Abstract
Cytophilic immunoglobulin (IgG) subclass responses (IgG1 and IgG3) to Plasmodium falciparum antigens have been associated with protection from malaria, yet the relative importance of transmission intensity and age in generation of subclass responses to pre-erythrocytic and blood-stage antigens have not been clearly defined. We analyzed IgG subclass responses to the pre-erythrocytic antigens CSP, LSA-1, and TRAP and the blood-stage antigens AMA-1, EBA-175, and MSP-1 in asymptomatic residents age 2 years or older in stable (n=116) and unstable (n=96) transmission areas in Western Kenya. In the area of stable malaria transmission, a high prevalence of cytophilic (IgG1 and IgG3) antibodies to each antigen was seen in all age groups. Prevalence and levels of cytophilic antibodies to pre-erythrocytic and blood-stage P. falciparum antigens increased with age in the unstable transmission area, yet IgG1 and IgG3 responses to most antigens for all ages in the unstable transmission area were less prevalent and lower in magnitude than even the youngest age group from the stable transmission area. The dominance of cytophilic responses over non-cytophilic (IgG2 and IgG4) was more pronounced in the stable transmission area, and the ratio of IgG3 over IgG1 generally increased with age. In the unstable transmission area, the ratio of cytophilic to non-cytophilic antibodies did not increase with age, and tended to be IgG3-biased for pre-erythrocytic antigens yet IgG1-biased for blood-stage antigens. The differences between areas could not be attributed to active parasitemia status, as there were minimal differences in antibody responses between those positive and negative for Plasmodium infection by microscopy in the stable transmission area. Individuals in areas of unstable transmission have low cytophilic to non-cytophilic IgG subclass ratios and low IgG3:IgG1 ratios to P. falciparum antigens. These imbalances could contribute to the persistent risk of clinical malaria in these areas and serve as population-level, age-specific biomarkers of transmission.
Keywords: malaria, antibodies, subclass, immunity, pre-erythrocytic antigen, blood-stage antigen
1. INTRODUCTION
The incidence of severe malaria markedly decreases after age 5 years of age in areas of high and stable P. falciparum transmission (Snow et al., 1997) as frequent parasite exposure leads to partial protective immunity against disease. In contrast, older individuals remain at risk for clinical malaria in areas of low or unstable transmission (Okiro et al., 2009; Reyburn et al., 2005). We previously observed (Noland et al., 2008) that asymptomatic residents of an unstable transmission area of Western Kenya had significantly lower total IgG antibody responses to the pre-erythrocytic antigens circumsporozoite protein (CSP), liver-stage antigen 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP), as well as to apical membrane antigen 1 (AMA-1), which is expressed in pre-erythrocytic and blood-stages of infection (Silvie et al., 2004), compared to individuals from a stable, high transmission area. In contrast, prevalence and levels of IgG antibody to the blood-stage antigens merozoite surface protein 1 (MSP-1) and erythrocyte binding antigen 175 (EBA-175), which is also expressed in pre-erythrocytic stages of infection (Gruner et al., 2001), were not significantly different between areas. As antibodies to pre-erythrocytic antigens have been found to associate with protection from infection and disease in high-transmission areas of Western Kenya (John et al., 2005b; John et al., 2008), the lack of antibodies to these antigens may explain in part the persistent risk for severe clinical malaria in residents of unstable transmission areas.
Examination of antibody isotype and subclass profile is critical to interpreting functional anti-malarial immunity. Studies from high-transmission areas consistently observe that cytophilic anti-parasite antibodies, i.e. those of the IgG1 and IgG3 subclasses, predominate in immune serum (Bouharoun-Tayoun and Druilhe, 1992; Chelimo et al., 2005; Egan et al., 1995; John et al., 2005b; Stanisic et al., 2009b; Wahlgren et al., 1983) and often correlate with protection from disease (Aribot et al., 1996; Metzger et al., 2003; Nebie et al., 2008; Sarthou et al., 1997; Shi et al., 1996; Soe et al., 2004; Taylor et al., 1998). The γ-globulin fraction of immune serum is clinically effective in passive transfer experiments (Cohen et al., 1961) and able to inhibit parasite growth in vitro when incubated in the presence of mononuclear cells (Bouharoun-Tayoun et al., 1990). Cytophilic subclass IgG antibodies limit pathogen growth by promoting complement activation, opsonizing phagocytosis, and antibody dependent cellular inhibition (Bouharoun-Tayoun et al., 1990; Ferrante and Rzepczyk, 1997; Tebo et al., 2001), with more recent work suggesting a role for reactive oxygen species release from activated polymorphonuclear neutrophils (Joos et al., 2010). Both IgG1 and IgG3 are capable of mediating these functions, as there is significant overlap in affinities to leukocyte-bound Fcγ receptors (Pleass and Woof, 2001). Cytophilic IgG3 antibodies to parasite antigens tend to be absent, however, in individuals with limited parasite exposure, for example those from a low transmission area of Senegal (Sarthou et al., 1997) or European adults following a primary malaria infection (Bouharoun-Tayoun and Druilhe, 1992; Wahlgren et al., 1983). Furthermore, sera from European individuals containing high levels of non-cytophilic IgG2 abrogated the in vitro growth inhibitory properties of IgG1- and IgG3-rich sera from immune Africans (Bouharoun-Tayoun and Druilhe, 1992). The view that non-cytophilic anti-parasite antibodies may interfere with the protective properties of cytophilic antibodies is further supported by in vivo studies demonstrating heightened susceptibility to disease in individuals with an abundance of non-cytophilic IgG2 or IgG4 antibodies (Ndungu et al., 2002; Soe et al., 2004). Thus development of protective immunity to malaria appears to depend not only on acquisition of parasite-specific antibodies, but also on the generation of cytophilic antibodies in particular.
To determine whether antigen-specific antibody responses from individuals living in unstable transmission area of Western Kenya are biased toward cytophilic or non-cytophilic antibodies, we compared the IgG subclass responses to six pre-erythrocytic and blood-stage P. falciparum antigens (CSP, LSA-1, TRAP, AMA-1, EBA-175, and MSP-1) in a subset of asymptomatic individuals previously evaluated for total IgG responses residing in stable and unstable transmission areas of Western Kenya (Noland et al., 2008).
2. MATERIALS AND METHODS
2.1. Study areas, recruitment, and sample collection
Healthy individuals two years of age and older were recruited as previously described (Noland et al., 2008) from Kanyawegi (population: ~3000; elevation: 1100 m), a holoendemic lowland area in Western Kenya with stable, year-round, intense malaria transmission, where entomological inoculation rates (EIR) have been recorded at >300 infectious bites per person per year (Beier et al., 1990), and Kipsamoite (population: ~3500; elevation: 1950—2100 m), an epidemic-prone highland area characterized by unstable malaria transmission, with an estimated EIR of <1 infectious bite per person per year (Rolfes et al., 2012). Malaria appears to have been introduced into highland areas by rail and travel of persons from endemic areas for tea planting and harvesting in the early 1900’s (Campbell, 1929; Matson, 1957). This likely explains the low prevalence of protective genetic polymorphisms hemoglobin AS (HbAS) and glucose-6-phosphate dehydrogenase deficiency among residents of Kipsamoite (Moormann et al., 2003).
This study was a cross-sectional survey conducted in August 2001, during a time of stable malaria incidence in the high transmission area and during a period of peak malaria incidence in the unstable transmission area (John et al., 2005a). Insecticide treated bed nets were not distributed or used commonly in either area at this time (Kanyawegi 6.0%, Kipsamoite, 0.0%).
Information about the study was provided through local barasas or meetings across the study sites. Field assistants then visited households chosen randomly from a list of households in each site and asked if individuals were interested in participating in the study. Written informed consent was obtained from the study participants or the parents or guardians of individuals under 18 years of age prior to sample collection. Blood was collected by venipuncture from adults (10 to 20 ml) and children (5 ml) to enable multiple types of immunologic investigation, including testing of peripheral blood mononuclear cells for antigen-specific cytokine responses. Plasma was separated by centrifugation from these blood samples and stored for the antibody testing conducted in this study. Blood-stage Plasmodium infection was determined by microscopy as previously described (John et al., 2004). A pre-specified number of households from villages within each site were enrolled to allow proportional geographic representation across each site. From the larger survey sample set, 116 samples from Kanyawegi (stable transmission area) and 96 samples from Kipsamoite (unstable transmission area) were randomly selected for anti-malaria antibody subclass analysis. Ethical approval was obtained from the Ethical Review Committee at the Kenya Medical Research Institute and the Institutional Review Board at Case Western Reserve University and University Hospital of Cleveland.
2.2. ELISA testing
Central repeat peptides of CSP (NANP)5 (Chougnet et al., 1991) and LSA-1 (LAKEKLQGQQSDLEQERLAKEKLQEQQ-SDLEQERLAKEKLQ) (LSA-Rep) (Fidock et al., 1994) as well as recombinant TRAP (3D7), AMA-1 (ectodomain 3D7, nonglycosylated), EBA-175 (3D7, non-glycosylated), and MSP-119 (E-KNG) antigens were used as previously described (Noland et al., 2008). To test for the presence and levels of IgG subclass antibodies, plasma samples diluted 1:100 were tested by enzyme-linked immunosorbent assay (ELISA) using biotinylated mouse anti-human IgG1 (clone HP6069), IgG2 (clone HP6002), IgG3 (clone HP6047), and IgG4 (clone HP6025; all from Zymed Laboratories, San Francisco, CA) as secondary detection antibodies as previously described (John et al., 2005b).
Antibody values were expressed in arbitrary units (AU), which were calculated by dividing the optical density (OD) generated by the test sample by the mean OD plus 3 standard deviations (SD) of samples from 40 North Americans never exposed to malaria. Individual sera from 9 North American control subjects with OD values representative of the 40 North American malaria-naïve samples were used on each plate. OD values greater than 1.0 AU were considered positive for antibody prevalence assessment. Two positive control samples were placed on each plate. The positive control samples were pooled plasma from adults from a Kenyan site with high malaria transmission. OD values corresponding to 1.0 AU for CSP, LSA-1, TRAP, AMA-1, EBA-175 and MSP-1 were 0.023, 0.028, 0.056, 0.054, 0.024, and 0.027 respectively for IgG1; 0.019, 0.037, 0.087, 0.176, 0.022, and 0.023 respectively for IgG2; 0.023, 0.019, 0.026, 0.033, 0.048, and 0.015 respectively for IgG3; and 0.022, 0.055, 0.015, 0.041, 0.23, and 0.011 respectively for IgG4. AU values less than 0, which occurred when sample OD values were less than blank wells, were converted to 0.00. Ratios of cytophilic to non-cytophilic antibodies were calculated by dividing the sum of AU’s for IgG1 and IgG3 by the sum of AU’s for IgG2 and IgG4 for individuals possessing positive cytophilic (either IgG1 and/or IgG3 greater than 1.0 AU) and non-cytophilic responses (either IgG2 and/or IgG4 greater than 1.0 AU).
2.3. Statistical analysis
To determine the significance of antibody prevalence and levels in each age group across the two transmission sites or in individuals with vs. without parasitemia, χ2 analysis and the Wilcoxon rank-sum test were used, respectively. To determine the significance of the trend of antibody prevalence and levels in populations across the four age groups for each site, the χ2 test for trend and a non-parametric test for trend across ordered groups, an extension of the Wilcoxon rank-sum test (Stata command: nptrend), were used, respectively. To determine the significance of difference in ratios of cytophilic to non-cytophilic antibodies and IgG3 to IgG1 antibodies in each age group across sites, the Wilcoxon rank-sum test was used. To assess trends in ratios across age groups within each site, the non-parametric test for trend was used. The non-parametric test for trend is superior to Kruskal-Wallis testing when a trend across groups is assessed, because it assesses for trend rather than a single outlier group. For this descriptive study, we chose individuals randomly from the population. A sample size of approximately 18 individuals in each age group had 90% power to detect a difference between antibody levels of 1.0 and 0.5 AU. All age groups were this size or greater except children 2–5 years of age in the area of stable transmission (n=12, power=65% for detection of the same level of difference) and children 6–15 years of age in the area of unstable transmission (n=14, power=75%). We aimed for a total sample size of approximately 100 in each site to allow for obtaining at least 20 children in each group. The random selection gave us slightly fewer children in two of the groups. All statistical analysis was done using Stata 10.0 software (Stata Corporation, College Station, TX).
3. RESULTS
3.1. Study populations and Plasmodium Prevalence
Population demographics and P. falciparum infection status by age group are shown in Table 1. Overall median age did not differ significantly between stable (22.6 yrs; range 2.8–84.7 yrs) and unstable transmission areas (26.6 yrs; range 2.3–78.6 yrs; P=0.68). Gender distribution was also similar between sites for each age group (P=0.50 overall).
Table 1.
| Table 1a. Population characteristics and P. falciparum (Pf) infection status of asymptomatic individuals from an area of stable malaria transmission by age group
| |||||
|---|---|---|---|---|---|
| Age group (yrs) | n | median age (yrs) | % female | No. Pf + (%) | Median (min, max) Pf parasitemia (parasites/μl) |
| 2–5 | 14 | 4.0 | 57.1 | 10 (71.4) | 2480 (80, 26,000) |
| 6–15 | 30 | 8.1 | 50.0 | 24 (80.0) | 4600 (80, 34,200) |
| 16–40 | 44 | 28.1 | 54.6 | 22 (50.0) | 240 (80, 6,240) |
| >40 | 28 | 56.1 | 60.7 | 13 (46.4) | 240 (80, 2,640) |
|
| |||||
| Total | 116 | 22.6 | 55.2 | 69 (59.5) | 880 (80, 34,200) |
| Table 1b. Population characteristics and P. falciparum (Pf) infection status of asymptomatic individuals from an area of unstable malaria transmission by age group
| |||||
|---|---|---|---|---|---|
| Age group (yrs) | n | median age (yrs) | % female | No. Pf + (%) | Median (min, max) Pf parasitemia (parasites/μl) |
| 2–5 | 19 | 4.5 | 66.7 | 0 (0) | -- |
| 6–15 | 12 | 8.3 | 66.7 | 0 (0) | -- |
| 16–40 | 37 | 26.6 | 40.5 | 0 (0) | -- |
| >40 | 28 | 48.7 | 46.4 | 1 (3.6) | 120 (120, 120) |
|
| |||||
| Total | 96 | 26.6 | 50.5 | 1 (1.0) | 120 (120, 120) |
In the area of stable transmission, 64 of the 116 asymptomatic individuals tested (58.7%) were positive for P. falciparum by microscopy, two (1.7%) were positive for Plasmodium malariae, and five (4.3%) were positive for both P. falciparum and P. malariae. In the area of unstable transmission, only one of the 96 individuals tested (1.0%) was positive for P. falciparum by microscopy and none were positive for P. malariae. No Plasmodium vivax or Plasmodium ovale infections were seen in either population.
3.2. Prevalence of IgG subclass antibodies in an area of stable malaria transmission
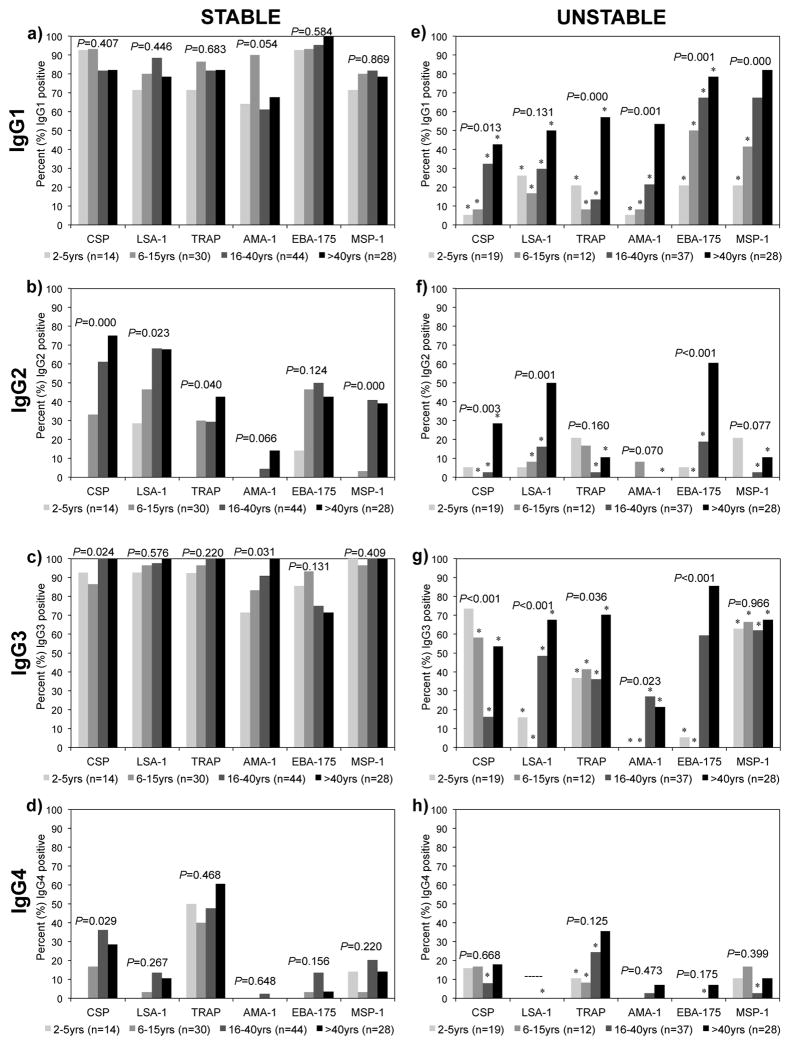
IgG subclass antibody responses to P. falciparum pre-erythrocytic (CSP, LSA-1, and TRAP), dual pre-erythrocytic-blood-stage (AMA-1, EBA-175), and blood-stage (MSP-1) antigens were evaluated in asymptomatic individuals from areas of Kenya with stable malaria transmission (Kanyawegi, n=116) and unstable malaria transmission (Kipsamoite, n=96). In the area of stable malaria transmission, a high prevalence of cytophilic IgG1 (>61%) and IgG3 (>71%) antibodies to each antigen was seen in all age groups (Figure 1a, c). Significant age-related increases in IgG1 or IgG3 antibodies were not seen for any antigens except for IgG3 to CSP and AMA-1. Antigen-specific IgG2 responses were less prevalent, but increased with age, such that >39% of individuals above 40 years of age had IgG2 antibodies to all antigens except AMA-1 (Figure 1b). IgG4 responses were infrequent to all antigens except CSP and TRAP, and increased with age only to CSP (Figure 1d).
Figure 1.
Prevalence of IgG1, IgG2, IgG3, and IgG4 subclass antibodies by age to P. falciparum antigens in areas of stable (a–d) and unstable (e–h) malaria transmission as measured by ELISA. Asterisk (*) indicates significant (P<0.05) difference in age groups between areas by χ2 analysis. P-values for trend for age by χ2 analysis for each antigen as indicated.
3.3. Prevalence of IgG subclass antibodies in an area of unstable malaria transmission
In the area of unstable transmission, IgG1 and IgG3 subclass antibody responses to P. falciparum antigens also predominated. Prevalence of IgG1 antibodies increased with age for all antigens (Figure 1e), such that ≥50% of individuals older than 40 years of age possessed IgG1 antibodies to LSA-1, TRAP, AMA-1, EBA-175, and MSP-1. However, IgG1 responses to all antigens in all age groups were lower in the unstable as compared to stable transmission area, with the exception of IgG1 to AMA-1 in those older than 40 years of age and IgG1 to MSP-1 in those older than 15 years of age.
Prevalence of IgG3 antibodies increased with age for LSA-1, TRAP, AMA-1, and EBA-175, with >67% of individuals older than 40 years of age possessing IgG3 antibodies to LSA-1, TRAP, and EBA-175 (Figure 1g). IgG3 responses to MSP-1 were also common (>62%) in all age groups 2 years and older, but surprisingly, IgG3 responses to CSP declined with age. Like IgG1 antibodies, IgG3 antibody prevalence was significantly lower in every age group in the unstable versus stable transmission areas for all antigens except EBA-175 in individuals older than 15 years and CSP in individuals 2–5 years of age.
IgG2 responses in the area of unstable malaria transmission were less prevalent than IgG1 or IgG3 responses. A significant increase with age was observed for IgG2 antibody prevalence to CSP, LSA-1, and EBA-175 (Figure 1f). In individuals older than 5 years of age, IgG2 antibody prevalence was generally lower in the unstable as compared to stable transmission area. Prevalence of IgG4 antibody responses in the unstable transmission area was low (<18%) in all age groups for all antigens except TRAP (Figure 1h).
3.4. Levels of IgG subclass antibodies according to age and transmission intensity
In the area of stable transmission, IgG1 antibody levels did not significantly increase with age for any antigen except TRAP (Table 2a), whereas IgG3 antibody levels increased with age for all antigens except LSA-1 and EBA-175 (Table 4a). In the area of unstable transmission, IgG1 antibody levels increased with age for all antigens (Table 2b), and IgG3 antibody levels increased with age for all antigens except CSP and MSP-1 (Table 4b). IgG2 antibody levels to most antigens also increased with age in both transmission areas though median levels were less than 1.0 AU for all antigens except CSP and LSA-1 in individuals older than 15 years in the stable transmission area, and LSA-1 and EBA-175 in individuals older than 40 years in the unstable transmission area (Table 3). IgG4 antibody levels were less than 1.0 AU for all antigens in both transmission areas, but increased with age in the area of unstable malaria transmission (Table 5).
Table 2.
| Table 2a. IgG1 Antibody levels across age groups in an area of stable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groups:
|
P value a (trend for age) | |||
| 2–5 yr (n=14) | 6–15 yr (n=30) | 16–40 yr (n=44) | >40 yr (n=28) | ||
| CSP | 2.54 (0.40,9.37) | 3.21 (0.33,12.00) | 1.85 (0.00,11.19) | 2.74 (0.06,18.42) | 0.259 |
| LSA-1 | 2.35 (0.16,16.05) | 2.19 (0.49,21.89) | 2.08 (0.49,41.30) | 2.38 (0.07,11.12) | 0.835 |
| TRAP | 1.32 (0.02,7.44) | 1.56 (0.31,6.28) | 1.98 (0.56,5.32) | 2.01 (0.31,9.20) | 0.010 |
| AMA-1 | 1.05 (0.08,4.78) | 2.31 (0.30,4.21) | 1.30 (0.00,4.87) | 1.36 (0.31,5.19) | 0.135 |
| EBA-175 | 2.27 (0.02,11.83) | 9.58 (0.87,64.52) | 8.58 (0.92,58.89) | 6.35 (1.30,55.83) | 0.123 |
| MSP-1 | 1.31 (0.15,2.51) | 1.64 (0.20,8.18) | 1.71 (0.40,9.93) | 1.74 (0.71,6.14) | 0.211 |
| Table 2b. IgG1 Antibody levels across age groups in an area of unstable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groupsb:
|
P value a (trend for age) | |||
| 2–5 yr (n=19) | 6–15 yr (n=12) | 16–40 yr (n=37) | >40 yr (n=28) | ||
| CSP | 0.05 (0.00,4.86)* | 0.00 (0.00,1.02)* | 0.85 (0.02,3.29)* | 0.90 (0.29,3.52)* | <0.001 |
| LSA-1 | 0.53 (0.24,10.97)* | 0.53 (0.03,2.40)* | 0.67 (0.20,3.91)* | 1.00 (0.12,15.95)* | 0.055 |
| TRAP | 0.45 (0.14,2.07)* | 0.26 (0.13,1.28)* | 0.52 (0.00,4.40)* | 1.12 (0.19,3.81)* | <0.001 |
| AMA-1 | 0.18 (0.00,2.46)* | 0.25 (0.00,2.32)* | 0.57 (0.01,2.91)* | 1.07 (0.24,4.08) | <0.001 |
| EBA-175 | 0.63 (0.08,3.90)* | 0.99 (0.16,18.69)* | 2.76 (0.12,63.50)* | 26.61 (0.44,60.07) | <0.001 |
| MSP-1 | 0.83 (0.24,1.62)* | 0.79 (0.16,1.69)* | 1.43 (0.30,5.52) | 2.31 (0.18,6.68) | <0.001 |
Non-parametric test for trend across ordered groups (see Materials and Methods)
Asterisk (*) indicates significantly different from antibody level for same antigen and age group in stable transmission area (P<0.05 by Wilcoxon rank-sum test).
Table 4.
| Table 4a. IgG3 Antibody levels across age groups in an area of stable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groups:
|
P value a (trend for age) | |||
| 2–5 yr (n=14) | 6–15 yr (n=30) | 16–40 yr (n=44) | >40 yr (n=28) | ||
| CSP | 2.74 (0.53,56.98) | 5.19 (0.62,81.76) | 15.07 (1.79,231.85) | 23.6 (3.56,223.93) | <0.001 |
| LSA-1 | 10.51 (0.41,125.84) | 26.06 (0.31,192.88) | 49.27 (0.15,179.06) | 35.87 (2.60,152.15) | 0.075 |
| TRAP | 7.29 (0.79,61.96) | 13.18 (0.85,103.07) | 53.32 (4.73,280.59) | 66.22 (12.71,241.89) | <0.001 |
| AMA-1 | 1.86 (0.05,37.37) | 2.55 (0.08,19.99) | 3.66 (0.88,39.53) | 4.38 (1.04,12.61) | 0.002 |
| EBA-175 | 4.02 (0.00,116.98) | 8.27 (0.00,113.69) | 5.35 (0.00,171.31) | 3.61 (0.00,34.94) | 0.117 |
| MSP-1 | 4.67 (1.19,27.19) | 5.05 (0.59,33.77) | 14.86 (1.13,93.56) | 14.39 (2.11,80.54) | <0.001 |
| Table 4b. IgG3 Antibody levels across age groups in an area of unstable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groupsb:
|
P value a (trend for age) | |||
| 2–5 yr (n=19) | 6–15 yr (n=12) | 16–40 yr (n=37) | >40 yr (n=28) | ||
| CSP | 1.29 (0.21,8.80)* | 1.07 (0.29,2.29)* | 0.58 (0.13,8.17)* | 1.13 (0.15,5.30)* | 0.214 |
| LSA-1 | 0.59 (0.19,15.52)* | 0.45 (0.00,0.72)* | 0.99 (0.00,179.06)* | 1.87 (0.03,185.69)* | <0.001 |
| TRAP | 0.66 (0.00,54.17)* | 0.59 (0.15,3.39)* | 0.78 (0.05,88.84)* | 2.10 (0.34,125.62)* | 0.006 |
| AMA-1 | 0.10 (0.00,0.26)* | 0.09 (0.00,0.25)* | 0.82 (0.07,10.47)* | 0.70 (0.27,6.86)* | <0.001 |
| EBA-175 | 0.06 (0.00,2.73)* | 0.05 (0.00,0.95)* | 1.35 (0.00,76.39)* | 2.50 (0.32,79.91) | <0.001 |
| MSP-1 | 1.15 (0.36,2.30)* | 1.30 (0.56,1.99)* | 1.36 (0.24,29.71)* | 1.35 (0.62,56.32)* | 0.132 |
Non-parametric test for trend across ordered groups (see Materials and Methods)
Asterisk (*) indicates significantly different from antibody level for same antigen and age group in stable transmission area (P<0.05 by Wilcoxon rank-sum test).
Table 3.
| Table 3a. IgG2 Antibody levels across age groups in an area of stable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groups:
|
P value a (trend for age) | |||
| 2–5 yr (n=14) | 6–15 yr (n=30) | 16–40 yr (n=44) | >40 yr (n=28) | ||
| CSP | 0.62 (0.00,0.95) | 0.80 (0.00,2.73) | 1.29 (0.00,16.88) | 3.46 (0.28,100.66) | <0.001 |
| LSA-1 | 0.37 (0.00,70.35) | 0.76 (0.04,100.16) | 5.66 (0.05,170.57) | 1.97 (0.09,120.90) | 0.004 |
| TRAP | 0.27 (0.00,0.51) | 0.49 (0.03,35.84) | 0.59 (0.01,21.14) | 0.83 (0.03,9.64) | 0.001 |
| AMA-1 | 0.07 (0.00,0.85) | 0.08 (0.01,0.37) | 0.13 (0.00,2.62) | 0.18 (0.03,9.72) | <0.001 |
| EBA-175 | 0.49 (0.00,2.93) | 0.76 (0.26,14.50) | 1.02 (0.00,33.59) | 0.77 (0.09,4.67) | 0.082 |
| MSP-1 | 0.16 (0.00,0.54) | 0.32 (0.00,1.39) | 0.85 (0.00,2.69) | 0.77 (0.17,2.95) | <0.001 |
| Table 3b. IgG2 Antibody levels across age groups in an area of unstable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groupsb:
|
P value a (trend for age) | |||
| 2–5 yr (n=19) | 6–15 yr (n=12) | 16–40 yr (n=37) | >40 yr (n=28) | ||
| CSP | 0.00 (0.00,1.36)* | 0.00 (0.00,0.54)* | 0.38 (0.00,1.04)* | 0.64 (0.13,3.20)* | <0.001 |
| LSA-1 | 0.16 (0.03,2.14) | 0.10 (0.00,2.46)* | 0.29 (0.00,10.49)* | 1.10 (0.00,28.90) | <0.001 |
| TRAP | 0.14 (0.00,11.62) | 0.06 (0.00,3.48)* | 0.16 (0.00,1.07)* | 0.31 (0.06,3.68)* | 0.113 |
| AMA-1 | 0.08 (0.00,0.50) | 0.05 (0.00,1.43) | 0.06 (0.00,0.82)* | 0.09 (0.00,0.71)* | 0.199 |
| EBA-175 | 0.28 (0.00,1.01)* | 0.17 (0.00,0.85)* | 0.43 (0.00,3.49)* | 1.77 (0.02,10.84) | <0.001 |
| MSP-1 | 0.31 (0.00,1.27) | 0.07 (0.00,0.77)* | 0.21 (0.00,1.01)* | 0.39 (0.00,1.54)* | 0.136 |
Non-parametric test for trend across ordered groups (see Materials and Methods)
Asterisk (*) indicates significantly different from antibody level for same antigen and age group in stable transmission area (P<0.05 by Wilcoxon rank-sum test).
Table 5.
| Table 5a. IgG4 Antibody levels across age groups in an area of stable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groups:
|
P value a (trend for age) | |||
| 2–5 yr (n=14) | 6–15 yr (n=30) | 16–40 yr (n=44) | >40 yr (n=28) | ||
| CSP | 0.64 (0.27,0.95) | 0.56 (0.00,1.69) | 0.88 (0.24,7.41) | 0.83 (0.23,3.03) | <0.001 |
| LSA-1 | 0.20 (0.03,0.87) | 0.19 (0.03,1.26) | 0.47 (0.00,3.09) | 0.42 (0.00,1.28) | 0.472 |
| TRAP | 1.04 (0.51,1.73) | 0.93 (0.21,1.64) | 0.98 (0.28,2.44) | 1.11 (0.35,2.55) | 0.395 |
| AMA-1 | 0.20 (0.00,0.73) | 0.22 (0.00,0.56) | 0.16 (0.00,1.06) | 0.18 (0.00,0.75) | 0.347 |
| EBA-175 | 0.51 (0.33,0.91) | 0.52 (0.09,1.92) | 0.44 (0.13,50.02) | 0.46 (0.06,1.10) | 0.231 |
| MSP-1 | 0.48 (0.15,3.00) | 0.27 (0.01,1.08) | 0.53 (0.03,5.88) | 0.54 (0.00,1.96) | 0.335 |
| Table 5b. IgG4 Antibody levels across age groups in an area of unstable malaria transmission
| |||||
|---|---|---|---|---|---|
| Antigen | Median level (AU) (min, max) for age groupsb:
|
P value a (trend for age) | |||
| 2–5 yr (n=19) | 6–15 yr (n=12) | 16–40 yr (n=37) | >40 yr (n=28) | ||
| CSP | 0.55 (0.13,1.08) | 0.43 (0.29,5.71) | 0.40 (0.02,2.15)* | 0.49 (0.00,3.38)* | 0.557 |
| LSA-1 | 0.02 (0.00,0.20)* | 0.03 (0.00,0.12)* | 0.20 (0.00,0.83)* | 0.28 (0.10,0.79) | <0.001 |
| TRAP | 0.44 (0.00,1.26)* | 0.29 (0.00,1.28)* | 0.85 (0.29,3.32)* | 0.89 (0.52,1.49) | <0.001 |
| AMA-1 | 0.05 (0.00,0.22)* | 0.07 (0.00,0.15)* | 0.19 (0.01,1.13) | 0.19 (0.00,10.94) | <0.001 |
| EBA-175 | 0.33 (0.10,0.57)* | 0.45 (0.22,0.64) | 0.41 (0.08,0.71) | 0.46 (0.21,1.02) | 0.001 |
| MSP-1 | 0.32 (0.00,1.31) | 0.31 (0.05,1.68) | 0.34 (0.00,1.53)* | 0.44 (0.00,4.24) | 0.357 |
Non-parametric test for trend across ordered groups (see Materials and Methods)
Asterisk (*) indicates significantly different from antibody level for same antigen and age group in stable transmission area (P<0.05 by Wilcoxon rank-sum test).
IgG1 antibody levels to the pre-erythrocytic antigens CSP, LSA-1, TRAP were significantly lower in every age group in the unstable as compared to stable transmission area (Table 2). However, levels of IgG1 antibodies to AMA-1 and EBA-175 in individuals older than 40 years as well as levels of IgG1 antibodies to MSP-1 in individuals older than 15 years were similar in the two areas. IgG3 antibody levels in the unstable transmission area were significantly lower for all antigens and ages except EBA-175 IgG3 antibodies in individuals older than 40 years (Table 4). In individuals older than 5 years of age, IgG2 levels were lower for most antigens in the unstable transmission area (Table 3). Differences in IgG4 levels were more heterogeneous due to the overall low magnitude of response (Table 5).
For a summary analysis, we compared natural log-transformed subclass antibody levels in the stable to unstable transmission site, adjusting for P. falciparum parasitemia status and age. Subclass antibody levels to all antigens were higher in the stable transmission than unstable transmission site, after adjustment for age and parasitemia status, except for IgG4 levels to AMA-1 and EBA-175 (Supplemental Table 1).
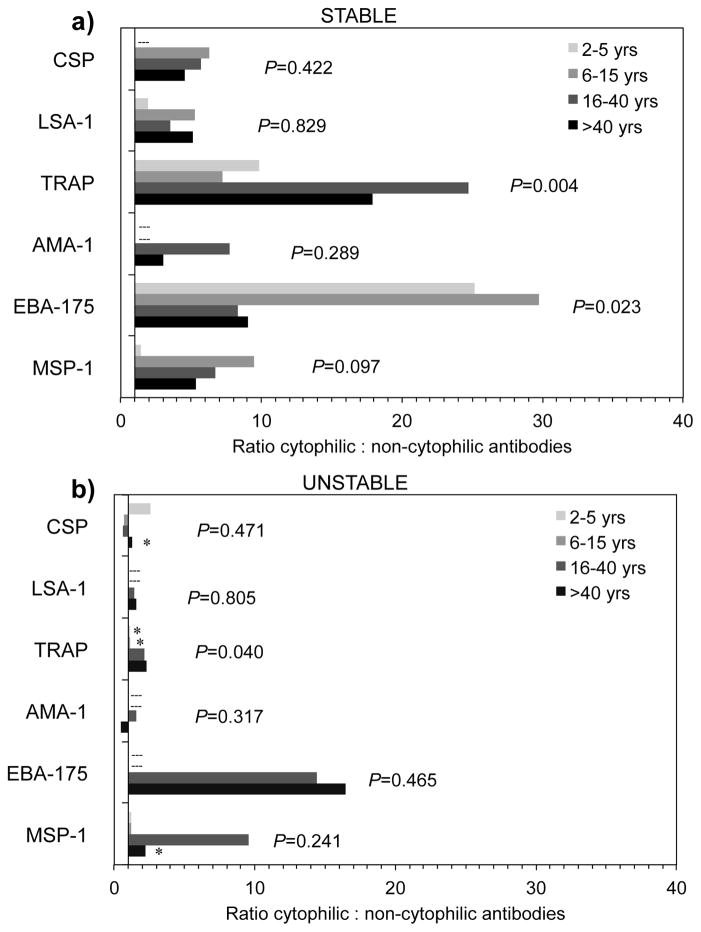
3.5. Ratio of cytophilic to non-cytophilic IgG antibody subclasses
To examine the relationship of antibody responses in terms of functional specificity across age groups and between transmission areas, we calculated the ratio of cytophilic (IgG1 + IgG3) to non-cytophilic (IgG2 + IgG4) antibodies among individuals with positive cytophilic (either IgG1 and/or IgG3 greater than 1.0 AU) and non-cytophilic responses (either IgG2 and/or IgG4 greater than 1.0 AU). The ratio of cytophilic to non-cytophilic antibodies significantly increased with age for TRAP in both stable (P=0.004) and unstable (P=0.040) transmission areas (Figure 2), while the ratio significantly decreased with age for EBA-175 in the stable transmission area (P=0.023). Ratios for other antigens did not significantly change with age in either area. Median ratios of cytophilic to non-cytophilic antibodies tended to be higher in the stable transmission area for all antigens except EBA-175, though the limited number of antibody-positive individuals in the unstable transmission area precluded detection of statistically significant differences in most instances (Figure 2).
Figure 2.
Median ratio of cytophilic (IgG1 + IgG3) to non-cytophilic (IgG2 + IgG4) antibodies by age to P. falciparum antigens in areas of stable (a) and unstable (b) malaria transmission among individuals with positive cytophilic (either IgG1 and/or IgG3 greater than 1.0 AU) and non-cytophilic responses (either IgG2 and/or IgG4 greater than 1.0 AU). Dashed line (---) indicates age groups without observations. Asterisk (*) indicates significant (P<0.05) difference in age groups between areas by Wilcoxon rank-sum analysis. P-values indicate trend for age by non-parametric test for trend across ordered groups.
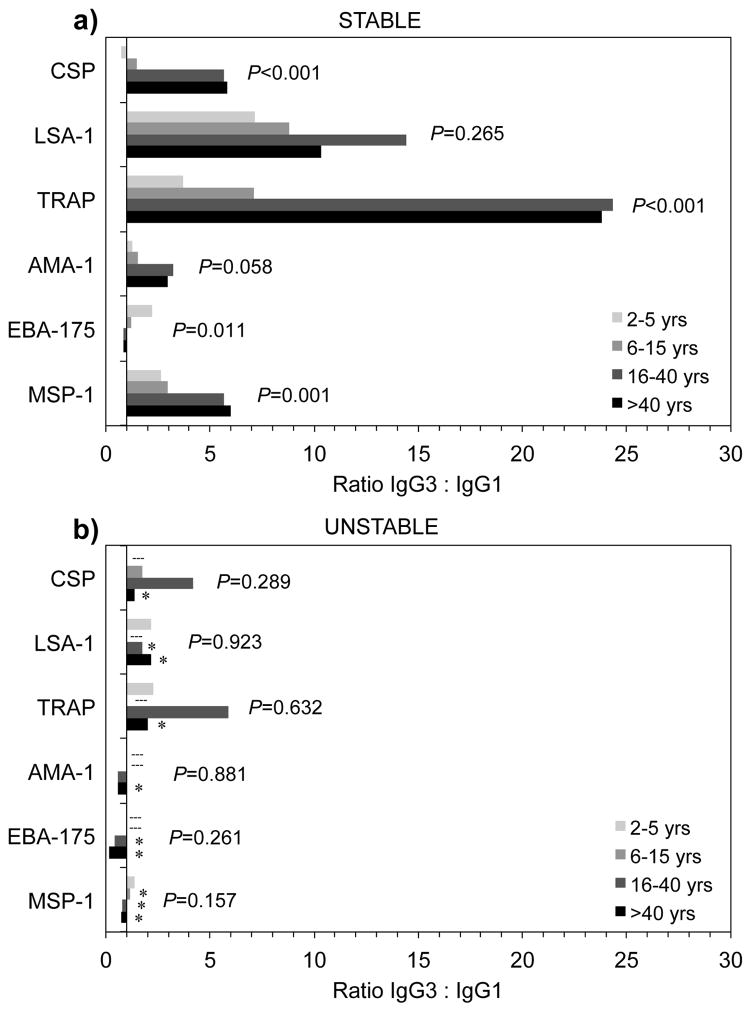
3.6. Ratio of IgG3 to IgG1 antibodies
We also examined the polarization between cytophilic IgG3 and IgG1 antibodies among individuals with positive IgG1 and IgG3 responses. In the stable transmission area, antigen-specific IgG3 generally predominated over IgG1 (Figure 3). The ratio of IgG3 to IgG1 tended to increase with age in the stable transmission area for all antigens except EBA-175, which significantly decreased with age. In contrast, IgG3 to IgG1 ratios did not increase with age in the unstable transmission area, and an IgG1 bias was detected for blood-stage antigens AMA-1, EBA-175, and MSP-1 in those older than 15 years of age. Median ratios of IgG3 to IgG1 were significantly lower in the unstable transmission area compared to the stable transmission area for all antigens among adults older than 40 years, as well as for LSA-1, EBA-175, and MSP-1 in those older than 15 years.
Figure 3.
Median ratio of IgG3 to IgG1 antibodies by age to P. falciparum antigens in areas of stable (a) and unstable (b) malaria transmission among individuals with positive IgG1 and IgG3 responses (greater than 1.0 AU). Dashed line (---) indicates age groups without observations. Asterisk (*) indicates significant (P<0.05) difference in age groups between areas by Wilcoxon rank-sum analysis. P-values indicate trend for age by non-parametric test for trend across ordered groups.
3.7. IgG subclass analysis according to parasitemia status
To examine whether differences in subclass levels were driven by current P. falciparum infection, we first compared levels between those with and without microscopically confirmed infection in the stable transmission area, bearing in mind that all were asymptomatic at the time of evaluation. Because age is a major confounder, we assessed differences in natural log-transformed levels in those with versus without P. falciparum peripheral blood parasitemia, adjusted for age. We found that age-adjusted antigen-specific antibody subclass levels did not differ between those with and without parasitemia (Table 6). Only levels of IgG4 to CSP differed between those with versus without parasitemia (P=0.04), but this difference was not considered biologically relevant, as levels in both parasitemic and non-parasitemic individuals were below the 1.0 AU cut-off. There was no significant difference in overall antigen-specific IgG3 to IgG1 ratios between those with P. falciparum parasitemia and those without parasitemia in the stable transmission area, whether considering all individuals, or only those with positive IgG1 and IgG3 responses (data not shown).
Table 6.
Adjusted mean difference in natural log-transformed antibody levels between P. falciparum positive vs. negative individuals in the stable transmission area, adjusted for agea.
| Antigen | Subclass | Adjusted mean difference (95% CI) | P-value |
|---|---|---|---|
| CSP | IgG1 | −0.13 (−0.48, 0.22) | 0.45 |
| IgG2 | 0.20 (−0.18, 0.59) | 0.30 | |
| IgG3 | −0.21 (−0.67, 0.25) | 0.38 | |
| IgG4 | −0.24 (−0.47, −0.01) | 0.04* | |
|
| |||
| LSA-1 | IgG1 | −0.15 (−0.55, 0.24) | 0.45 |
| IgG2 | −0.59 (−1.41, 0.24) | 0.16 | |
| IgG3 | −0.01 (−0.61, 0.58) | 0.97 | |
| IgG4 | −0.38 (−0.79, 0.02) | 0.06 | |
|
| |||
| TRAP | IgG1 | −0.30 (−0.60, 0.01) | 0.06 |
| IgG2 | −0.38 (−0.95, 0.20) | 0.19 | |
| IgG3 | −0.08 (−0.50, 0.35) | 0.71 | |
| IgG4 | −0.05 (−0.21, 0.11) | 0.52 | |
|
| |||
| AMA-1 | IgG1 | 0.11 (−0.16, 0.37) | 0.44 |
| IgG2 | −0.02 (−0.46, 0.43) | 0.95 | |
| IgG3 | 0.02 (−0.39, 0.43) | 0.93 | |
| IgG4 | −0.17 (−0.49, 0.15) | 0.31 | |
|
| |||
| EBA-175 | IgG1 | −0.21 (−0.71, 0.29) | 0.41 |
| IgG2 | −0.38 (−0.80, 0.04) | 0.08 | |
| IgG3 | −0.08 (−0.81, 0.66) | 0.84 | |
| IgG4 | −0.01 (−0.32, 0.30) | 0.94 | |
|
| |||
| MSP-1 | IgG1 | −0.23 (−0.50, 0.04) | 0.09 |
| IgG2 | −0.25 (−0.57, 0.07) | 0.12 | |
| IgG3 | 0.08 (−0.30, 0.46) | 0.69 | |
| IgG4 | −0.23 (−0.57, 0.11) | 0.18 | |
Linear regression analysis
P<0.05.
We then repeated comparison of prevalence and levels of antigen-specific antibody subclasses by age between stable and unstable transmission areas restricted only to microscopically negative individuals in the area of stable transmission and found that results were consistent with the full study cohort (data not shown).
4. DISCUSSION
A large body of evidence indicates that cytophilic IgG1 and IgG3 antibodies to malaria antigens predominate over non-cytophilic IgG2 and IgG4 responses in individuals from malaria endemic areas. However, the majority of such studies have been carried out in areas of high transmission, and it has been difficult to determine whether the development of cytophilic antibodies is an age- or exposure-dependent process, as the two parameters are often inseparable in highly endemic settings. Furthermore, most studies have focused on responses to blood-stage antigens since the morbidity and mortality arising from Plasmodium infection is attributable to this phase of disease. To address these gaps, we compared IgG subclass responses to both pre-erythrocytic and blood stage P. falciparum antigens in two areas of highly differing malaria endemicity. We found that in an area of unstable malaria transmission, cytophilic IgG1 and IgG3 antibodies increased with age to nearly all antigens, but were less prevalent and generally lower in magnitude compared to an area of stable transmission. In addition, the cytophilic response to all antigens in the area of stable transmission was dominated by IgG3 antibodies that increased with age in relation to IgG1 antibodies, whereas ratios of cytophilic antibodies to pre-erythrocytic antigens (IgG3-biased) and blood stage antigens (IgG1-biased) did not change with age in an area of unstable transmission.
The finding that age and exposure independently affect anti-malaria subclass antibody response is consistent with results from a previous study of subclass responses to blood-stage antigens across several transmission gradients in Tanzania (Tongren et al., 2006). Results from our study indicate that this applies to pre-erythrocytic as well as blood-stage antigens. Interestingly, the Tanzanian study and several others (Dobano et al., 2012; Stanisic et al., 2009a) document a predominance of IgG1 over IgG3 for MSP-1 and AMA-1 in areas of moderate and high transmission, whereas we observed a clear IgG3 bias to these as well as to pre-erythrocytic antigens in the area of high, stable transmission. A number of other studies (Courtin et al., 2009; Dodoo et al., 2008; Duah et al., 2010; Duah et al., 2009; Kinyanjui et al., 2003; Richards et al., 2010), including our previous evaluations of CSP, TRAP and LSA-1 in another unstable transmission area of Kenya (John et al., 2003), have also shown an IgG3 bias over IgG1 to P. falciparum antigens. Such bias is somewhat unexpected, as IgG3 antibodies have a shorter half-life than other subclasses. It has been suggested that repeated parasite exposure may sustain high-levels of IgG3 antibodies (Kinyanjui et al., 2003; Sarr et al., 2012), while recent evidence reveals a genetic contribution to differential subclass production against malaria (Afridi et al., 2012). It is also possible the higher sensitivity of commercial anti-IgG3 detection antibodies may have led to an apparent IgG3 bias. However, the monoclonal antibody clones used in this study have been widely used for decades in research applications and have been accepted as reliable indicators of relative human IgG subclass concentrations.
Antibody profiles of residents from the unstable transmission area lacked the magnitude of responses present in individuals from a highly endemic area to the extent that IgG1 and IgG3 responses in adults from the unstable area to nearly all studied antigens were lower than responses in the youngest age group in the stable transmission area. Even allowing for occasional travel exposure in adults, this result suggests that multiple exposures over a relatively short period of time (several years) leads to a more robust response than an equivalent number of exposures over an extended period of time (several decades). This assumes a steady, low rate of exposure in the unstable transmission area. Surveillance data from the unstable transmission area show that malaria has been present with seasonal peaks for several years surrounding the present survey (John et al., 2009), meaning that adults and children likely experienced malaria in the recent past. In addition, health facility data from a nearby highland tea estate dating back to the late 1960s indicates that historical incidence rates were even lower until the advent of chloroquine resistance in the area around 1990 (Shanks et al., 2000). Results presented here may not capture the absolute levels of biologically active antibodies due to factors like differential avidity and competitive binding between subclasses, as well as conformational structure of recombinant antigens. Yet the relative differences in antibodies detected across sites still likely reflects true differences since the method of analysis was the same for each site. A recent publication (Baum et al., 2013) using protein microarray to examine total IgG antibody response in other areas of western Kenya with high versus lower malaria transmission showed that antibodies to some antigens were acquired with much less exposure requirement than antibodies to other antigens, similar to the findings in this study. Assessment of subclass antibodies by high-throughput microarray might shed further light on differences in antibody acquisition and seroconversion in areas of differing transmission.
The cross-sectional nature of this study posed several limitations. As mentioned above, detailed infection history was not collected. Even though all patients were asymptomatic at the time of sample collection, 59% of individuals in the stable transmission area were positive for P. falciparum by microscopy. These results could thus reflect active or recent infection rather than cumulative exposure. To address this, we compared responses between stable and unstable transmission areas only among non-parasitemic individuals. We found that results were similar to the findings of the full study population and that within the stable transmission area, differences between parasitemic and non-parasitemic individuals were primarily limited to deficiencies of cytophilic responses to the pre-erythrocytic antigens TRAP (IgG1 and IgG3) and CSP (IgG3) in parasitemic versus non-parasitemic individuals. This further reinforces the view that cytophilic antibodies to pre-erythrocytic antigens are associated with protection from malaria. However, the cross sectional design limited this study’s ability to make causal inferences regarding antibody responses and protection from infection. Other studies have found that cytophilic IgG1 (Shi et al., 1996) and IgG3 (Aribot et al., 1996; Metzger et al., 2003; Roussilhon et al., 2007; Sarthou et al., 1997; Soe et al., 2004; Taylor et al., 1998) subclass responses to P. falciparum and high ratios of cytophilic to non-cytophilic IgG (Bouharoun-Tayoun and Druilhe, 1992; Roussilhon et al., 2007) have been associated with protection from malaria. Adults in highland areas of unstable transmission remain at risk for malaria, though evidence suggests that they are at lower risk than children in the same area (Hay et al., 2002). Results from this study suggest that persistently low cytophilic antibody responses may contribute to the partial but limited protection from clinical malaria seen in adults in these areas, though longitudinal cohort studies are required.
Additionally, this study included a wide age range in order to examine the contribution of age in the development of anti-malaria subclass antibodies, but children less than 2 years of age were not recruited. In order to fully evaluate the contribution of age and exposures in development of antigen-specific immunity, it would be valuable to compare IgG subclass distribution in infants in areas of stable and unstable transmission. Results from a birth cohort from an area of seasonal transmission in the Gambia indicate that IgG1 responses to blood-stage antigens precede IgG3 responses (Duah et al., 2010), while previous work from a holoendemic area of Tanzania did not detect clear patterns of sequential IgG1 or IgG3 antibody development in response to pre-erythrocytic or blood-stage antigens (Kitua et al., 1999). How this compares to infants in areas of unstable transmission is not known. The preferential development of cytophilic IgG subclasses remains a poorly understood process, though evidence from this study and others indicates that induction depends on multiple factors including host age, host genetic background, level of antigen exposure and the nature of the antigen (Duah et al., 2009; Tongren et al., 2006).
5. CONCLUSIONS
These findings provide new evidence that ratios of cytophilic to non-cytophilic IgG subclass responses and development of IgG3-biased responses to both pre-erythrocytic and blood-stage antigens are reduced in populations of unstable as compared to stable malaria transmission even into late adulthood. The lower ratios, which generally did not increase with age in the unstable transmission area, may contribute to the persistent risk of clinical malaria for older individuals in such areas. As interventions reduce malaria transmission in areas of previously high transmission, it will be important to assess if a loss of cytophilic subclass responses occurs in these populations, and if the ratios of cytophilic to non-cytophilic subclass responses change over time, as these may provide valuable markers of population-level, age-specific risk of clinical disease from malaria.
Supplementary Material
HIGHLIGHTS.
IgG1 and IgG3 to P. falciparum are lower in unstable than stable transmission areas.
Cytophilic:non-cytophilic antibody ratios are decreased in unstable transmission.
IgG3:IgG1 ratios for blood-stage antigens are decreased in unstable transmission.
Low IgG3:IgG1 and cytophilic:non-cytophilic ratios may contribute to malaria risk.
Acknowledgments
We thank the study participants for their involvement in this study. We thank David Koech, Jackson Abuya and the late Livingstone Wanyama for their work in the field collection of these samples and microscopy testing. We gratefully acknowledge the Malaria Research and Reference Reagent Resource Center (Manassas, VA) for provision of MSP-119 antigen.
This work is published with the permission of the Office of the Director of the Kenya Medical Research Institute.
This study was supported by grants from the National Institutes of Allergy and Infectious Diseases AI01572 and AI056270 (CCJ) and AI43906 (JWK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gregory S. Noland, Email: gregory.noland@emory.edu.
Paul Jansen, Email: jansenpa@gmail.com.
John M. Vulule, Email: vulule@yahoo.com.
Gregory S. Park, Email: parkx479@umn.edu.
Bartholomew N. Ondigo, Email: ondigo2002@yahoo.com.
James W. Kazura, Email: james.kazura@case.edu.
Ann M. Moormann, Email: ann.moormann@umassmed.edu.
Chandy C. John, Email: ccj@umn.edu.
References
- Afridi S, Atkinson A, Garnier S, Fumoux F, Rihet P. Malaria resistance genes are associated with the levels of IgG subclasses directed against Plasmodium falciparum blood-stage antigens in Burkina Faso. Malar J. 2012;11:308. doi: 10.1186/1475-2875-11-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribot G, Rogier C, Sarthou JL, Trape JF, Balde AT, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa) Am J Trop Med Hyg. 1996;54:449–457. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- Baum E, Badu K, Molina DM, Liang X, Felgner PL, Yan G. Protein microarray analysis of antibody responses to Plasmodium falciparum in western Kenya highland sites with differing transmission levels. PLoS One. 2013;8(12):e82246. doi: 10.1371/journal.pone.0082246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Perkins PV, Onyango FK, Gargan TP, Oster CN, Whitmire RE, Koech DK, Roberts CR. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J Med Entomol. 1990;27:570–577. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JM. Malaria in the Uasin Gishu and the Trans Nzoia, Kenya. East Afr Med J. 1929;6:32–43. [Google Scholar]
- Chelimo K, Ofulla AV, Narum DL, Kazura JW, Lanar DE, John CC. Antibodies to Plasmodium falciparum antigens vary by age and antigen in children in a malaria-holoendemic area of Kenya. Pediatr Infect Dis J. 2005;24:680–684. doi: 10.1097/01.inf.0000172151.28851.fd. [DOI] [PubMed] [Google Scholar]
- Chougnet C, Lepers JP, Astagneau P, Rason MD, Savel J, Deloron P. Lymphoproliferative responses to synthetic peptides from merozoite ring-infected erythrocyte surface antigen and circumsporozoite protein: a longitudinal study during a falciparum malaria episode. Am J Trop Med Hyg. 1991;45:560–566. doi: 10.4269/ajtmh.1991.45.560. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, Gaye O, Roeffen W, Remarque EJ, Sauerwein R, Garcia A, Luty AJ. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One. 2009;4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobano C, Quelhas D, Quinto L, Puyol L, Serra-Casas E, Mayor A, Nhampossa T, Macete E, Aide P, Mandomando I, Sanz S, Puniya SK, Singh B, Gupta P, Bhattacharya A, Chauhan VS, Aponte JJ, Chitnis CE, Alonso PL, Menendez C. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin Vaccine Immunol. 2012;19:157–166. doi: 10.1128/CVI.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodoo D, Aikins A, Kusi KA, Lamptey H, Remarque E, Milligan P, Bosomprah S, Chilengi R, Osei YD, Akanmori BD, Theisen M. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Miles DJ, Whittle HC, Conway DJ. Acquisition of antibody isotypes against Plasmodium falciparum blood stage antigens in a birth cohort. Parasite Immunol. 2010;32:125–134. doi: 10.1111/j.1365-3024.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Weiss HA, Jepson A, Tetteh KK, Whittle HC, Conway DJ. Heritability of antibody isotype and subclass responses to Plasmodium falciparum antigens. PLoS One. 2009;4:e7381. doi: 10.1371/journal.pone.0007381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AF, Chappel JA, Burghaus PA, Morris JS, McBride JS, Holder AA, Kaslow DC, Riley EM. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Rzepczyk CM. Atypical IgG subclass antibody responses to Plasmodium falciparum asexual stage antigens. Parasitol Today. 1997;13:145–148. doi: 10.1016/s0169-4758(97)89812-2. [DOI] [PubMed] [Google Scholar]
- Fidock DA, Gras-Masse H, Lepers JP, Brahimi K, Benmohamed L, Mellouk S, Guerin-Marchand C, Londono A, Raharimalala L, Meis JF, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- Gruner AC, Brahimi K, Letourneur F, Renia L, Eling W, Snounou G, Druilhe P. Expression of the erythrocyte-binding antigen 175 in sporozoites and in liver stages of Plasmodium falciparum. J Infect Dis. 2001;184:892–897. doi: 10.1086/323394. [DOI] [PubMed] [Google Scholar]
- Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543–548. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005a;99:780–786. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005b;73:222–228. [PubMed] [Google Scholar]
- John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun. 2004;72:5135–5142. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, Riedesel MA, Magak NG, Lindblade KA, Menge DM, Hodges JS, Vulule JM, Akhwale W. Possible interruption of malaria transmission, highland Kenya, 2007–2008. Emerg Infect Dis. 2009;15:1917–1924. doi: 10.3201/eid1512.090627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, Zickafoose JS, Sumba PO, King CL, Kazura JW. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect Immun. 2003;71:4320–4325. doi: 10.1128/IAI.71.8.4320-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B, Trape JF, Tall A, Longacre S, Perraut R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One. 2010;5:e9871. doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanjui SM, Bull P, Newbold CI, Marsh K. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J Infect Dis. 2003;187:667–674. doi: 10.1086/373994. [DOI] [PubMed] [Google Scholar]
- Kitua AY, Urassa H, Wechsler M, Smith T, Vounatsou P, Weiss NA, Alonso PL, Tanner M. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 1999;21:307–317. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- Matson AT. The history of malaria in Nandi. East Afr Med J. 1957;34:431–441. [PubMed] [Google Scholar]
- Metzger WG, Okenu DM, Cavanagh DR, Robinson JV, Bojang KA, Weiss HA, McBride JS, Greenwood BM, Conway DJ. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 2003;25:307–312. doi: 10.1046/j.1365-3024.2003.00636.x. [DOI] [PubMed] [Google Scholar]
- Moormann AM, Embury PE, Opondo J, Sumba OP, Ouma JH, Kazura JW, John CC. Frequencies of sickle cell trait and glucose-6-phosphate dehydrogenase deficiency differ in highland and nearby lowland malaria-endemic areas of Kenya. Trans R Soc Trop Med Hyg. 2003;97:513–514. doi: 10.1016/s0035-9203(03)80010-x. [DOI] [PubMed] [Google Scholar]
- Ndungu FM, Bull PC, Ross A, Lowe BS, Kabiru E, Marsh K. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 2002;24:77–82. doi: 10.1046/j.0141-9838.2001.00440.x. [DOI] [PubMed] [Google Scholar]
- Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma EC, Tiono AB, Konate AT, Chilengi R, Theisen M, Dodoo D, Remarque E, Bosomprah S, Milligan P, Sirima SB. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2008;76:759–766. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland GS, Hendel-Paterson B, Min XM, Moormann AM, Vulule JM, Narum DL, Lanar DE, Kazura JW, John CC. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun. 2008;76:5721–5728. doi: 10.1128/IAI.00591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J. 2009;8:4. doi: 10.1186/1475-2875-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleass RJ, Woof JM. Fc receptors and immunity to parasites. Trends Parasitol. 2001;17:545–551. doi: 10.1016/s1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. Jama. 2005;293:1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, Kumar S, Chitnis CE, Narum DL, Michon P, Siba PM, Cowman AF, Mueller I, Beeson JG. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- Rolfes MA, McCarra M, Magak NG, Ernst KC, Dent AE, Lindblade KA, John CC. Development of clinical immunity to malaria in highland areas of low and unstable transmission. Am J Trop Med Hyg. 2012;87:806–812. doi: 10.4269/ajtmh.2012.11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Perignon JL, Druilhe P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr JB, Samb B, Sagna AB, Fortin S, Doucoure S, Sow C, Senghor S, Gaayeb L, Guindo S, Schacht AM, Rogerie F, Hermann E, Dia I, Konate L, Riveau G, Remoue F. Differential acquisition of human antibody responses to Plasmodium falciparum according to intensity of exposure to Anopheles bites. Trans R Soc Trop Med Hyg. 2012;106:460–467. doi: 10.1016/j.trstmh.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Sarthou JL, Angel G, Aribot G, Rogier C, Dieye A, Toure Balde A, Diatta B, Seignot P, Roussilhon C. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–3276. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks GD, Biomndo K, Hay SI, Snow RW. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans R Soc Trop Med Hyg. 2000;94:253–255. doi: 10.1016/s0035-9203(00)90310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YP, Sayed U, Qari SH, Roberts JM, Udhayakumar V, Oloo AJ, Hawley WA, Kaslow DC, Nahlen BL, Lal AA. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken CH, Thomas AW, Van Gemert GJ, Sauerwein RW, Blackman MJ, Anders RF, Pluschke G, Mazier D. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun. 2004;72:247–252. doi: 10.1128/IAI.72.1.247-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009a;77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009b;77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- Tebo AE, Kremsner PG, Luty AJF. Plasmodium falciparum: A Major Role for IgG3 in Antibody-Dependent Monocyte-Mediated Cellular Inhibition of Parasite Growth in Vitro. Exp Parasit. 2001;98:20–28. doi: 10.1006/expr.2001.4619. [DOI] [PubMed] [Google Scholar]
- Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, Lemnge MM, Gowda CD, Todd JE, Corran PH, Riley EM. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–264. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M, Berzins K, Perlmann P, Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983;54:135–142. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.