Abstract
For isolation of environmental mycobacteria, a decontamination procedure has been standardized by which treatment with 3% sodium dodecyl sulfate plus 4% NaOH (15 and 30 min for rapid and slow growers, respectively) is followed by incubation with 2% cetrimide (5 and 15 min for fast- and slow-growing mycobacteria, respectively); this procedure was found to completely eliminate contamination with other organisms and resulted in the isolation of only mycobacteria.
Several species of environmental mycobacteria have been known to be important human pathogens (12). Further exposure to them is believed to alter immunity to vaccines like Mycobacterium bovis BCG (11). Isolation of mycobacteria from environmental samples is difficult because other microbes are also present in the environment. All mycobacterial species are not equally resistant to the different decontamination procedures. For the isolation of mycobacteria from environmental samples, such as soil and water, different methods have been described by various workers (1-10). These are not universally applicable because of differences between floras. No studies of this issue had previously been carried out in the northern parts of India. For this reason, the present study was undertaken to select or develop an improved, appropriate decontamination method(s) for the isolation of mycobacteria from the predominantly hot, dry environment of Agra, India (annual temperature, maximum of 16 to 47°C and minimum of 3 to 30°C; humidity, maximum of 49 to 100% and minimum of 28 to 70%).
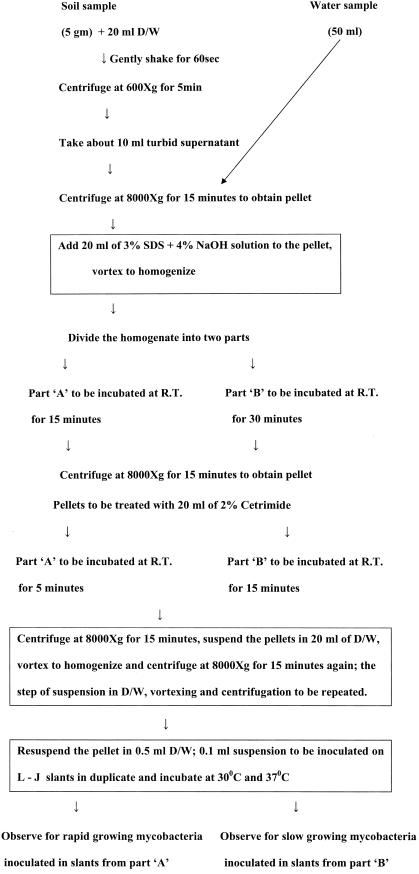
After we tried different permutations and combinations in controlled experiments, the following procedure was standardized (Fig. 1). Wet soil samples of approximately 5 g were collected from a depth of 3 cm, and 50-ml water samples were collected from ditches, ponds, lakes, and rivers in the Agra region throughout the year. Soil was suspended in 20 ml of double-distilled autoclaved water (D/W) in polycarbonate centrifuge tubes. After being shaken manually for 60 s, the suspension was centrifuged at 600 × g for 5 min at 4°C to pellet the soil particles. The turbid supernatant (10 ml) was transferred into other sterile centrifuge tubes and centrifuged at 8,000 × g for 15 min at 4°C. Water samples were centrifuged at 8,000 × g for 15 min at 4°C. Pellets from the soil and water samples were resuspended in 20 ml of treatment solution (3% sodium dodecyl sulfate [SDS] plus 4% NaOH) and then divided into two parts: A and B. Part A was incubated at room temperature (RT) for 15 min to obtain the growth of rapid growers, and part B was incubated at RT for 30 min to obtain the growth of slow growers. After incubation, both the suspensions were centrifuged at 8,000 × g for 15 min at 4°C, and then the supernatants were decanted. Sediments were processed for cetrimide treatment. In the initial pilot experiments, various incubation periods with 2% cetrimide treatment were tried for slow and rapid growers. The pellets were resuspended in 20 ml of 2% cetrimide. Part A was incubated at RT for 5 min to obtain the growth of rapid growers, and part B was incubated at RT for 15 min to obtain the growth of slow-growing mycobacteria, following which the suspensions were centrifuged at 8,000 × g for 15 min at 4°C. Subsequently the pellets were washed twice with 20 ml of D/W and finally resuspended in 0.5 ml of D/W. A 0.1-ml sample of the suspension was inoculated on Lowenstein-Jensen (L-J) slants in duplicate and incubated at 30 and 37°C.
FIG. 1.
Flowchart showing decontamination procedure used for mycobacteria isolated from environmental samples.
Isolation of mycobacteria from water.
When 3% SDS plus 1% NaOH was used with water samples, no mycobacteria could be isolated because all of the samples showed contamination with other organisms. With 3% SDS plus 2% NaOH and with 3% SDS plus 4% NaOH, more than 50% of the samples were found to be contaminated. Finally, treatment with 3% SDS plus 4% NaOH followed by 2% cetrimide was found to be best, as it succeeded in totally eliminating contamination, and both slow and rapid growers could be isolated. M. avium, M. kansasii, M. terrae, M. marinum, M. fortuitum, and M. chelonae were isolated from these specimens (Table 1).
TABLE 1.
Effects of different concentrations of NaOH and cetrimide with 3% SDS on isolation of mycobacteria from water samples
| Expt | Decontaminating treatment | No. of samples | Contamination rate (%)a | Species isolated |
|---|---|---|---|---|
| 1 | 3% SDS + 1% NaOH | 4 | 4/4 (100) | |
| 2 | 3% SDS + 2% NaOH | 16 | 10/16 (62.5) | M. terrae, M. marinum |
| 3 | 3% SDS + 4% NaOH | 16 | 9/16 (56.2) | M. chelonae, M. phlei |
| 4 | 3% SDS + 4% NaOH, 1% cetrimide | 24 | 12/24 (50) | M. avium, M. chelonae, M. flavescens |
| 5 | 3% SDS + 4% NaOH, 2% cetrimide | 24 | 0/24 (0) | M. kansasii, M. terrae, M. avium, M. marinum, M. fortuitum, M. chelonae |
Number of samples in which organisms other than mycobacteria grew after a particular treatment, leading to spoilage of slants and no isolation of mycobacteria, over the total number of samples tested.
Isolation of mycobacteria from soil.
As with the water samples, when 3% SDS plus 1% NaOH was used, all the soil samples showed contamination. Treatments with 3% SDS plus 2% NaOH and with 3% SDS plus 4% NaOH were found to be successful for 62.5 and 75% of the samples, respectively. When the same eight samples were processed by treatment with 3% SDS plus 4% NaOH and 1% cetrimide and with 3% SDS plus 4% NaOH and 2% cetrimide, the success rates were found to be 87.5 and 100%, respectively, and both the slow-growing mycobacteria M. avium and M. terrae and the fast-growing mycobacteria M. fortuitum and M. chelonae were isolated (Table 2).
TABLE 2.
Effects of different concentrations of NaOH and cetrimide with 3% SDS on isolation of mycobacteria from soil samples
| Expt | Decontaminating treatment | No. of samples | Contamination rate (%)a | Species isolated |
|---|---|---|---|---|
| 1 | 3% SDS + 1% NaOH | 4 | 4/4 (100) | |
| 2 | 3% SDS + 2% NaOH | 8 | 3/8 (37.5) | M. terrae |
| 3 | 3% SDS + 4% NaOH | 8 | 2/8 (25) | M. chelonae |
| 4 | 3% SDS + 4% NaOH, 1% cetrimide | 8 | 1/8 (12.5) | M. avium, M. chelonae |
| 5 | 3% SDS + 4% NaOH, 2% cetrimide | 8 | 0/8 (0) | M. terrae, M. avium, M. fortuitum, M. chelonae |
Number of samples in which organisms other than mycobacteria grew after a particular treatment, leading to spoilage of slants and no isolation of mycobacteria, over the total number of samples tested.
It is known that all mycobacterial species are not equally resistant to the different decontamination procedures (5). Falkinham et al. (4) and Reznikov and Leggo (9) had originally developed the methods for the isolation of mycobacteria, particularly those belonging to the M. avium-M. intracellulare-M. scrofulaceum complex from soil. Engbaek et al. (2) have used five methods for decontamination, and the sodium lauryl sulfate method was reported as most suitable. Kamala et al. (7) found treatment with 3% SDS in combination with 1% NaOH to be the most effective decontamination method for soil as well as water samples. When this procedure was followed in our study, contamination could not be removed from any of specimens, which is obviously due to the differences between the range of contaminants present in the samples collected for the present study and that in samples from southern India. In an attempt to further improve success rates, the technique was modified by trying different concentrations of reagents (from 1 to 4% NaOH and 1 to 2% cetrimide) for decontamination. Finally, 3% SDS plus 4% NaOH with 2% cetrimide appears to be more useful, at least in our cases. The 100% success rate obtained by this procedure implies that either only mycobacteria were isolated or no contamination occurred. However, different procedures were followed for rapid and slow growers, because if the same method was followed for both, the rapid growers survived, the L-J slants were full with their growth, and no zone on the L-J slants was left for the slow growers to survive. For this reason, the time of decontamination treatment was increased. When the time of treatment with 3% SDS plus 4% NaOH was increased from 15 to 30 min and the time of 2% cetrimide treatment was increased from 5 to 15 min, the rapid growers were killed or inhibited, but the slow growers survived. One spiking experiment in which water and soil samples (which were positive for fast-growing mycobacteria) were spiked with M. avium (a slow grower) and decontaminated with the method employing increased NaOH and cetrimide treatment times (30 and 15 min, respectively) yielded a 100% rate of isolation of M. avium.
The experience of our study indicates that in-house methods should be developed for the efficient recovery of environmental mycobacteria from various settings in different parts of the world. In the case of water and soil samples, 3% SDS plus 4% NaOH followed by 2% cetrimide treatment yielded more mycobacterial isolates than 3% SDS plus 4% NaOH and 1% cetrimide. The information generated from this study will have a wider application value for the development or optimization of methods for undertaking such studies of similar environmental conditions in other parts of the world.
Acknowledgments
This work was supported by grants from the Department of Biotechnology of the Government of India (grant no. BT/PR/1253/MED/09/203/98) and LEPRA, Colchester, United Kingdom.
We thank Sri Ram and Harishankar for their technical support.
REFERENCES
- 1.Conville, P. S., J. W. B. Andrews, and F. G. Witebsky. 1995. Effect of PANTA on growth of Mycobacterium kansasii in BACTEC 12B medium. J. Clin. Microbiol. 33:2012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engbaek, H. C., B. Vergmann, and B. Weis Bentzon. 1967. The sodium lauryl sulphate method in culturing sputum for mycobacteria. Scand. J. Respir. Dis. 48:268-284. [Google Scholar]
- 3.Engel, H. W. B., L. G. Berwald, and A. H. Havelaar. 1980. The occurrence of M. kansasii in tap water. Tubercle 61:21-26. [DOI] [PubMed] [Google Scholar]
- 4.Falkinham, J. O., III, B. C. Parker, and H. Gruft. 1980. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am. Rev. Respir. Dis. 121:931-939. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins, P. A., S. R. Pattyn, and F. Portaels. 1982. Diagnostic bacteriology, p. 441-470. In C. Ratledge and J. Stanford (ed.), The biology of the mycobacteria, vol. 1. Academic Press, London, England. [Google Scholar]
- 6.Joseph, S., N. G. K. Nair, and P. R. J. Gangadharam. 1969. A sputum swab culture method for tubercle bacilli using cetrimide compared with two other swab culture methods and the concentration culture method. Tubercle 50:299-303. [DOI] [PubMed] [Google Scholar]
- 7.Kamala, T., C. N. Paramasivan, D. Herbert, P. Venkatesan, and R. Prabhakar. 1994. Evaluation of procedure for isolation of nontuberculous mycobacteria from soil and water. Appl. Environ. Microbiol. 60:1021-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portaels, F., A. D. Muynk, and M. P. Sylla. 1988. Selective isolation of mycobacteria from soil: a statistical analysis approach. J. Gen. Microbiol. 134:849-855. [DOI] [PubMed] [Google Scholar]
- 9.Reznikov, M., and J. H. Leggo. 1974. Examination of soil in the Brisbane area for organisms of the Mycobacterium avium-intracellulare-scrofulaceum complex. Pathology 6:269-273. [DOI] [PubMed] [Google Scholar]
- 10.Songer, J. G. 1981. Methods for selective isolation of mycobacteria from the environment. Can. J. Microbiol. 22:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Stanford, J. L., M. J. Shield, and G. W. Rook. 1981. Hypothesis: how environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle 62:55-62. [DOI] [PubMed] [Google Scholar]
- 12.Wallace, R. J., Jr., R. O'Brien, J. Glassroth, J. Raleigh, and A. Dutta. 1990. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. Rev. Respir. Dis. 142:940-953. [DOI] [PubMed] [Google Scholar]