequent fixation under non-permeabilized conditions. Scale bars, 10 μm.
(B–C) Quantitative analysis of surface GluA1 labeling on hippocampal neurons expressing GFPTriad3A (B) or Triad3-shRNA (C). Data represent means ± SEM. *p < 0.05, n = 6–23. **p < 0.005, n = 6–23.
(D) mEPSC recordings from DIV17–21 neurons expressing GFP, GFP-Triad3A or Triad3-shRNA treated with 1 μM TTX for 48 h. Note the increase in mEPSC amplitudes in GFP-expressing neurons upon TTX treatment that is prevented by Triad3A-shRNA and occluded by Triad3A overexpression.
(E) Cumulative mEPSC amplitude distribution summarizing data from GFP (top), GFP-Triad3A (middle), and Triad3-shRNA (bottom) expressing cells in control and following TTX treatment.
(F) Cumulative mEPSC inter-event interval distribution summarizing data from GFP (top), GFPTriad3A (middle), and Triad3-shRNA (bottom) expressing cells in control and following TTX treatment. Note that mEPSC frequency is not affected by GFP-Triad3A or Triad3-shRNA.
(G) Data represent means ± SEM of mEPSC amplitudes. *p < 0.05, n = 11–13.
(H) Data represent means ± SEM of mEPSC frequencies, n = 11–13.
Arc synthesis is required for long-term synaptic depression (LTD) elicited by the mGluR1/5 agonist 3,5-dihydroxyphenylglycine (DHPG) in CA1 hippocampus, a form of synaptic plasticity termed mGluR-LTD (Waung et al., 2008). We reasoned that by targeting Arc for degradation, Triad3A might act to dampen or limit mGluR-LTD. In control neurons expressing GFP, bath application of RS-DHPG (100 μM, 10 min) led to a robust depression of mEPSC amplitudes and frequency (Figures 8A and 8E). Overexpression of GFP-Triad3A prevented this mGluR-induced synaptic depression (Figures 8A and 8E; percent reduction: GFP, 22 ± 1 %; GFP-Triad3A, −2 ± 3 %), consistent with a reduction in activity-induced Arc (Figures 4E and 4F). We then knocked down Triad3A by expressing Triad3-shRNA in hippocampal neurons (Figure 8D). In control neurons expressing a scrambled shRNA, DHPG application produced a significant depression of mEPSC amplitude and frequency (Figures 8C and 8F). Notably, in neurons expressing Triad3-shRNA, we observed a larger decrease in mGluR-dependent mEPSC amplitude, with a nearly two-fold greater reduction in mEPSC amplitudes relative to Scr control (Figures 8C, 8D, and 8F; percent reduction: Scr, 12 ± 3 %; Triad3-shRNA, 21 ± 2 %), supporting the hypothesis that control of Arc levels by Triad3A determines the degree or extent of synaptic depression induced by mGluR activation.
Figure 8. Triad3A Limits mGluR-Mediated Synaptic Depression.
(A) Representative AMPAR-dependent mEPSC traces from a neuron expressing GFP recorded during baseline and after application of DHPG (100 μM). (Ai) mEPSC amplitude distributions from the same cell as in (A). Note the reduction in unitary quantal size after DHPG exposure (the red trace is shifted to the left and there is an increase in main peak amplitude). Inset, superimposed average mEPSC waveforms recorded at baseline and after DHPG exposure. (Aii) Cumulative mEPSC amplitude distribution summarizing data from GFP expressing cells at baseline and after DHPG exposure. *** p < 0.0001. (Aiii) Cumulative mEPSC interval distribution summarizing data from the same cells as (Aii). *** p < 0.0001. Data is from 5 cells: baseline, 1574 events; DHPG, 1957 events.
(B) Representative AMPAR-dependent mEPSC traces from a neuron expressing GFP-Triad3A recorded during baseline and after DHPG (100 μM) exposure. (Bi) mEPSC amplitude distributions from the same cell as in (B). Note that DHPG has little effect on quantal size. Inset, superimposed average mEPSC waveforms recorded at baseline and after DHPG exposure. (Bii) Cumulative mEPSC amplitude distribution summarizing data from GFP-Triad3A expressing cells at baseline and after DHPG exposure. p = 0.073. (Biii) Cumulative mEPSC interval distribution summarizing data from the same cells as (Bii). p = 0.006. Data is from 5 cells: baseline, 1072 events; DHPG, 1913 events.
(C) Representative AMPAR-dependent mEPSC traces from a neuron expressing scrambled RNA (Scr) recorded during baseline and after DHPG (100 μM) exposure. (Ci) mEPSC amplitude distributions from the same cell as in (C). Inset, superimposed average mEPSC waveforms recorded at baseline and after DHPG exposure. (Cii) Cumulative mEPSC amplitude distribution summarizing data from scrambled RNA expressing cells at baseline and after DHPG exposure. ** p = 0.00042. (Ciii) Cumulative mEPSC interval distribution summarizing data from the same cells as (Cii). *** p < 0.0001. Data is from 5 cells: baseline, 832 events; DHPG, 836 events.
(D) Representative AMPAR-dependent mEPSC traces from a neuron expressing Triad3-shRNA recorded during baseline and after DHPG (100 μM) exposure. (Di) mEPSC amplitude distributions from the same cell as in (D). Note the larger reduction in unitary quantal size after DHPG addition compared to the cell expressing Scr shRNA (Cii). Inset, superimposed average mEPSC waveforms recorded at baseline and after DHPG exposure. (Dii) Cumulative mEPSC amplitude distribution summarizing data from Triad3-shRNA expressing cells in control solution and in DHPG. *** p < 0.0001. (Diii) Cumulative mEPSC amplitude distribution summarizing data from the same cells as (Dii). *** p < 0.0001. Data is from 5 cells: baseline, 559 events; DHPG, 691 events. For the insets, the mEPSCs were aligned on the rise and are averages of 100 mEPSCs for each condition. Statistical significance was measure using one-way ANOVA and data are from at least 3 independent experiments.
(E) Bar graphs summarizing the effects of DHPG on mEPSCs from cells expressing GFP (n=5 cells) and GFP-Triad3A (n = 5 cells). Left, graph plotting reduction in mean mEPSC amplitude (p = 0.000141). Right, graph plotting mean increase in the interval between mEPSCs (p = 0.02719).
(F) Bar graphs summarizing the effects of DHPG on mEPSCs from cells expressing scrambled RNA (n = 5 cells) and Triad3-shRNA (n = 5 cells). Left, graph plotting reduction in mean mEPSC amplitude (p = 0.03212). Right, graph plotting mean increase in the interval between mEPSCs (p = 0.953). Statistical significance was measured using an unpaired t-test.
Discussion
In the present study, we have identified Triad3A/RNF216 as a novel E3 ubiquitin ligase targeting Arc – an activity-regulated gene product that regulates AMPA receptor endocytosis and synaptic plasticity. We have demonstrated that Triad3A is present at clathrin-coated pits (CCPs), including endocytic zones of dendritic spines. By limiting Arc protein levels induced by synaptic activity, Triad3A regulates Arc-dependent forms of synaptic plasticity. Our findings support a model, in which the upregulation and recruitment of Arc to the endocytic machinery is coupled to its ubiquitination and degradation by Triad3A.
An Ubiquitin-Dependent Brake on Arc-Induced Plasticity
Many forms of synaptic plasticity are expressed by the insertion or removal of AMPA receptors from the postsynaptic membrane (Kessels and Malinow, 2009; Malenka and Bear, 2004; Newpher and Ehlers, 2008). In response to learning-related synaptic activity and protein-synthesis dependent LTD, Arc is rapidly induced (Guzowski et al., 1999; Luscher and Huber, 2010; Lyford et al., 1995; Steward et al., 1998) and facilitates endocytosis of synaptic AMPA receptors (Waung et al., 2008). Arc-mediated endocytosis of AMPA receptors dampens excitatory synaptic transmission and this compensatory decrease in synaptic strength mediates homeostatic plasticity (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006). Here we have shown that Arc half-life and hence Arc levels are determined by ubiquitindependent degradation mediated by Triad3A. Triad3A binds to Arc and ubiquitinates Arc at lysines 268 and 269. Furthermore, Triad3A is required for homeostatic synaptic upscaling and mGluR-induced synaptic depression. Although Triad3A is required for TTX-induced upscaling of synaptic strength, manipulations of Triad3A did not significantly influence downscaling of AMPA receptors following bicuculline treatment (data not shown). These findings are consistent with Shepherd et al who found a larger effect of Arc on TTX-induced upscaling whereas Arc knockout neurons were still capable of synaptic depression induced by bicuculline (Shepherd et al., 2006). Thus, Triad3A and Arc share similar properties for synaptic scaling. In addition, we have shown that Triad3A exerts tonic negative regulation of mGluR-induced synaptic depression, consistent with previous work demonstrating that Arc synthesis is required for mGluR-LTD (Waung et al., 2008), and further supporting Triad3A’s role in mediating Arc. By controlling Arc levels via ubiquitination and UPS-dependent degradation, Triad3A limits AMPA receptor endocytosis and associated synaptic depression.
In addition to regulating rapid forms of synaptic plasticity, loss of Arc or deletion of the endophilin binding domain of Arc both prevent forms of homeostatic synaptic plasticity (Beique et al., 2011; Gao et al., 2010; Shepherd et al., 2006) that globally scale AMPA receptor-dependent synaptic currents (Turrigiano, 2008). Such slow forms of homeostatic plasticity are known to require ubiquitin-dependent degradation and remodeling of the PSD. The translation of Arc mRNA is rapidly induced by activation of group I mGluRs, and this synthesis of Arc is coupled to depression of synaptic strength in a manner regulated by the fragile X mental retardation protein (FMRP) (Niere et al., 2012; Park et al., 2008; Waung et al., 2008). We found that disruption of the dynamic cycle of Arc induction and subsequent degradation by knockdown of Triad3A prevents mGluR-dependent synaptic depression. Given the role of Arc in executing both rapid and more chronic forms of synaptic plasticity, an attractive model is that Arc protein levels are under continuous online regulation via regulated synthesis and degradation, the latter of which is controlled by Triad3A. These findings are supported by recent work, which demonstrates that Arc levels dictate the ability of a cell to elicit mGluR-LTD (Jakkamsetti et al., 2013). One prediction of this model is that alteration of Triad3A can trigger both immediate and longer-term effects on synaptic strength that will depend on ongoing synaptic activity, patterns of receptor activation, and homeostatic compensation. Our findings strongly suggest that Triad3A couples protein synthesis to protein turnover by targeting Arc for ubiquitin-dependent degradation at excitatory synapses.
Triad3A as a Ubiquitin Ligase in the Endocytic Pathway
Arc interacts with dynamin-2 and endophilin-3, and this interaction is necessary for Arc-mediated endocytosis of AMPA receptors (Bramham et al., 2008; Chowdhury et al., 2006). When Arc is expressed in HeLa cells, it localizes to both the cytoplasm and nucleus with partial enrichment at the plasma membrane (Chowdhury et al., 2006). This localization pattern is altered upon coexpression with endophilin and dynamin, which results in a redistribution of Arc to endosomal structures (Chowdhury et al., 2006). Using TIRF microscopy, we found that a pool of Arc localizes to CCPs in COS7 cells. This finding, along with an increased surface lifetime of Arc-KR, suggests that upon activity-induced synthesis, Arc is recruited to the cell surface by molecular interactions with endophilin-3 and dynamin-2 where it encounters Triad3A residing at CCPs for subsequent ubiquitination and UPS-mediated degradation. Although our findings support a temporal relationship between Arc synthesis, coated pit association, and Triad3A-mediated ubiquitination, it will be important for future studies to elucidate the precise timing and kinetics of Triad3A-mediated Arc ubiquitination before, during, and after a period of facilitated endocytosis.
Similar to other UPS targets, the addition and removal of ubiquitin chains on Arc likely reflect the competing activity of other ubiquitin ligases and de-ubiquitinating enzymes (DUBs) as well as other ubiquitin-like modifications such as SUMOylation (Craig et al., 2012). Moreover, ubiquitination of Arc by Triad3A at CCPs could facilitate interactions with other endocytic proteins, such as Eps15, Eps15R, and epsin that normally localize to CCPs and contain ubiquitin-binding motifs (Di Fiore et al., 2003). We observed a large fraction of Arc events at the cell surface that did not colocalize with CCPs but did colocalize with Triad3A, suggesting that Arc may also function in dynamin-dependent but clathrin-independent pathways to regulate endocytosis (Hansen and Nichols, 2009). Overall, the Triad3A-Arc pathway defined here reveals a mechanistic link between the UPS, AMPA receptor endocytosis, and activity-dependent plasticity. Beyond the nervous system, such a mechanism may provide a general paradigm for the transient modification of endocytosis in diverse tissues.
Regulating the Stability of Arc
In a recent study, the HECT domain E3 ligase Ube3a, whose maternal copy loss causes Angelman syndrome (AS) (Kishino et al., 1997), was found to be a direct modulator of Arc (Greer et al., 2010). We were unable to detect Arc ubiquitination by Ube3a in HEK cells, even though we found that Ube3a overexpression reduced Arc protein levels in the same system as previously reported (Greer et al., 2010). Furthermore, we failed to detect Ube3a-dependent poly-ubiquitination of full-length Arc, but could detect Ube3a-dependent mono-ubiquitination on a recombinant C-terminal Arc fragment (132–396) as previously reported (Greer et al., 2010). Finally, although we could detect a weak interaction between Arc and Ube3a in neurons, we were unable to detect a loss of Arc ubiquitination in Ube3a null neurons. Our findings are consistent with a recent study showing that Ube3a does not promote ubiquitination of Arc (Kuhnle et al., 2013). Intriguingly, we found that the same two lysine residues on Arc (K268 and K269) reported to be ubiquitinated by Ube3a (Greer et al., 2010) are direct acceptor sites for ubiquitination by Triad3A. Ube3a may regulate Arc degradation following prolonged changes in synaptic activity, whereas Triad3A may be involved in the steady-state turnover of Arc under basal synaptic transmission and upon more immediate increases or decreases in synaptic drive. The exact molecular interplay between Arc, Triad3A, and Ube3a remains to be elucidated.
Intriguingly, like Ube3a, there may be a link between Triad3A and neurodevelopmental disorders. A recent report described an autistic patient carrying a t(7;16)(22.1;p11.2) reciprocal translocation that results in a highly selective 30-fold increase in the expression of the human Triad3 ortholog RNF216 (also called Q6NUR6, U7I1, UBCE7IP1, and MMD2) at chromosome 7p22.1 (Bayou et al., 2010). The RNF216 gene is one of the genes deleted in a patient with severe colorectal cancer who also exhibits intellectual impairment, but lacks a brain tumor (Will et al., 2007). Moreover, mutations and disruptions in the Triad3/RNF216 gene have recently been described in patients with Gordon Holmes syndrome characterized by ataxia, dementia, and hypogonadotropism accompanied by neuronal loss and intranuclear inclusions in the cerebellum and hippocampus (Margolin et al., 2013). These findings suggest that increasing or decreasing levels of Triad3A could contribute to human intellectual disorders, potentially via modulation of Arc. More broadly, local regulation of Arc by Triad3A may provide a general paradigm for tuning the timing and duration of activity-dependent plasticity in diverse neural circuits.
Experimental Procedures
Detailed methods regarding DNA constructs, cell culture, antibodies, immunocytochemistry, protein purification, in vitro ubiquitin assays, electron microscopy, image acquisition, and immunoprecipitation are included in Supplemental Methods.
Immunoprecipitation
Constructs were transfected in HEK293 cells by Lipofectamine (Invitrogen). Cell extracts were prepared 24 h after transfection in IP buffer. For endogenous interaction of Arc and Ube3a in neurons, Arc protein was immunoprecipitated with Gamma Bind G-Sepharose beads conjugated with an anti-Arc antibody (Santa Cruz, C-7). For details, see supplemental methods.
Metabolic Pulse-Chase Assays
For pulse-chase analysis, DIV14–17 cortical neurons (1.25 × 106 cells per 60 mm dish) were pretreated with 2 μM TTX for 24 h. For metabolic labeling with 35S-cysteine/methionine, cells were washed three times in Neurobasal media (Invitrogen) lacking methionine and cysteine (Invitrogen) and incubated for 15 min at 37°C in the presence of 2 μM TTX. Cells were then incubated for 4 h in methionine-cysteine free media containing 0.3 mCi/mL of EXPRE35S35S methionine/cysteine (Perkin Elmer) in the presence of 2 μM TTX or 40 μM 4-AP plus 50 μM bicuculline to promote activity-dependent Arc protein induction. For details, see supplemental methods.
In Vitro Ubiquitin Assays
In vitro ubiquitination of Arc was performed using 0.1 μg of recombinant ubiquitin E1 (UBE1) (Boston Biochem), 0.5 μg recombinant ubiquitin E2 (UbcH7) (Boston Biochem), 10 μg of recombinant ubiquitin (Boston Biochem), and 0.1 μg immunopurified Flag-Triad3A added to 0.25 μg of His-tagged Arc (or 0.15 μg of GST-Arc full-length or GST-Arc 132–396) in the presence of an ATP regenerating system (10 mM creatine phosphate, 10 units of creatine kinase, 1 unit inorganic pyrophosphatase, and 2 mM ATP) in 50 mM Tris (pH 7.6) and 5 mM MgCl2 in a 120 μl reaction with 1 μg/mL aprotinin and 1 mM PMSF. GST-Arc was purchased from BD Pharmingen. GST-Arc (132–396) was expressed and purified as previously described (Chowdhury et al., 2006; Greer et al., 2010). The reaction was incubated at 30°C for 120 min. Reactions were terminated by boiling samples in SDS-PAGE loading buffer, polypeptides resolved by SDS-PAGE gel, and immunoblot analysis performed with an anti-His antibody to detect ubiquitinated bands or with anti-Flag antibody to detect immunopurified Flag-Triad3A. For additional assay information, see supplemental methods.
Electrophysiology
Homeostatic scaling and baseline mEPSC recordings
Neurons were held at −65mV using a MultiClamp 700B amplifier (Molecular Devices) controlled with a computer running MultiClamp Commander and pClamp (Molecular Devices), filtered at 2 kHz and digitized at 40 kHz (Digidata 1440A, Molecular Devices). Only cells with stable series resistance < 30 MΩ were included for analysis. Data were analyzed offline using ClampFit (Molecular Devices) or MiniAnalysis software (Synaptosoft). Detection criteria for mEPSC included amplitude greater than 8 pA and rise times from the onset to the peak of less than 5 msec. Only recordings maintained more than 10 min were analyzed. For details, see supplemental methods.
DHPG-induced synaptic depression
Recordings of mEPSCs were performed in hippocampal neurons obtained at a holding potential of −75 mV using an Axon Multiclamp 700B amplifier (Molecular Devices, USA), filtered at 3 kHz and digitized at 20 kHz (Digidata 1440A, Molecular Devices). Data acquisition was performed using pClamp 10 (Molecular Devices). Analysis of mEPSCs was performed using MiniAnalysis software (SynaptoSoft, Decatur, GA). Events were manually analyzed and were accepted if they had an amplitude >6 pA (events below this amplitude were difficult to distinguish from baseline noise) and a faster rise than decay. Cumulative probability curves for mEPSC amplitude and interval were constructed using Origin (MicroCal). Statistical significance was measured using a one-way ANOVA with 0.05% taken as significant. For details, see supplemental methods.
Animals
All studies were conducted with protocols approved by Duke University Institutional Animal Care and Use Committee.
Statistical Analysis
Unless otherwise stated, error bars represent the standard error of the mean. Statistical analyses applied were the post-hoc Student’s t test or repeated-measures ANOVA with pairwise multiple comparisons where the significance criterion was p = 0.05.
Supplementary Material
Highlights.
Triad3A ubiquitinates Arc for subsequent degradation.
Triad3A localizes to clathrin-coated pits in dendrites and spines.
Triad3A limits Arc-dependent endocytosis of AMPA receptors.
Triad3A regulates Arc-mediated forms of synaptic plasticity.
Acknowledgments
We thank Dr. Tsung-Hsien Chuang for supplying Triad3A, B, C constructs and Triad3A-specific antibody, Dr. John Asara at the Beth Israel Deaconess Medical center (BIDMC) Mass Spectrometry Core Facility for mass spectrometry assistance, Dr. Chandra Tucker at the Duke University Yeast Model Systems Genomics Facility for performing the yeast-two-hybrid screen, and Dr. Serena Dudek for piloting electrophysiology. We also thank Dr. Anne Taylor for providing rat hippocampal neurons, Gul Dolen, Matthew Judson, Marguerita Klein, Sarah Lancaster, Irina Lebedeva, and JayaLakshmi Miriyala for excellent technical assistance. We thank Ben Arenkiel, Cyril Hanus, Juliet Hernandez, Matthew Kennedy, Richard Mooney, Thomas Newpher, and Rui Peixoto for their critical comments on the manuscript. This work was supported by NIH grants R01 NS047574, R01 MH064748, and the Howard Hughes Medical Institute (to M.D.E.). A.M.M. was supported by an NRSA postdoctoral fellowship from the NINDS F32NS067712. H.S.J. was supported by a fundamental and translational neuroscience NIH training grant fellowship to Duke University Medical Center. Y.Q. was supported by the Singapore ministry of education academic research fund (MOE2012-T2010921). R.S.L. was supported by an NRSA predoctoral fellowship from the NIMH F31MH091817. Work in the S.A.L.C. lab is supported by the BBSRC (BB/H018344/1). Work in the lab of M.D.E. is supported by Pfizer, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayou N, Belhadj A, Daoud H, Briault S, Helayem MB, Chaabouni H, M’Rad R. Exploring the 7p22.1 chromosome as a candidate region for autism. J Biomed Biotechnol. 2010;2010:423894. doi: 10.1155/2010/423894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci U S A. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TJ, Jaafari N, Petrovic MM, Rubin PP, Mellor JR, Henley JM. Homeostatic synaptic scaling is regulated by protein SUMOylation. The Journal of biological chemistry. 2012;287:22781–22788. doi: 10.1074/jbc.M112.356337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol. 2003;4:491–497. doi: 10.1038/nrm1124. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci. 2010;30:7168–7178. doi: 10.1523/JNEUROSCI.1067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122:1713–1721. doi: 10.1242/jcs.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F, Wang KH, Osten P, Bassell GJ, Gibson JR, et al. Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron. 2013;80:72–79. doi: 10.1016/j.neuron.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A. 2009;106:316–321. doi: 10.1073/pnas.0806518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- Korb E, Wilkinson CL, Delgado RN, Lovero KL, Finkbeiner S. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nature neuroscience. 2013;16:874–883. doi: 10.1038/nn.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnle S, Mothes B, Matentzoglu K, Scheffner M. Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8888–8893. doi: 10.1073/pnas.1302792110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches Synaptic plasticity: LTP and LTD. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Margolin DH, Kousi M, Chan YM, Lim ET, Schmahmann JD, Hadjivassiliou M, Hall JE, Adam I, Dwyer A, Plummer L, et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N Engl J Med. 2013;368:1992–2003. doi: 10.1056/NEJMoa1215993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, Huber KM. Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:5924–5936. doi: 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Merrifield CJ. Dynamics of endocytic vesicle creation. Dev Cell. 2005;9:581–592. doi: 10.1016/j.devcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will O, Carvajal-Carmona LG, Gorman P, Howarth KM, Jones AM, Polanco-Echeverry GM, Chinaleong JA, Gunther T, Silver A, Clark SK, et al. Homozygous PMS2 deletion causes a severe colorectal cancer and multiple adenoma phenotype without extraintestinal cancer. Gastroenterology. 2007;132:527–530. doi: 10.1053/j.gastro.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, Chowdhury S, Shepherd JD, Dehoff M, Li Y, et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent beta-amyloid generation. Cell. 2011;147:615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.