Abstract
Background
Impairments in structural and functional connections are demonstrated in schizophrenia. Certain disconnectional patterns may be biomarkers of elevated risk for schizophrenia. Convergent examination of multiple diffusion parameters and cognitive performance better illustrates pathophysiological significance of such disconnectional patterns.
Methods
Diffusion Tensor Imaging data on 39 early-course schizophrenia subjects, 21 adolescent/young adult first-degree relatives (FDR) of schizophrenia subjects and 29 healthy controls (HC) were examined for threshold-free clusters of fractional anisotropy (FA) and radial diffusivity (RD) differences correcting for multiple comparisons. Regression models examined the variance contributed by anisotropy differences, age and sex. Group-wise differences on sustained attention, verbal memory and executive functions were examined and correlated with diffusivity measures controlling for age and sex.
Results
Schizophrenia subjects showed significantly decreased FA and increased RD in the forceps minor and superior longitudinal fasciculus (SLF) compared to HC. FDR showed decreased forceps minor FA compared to HC, and decreased SLF RD compared to HC and schizophrenia subjects. Quantitative RD differences were 2–3 fold higher compared to FA. Besides, forceps minor RD was inversely correlated with sustained attention in schizophrenia.
Conclusions
Schizophrenia and FDR subjects show different patterns of white matter diffusivity compared to HC. While forceps minor changes may be a disease marker, SLF changes may be risk markers. In addition, RD may be a more robust risk marker than FA.
Keywords: Schizophrenia, High risk, White matter, Connectivity, Genetics, Cognition
1. Background
Schizophrenia is proposed as a ‘disconnection syndrome’ (Friston and Frith, 1995). Systematic mapping of the ‘disconnection’ could elucidate pathophysiology, risk for psychoses and endophenotypes. Diffusion tensor imaging (DTI) is a promising non-invasive approach to characterize anisotropy of water diffusion reflecting tissue microstructure. DTI relies on the principle that water tends to diffuse more freely along the longitudinal axis (λ1) than along the transverse axes (λ2 and λ3) of axons. Fractional anisotropy (FA; relative changes in diffusion along the λ1 compared to λ2 and λ3) and radial diffusivity (RD; mean diffusion perpendicular to the axonal orientation) are frequently used to characterize altered anisotropy (Beaulieu, 2002), myelination (Kubicki et al., 2005; Klawiter et al., 2011) and tract coherence (Kubicki et al., 2005). Altered FA alone does not clearly depict changes in white matter microarchitecture. Simultaneously examining multiple diffusivity measures more clearly suggest white matter pathology (Hasan, 2006).
Functional and DTI studies suggest ‘disconnected’ networks among schizophrenia subjects and first-degree relatives of schizophrenia subjects (FDR) who have about 10% risk for schizophrenia (Gottesman, 1991) compared to healthy controls (HC). Increased prefrontal and thalamic activations during episodic memory processing among schizophrenia subjects and FDR compared to HC suggest an impaired fronto-temporal and fronto-thalamic connectivity (Stolz et al., 2012). Resting fMRI studies report altered functional connectivity among schizophrenia subjects and FDR (Whitfield-Gabrieli and Ford, 2012). DTI studies report altered anisotropy in the association (superior longitudinal fasciculus (SLF), uncinate fasciculus) (Friedman et al., 2008), commissural (corpus callosum, forceps minor) (Friedman et al., 2008) and projection (internal capsule and cingulum) (Ellison-Wright and Bullmore, 2009) fibers in schizophrenia compared to controls. A combined structural and functional connectivity showed lower coherence between the two although there was globally decreased anatomical connectivity (Skudlarski et al., 2010). The nature of diffusivity differences suggests that schizophrenia subjects in general show decreased FA (Bora et al., 2011; Fitzsimmons et al., 2013) and increased mean diffusivity (Fitzsimmons et al., 2013) that may be contributed by increased RD but not axial diffusivity (Seal et al., 2008; Scheel et al., 2013). Anisotropy differences among FDR were intermediate between schizophrenia and HC (Skudlarski et al., 2013). Decreased FA in the SLF among ultra-high risk (UHR) subjects at baseline (Karlsgodt et al., 2009) and in those who later developed psychosis (Bloemen et al., 2010) suggest that reduced anisotropy in the SLF may be a biomarker of risk for psychosis. Another DTI study on UHR subjects reported decreased FA and increased RD among UHR subjects compared to HC in the SLF along with other regions; progressive reduction in FA in the frontal white matter was noted among UHR subjects who later developed psychosis compared to those who did not suggesting that the frontal white matter may be a risk biomarker (Carletti et al., 2012). It is unclear whether the nature of changes in diffusivity patterns or variations in specific tracts among the diagnostic groups contributes to the heterogeneity of the disorder.
We comprehensively examined multiple diffusivity measures among early-course schizophrenia subjects, FDR and HC. We hypothesized that the: (a) fronto-temporal, fronto-parietal and frontothalamic tracts would show reduced FA and increased RD within the same regions among schizophrenia subjects compared to HC, (b) FA and RD in the above tracts among FDR subjects would be intermediate between that of schizophrenia and HC subjects, and (c) diffusivity differences in these tracts would correlate with cognitive performance.
2. Methods
2.1. Clinical
We enrolled 89 adolescents/young adults with early-course schizophrenia/schizoaffective disorder (n = 39), FDR of schizophrenia/ schizoaffective disorder subjects (n = 21) and HC (n = 29) at the University of Pittsburgh, Pittsburgh, PA. FDR had at least one parent/sibling with DSM-IV schizophrenia/schizoaffective disorder who were not participants in this study. Consensus diagnosis was made by collating data from the Structured Clinical Interview for DSM-IV (SCID) (First, 1997), follow-up evaluations and medical charts. Substance abuse in the previous month or dependence 6 months prior to enrolment, mental retardation per DSM-IV, serious neurological (e.g. epilepsy, encephalitis/ meningitis) or medical illnesses were exclusion criteria. After fully explaining the experimental procedures, subjects provided informed consents. The University of Pittsburgh IRB approved the study.
Sustained attention, executive functions and verbal memory were evaluated within a week of imaging using the Continuous Performance Test (CPT-IP) (Cornblatt and Keilp, 1994), the Wisconsin Card Sorting Test (WCST) (Berg, 1948) and the Word List Memory Test (WLMT) (Sharma, 2003), respectively. Verbal d’ from the CPT-IP was used as a sensitivity measure of discrimination of signal from the false alarms. The percentage of perseverative errors in the WCST indexed executive functions. Within the WLMT, trial-to-trial transfer measured verbal memory and learning.
2.2. Imaging
DTI data were acquired on a 3T Siemens Tim Trio whole-body scanner in 30 directions (b = 1000 s/mm2) with 2 averages (slices = 48, thickness = 3.2 mm, TE = 90 ms, TR = 6300 ms, flip angle = 90°, matrix = 128 × 128, FOV = 240 mm). For each average, one b = 0 reference image was acquired.
The FA and RD maps were separately analyzed using FSL 4.1 (Smith et al., 2006). Diffusion scans were skull-stripped and manually checked for optimum brain extraction using the brain extraction tool (BET)(Hua et al., 2008). Eddy current and motion artefacts were corrected before all subjects' FA data were aligned into the MNI152 FA template using nonlinear registration. The diffusion tensors were fitted at each voxel on the corrected data. Voxelwise statistical analyses in these FA/RD maps were carried out using the Tract-Based Spatial Statistics (TBSS) applying 10,000 permutations. The mean FA image was created and thinned to generate a mean FA skeleton which represents the centers of all tracts common to the group. Each subject's aligned FA data was then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics. Threshold-free cluster enhancements (TFCE) were examined among the study groups correcting for multiple comparisons using the familywise error (FWE) correction at p b 0.05 within Randomise 4.1.9. The Johns Hopkins University (JHU) White Matter Tractography Atlas (Mori et al., 2008) was used to identify regions with FA/RD changes.
The FA/RD were extracted for each subject from the regions with significant diffusion changes within each contrast. Using the co-ordinates from the contrasts that showed diffusion changes, diffusion data were extracted from the contrasts that did not show changes within FSL for posthoc analyses. We conducted a group-wise comparison of primary diffusion direction (PDD) dispersion within these clusters to investigate complex fiber architecture (e.g. crossing fibers).
2.3. Statistical plan
Within FSL, three independent contrasts (HC/SZ, HC/FDR and FDR/SZ) examined FA/RD differences separately using ANCOVA models by including age and sex as covariates. FA/RD extracted from significant clusters were examined within forward stepwise linear regression models for the variance contributed by age, sex and FA/RD. FA/RD changes that remained significant after removing the effects of age and sex are reported. Using ANCOVA, we compared cognitive performances among the groups controlling for age and sex, and then correlated them with the FA/RD using partial correlations correcting for multiple testing. Only corrected p-values are reported.
3. Results
3.1. Demographic and clinical
Although the main effect of group on age was not significant (p = 0.08), FDR were younger compared to both schizophrenia (p = 0.048) and HC (p = 0.043). There were more females among FDR and HC compared to schizophrenia (χ2 = 13.54, df 2, p = 0.001). Hence, age and sex were covaried in all our analyses and variance contributed by age and sex for the FA/RD changes was estimated. Mean illness duration of patients was 3.35 ± 2.84 years (Table 1).
Table 1.
Demographic and clinical characteristics.
| Schizophrenia | First-degree relatives | Healthy controls | Statistics | Significance | |
|---|---|---|---|---|---|
| Age | |||||
| Mean ± SD | 26.83 ± 8.53 | 22.95 ± 4.10 | 27.14 ± 6.75 | F(2,88) = 2.56 | 0.084 |
| Min-Max | 18.21-50.38 | 18.44-34.07 | 18.05-42.10 | ||
| Median | 24.87 | 21.79 | 26.12 | ||
| Sex | |||||
| Male | 22 | 2 | 9 | χ2 = 13.54 | 0.001 |
| Female | 17 | 19 | 20 | ||
| Duration of illness | |||||
| From first psychotic symptom | 3.35 ± 2.84 years | - | - |
3.2. FSL analysis
3.2.1. Diffusion measures
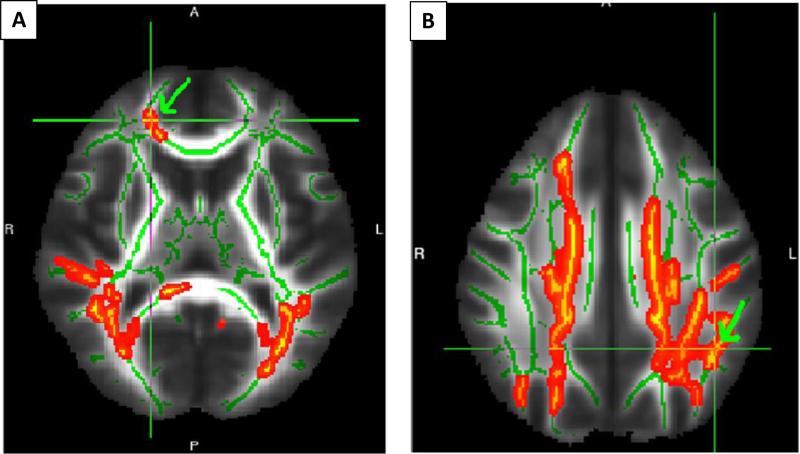
Schizophrenia subjects showed significant FA reductions compared to HC in the left SLF (p = 0.022), the right SLF (p = 0.016), forceps minor (p = 0.04), and the right uncinate fasciculus (p = 0.04) regions (Fig. 1). FA did not differ in the FDR and schizophrenia, and FDR and HC contrasts. No regions showed increased FA in any of the contrasts.
Fig. 1.
FA reduction among schizophrenia subjects compared to controls in the forceps minor (A), and the SLF (B). Regions that met statistical threshold (whole-brain FWE p < 0.05) are shown with green arrow and overlaid cross-hairs. Extracted FA and RD were. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Schizophrenia subjects showed increased RD compared to HC in the forceps minor (p = 0.0025), the left (p = 0.008) and the right SLF (p = 0.0055) regions approximately within the same coordinates as FA was reduced. Like FA, RD did not differ in the FDR/schizophrenia and FDR/HC contrasts. No regions with decreased RD were detected.
3.2.2. Extracted FA
3.2.2.1. Schizophrenia Vs HC
Forward stepwise regression models noted FA reduction in the forceps minor region, sex and left SLF region (in that order) accounting for 25% of the variance (t = 2.11, p = 0.039; goodness-of-fit, F(3, 67) = 8.03, p = 0.001). The forceps minor and left SLF FA among schizophrenia subjects each showed 8% reduction compared to HC (Table 2).
Table 2.
Results of forward stepwise linear regression analyses of FA and RD changes.
| Contrasts | Tracts | Coordinates (mm) | Coefficients |
Variance |
Model fit |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | t | p | R2 | R2 change | F | Df | p | |||||
| FA | ||||||||||||
| Schizophrenia vs healthy controls | Forceps minor | 20 | 41 | 10 | 0.318 | 2.87 | 0.006 | 0.130 | 0.130 | 7.12 | 3, 67 | 0.0003 |
| Sex | 0.243 | 2.24 | 0.028 | 0.198 | 0.068 | |||||||
| Left SLF | –35 | –29 | 35 | 0.232 | 2.04 | 0.045 | 0.250 | 0.052 | ||||
| Posthoc comparison | ||||||||||||
| FDR vs healthy controls | Age | 0.387 | 2.912 | 0.005 | 0.117 | 0.117 | 5.54 | 2,49 | 0.007 | |||
| Forceps minor | 20 | 41 | 10 | 0.275 | 2.068 | 0.044 | 0.191 | 0.074 | ||||
| RD | ||||||||||||
| schizophrenia vs healthy controls | Forceps minor | 19 | 40 | 16 | –0.27 | –2.28 | 0.026 | 0.073 | - | 5.21 | 1,67 | 0.026 |
| Posthoc comparison | ||||||||||||
| FDR vs healthy controls | Left SLF | –33 | –20 | 35 | 0.412 | 3.35 | 0.002 | 0.267 | 0.267 | 13.27 | 2,49 | 0.00003 |
| Forceps minor | 19 | 40 | 16 | 0.241 | 2.63 | 0.011 | 0.334 | 0.094 | ||||
| FDR vs schizophrenia | Left SLF | –33 | –20 | 35 | –0.405 | –3.85 | 0.0003 | 0.290 | 0.290 | 18.19 | 3,59 | 2 × 10–7 |
| Sex | 0.361 | 3.69 | 0.001 | 0.453 | 0.163 | |||||||
| Forceps minor | 19 | 40 | 16 | –0.227 | –2.11 | 0.039 | 0.494 | 0.040 | ||||
3.2.2.2. Posthoc comparisons
Age contributed 11.7% of the variance between FDR and HC. After removing the effects of age, FA reduction in the left SLF region among FDR compared to HC accounted for an additional 7.4% of the variance (t = 2.07, p = 0.04; goodness-of-fit, F(2, 49) = 5.54, p = 0.007). FDR showed 6% reduction in the left SLF FA region compared to HC. Schizophrenia subjects and FDR did not differ in FA in the forceps minor and SLF regions (p N 0.1).
3.2.3. Extracted RD
3.2.3.1. Schizophrenia Vs HC
The RD within the forceps minor region accounted for 7.3% of the variance (t = 2.28, p = 0.026; goodness-of-fit, F(1, 67) = 5.21, p = 0.026) for the differences between schizophrenia and HC with a 15% increase in schizophrenia compared to HC. Age and sex did not contribute to the model (Table 1).
3.2.3.2. Posthoc comparisons
FDR and HC comparison showed that the RD in the left SLF and forceps minor regions together contributed 33% of the variance (t = 2.63, p = 0.01; goodness-of-fit, F(2, 49) = 13.27, p = 0.00003). RD among FDR was decreased by 23.6% in the left SLF region and 17.4% in the forceps minor region compared to HC. Age and sex did not contribute significantly.
Further, the left SLF, sex, forceps minor RD (in that order) contributed to about 49% of the variance for the differences between FDR and schizophrenia (t = 2.11, p = 0.039; goodness-of-fit, F(3, 59) = 18.19, p < 0.001). Schizophrenia subjects showed about 18% and 29% increase in RD in the left SLF and forceps minor regions, respectively compared to FDR.
3.3. Crossing fiber analysis
PDD dispersion in the voxel clusters that showed significant group differences was not different among the groups (all p > 0.1). Mean PDD dispersion was closer to 0 suggesting a coherent alignment of the fibers.
3.4. Cognitive measures and diffusion metrics
We observed group main effect on verbal d' on CPT-IP (Wilk's λ = 0.84, F = 2.14, p = 0.052; between-subjects effects, F = (2,76) = 5.57, p = 0.006 for verbal d’) in the entire sample of schizophrenia, FDR and HC subjects. Posthoc Bonferroni tests revealed that the verbal d’ was reduced among schizophrenia subjects compared to FDR (p = 0.0002) and HC (p = 0.0003) but not between FDR and HC. Likewise, verbal memory performance was poorer among schizophrenia subjects compared to HC (p = 0.0003) but FDR and SZ, and FDR and HC did not differ in performance. Schizophrenia subjects committed higher percentage of perseverative errors compared to FDR (p = 0.028) and HC (p = 0.045). FDR and HC did not differ. Thus, FDR did not differ from HC in performance on these tasks.
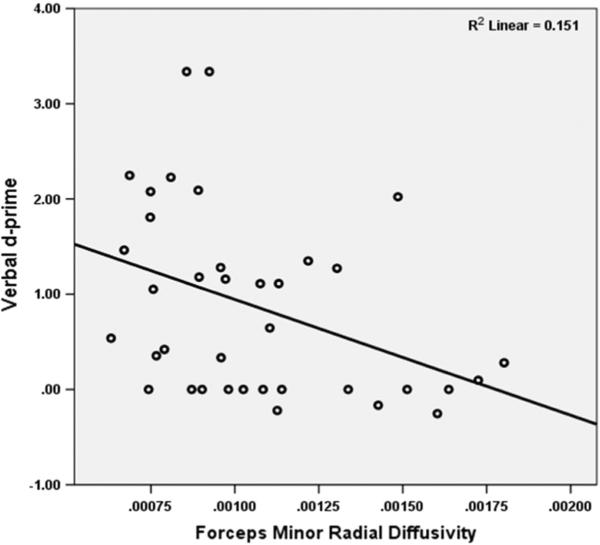
There was an inverse correlation between forceps minor RD with verbal d' in the entire sample controlling for age, sex and caseness (partial r = −0.24, p = 0.027). Verbal memory and executive functions were not correlated with either FA or RD changes. Within-group analyses showed inverse correlation of forceps minor RD with verbal d’ among schizophrenia subjects (partial r = −0.41, p = 0.014) but not FDR or HC (Fig. 2).
Fig. 2.
Correlation of forceps minor RD with verbal d' from CPT-IP.
4. Discussion
Our results suggest anisotropy differences in the forceps minor region that correlated with sustained attention deficits suggesting impaired prefrontal interhemispheric connectivity in schizophrenia. Altered anisotropy in the SLF region suggests impaired translobar connectivity between the fronto-parieto-limbic regions in schizophrenia. We did not observe changes in the fronto-thalamic tracts as hypothesized. Additionally, FA reduction was accompanied by RD increase within the same regions among schizophrenia subjects suggesting an increased isotropic diffusion in these tracts. The observed anisotropy differences were not associated with underlying complex fiber architecture. Such diffusion dynamics may reflect altered tract coherence and myelination (Pierpaoli and Basser, 1996; Kubicki et al., 2005). These observations support selective impairments in commissural and association tracts but not the projection fibers; commissural connectivity may underlie impairment of cognitive domains regulated by the PFC in schizophrenia.
The forceps minor connects a broad swath of prefrontal regions from the Brodmann area 8 dorsally to area 10 ventrally, and area 9 superiorly to area 44 inferiorly of each hemisphere that regulate attention (Langner and Eickhoff, 2012) and executive functions (Kraus et al., 2007; Perez-Iglesias et al., 2010) among others. Decreased forceps minor anisotropy may affect interhemispheric communications and may contribute to sustained attention deficits. A meta-analysis of subjects with attention-deficit hyperactivity disorder reported significantly reduced forceps minor FA (van Ewijk et al., 2012). Another study reported correlation of intra-individual variability in attention with forceps minor mean diffusivity (Tamnes et al., 2012). The left SLF did not show such differences in schizophrenia suggesting that impaired forceps minor integrity may be a disease marker.
FDR showed decreased FA and RD compared to HC in the left SLF suggesting poorer connection between PFC and posterior parietal but not with the temporal cortex suggesting that impaired SLF integrity may be a risk marker. Previous studies reported sustained attention deficits with impaired SLF integrity (Konrad et al., 2010; Klarborg et al., 2012); however, we did not note such correlations. There were no forceps minor abnormalities among FDR. Besides, FDR and schizophrenia were not dissimilar in the SLF diffusivity but FDR performed similar to HC on cognitive tasks. This lack of coherence between decreased anisotropy and cognitive performance may be due to compensatory mechanisms through better functional connectivity. Such an observation was made in SZ-HC comparison (Skudlarski et al., 2010); it is likely that such a mechanism may underlie our observation. Such compensatory reserve may be limited in schizophrenia due to ‘dysconnection’ across multiple regions. Besides, anisotropy may need to reach a critical threshold to manifest cognitive deficits. Characterizing such a threshold may be critical to quantifying future risk for psychoses.
FDR showed decreased RD compared to both schizophrenia and HC, and decreased FA compared to HC but not schizophrenia. Posthoc quantification of RD and FA differences showed that RD was 2–3 folds different between SZ/HC, FDR/HC and FDR/SZ comparisons compared to FA. During development, progressive RD reductions (Asato et al., 2010) associated with FA increase (Gao et al., 2009; Geng et al., 2012) are reported. The association and projection fibers continue to mature beyond adolescence (Asato et al., 2010). A meta-analysis observed a robust increase in left SLF FA during development among adolescents (Peters et al., 2012). We noted RD reductions among FDR in both tracts without concomitant increase in FA after controlling for age and sex. Further, PDD dispersion among FDR was no different from other groups suggesting that the decreased FA and RD among FDR were not due to complex fiber architecture. Thus, FDR subjects in this study show decreased RD with no concomitant increase in FA suggesting that an impaired integrity of SLF and forceps minor may be due to altered development. However, the relationship between age and FA is non-linear and requires longitudinal data to appropriately model developmental changes. Previous studies reported decreased FA in the SLF among UHR subjects (Karlsgodt et al., 2009) and predicted later development of psychosis (Bloemen et al., 2010). Our data suggest that decreased RD may be a more robust risk marker than FA, and that developmental delay particularly in the SLF may be a marker of psychosis risk.
The forceps minor FA may progressively decrease over the illness course. A prior study reported prominent reduction in forceps minor FA in chronic schizophrenia (>20 years illness) compared to first-episode schizophrenia (illness <1 year) (Friedman et al., 2008). We noted robust reduction in forceps minor integrity and correlation with sustained attention deficits among schizophrenia subjects with an illness duration of 3.35 ± 2.84 years suggesting that FA reduction may occur early in the illness. The two studies differed in sample characteristics and methods. Prior study examined hypothesized tracts (Friedman et al., 2008) whereas we examined whole-brain differences using a stringent statistical threshold to identify affected tracts. Our sample had more females with no group-differences in age; however, diffusivity differences emerged significant even after controlling for age and sex. Longitudinal studies could clarify progressive changes in these tracts.
Reciprocal changes in FA and RD within the same coordinates on the forceps minor and the SLF suggest that the diffusion ellipsoid is more spherical and underscores the need to measure multiple diffusivity metrics to obtain convergent evidence of white matter pathology (Hasan, 2006). Concurrent increase of RD may suggest impaired myelination (Klawiter et al., 2011) and axonal pathology (Basser and Pierpaoli, 1996; Pierpaoli et al., 1996; Beaulieu, 2002) that may affect neural communication (Westlye et al., 2009) and neural firing patterns in the cortex (Daselaar et al., 2013). FA and RD hold mathematically complex and non-linear relationship. FA is a scaled measure of square root of sum of squares of diffusivity differences divided by square root of sum of squares of diffusivity differences along three axes whereas RD is the mean of the diffusion in two minor axes (Mori, 2007). Appropriate modeling of these metrics may characterize relative diffusion directionality changes among the groups.
Our study was limited by relatively small sample of FDR, and uneven sex distribution across the groups. Although TBSS is a robust method to examine anisotropy changes, the TBSS skeleton discontinuity at crossing fibers might make interpretation of FA and RD changes ambiguous. Implementing B-matrix rotation could have provided better correction for head motion (Leemans and Jones, 2009). Since the scanning duration was about 10 min in this study, head motion is unlikely to be a major source of error. Potential effects of psychotropic medications cannot be fully resolved unless antipsychotic-naïve first-episode subjects are examined (Wang et al., 2013). Controlling for and examining the variance contributed by demographic factors revealed that diffusion measures were still important. We examined early course schizophrenia subjects to avoid inherent confounds of illness chronicity.
In summary, we report decreased FA and increased RD of forceps minor that correlated with sustained attention deficits in schizophrenia. Impaired left SLF integrity in schizophrenia did not correlate with cognitive performance. The left SLF RD among FDR was intermediate between schizophrenia and HC suggesting that this may be a risk marker.
Acknowledgments
This work was supported by research grants from the National Institute of Mental Health MH72995 and MH93540 (KMP). We thank Dr. Debra Montrose, Dr. Elizabeth Radomsky, Mr. Kevin Eklund, Ms. Diana Dworakowski and Ms. Alicia Thomas for enrolment and characterization of research participants. We also thank Mr. Dhruman Goradia, Ms, Krishna Pancholi, Mr. Steven Goodnow, Ms. Jean Miewald and Ms. Karol Rosengarth for their help in data analysis, data management and quality assurance. Funding agencies did not have any further role in the analysis and reporting of the results.
Role of funding source
This work was supported by research grants from the National Institute of Mental Health MH72995, MH93540 and the Stanley Medical Research Institute (07T-722) (KMP). Funding agencies did not have any further role in the analysis and reporting of the results.
Footnotes
Contributors
Dr. Konasale Prasad conceived the idea and designed the study. Ms. Catherine Upton conducted the data processing and analysis under Dr. Prasad's supervision. Drs. Nimgaonkar and Keshavan discussed the concept, reviewed the manuscript, suggested further analyses and interpreted the results along with other authors. All authors took part in the preparation, editing and reviewing the manuscript carefully.
Conflict of interest
None of the authors disclose any conflict of interest to declare that would impinge on the design, execution, analysis or reporting and discussion of the results
References
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, Dingemans P, Linszen DH, van Amelsvoort TA. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol. Med. 2010;40(8):1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 2011;127(1–3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Fusar Poli P, Valmaggia L, Broome MR, Bramon E, Johns L, Giampietro V, Williams SC, Barker GJ, McGuire PK. Alterations in white matter evident before the onset of psychosis. Schizophr. Bull. 2012 doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr. Bull. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: Low-performing older adults compensate for impaired white matter with greater neural activity. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version. Administration Booklet. American Psychiatric Press; Washington, DC.: 1997. [Google Scholar]
- Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr. Opin. Psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am. J. Psychiatry. 2008;165(8):1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am. J. Neuroradiol. 2009;30(2):290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gerig G, Gilmore JH. Quantitative tract-based white matter development from birth to age 2 years. Neuroimage. 2012;61(3):542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia Genesis: The Origins of Madness. W. H. Freeman; New York: 1991. [Google Scholar]
- Hasan KM. Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: fractional anisotropy alone is not sufficient. Radiology. 2006;239(2):611–612. doi: 10.1148/radiol.2392051172. (author reply 612–613) [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol. Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarborg B, Skak Madsen K, Vestergaard M, Skimminge A, Jernigan TL, Baare WF. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, Vucurevic G, Stoeter P, Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur. J. Neurosci. 2010;31(5):912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;30(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2012 doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to Diffusion Tensor Imaging. Elsevier; Oxford: 2007. [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am. J. Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, Derosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr. Bull. 2012 doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Scheel M, Prokscha T, Bayerl M, Gallinat J, Montag C. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct. Funct. 2013;218(1):151–156. doi: 10.1007/s00429-012-0389-2. [DOI] [PubMed] [Google Scholar]
- Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, Walterfang M, Pell GS, Pantelis C. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr. Res. 2008;101(1–3):106–110. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- Sharma T. Cogtest: Computerized Neurocognitive Test Battery for Clinical Trials. 2003 [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68(1):61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O'Neil K, Pearlson GD. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry. 2013;170(8):886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stolz E, Pancholi KM, Goradia DD, Paul S, Keshavan MS, Nimgaonkar VL, Prasad KM. Brain activation patterns during visual episodic memory processing among first-degree relatives of schizophrenia subjects. Neuroimage. 2012;63(3):1154–1161. doi: 10.1016/j.neuroimage.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Ostby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J. Neurosci. 2012;32(3):972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, Wang Y, Jiang L, Sham PC, Collier DA, Gong Q, Chua SE, McAlonan GM, Li T. White-matter micro-structure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol. Med. 2013;43(11):2301–2309. doi: 10.1017/S0033291713000238. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjørnerud A, Due-Tønnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus—a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb. Cortex. 2009;19(2):293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]