Abstract
In a previous study we have demonstrated, using a novel diffusion MRI analysis called free-water imaging, that the early stages of schizophrenia are more likely associated with a neuroinflammatory response and less so with a white matter deterioration or a demyelination process. What is not known is how neuroinflammation and white matter deterioration change along the progression of the disorder. In this study we apply the free-water measures on a population of 29 chronic schizophrenia subjects and compare with 25 matching controls. Our aim was to compare the extent of free-water imaging abnormalities in chronic subjects with the ones previously obtained for subjects at their first psychotic episode. We find that chronic subjects showed a limited extent of abnormal increase in the volume of the extracellular space, indicative of a less extensive neuroinflammatory response relative to patients at the onset of schizophrenia. At the same time, the chronic schizophrenia subjects had greater extent of reduced fractional anisotropy compared to the previous study, suggesting increased white matter deterioration along the progression of the disease. Our findings substantiate the role of neuroinflammation in the earlier stages of the disorder, and the effect of neurodegeneration that is worsening in the chronic phase.
Keywords: schizophrenia, neuroinflammation, free-water, degeneration, white matter
1. Introduction
The development of diffusion MRI, and its most common analysis method, diffusion tensor imaging (DTI) (Basser et al., 1994), have made it possible to study imaging correlates of white matter pathologies in schizophrenia. Many DTI studies have found decreased fractional anisotropy (FA), and some have found increased mean diffusivity (MD) in different populations of schizophrenia subjects (see Fitzsimmons et al. (2013) for a recent review.) Despite non-specificity of the FA and MD measures, the DTI findings are often considered evidence of a white matter pathology that is likely related to demyelination (Kubicki et al., 2007). Myelin related deficiencies were also inferred from histopathological studies (Uranova et al., 2011), and genetics studies (Davis et al., 2003). An alternative interpretation of the DTI results associates the abnormalities with a neuroinflammatory response, further supported by increased cytokine levels, microglial activation measured with PET, genetic association, and upregulation of inflammatory pathways (see Najjar and Pearlman (in print), for a recent review of neuroinflammation related findings in schizophrenia.)
There is a reciprocal causative relation between neuroinflammation and degeneration, with prolonged inflammatory response that can lead to deterioration, and, on the other hand, inflammation that may be triggered by cellular deterioration (Streit, 2006). Distinguishing between neuroinflammation and deterioration is therefore important to understand the etiology of schizophrenia, and to better target potential treatments. Recently, free-water imaging (Pasternak et al., 2009) was proposed as an analysis method of diffusion MRI that can differentiate the contribution of water molecules diffusing freely in the extracellular space from the contribution of water molecules that diffuse close to tissue membranes. Therefore free-water imaging can help to differentiate between neuroinflammation that is expected to affect the water content in the extracellular space, and white matter deterioration that is expected to affect the tissue itself.
In our previous free-water imaging study, patients diagnosed with schizophrenia were scanned following their first psychotic episode (FE) (Pasternak et al., 2012). It was found that a regular DTI analysis comparing the FE group with matched controls shows a widespread global decrease in FA and overlapping increase in MD. However, applying the free-water imaging analysis revealed that the majority of differences between these groups could be explained as an increase in the extracellular space, and that anisotropy differences in the tissue were only limited to focal areas in the frontal lobe. This disambiguation of the source of differences between the groups led to a conclusion that the early stages of schizophrenia are more likely associated with a neuroinflammatory response and less so with a white matter deterioration or a demyelination process.
What is not known is how neuroinflammation and white matter deterioration change along the progression of the disorder. If schizophrenia has a neurodegenerative component, an increased extent of deterioration is expected as the disease progresses. Furthermore, it is not known whether neuroinflammation also plays a role in the chronic stages of schizophrenia. To address these questions, in this study we apply the free-water imaging method on a cohort of chronic schizophrenia (CHR) patients, and compare the extents of deterioration and neuroinflammation with the ones previously obtained for FE patients.
2. Methods
2.1 Subjects
The subjects were 29 patients diagnosed with CHR and 25 controls matched for age, gender, handedness, PSES and premorbid IQ. Patients with CHR were recruited from in-patient, day treatment, out-patient, and foster care programs. DSM-IV diagnoses were based on SCID-P interviews, and information from patient medical records. The CHR subjects were included if they were at least 1 year following diagnosis, however the average duration of illness for this cohort was 15 years (see table 1). The Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS) were used to evaluate positive and negative symptoms in schizophrenia (Andreasen, 1983, 1984). The severity of clinical symptoms was also assessed using the positive and negative symptom scale (PANSS) (Kay et al., 1987). In our cohort there was an average score of 10.8 for SANS, 10.2 for SAPS and 22.9 for positive PANSS, 21.7 for negative PANSS and 42.4 for general PANSS. Comparison subjects (group-matched to patients on age, sex, handedness (Edinburgh Handedness Inventory), and parental social economic-status (PSES)) were recruited through advertisements in local newspapers, and tested with SCID-NP interviews. All subjects were included in the study if they met the following criteria: ages between 18 and 55, no history of neurological illness, no alcohol or drug dependence in the last 5 years and no abuse in the past year, right handedness and an ability and desire to cooperate with the procedures. Normal comparison subjects were also screened to exclude first-degree relatives with an Axis I disorder. For demographic details of both patients and controls see Table 1. The study was approved by the local IRB committees. All subjects signed informed consent forms prior to study participation.
Table 1.
Clinical and experimental parameters
| Controls | Schizophrenia | ||
|---|---|---|---|
| n | 25 | 29 | |
| female | 5 | 4 | |
| male | 20 | 25 | |
| CPZ (mg/day) | 451 ± 273 | ||
| duration of illness (months) | 15 ± 10.5 | ||
| IQ | 98.92 10.12 | 101.1 14.7 | p=0.64 |
| PSES | 2.79 1.38 | 2.81 0.94 | p=0.96 |
| age (years) | 43.68 ± 7.62 | 46.59 ± 9.504 | p=0.30 |
| education (years) | 14.35 ± 1.843 | 13.75 ± 2.51 | p=0.16 |
| motion (mm) | 0.777 ± 0.13 | 0.786 ± 0.184 | p=0.84 |
CPZ = chlorpromazine equivalent daily dosage; PSES = Parental social economic status
2.2 MRI Acquisition
All subjects were scanned on a single 3 Tesla General Electric Signa system (GE Medical Systems). This cohort was scanned using the same scanner and protocol as we previously used to scan the FE patients (Pasternak et al., 2012). However, the scans of the current cohort were acquired following a software upgrade, which may have slightly shifted the measurement. Therefore, in this paper we refrain from quantitatively comparing the results previously obtained for the FE subjects with those reported here for the CHR subjects, and instead compare the spatial extent of findings across studies. The complete MRI acquisition is reported in a previous publication (Pasternak et al., 2012). In short, the acquisition included a high-resolution 1.7x1.7x1.7 mm3 diffusion MRI sequence, with 51 gradient directions with b=900 s/mm2, and eight additional b=0 images, in addition to several other anatomical acquisitions.
2.3 Free-Water Imaging
The analysis pipeline replicated the analysis reported in (Pasternak et al., 2012), including motion and eddy currents artifact corrections and masking of the diffusion MRI. The free-water imaging maps were obtained by fitting the aligned diffusion MRI data with the free-water model (Pasternak et al., 2009). The model includes two compartments, a free-water compartment and a tissue compartment. The free-water compartment accounts for water molecules that diffuse freely (only likely to be found in the extracellular space), and is described by a single parameter, which is the fractional volume of free-water (FW). The second compartment accounts for water molecules that are in proximity to tissue membranes, which is also the signal that is left following the elimination of freely diffusing water molecules. The tissue compartment is modeled using a diffusion tensor and the tensors are converted to scalar measures by calculating their eigenvalue decomposition. The free-water model can be estimated from a conventional diffusion MRI acquisition, in which case additional mathematical restrictions of continuity are enforced to allow the estimation of the two compartments. The model, as well as the continuity restriction, forms a cost function, and the fitting is done using an iterative process that simultaneously minimizes this function for all of the free parameters, namely the FW parameter and the diffusion tensor representing the tissue compartment. As a result, voxel-wise maps of FA corrected for free-water (FAT) are obtained, in addition to the voxel-wise FW map. This FAT parameter is equivalent to the regular FA parameter, yet since the signal from the extracellular space is attenuated, FAT is more sensitive and specific to geometrical changes that occur in the tissue (Metzler-Baddeley et al., 2012).
2.4 Statistical analysis
To compare between groups we first applied the tract based spatial statistics (TBSS) method (Smith et al., 2006), which uses FA images from all subjects to coregister onto a common space (MNI), and to generate a common white matter skeleton. The different scalar measures (FW and FAT) were then projected onto the skeleton. Group comparisons were carried using a permutation-based test (Randomise, FSL), with a threshold free cluster enhancement, fully accounting for family-wise errors (Smith and Nichols, 2009). The permutation tests included age, gender and motion as covariates. The resulted voxel-wise significance maps were visualized using the FSL software package in 2D (TBSS-fill and FSLview).
Further analysis was performed in selected regions of interest (ROI), which were defined on the white matter skeleton. Diffusivity values were averaged across all voxels in the ROI. In addition, the correlation coefficients of the average values and the total SANS or SAPS scores were calculated using Pearson correlation. In a secondary analysis we looked at each SANS or SAPS socre separately, and compared the averaged diffusivity values between symptomtatic (score >0) and non-symptomatic (score=0) patients using a t-test.
3. Results
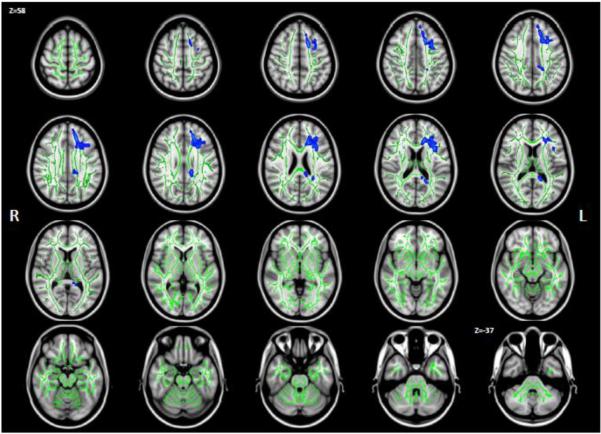
Comparing the FW measure between the schizophrenia patients and controls showed localized increased FW (Fig. 1). Voxels with significantly increased FW (p<0.05 in blue) were found on 2.7% of the white matter skeleton (green) in the left hemisphere, and none on the right side. This is a much smaller extent compared to the widespread FW increase of 41% in the left hemisphere and 38% in the right hemisphere white matter of FE patients (Pasternak et al., 2012). The brain areas showing significantly increased FW in the CHR patients were in the left frontal, parietal and temporal white matter, identified by an atlas (Mori et al., 2005 The Netherlands (2005)) as the anterior, superior and posterior corona radiata, as well as parts of the genu and splenium of the corpus callosum (forceps minor and major). The average FW in the significant voxels was 0.18 for the control group and 0.22 for the CHR group. These average values over the CHR group did not correlate with any of the summarized clinical measures. A secondary analysis revealed that subjects with the following positive symptoms had significantly higher FW than asymptomatic subjects: Olfactory hallucinations, circumstantiality, pressure of speech, clanging and inappropriate affect.
Figure 1.
Group differences in FW. Statistically significant (p<0.05) Increased FW in chronic schizophrenia patients compared with controls is shown in blue over the white matter skeleton (green). FW was increased on 2.7% of the left hemisphere skeleton. Presented are axial slices equally spaced between z=58mm to z=-37mm on the MNI template.
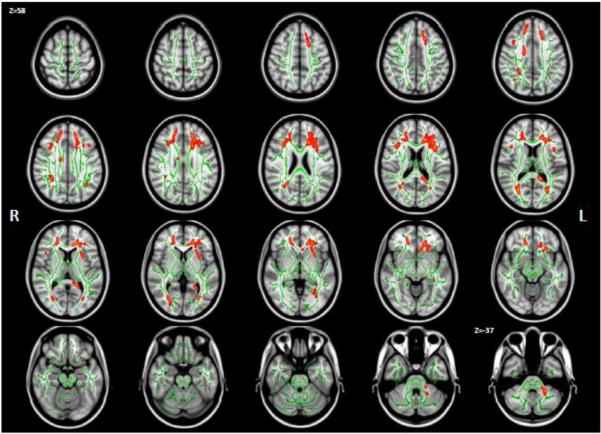
Comparing the FAT measure between the groups showed decreased FAT (Fig 2; p<0.05 in red) for the patient group on 2.1% of the white matter skeleton in the right hemisphere, and 4.3% of the skeleton in the left hemisphere. This is an increase in extent compared to 1.65% and 1.25% of voxels with decreased FAT on the right and left hemisphere skeletons, respectively, reported for FE (Pasternak et al., 2012). The areas where decreased FAT were found for the CHR patients largely overlapped with areas showing increased FW on the left hemisphere, but were also found on the corona radiata on the right hemisphere. In addition there were areas with decreased FAT in parts of the thalamic radiation and SLF in both sides, the left external capsule and left middle cerebellar peduncle.
Figure 2.
Group differences in FAT . Statistically significant (p<0.05) decreased FAT (red) in chronic schizophrenia patients compared with healthy controls was found on 2.1% of the right hemisphere skeleton, and on 4.3% of the left hemisphere skeleton.
Figure 3 compares the extent of decreased FAT for the CHR subjects (peach) with the extent of decreased FAT previously reported for FE patients (cyan), and areas in the brain where the two reports overlapped (red). There was a small overlap of 5.3% (7.5% on the right and 2.3% on the left hemispheres) between the two results, i.e., only 5.3% of the voxels that had significantly decreased FAT for the FE patients also had significantly decreased FAT for the CHR patients. To further compare the results between the two reports we defined two regions of interest: ROI-CHR was composed of all voxels that showed decreased FAT in the CHR patients (red and peach in figure 3); ROI-FE was composed of all voxels that showed decreased FAT in the previous report of first episode patients (red and cyan in figure 3).
Figure 3.
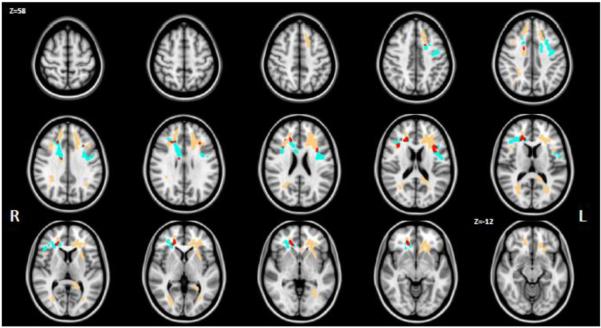
Comparison of decreased FAT in chronic schizophrenia subjects with decreased FAT in first-episode schizophrenia subjects. Voxels previously reported to have lower FAT in first episode are presented in cyan, whereas voxels reported here with lower FAT values in chronic schizophrenia subjects are presented in peach. The overlap (i.e., areas where FAT was significantly decreased in both populations) is colored in red.
The average FAT over ROI-CHR was 0.578 for the controls and 0.534 for the CHR patients. Plotting the values of each subject against age (Fig. 4) shows that group differences were driven by the older subjects. Furthermore, in ROI-CHR there was a negative correlation between FAT and age for the CHR subjects (R=−0.38; p=0.0409), but not for the controls (R=−0.06; p=0.7580). The average FAT in ROI-CHR was negatively correlated (R=−0.47; p=0.014) with negative symptoms as measured by SANS (Fig. 5), and had a trend level negative correlation (R=−0.34; p=0.086) with the general psychopathology PANS scale. A secondary analysis did not reveal any significant group differences between symptomatic and asymptomatic subjects for any of the SANS or SAPS scores.
Figure 4.
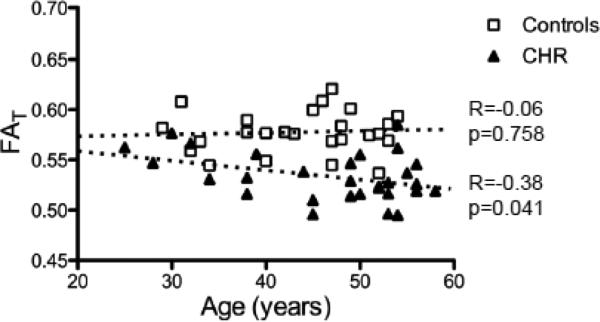
Correlation with Age in ROI-CHR. When averaging FAT over all reduced FAT voxels (red and peach in figure 3) there was a negative correlation with age for the chronic schizophrenia (CHR) patients, but not for the controls. The largest group differences can be seen for the older subjects.
Figure 5.
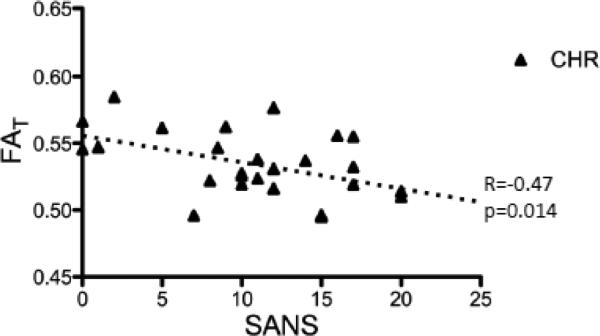
Correlation with negative symptoms. The average FAT over all reduced FAT voxels was negatively correlated with SANS, for the chronic schizophrenia (CHR) patients.
Figure 6 presents the FAT values measured in ROI-FE (Pasternak et al., 2012). Here, for the CHR subjects, within this ROI, the decrease in FAT was no longer significant compared with controls (p =0.102; t(49) = −1.66). The mean FAT across the control subjects was 0.3931, and for the CHR patients 0.3835. Within ROI-FE the CHR patients had a negative correlation between FAT and age (R= −0.42; p=0.022). The correlation coefficient for the controls and age within this ROI was not statistically significant (R= −0.28; p=0.18).
Figure 6.
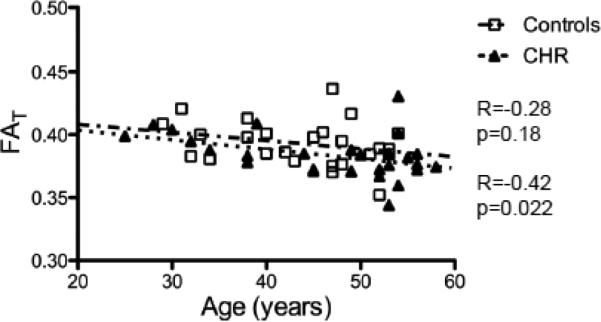
Correlation with Age in ROI-FE. The average FAT over voxels that previously showed reduced FAT in First-episode patients (cyan and peach in figure 3) was negatively correlated with age for both the chronic schizophrenia patients and for the healthy controls.
4. Discussion
Our results demonstrate that free-water imaging identifies abnormalities in the chronic stage of schizophrenia. There were limited areas in the brain that showed increased free-water, and larger areas in the brain that showed decreased FAT. Taken together with previous results obtained for subjects in the early stages of the disease, our findings suggest that white matter deterioration may play a larger role than neuroinflammation in the chronic stages of schizophrenia.
For typical DTI settings, such as those used here, freely diffusing water molecules require spaces larger than a few tens of microns, which are not likely to be found within brain cells, since those typically have smaller sizes (Kandel et al., 2013). Therefore the FW contrast provides a voxel-wise measure of extracellular fractional volume that is expected to be increased in neuroinflammation due to modulation of the blood brain barrier permeability (Whitney et al., 2009). We report here that the extent of abnormally increased FW was much lower in CHR patients, compared with FE patients in our previous report (Pasternak et al., 2012), suggesting that neuroinflammation diminishes in extent at the chronic phases of schizophrenia. This is aligned with findings from other studies, where neuroinflammation was mainly found close to the onset of the disorder (Najjar and Pearlman, in print), or during psychotic episodes (Steiner et al., 2008). Nevertheless, signs of neuroinflammation were also found in post-mortem studies of chronic patients (Fillman et al., 2013). It is interesting to note that peripheral inflammation was also found in the early stages of schizophrenia, which may suggest a chronic inflammatory disorder affecting not just the brain (Dickerson et al., 2012). In our results, we do not find a correlation between FW and summary scores of positive and negative symptoms, but we do find increased FW in patients with specific positive symptoms.
Neuroinflammation is a reversible process; being a response triggered by the innate immune system, neuroinflammation is an important part in the brain's defense mechanism as well as in the healing process of injuries or insults, and is usually resolved autonomously (Streit, 2006). In schizophrenia, one of the recent hypotheses assumes that neuroinflammation is triggered, but is not terminated, much like an immune response in autoimmune diseases (Najjar et al., 2013; Streit, 2006). As a result, toxins that are released during the neuroinflammatory process cause collateral damage to the surrounding tissue (Whitney et al., 2009), which could lead to cellular deterioration. On the other hand, it has also been found that many of the psychiatric drugs have strong anti-inflammatory properties (Sommer et al., 2012), which could diminish neuroinflammation in subjects who respond well to drugs. In our cohort, all of the CHR subjects were medicated, and not currently experiencing a psychotic episode. Therefore medication could be one possible explanation for the reduction in the extent of increased FW from the first episode stage to the chronic stage.
There was a greater extent of decreased FAT reported here for CHR patients, compared with the extent previously reported for FE patients (Pasternak et al., 2012). Further, the location of abnormal FAT has spread from initially being only in the frontal lobe for FE, to the frontal, temporal and occipital lobes bilaterally in the CHR subjects. These findings are in line with an expected neurodegenerative pattern in which more brain areas are affected as the disease progresses (Keshavan, 1999; Lieberman, 1999). There were more FAT findings, as well as FW findings, on the left and frontal areas of the brain. Since the FE results were more symmetric, we can assume that more deterioration develops in the left hemisphere in later stages of schizophrenia. This is consistent with findings in many other studies, showing more tendencies for left and frontal abnormalities in CHR (Ellison-Wright and Bullmore, 2009; Fitzsimmons et al., 2013). Further supporting a neurodegenerative pattern is the fact that the decreased FAT was correlated both with age and with negative symptoms, associating the extent of FAT decreases with the period of illness and its severity (Voineskos et al., 2013). FAT, however, did not show group differences for any specific negative symptoms.
Only 5.3% of the voxels that had decreased FAT in FE patients (ROI-FE) also had decreased FAT in CHR patients (ROI-CHR). The overlapping area included relatively small regions in the frontal lobe that showed similar FAT differences for both the FE and CHR populations. The FAT values observed in ROI-FE for the CHR population appear to gradually decrease with age and are lower than those reported in the FE population. These FAT values were also lower comparing with the FAT values in ROI-CHR on the same CHR population. Taken together, this suggests that ROI-FE may point to areas where the degeneration process starts early in schizophrenia. On the other hand, the ROICHR areas included much larger brain areas, which may be involved in the later stages of the disease. However, it could also be that differences in locations are population specific, since clinical symptomatology varies between individuals in schizophrenia, as do white matter differences (Melonakos et al., 2011). Furthermore, since age, duration of illness, and duration of medication are highly correlated in CHR subjects, it is not possible to definitively state the cause of decreased FAT, as well as the relation between neuroinflammation and white matter deterioration. It would therefore be important to replicate our findings in a longitudinal design, where the same population is compared in various stages of the disease, rather than a cross-sectional design such as the one used here.
We note that in the previous study increased FW was also found in gray matter (Pasternak et al., 2012). Volumetric gray matter changes (Honea et al., 2005), as well as functional MRI abnormalities (Gur and Gur, 2010) are often reported in CHR subjects. However, studying the gray matter using free-water imaging in the CHR population is more challenging since the increased FW found in gray matter (data not shown) is highly affected by gray matter atrophy. Future work separating the effect of atrophy from the FW measure may be useful for gray matter studies in chronic schizophrenia. In addition, a more direct validation of the association of the free-water measure with neuroinflammation is needed. Our findings also promote the need to search for free-water imaging abnormalities in prodromal stages of schizophrenia, in order to better understand the roles of neuroinflammation and white matter deterioration in the etiology of schizophrenia.
Acknowledgments
This work was partially funded by grants from the NIH (nos. R01MH102377-01, R01MH074794, R01MH082918, P41RR013218, and P41EB015902). OP was partially supported by a NARSAD (National Alliance for Research on Schizophrenia and Depression) Young Investigator grant from the Brain & Behavior Research Foundation.
Role of funding source
The identified funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Ofer Pasternak and Marek Kubicki designed the study. Sylvain Bouix and Marek Kubicki collected the data. Ofer Pasternak and Carl-Fredrik Westin implemented algorithmic tools to analyze the data. Ofer Pasternak, Brian Dahlben and Sylvain Bouix processed the data. Ofer Pasternak and Brian Dahlben performed the statistical analysis. Ofer Pasternak wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that there are no competing, financial, or potential conflicts of interests.
References
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA.: 1983. [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) The University of Iowa; Iowa City, IA.: 1984. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Khushalani S, Yang S, Yolken R. C-reactive protein is elevated in schizophrenia. Schizophrenia Research. 2012;143(1):198–202. doi: 10.1016/j.schres.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia research. 2009;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Molecular Psychiatry. 2013;18(2):206–14. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues in clinical neuroscience. 2010;12(3):333–343. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. The American journal of psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth AJ. Principles of Neural Science. Fifth Edition. McGraw-Hill Education; 2013. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. Journal of psychiatric research. 1999;33(6):513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of psychiatric research. 2007;41(1-2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46(6):729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel-based morphometry (VBM) studies in schizophrenia-can white matter changes be reliably detected with VBM? Psychiatry Res. 2011;193(2):65–70. doi: 10.1016/j.pscychresns.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK. How and how not to correct for CSF-contamination in diffusion MRI. NeuroImage. 2012;59(2):1394–1403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. 1st Edition. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- Najjar S, Pearlman DM. Association between neuroinflammation and white matter pathology in schizophrenia: a systematic review. Schiz Research. doi: 10.1016/j.schres.2014.04.041. in print. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. Journal of neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011;2011:325789. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA psychiatry. 2013;70(5):472–480. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]