Abstract
Background
The pathophysiological underpinnings of impaired anatomical and functional connectivity are not precisely known. Emerging data suggest that immune mediators may underlie such dysconnectivity. We examined anatomical brain connections using diffusion tensor imaging (DTI) data in relation to interleukin-6 (IL-6) and C-reactive protein (CRP) levels among early-course clinically stable schizophrenia subjects compared to healthy controls (HC).
Methods
DTI data were acquired in 30 directions with 2 averages. Fractional anisotropy (FA) and radial diffusivity (RD) maps were separately processed using FSL4.1.9 and Tract-Based Spatial Statistics (TBSS). Threshold free cluster enhancements (TFCE) were examined employing familywise error (FWE) corrections for multiple testing within linear regression models including age, sex and socioeconomic status as covariates. IL-6 and CRP were assayed using highly sensitive and specific sandwich immunosorbent assays.
Results
The groups did not differ in age and sex as well as in the IL-6 and CRP levels. IL-6 levels were negatively correlated with the FA and positively correlated with RD among schizophrenia subjects but not HC. The voxel clusters that showed significant correlations were localized to the forceps major, the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus. CRP levels showed similar pattern except for lack of correlation with RD on any cluster that corresponded to the forceps major.
Discussion
Our results suggest that the IL-6 and CRP contribute to impaired anisotropy of water diffusion in selected pathways that have been previously associated with schizophrenia suggesting differential susceptibility of selected neural pathways to immune mediators.
Keywords: Schizophrenia, Inflammation, Immune mediators, Cytokines, White matter, Connectivity
1. Introduction
Impairments in both anatomical (Ellison-Wright and Bullmore, 2009) and functional (Fitzsimmons et al., 2013) connectivity suggest that schizophrenia may be a dysconnection syndrome. Elucidating the factors that contribute to the dysconnection is critical to identify novel targets for drug discovery.
Several lines of evidence strongly suggest the role of immune mediators in the pathophysiology of schizophrenia (Heath et al., 1967; Muller and Schwarz, 2010; Rothermundt et al., 2001; Saetre et al., 2007). Postmortem studies note activated microglia/macrophages (Bayer et al., 1999; Radewicz et al., 2000; Wierzba-Bobrowicz et al., 2005), elevated expression of inflammatory markers in the prefrontal cortex (PFC) neurons (Fillman et al., 2013) and vasculature (Harris et al., 2008), and autoantibodies against frontal (Henneberg et al., 1994), cingulate (Ganguli et al., 1987; Henneberg et al., 1994; Kelly et al., 1987), hippocampal cortices (Ganguli et al., 1987), and glutamate receptors (Tsutsui et al., 2012) in schizophrenia. Autoimmune disorders may elevate the risk for schizophrenia both independently (Benros et al., 2011; Chen et al., 2012) and in combination with exposure to infectious agents (Benros et al., 2011). A meta-analysis noted elevated peripheral blood inflammatory cytokines in schizophrenia compared to healthy controls (HC) (Potvin et al., 2008). Genome-wide association studies replicate the association of Major Histocompatibility Complex (MHC) region variants (where a majority of immune genes are located) with schizophrenia (Purcell et al., 2009; Ripke et al., 2011; Shi et al., 2009; Stefansson et al., 2009). Non-steroidal anti-inflammatory drugs may reduce psychotic symptom severity (Muller et al., 2010; Muller et al., 2002; Sommer et al., 2011). However, the mechanisms through which immune mediators affect cognitive impairments and psychopathology are under active investigation.
Characterizing peripheral immune markers that are relevant to the pathophysiology of schizophrenia is critical for early diagnosis, novel drug discovery, monitoring treatment response and predicting risk. We examined the association of the C-reactive protein (CRP)—an acute phase reactant, and interleukin-6 (IL-6) - a predominantly pro-inflammatory cytokine with the anisotropy of water diffusion using diffusion tensor imaging (DTI). CRP is a pattern recognition molecule that is phylogenetically highly conserved and is primarily expressed in liver following induction by IL-6 (Black et al., 2004). Although extrahepatic expression of CRP in the neurons (Yasojima et al., 2000) and lymphocytes (Kuta and Baum, 1986) are described, plasma levels mainly reflect hepatic synthesis (Black et al., 2004). CRP may be either pro- or anti-inflammatory depending on the context (Eisenhardt et al., 2009). IL-6 is mainly a pro-inflammatory cytokine secreted by T cells and macrophages (Kato et al., 2009). Quantitative reviews have reported elevated levels of IL-6 (Potvin et al., 2008) and CRP (Miller et al., 2014) in schizophrenia with variations related to antipsychotic use, associated comorbidities and stage of illness.
Elevated CRP is associated with cognitive deficits in schizophrenia both independently (Dickerson et al., 2007) and through interaction with exposure to Herpes Simplex Virus 1 - a neurotropic virus (Dickerson et al., 2012). Although quantitative reviews suggest elevated IL-6 in schizophrenia, few studies have examined the association of IL-6 with in vivo neurobiology of schizophrenia. Among middle-aged and elderly healthy subjects, elevated IL-6 was associated with cognitive impairments (Marsland et al., 2006; Weaver et al., 2002) and hippocampal volume reductions (Marsland et al., 2008). However, the association of CRP and IL-6 with brain connections in schizophrenia is not examined adequately.
DTI offers insights into axonal integrity by characterizing anisotropy of water diffusion. DTI is based on the principle that water tends to diffuse more freely along the longitudinal axis (λ1) than along the transverse axes (λ2, λ3) of axons. The fractional anisotropy (FA) - diffusion along the λ1 compared to λ2 and λ3, and the radial diffusivity (RD) - the diffusion perpendicular to the axon orientation-index white matter integrity (Beaulieu, 2002). Factors affecting impaired anisotropy of water diffusion that could impact connectivity are unclear. DTI studies report altered diffusivity in the association (superior longitudinal fasciculus (SLF), uncinate fasciculus) (Friedman et al., 2008), commissural (corpus callosum, forceps minor) (Friedman et al., 2008) and projection (internal capsule and cingulum) (Cheung et al., 2008; Kubicki et al., 2005) fibers in schizophrenia compared to controls. A meta-analysis noted reduced anisotropy in the left frontal and left temporal deep white matter (Ellison-Wright and Bullmore, 2009) suggesting impaired prefrontal-thalamic and fronto-temporo-occipital connections. Our functional imaging study revealed increased activation in the PFC and thalamus among schizophrenia subjects compared to HC during episodic memory challenge (Stolz et al., 2012).
Our goal was to examine whether selected immune mediators (IL-6 and CRP) affected the anisotropy measures in these tracts. Specifically, we predicted that the IL-6 and CRP levels would be negatively correlated with FA and positively correlated with RD in the fronto-thalamic and fronto-temporo-occipital white matter tracts. Since FA and RD represent diffusion in orthogonal directions and may be negatively correlated with each other, we explored the correlation between the FA and RD extracted from the significant voxel clusters that show correlation with IL-6 and CRP within FSL.
2. Methods
2.1. Clinical Evaluations
We enrolled 68 young adults (schizophrenia/schizoaffective disorder=39, HC=29). Of these participants, 64 subjects were included in the CRP analysis (SZ=37; HC=27) and 56 (SZ=33, HC=23) in the IL-6 analysis based on the availability of blood samples and assay qualities. The diagnosis of schizophrenia/schizoaffective disorder was obtained by administering the Structured Clinical Interview for DSM-IV (SCID)(First, 1997) and confirmed through consensus diagnosis by reviewing follow-up and medical chart data. The socioeconomic status (SES) was assessed using Hollingshead Index (Hollingshead, 1975). All patients were on stable doses of antipsychotics. The exclusion criteria were substance abuse in the previous month or dependence 6 months prior to enrollment, mental retardation per the DSM-IV, and/or serious neurological or other medical illnesses. After fully explaining the experimental procedures, subjects provided informed consents. The University of Pittsburgh IRB approved the study.
2.2. Imaging procedures
The DTI data was acquired in 30 directions (b=1000s/mm2) with 2 averages on a 3T Siemens Tim Trio scanner. For each average, one b=0 reference image was acquired. Scanning parameters were as follows: slices 48, thickness 3.2mm, TE=90ms, TR=6300ms, flip angle=900, matrix 128×128, FOV=240mm. Scans with motion artefacts were not included in the analysis. The diffusion data were processed using FSL 4.1 tools (Smith et al., 2006). The FA and RD maps were separately analysed. Diffusion scans were skull-stripped using FSL’s brain extraction tool and manually checked for optimum brain extraction. Eddy current and motion artefacts were then corrected before nonlinear registration of the skull-stripped FA maps to the MNI152 FA template. These co-registered images were processed using FSL’s Tract-Based Spatial Statistics (TBSS) tool. Randomize 4.1.9 was used to compare study groups. Threshold free clusters enhancements (TFCE) were examined correcting for multiple comparisons using the familywise error (FWE) correction at p ≤ 0.05 and 10000 permutations. The JHU White-Matter Tractography Atlas tool was used to identify significant regions-of-interest. The RD images were also processed using the same pipeline.
Using regression, CRP and Il-6 values were regressed to the FA and RD separately controlling for age, sex and SES. Fslmeants tool was used to extract FA values for each subject from the voxel clusters that showed significant differences in each model and identified using the JHU White-Matter Tractography Atlas.
2.3. Immunoassay
Highly specific singleplex quantitative ultrasensitive sandwich immunosorbent bead assay for IL-6 and CRP were conducted at the Luminex facility of the University of Pittsburgh Cancer Institute. Sandwich immunoassay uses the magnetic bead technology employing polystyrene microsphere beads that are internally dyed with red and infrared fluorospheres of differing intensities. After the sandwich immunoassay, beads are read for fluorescence signal intensity using Luminex detection systems.
2.4. Neuropsychological assessments
Sustained attention and executive functions were evaluated within a week of imaging using the Continuous Performance Test (CPT-IP) (Cornblatt and Keilp, 1994) and the Wisconsin Card Sorting Test (WCST) (Sharma, 2003), respectively. Verbal d′ from the CPT-IP was used as a sensitivity measure of discrimination of signal from the false alarms. Within the WCST, we used percentage of perseverative errors to index executive functions.
2.5. Plan of analysis
After examining the group main effect on FA and RD controlling for age, sex and SES within FSL, we investigated the correlation of CRP and IL-6 levels with anisotropy of water diffusion. General Linear Model linear regressions were set up within the FSL to regress the CRP and IL-6 levels to the whole-brain FA and RD separately controlling for age, sex and SES. Statistical thresholds were applied as stated above. Next, we examined the correlation between the FA and RD extracted from the white matter regions with voxel clusters that showed significant correlation with IL-6 and CRP within FSL. The diagnostic groups were compared for differences in cognitive performances controlling for age and sex using ANCOVA models. We, then, examined the correlation of cognitive performance with immune mediator levels and diffusion measures using partial correlation tests controlling for diagnosis, age and sex in the entire sample of schizophrenia and HC. Follow-up posthoc correlation tests were conducted for the cognitive measure that showed significant correlation with either FA or RD correcting for multiple comparisons within each diagnostic group.
3. Results
3.1. Demographic and clinical characteristics
The distribution of sex and mean age of subjects within the groups were not significantly different. The IL-6 and CRP levels were not significantly different between the groups. Mean duration of illness of schizophrenia subjects was about 3 years from the onset of first psychotic symptoms (Table 1).
Table 1.
Demographic and clinical characteristics of the study sample
| IL 6 | CRP | |||||||
|---|---|---|---|---|---|---|---|---|
| SZ | HC | Statistic | Sig | SZ | HC | Statistic | Sig | |
| Mean age±SD (years) | 26.39±8.57 | 25.44±5.50 | t=0.46 | 0.64 | 26.49±8.21 | 26.68±6.41 | t=0.10 | 0.92 |
| Sex | ||||||||
| Male | 18 | 7 | χ2=3.19 | 0.074 | 21 | 9 | χ2=3.44 | 0.064 |
| Female | 15 | 16 | 16 | 18 | ||||
| Immune marker levels | 1.14±2.66 ng/ml | 0.96±2.17 ng/ml | t=0.27 | 0.79 | 19.78±23.53 μg/ml | 15.69±21.69 μg/ml | t=0.71 | 0.48 |
| Duration of illness | 3.17±2.42 years | ----- | ----- | ----- | 3.12±2.40 years | ----- | ----- | ----- |
| BPRS positive symptoms | 8.79±3.80 | ----- | ----- | ----- | 8.93±3.88 | ----- | ----- | ----- |
| BPRS negative symptoms | 8.33±3.40 | ----- | ----- | ----- | 8.22±3.33 | ----- | ----- | ----- |
3.2. Comparison of diffusion measures
Schizophrenia subjects showed significant reductions in FA in the left (p=0.022) and right (p=0.016) SLF, forceps minor (p=0.04), and the right uncinate fasciculus (p=0.04) regions compared to HC. No regions showed increased FA. In addition, schizophrenia subjects showed increased RD compared to HC in the forceps minor (p=0.0025), the left (p=0.008) and the right SLF (p=0.0055) regions with no regions showing decreased RD.
3.3. Association of IL-6 levels with diffusivity measures
Using within-groups voxel-wise regression, we noted significant negative correlation of FA and positive correlations of RD with IL-6 levels in the forceps major among schizophrenia subjects (both FWE p<0.001) but not among HC. The extracted FA and RD from these voxel clusters were negatively correlated with each other among patients (Pearson correlation, r= −0.54, p=0.001; R2=0.29) but not HC (Fig 1).
Fig 1.
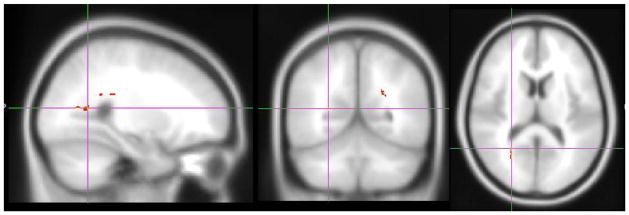
IL-6 levels and FA among SZ in the Forceps Major region. GLM linear regression showing significant negative correlation of FA with IL-6 levels. RD was positively correlated with IL-6 in the same region in the forceps major (both FWE p<0.001)
A similar pattern of negative correlation of the FA of the inferior longitudinal fasciculus (ILF) and the inferior fronto-occipital fasciculus (IFOF) among schizophrenia subjects with IL-6 levels (FWE p<0.05) but not HC was noted. In both these tracts, the extracted FA and RD were inversely correlated between each other among patients but not in controls (IFOF, r= −0.55, p=0.001; ILF, r= −0.54, p=0.001) (Fig 2).
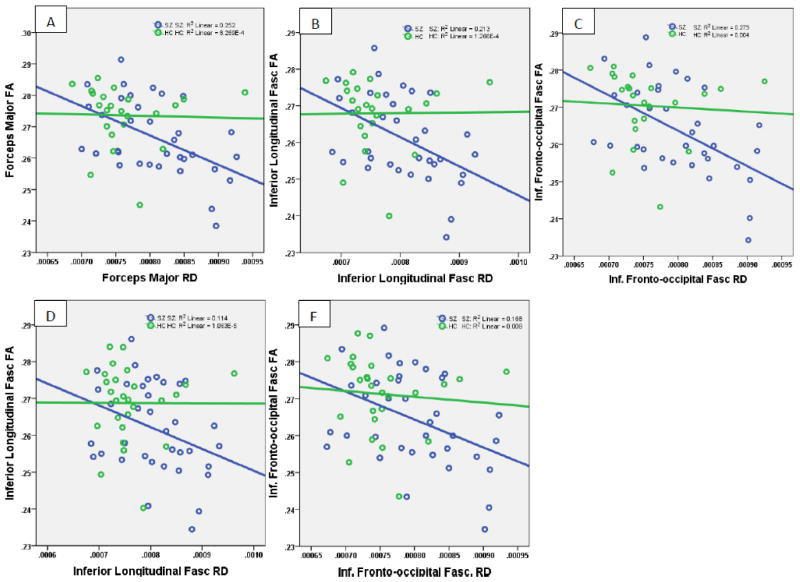
Fig 2.
Correlation of extracted FA and RD from the voxel clusters that showed correlation of diffusivity measures with immune mediator levels. Scatterplots A, B and C depict the correlation between FA and RD within the voxel clusters corresponding to the forceps major, the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus, respectively that showed significant correlation between diffusivity measures and IL-6. Scatterplots D and E depict similar correlations at the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus with CRP. CRP did not correlate with RD on any cluster that corresponded to the forceps major.
3.3. Association of CRP levels with diffusivity measures
Similar to IL-6, we observed significant negative correlation of FA within the forceps major with CRP levels among SZ subjects (FWE p=0.001) but not HC. However, RD did not show differences related to CRP levels. In addition, CRP levels negatively correlated with FA (FWE p<0.05) and positively correlated with RD of the IFOF and the ILF within the FSL among schizophrenia subjects but not controls. The extracted FA and RD from the ILF and IFOF regions also showed significant negative correlation among patients but not in controls (ILF, r= −0.39, p=0.019; IFOF, r= −0.41, p=0.013).
3.4. Neuropsychological data
Sustained attention data were available for 34 SZ and 27 HC whereas WCST data were available for 28 SZ and 26 HC. Schizophrenia patients showed impaired performance on both the sustained attention (verbal d′, SZ=1.009±1.03, HC=2.148±1.06, F(1,60)=19.00, p=5×10−5) and executive function measures (percent perseverative errors, SZ=17.25±10.08, HC=12.11±9.10, F(1,53)=3.97, p=0.052, and concept formation, SZ=54.89±20.54, HC=63.23±12.35, F(1,53)=3.75, p=0.058). Partial correlation tests controlling for age and sex revealed that the forceps major FA and RD were correlated with executive functions that did not survive corrections for multiple comparisons.
4. DISCUSSION
Our main finding is that the CRP and IL-6 levels correlated with anisotropy of water diffusion in selected tracts among schizophrenia subjects. Although the case-control differences in anisotropy measures was observed in the forceps minor, uncinate fasciculus and SLF, the CRP and IL-6 levels were correlated with FA and RD in distinctly different fiber tracts in the dorsal brain. The mean CRP and IL-6 levels did not differ between the groups; however, higher levels of each immune mediator was associated with decreased FA and higher RD suggesting that anisotropy of water diffusion within certain white matter tracts may be more vulnerable to IL-6 and CRP among schizophrenia subjects but not among HC. Further, such alterations may not be in the tracts that show case-control differences in diffusivity measures.
Forceps major is a major fiber bundle that crosses the midline through the splenium of the corpus callosum connecting the occipital lobes of each side. The forceps major system has been associated with the dorsal hippocampal commissural system that is well developed in humans compared to primates connecting the presubiculum, entorhinal cortex and parahippocampal gyrus of one side with the contralateral entorhinal cortex (Gloor et al., 1993). Lesions of the forceps major is associated with deficits in multi-tasking (Burgess et al., 2000), attention (Rossi et al., 2012) and allocation of attentional resources. Prior DTI studies on schizophrenia have reported impaired integrity of forceps major (Friedman et al., 2008; Perez-Iglesias et al., 2010a) that was associated with hallucinations (Curcic-Blake et al., 2013), especially of auditory-visual modality (Amad et al., 2014). However, our cohort did not show case-control differences in this tract.
Although we did not note case-control differences in anisotropy of water diffusion in the ILF and IFOF in this cohort, others have reported such changes (Liu et al., 2013, 2014). The ILF and IFOF overlap functionally in regulating object recognition, visuo-perceptual abilities (Ortibus et al., 2012; Tusa and Ungerleider, 1985), and emotion recognition (Philippi et al., 2009). Decreased FA in the IFOF was correlated with positive symptoms, negative symptoms and, and hallucinations among first-episode schizophrenia subjects (Curcic-Blake et al., 2013; Liu et al., 2014). Further, these tracts were associated with prolonged processing speed, verbal and visual learning deficits among chronic (Liu et al., 2013) and executive functions among first-episode (Lee et al., 2013; Mitelman et al., 2007; Perez-Iglesias et al., 2010b) schizophrenia subjects. The IFOF FA reduction is also associated with deterioration of social role function among individuals at ultra-high risk for psychosis (Karlsgodt et al., 2009).
It is precisely not known why anisotropy of water diffusion in the dorsal networks but not the ventral tracts correlated with immune mediator levels. It is likely that our sample did not have adequate power to detect such correlations in the ventral tracts. It is also possible that the dorsal networks are more susceptible to immune mediators compared to the ventral networks. Altered anisotropy of water diffusion can be attributed to pathology in the microtubules, neurofilaments, structural integrity of axonal membranes, axonal transport and myelin (Beaulieu, 2002). At present, the literature is confusing with regard to the association of IL-6 and CRP with myelin. One study reported an association of elevated IL-6 levels with demyelination (Leibinger et al., 2013). Another study noted that IL-6 induced neurite outgrowth while reducing myelin (Neville, 2006). Others have noted that the IL-6 levels did not correlate with oligodendrocyte death (Lisak et al., 2012), and that IL-6 increased oligodendrocyte progenitor cell differentiation to mature oligodendrocytes and promoted their survival (Valerio et al., 2002). Age-related myelin changes show that late developing myelin in the frontal regions of the brain is more susceptible to pathology affecting cognitive decline with advancing age (Bartzokis, 2004). Post mortem (Tang et al., 1997) and neuroimaging (Bartzokis, 2004) studies show that the myelin in the dorsal regions of the brain show linear decline from age 19 years whereas the ventral regions show continued myelination until age 31 followed by decline. Oligodendrocytes from different brain regions are intrinsically different in terms of ability to regenerate and other properties (Power et al., 2002). These factors could explain differential vulnerability of myelin in different brain regions to immune mediator levels. Nevertheless, it is known that abnormalities in myelin alone may not contribute to the anisotropy differences (Beaulieu and Allen, 1994). Therefore, IL-6 may additionally affect other cellular components that contribute to anisotropy. IL-6 was also associated with neurofilament damage (Leibinger et al., 2013). The impact of IL-6 specifically on the axonal microtubules is unknown. Likewise, CRP was associated with reduced global FA especially in the frontal lobes, corpus callosum and corona radiate and with executive functions among healthy elderly individuals (di Penta et al., 2013). However, specific molecular pathways associated with anisotropic changes that correlate with CRP are lacking. Future studies that measure myelin water fraction may relatively more specifically suggest inflammation in these tracts compared to the others. Il-6 and CRP are one of the factors within an inflammatory cascade involving many other immune mediators. It is possible that other mediators in the cascade may have more direct impact on cellular components that affect anisotropy.
As observed in the regression models, we noted significant correlations between FA and RD among schizophrenia subjects but not in controls. These correlations are biologically important because relying on a single measure of anisotropy may be insufficient to index white matter integrity (Hasan, 2006). Such reciprocal changes in FA and RD further affirm that the IL-6 or CRP might affect a fundamental biological process underlying altered anisotropy of water diffusion. In addition, IL-6 and CRP are one of the factors in the pathway consisting of multiple intermediaries that affect white matter integrity. Therefore, future studies need to examine these pathways that affect white matter connections.
Some limitations of our study include the relatively small sample using a cross-sectional examination of selected immune mediators. Potential confounds due to medication effects cannot be excluded fully. However, the sample was matched for age and sex. Blood sample for immune assays were collected very close to imaging data acquisition. In summary, the results of our study suggest that IL-6 and CRP may affect white matter structure in schizophrenia subjects but not in controls. Future studies should address these shortcomings and focus on specific molecular pathways that lead to dysconnection.
Acknowledgments
This work was supported by research grants from the National Institute of Mental Health MH72995, MH93540 and the Stanley Medical Research Institute (07T-722) (KMP). We thank Dr. Debra Montrose, Dr. Elizabeth Radomsky, Mr. Kevin Eklund, Ms. Diana Dworakowski and Ms. Alicia Thomas for enrolment and characterization of research participants. We also thank Ms. Jean Miewald and Ms. Karol Rosengarth for their help in data management and quality assurance. Funding agencies did not have any further role in the analysis and reporting of the results.
Footnotes
Contributors
Dr. Konasale Prasad conceived the idea and designed the study. Mr. Konasale and Ms. Catherine Upton conducted the data processing and analysis. Drs. Nimgaonkar and Keshavan discussed the concept, reviewed the manuscript, suggested further analyses and interpreted the results along with other authors. All authors took part in the preparation, editing and reviewing the manuscript carefully.
Conflict of Interest
None of the authors disclose any conflict of interest to declare that would impinge on the design, execution, analysis or reporting and discussion of the results
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, Mondino M, Thomas P, Jardri R. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 2014;19(2):184–191. doi: 10.1038/mp.2012.181. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271(2):126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31(4):394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168(12):1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Chao YL, Chen CY, Chang CM, Wu ECH, Wu CS, Yeh HH, Chen CH, Tsai HJ. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. The British Journal of Psychiatry. 2012;200(5):374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38(6):877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Curcic-Blake B, Nanetti L, van der Meer L, Cerliani L, Renken R, Pijnenborg GH, Aleman A. Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain structure & function. 2013 doi: 10.1007/s00429-013-0663-y. Epub ahead of print 2013 Nov 2. [DOI] [PubMed] [Google Scholar]
- di Penta A, Moreno B, Reix S, Fernandez-Diez B, Villanueva M, Errea O, Escala N, Vandenbroeck K, Comella JX, Villoslada P. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One. 2013;8(2):e54722. doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1–3):261–265. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83–88. doi: 10.1016/j.schres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 2009;8(23):3885–3892. doi: 10.4161/cc.8.23.10068. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165(8):1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Rabin BS, Kelly RH, Lyte M, Ragu U. Clinical and laboratory evidence of autoimmunity in acute schizophrenia. Ann N Y Acad Sci. 1987;496:676–685. doi: 10.1111/j.1749-6632.1987.tb35829.x. [DOI] [PubMed] [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116 (Pt 5):1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Harris LW, Wayland M, Lan M, Ryan M, Giger T, Lockstone H, Wuethrich I, Mimmack M, Wang L, Kotter M, Craddock R, Bahn S. The cerebral microvasculature in schizophrenia: a laser capture microdissection study. PLoS ONE. 2008;3(12):e3964. doi: 10.1371/journal.pone.0003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM. Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: fractional anisotropy alone is not sufficient. Radiology. 2006;239(2):611–612. doi: 10.1148/radiol.2392051172. author reply 612–613. [DOI] [PubMed] [Google Scholar]
- Heath RG, Krupp IM, Byers LW, Lijekvist JI. Schizophrenia as an immunologic disorder. 3. Effects of antimonkey and antihuman brain antibody on brain function. Arch Gen Psychiatry. 1967;16(1):24–33. doi: 10.1001/archpsyc.1967.01730190026003. [DOI] [PubMed] [Google Scholar]
- Henneberg AE, Horter S, Ruffert S. Increased prevalence of antibrain antibodies in the sera from schizophrenic patients. Schizophr Res. 1994;14(1):15–22. doi: 10.1016/0920-9964(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Watanabe T, Yamazaki M, Deki T, Suzuki M. IL–6R distribution in normal human and cynomolgus monkey tissues. Regulatory toxicology and pharmacology : RTP. 2009;53(1):46–51. doi: 10.1016/j.yrtph.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Kelly RH, Ganguli R, Rabin BS. Antibody to discrete areas of the brain in normal individuals and patients with schizophrenia. Biol Psychiatry. 1987;22(12):1488–1491. doi: 10.1016/0006-3223(87)90110-7. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuta AE, Baum LL. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. The Journal of experimental medicine. 1986;164(1):321–326. doi: 10.1084/jem.164.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Iimaging (DTI) study. Schizophr Res. 2013;143(2–3):231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Gobrecht P, Diekmann H, Andreadaki A, Fischer D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013;4:e609. doi: 10.1038/cddis.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak RP, Benjamins JA, Nedelkoska L, Barger JL, Ragheb S, Fan B, Ouamara N, Johnson TA, Rajasekharan S, Bar-Or A. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. Journal of Neuroimmunology. 2012;246(1–2):85–95. doi: 10.1016/j.jneuroim.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, Yu X, Hong N. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: A diffusion tensor study using TBSS. Behavioural Brain Research. 2013;252(0):157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, Yu X, Hong N. A combined DTI and structural MRI study in medicated-naïve chronic schizophrenia. Magnetic Resonance Imaging. 2014;32(1):1–8. doi: 10.1016/j.mri.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64(6):484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68(6):895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH. C-Reactive Protein Levels in Schizophrenia: A review and meta-analysis. Clinical Schizophrenia & Related Psychoses. 2014;7(4):223–230. doi: 10.3371/CSRP.MICU.020813. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: A diffusion tensor imaging survey. Schizophrenia Research. 2007;92(1–3):211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, Moller HJ, Klauss V, Schwarz MJ, Riedel M. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121(1–3):118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Moller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159(6):1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. Immune System and Schizophrenia. Current immunology reviews. 2010;6(3):213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville HJ. In: Flexibility and plasticity in cortical development. Munakata Y, Johnson MH, editors. Oxford University Press; Oxford: 2006. pp. 287–314. [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L. Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol. 2012;54(1):38–43. doi: 10.1111/j.1469-8749.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, McGuire PK, Roiz-Santianez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage. 2010a;49(1):199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010b;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory Cytokine Alterations in Schizophrenia: A Systematic Quantitative Review. Biological Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Developmental biology. 2002;245(2):362–375. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59(2):137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lonnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nothen MM, O’Dushlaine CT, Olincy A, Olsen L, O’Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Rethelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O’Donovan MC, Daly MJ, Gejman PV. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Giorgio A, Battaglini M, Stromillo ML, Portaccio E, Goretti B, Federico A, Hakiki B, Amato MP, De Stefano N. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS One. 2012;7(11):e44826. doi: 10.1371/journal.pone.0044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav Immun. 2001;15(4):319–339. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T. Cogtest: Computerized Neurocognitive Test Battery for Clinical Trials. 2003. [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? a meta-analysis. J Clin Psychiatry. 2011 doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz E, Pancholi KM, Goradia DD, Paul S, Keshavan MS, Nimgaonkar VL, Prasad KM. Brain activation patterns during visual episodic memory processing among first-degree relatives of schizophrenia subjects. Neuroimage. 2012;63(3):1154–1161. doi: 10.1016/j.neuroimage.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J, Mori A, Hishikawa Y, Shimizu T, Nishino S. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 2012;12(1):37. doi: 10.1186/1471-244X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985;18(5):583–591. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- Valerio A, Ferrario M, Dreano M, Garotta G, Spano P, Pizzi M. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Molecular and cellular neurosciences. 2002;21(4):602–615. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2005;43(2):81–89. [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer’s disease. Brain Res. 2000;887(1):80–89. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]