Abstract
In vertebrates, soluble epoxide hydrolase (sEH) hydrolyzes natural epoxy-fatty acids (EpFAs), which are chemical mediators modulating inflammation, pain, and angiogenesis. Chick embryos are used to study angiogenesis, particularly its role in cardiovascular biology and pathology. To find potent and bio-stable inhibitors of the chicken sEH (chxEH) a library of human sEH inhibitors was screened. Derivatives of 1(adamantan-1-yl)-3-(trans-4-phenoxycyclohexyl) urea were found to be very potent tight binding inhibitors (KI < 150 pM) of chxEH while being relatively stable in chicken liver microsomes, suggesting their usefulness to study the role of EpFAs in chickens.
Keywords: epoxy-eicosatrienoic acids, angiogenesis, liver microsomes, high throughput screening, di-substituted ureas
In mammals, epoxides of arachidonic acids (called epoxy-eicosatrienoic acids or EETs) and of other fatty acids are important lipid mediators that have key roles in the regulation of hypertension, inflammation, and many cardiovascular related diseases as well as in modulating both inflammatory and neuropathic pain.1,2 However, endogenous metabolism of these epoxy-fatty acids to their corresponding hydrated products by soluble epoxide hydrolase (sEH EC 3.3.2.10) generally reduces these biological activities.3–5 Both in vitro and in vivo studies have demonstrated that the anti-hypertensive and cardio protective effects mediated by the EETs are inversely dependent on the extent of their hydrolysis by sEH.3-5 Thus, maintaining a high in vivo concentration of EETs through sEH inhibition is a promising therapeutic pathway to treat cardiovascular and other diseases.3–5 Recently, EETs have been shown to promote angiogenesis in humans,2,6 while epoxides of DHA have been shown to reduce angiogenesis,7 underlying the importance of epoxy-fatty acids in the regulation of this biological process and the potential of human sEH inhibitors to treat diseases associated with angiogenesis.
These beneficial therapeutic effects of epoxy-fatty acids have shown a potential for veterinary applications.8 Currently, the only classes of drugs used to reduce pain and inflammation in animals are opioids and nonsteroidal anti-inflammatory drugs (NSAIDs). Testing sEHIs for veterinary purposes could increase the limited veterinary drug armamentarium. There has already been some success using sEHIs as an analgesic and anti-inflammatory for horses with laminitis.9 Testing potential human drugs and therapies on animals is an effective way to increase the variety of available veterinary pharmaceuticals and can also give researchers insight into the potential effects of these drugs on humans.
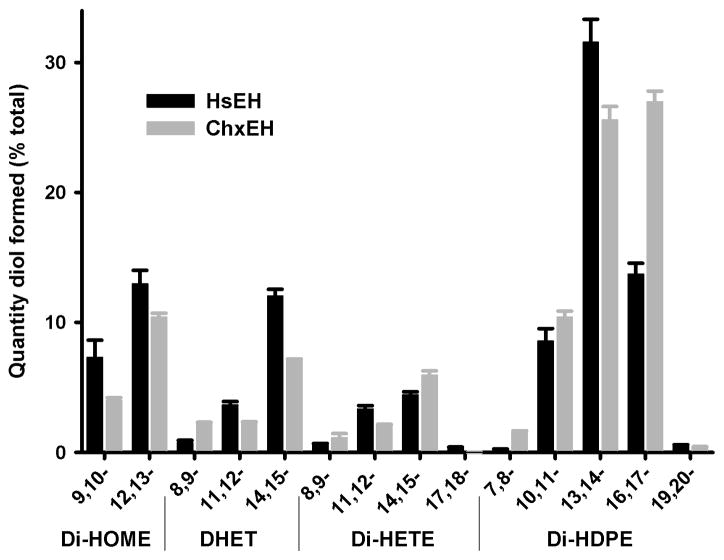
Animal models are effective tools for the study of diseases but the high cost associated with mammalian models makes their use impractical in initial studies. Therefore, utilizing non-mammalian animal models can provide a cost effective way to study human diseases.10 The chicken and chick embryo model has been used in research since the time of Aristotle.11 More recently, chickens have been successfully used as a model for various human diseases.11 Beside being classically used for immunology, genetics, virology, cancer, and cell biology, chick embryos are currently also being used as a human model for angiogenesis and its role in cardiovascular biology and pathology.12 Interestingly, a dose dependent vascular response to EETs was observed in chickens.13 In addition, chicken sEH (chxEH) is active on EETs and the catalytic residues between chxEH and human sEH are conserved.14 The overall selectivity of chxEH for a series of epoxy-fatty acids (Figure 1) is similar to the human sEH,15 with a clear preference for the epoxide of DHA over the epoxides of EPA, ARA or linoleic acid. The kinetic constants for chxEH’s best substrate, 16,17-epoxy-docosapentaenoic acid, yield a Km (12 ± 3 μM) that is similar to the one of the human sEH, but a Vmax (728 ± 97 nmol.min−1.mg−1) that is roughly 10-fold lower than the one measured for the human sEH.16 Finally, epoxy-fatty acids were detected in the plasma and liver extracts of chicken.14 Put together, these data support using the chick embryo model to study the role of epoxy-fatty acids in cardiovascular angiogenesis, especially to quickly and cheaply test the pharmacological action of sEH inhibitors.
Figure 1.
Substrate preferences of human and chicken sEH. Selectivity was measured using a mixture of 14 epoxy-fatty acids each at a concentration of 1 μM, and the diols formed were quantified by LC/MS-MS.15 Di-HOME: diols from linoleic acid epoxides; DHET: diol from arachidonic acid epoxides; Di-HETE: diols from EPA epoxides; Di-HPDE: diols from DHA epoxides.
A small series of sEH inhibitors were previously tested on chxEH,14 however the more potent inhibitors found are either metabolically unstable in vivo or have low solubility limiting their usefulness, though as compounds become more potent, solubility is less important, of course. Thus, toward finding more potent and more useful chxEH inhibitors, we herein report the screening of a chemical library of EH inhibitors.17 This library is a unique collection of over 2,200 chemicals (26 plates of 88 compounds at 10 mM in DMSO) that were synthesized with the aim of inhibiting mammalian soluble epoxide hydrolases.
Using recombinant purified chicken sEH and the fluorescent substrate PHOME ((3-phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester; Km= 1.5 ± 0.3 μM, and Vmax= 60 ± 4 nmol.min−1.mg−1), we screened the library at a final concentration of inhibitor at 100 nM and a chxEH concentration of 1.4 nM (84 ng/mL), following a methodology previously described for the human sEH.18,19 Overall, we obtained on average for the 26 plates S/B = 2.9 ± 0.3, S/N = 100 ± 60 and Z′ = 0.81 ± 0.07 indicating that the assay performed very well.
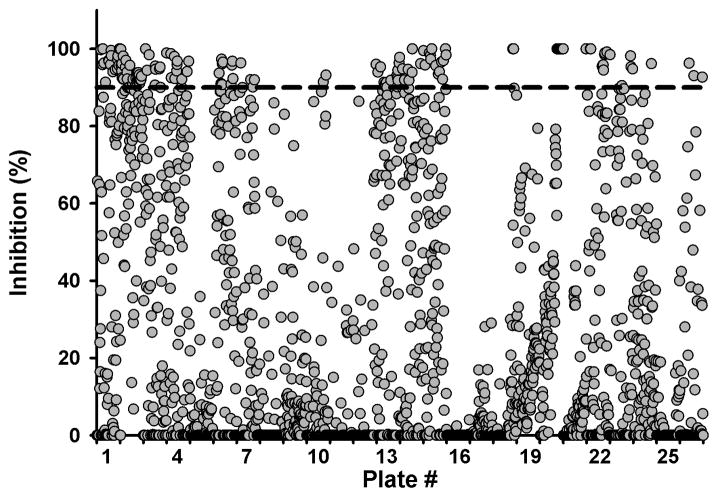
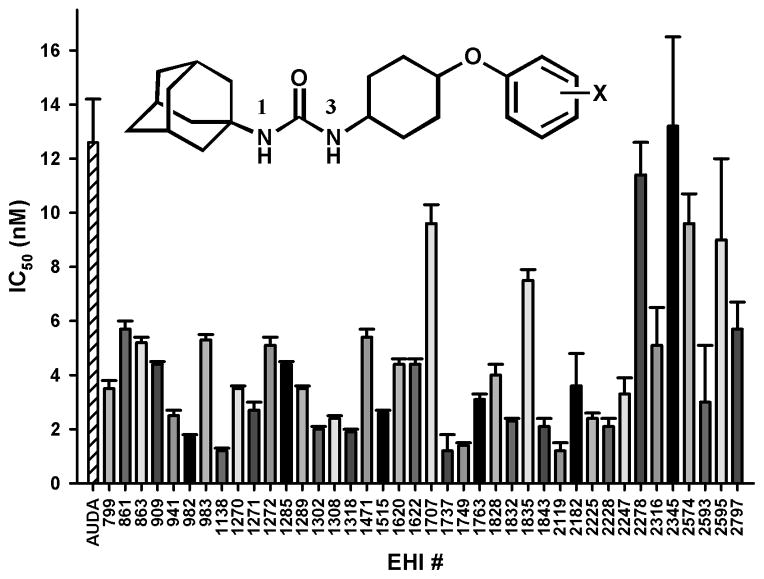
As shown in Figure 2, out of the 2,288 compounds that composed the EH inhibitor library, 200 showed greater than 90% inhibition for chxEH. To confirm the potency of these compounds, fresh solutions in DMSO were prepared, and their ability to inhibit the chicken sEH was tested at 100, 10 and 1 nM using PHOME as substrate. Instead of the endpoint mode used for the primary screening, a kinetic mode was used to eliminate compounds that gave false positive responses by altering the fluorescent signal.19 Out of 196 compounds tested, 99 gave high inhibition (> 75%) at 100 nM, suggesting a rate of false positive around 50% in the primary screen. Of these, around 40 yielded more than 50% inhibition at 10 nM, which is roughly the inhibitory potency (IC50) of AUDA, the best chxEH previously described.14 The IC50s of these 40 compounds were determined using PHOME as substrate for chxEH (Figure 3, structures are given in table S1).19
Figure 2.
Primary screening results of the EH inhibitor library. Percent of sEH inhibition for each compound tested at 100 nM. Compounds that gave more than 90% inhibition (dashed line) were selected for secondary screening.
Figure 3.
Secondary screening results. IC50s were measured using PHOME as substrate.19 Results are mean ± standard deviation of at least three separate measurements. The general structure of the most common inhibitor is given.
The IC50s of these 40 compounds for chxEH were compared with the ones obtained previously for the human sEH. A Spearman’s ranking correlation coefficient (ρ) of only 0.17 was found, suggesting significant differences in the actives sites of the two enzymes. As shown in Figure 3, most of the compounds tested yielded IC50s significantly smaller than AUDA’s 12 nM, indicating that we successfully identified more potent chxEH inhibitors. When looking at the structure of the 40 compounds, general structural features necessary to yield potent chxEH inhibitors emerged. The library contains mostly ureas (1602 out of 2288) and amides (450 out of 2288). However, mostly urea compounds (37 out of 40) were obtained as potent chxEH inhibitors, suggesting that, as observed for the human sEH,20 a central urea pharmacophore is needed to yield more potent chxEH inhibitors. The other 3 compounds are amides, suggesting this functionallity as an alternative central pharmacophore for chxEH inhibitors. On the 1-position (the smaller side) of the urea function, the compounds in the library have mostly aryl (954 out of 1602) or adamantyl (403 out of 1602) substituents folowed by cycloalkyl (144 out of 1602) and alkyl chains (101 out of 1602). Interestingly, of the potent chxEH inhibitors obtained in this screen, admantyl functionality was the most frequent (20 out of 37 ureas), followed by aryl substituents (13/37) and cycloalkyl (4/37). These suggest that, contrary to the human sEH,20 an adamantyl group is more likely to yield potent compounds for chxEH inhibition than phenyl groups. However, exceptions exist such as compound 1749. On the 3-position of the urea function, the library contains a similar amount of compounds with alkyl (280), phenyl (358), cyclohexyl (364) or piperidyl (280) substituents. However, for the chxEH, a cyclohexyl group was the most frequent (25/37), while alkyl, piperidyl and phenyl derivatives were less common (7/37, 2/37 and 3/37, respectively). In general, piperidine ureas are potent human sEH inhibitors.20 Remarkably, this series of compounds are not potent against chxEH, except for 1749, underlying difference in selectivity between the chicken and human enzymes. For example, the commonly used piperidine urea TPPU (aka 1770)5 showed only 15% inhibition fo the chxEH at 100 nM. Interestingly, most of the inhibitors (29/37) for chxEH found (table S1) have an ether function as secondary pharmacophore independently of the nature of the group between the urea and the secondary polar function. The library contains roughly the same amount of urea compounds with an ether (454) or a carboxylate derivatives (383) as secondary pharmacophore. Finally, for the chxEH inhibitors, on the other side of this ether, aryl groups (24/30) were the more frequent followed by alkyl groups, which is similar to the compounds in the library. The resulting general structure for the most potent chxEH inhibitors is shown in Figure 3. While the structure depicted in Figure 3 is similar to a series of inhibitors developed for the human sEH,21 it does not depict the most potent human sEH inhibitors,20 underlying significant differences between the selectivity of the human and chicken enzymes.
While it is important to identify novel potent inhibitors of chxEH in an in vitro screen, the compounds need also to be soluble and metabolically stable for in vivo use. These parameters were previously shown to greatly influence the in vivo potency of sEH inhibitors in rodents.22 Thus, five newly identified chxEH inhibitors with distinct structural features were chosen, along with AUDA as a positive control, for further testing.
The cLogP, solubility and stability were determined for all seven compounds (Table 1). The cLogPs were calculated using ChemBioDraw (v.12.0, CambridgeSoft). The cLogP is a measurement of both relative solubility and bioavailability. For AUDA and 1471 (a.k.a. t-AUCB), the charged form (a.k.a. base form) of the acid function yields significantly lower cLogP, suggesting higher water solubility for the base form, as expected. Otherwise, compounds 1749, 2119, 2225 and 2228 have cLogP between 3 and 4, suggesting reasonable solubility and good bioavailability.23 To support these results, the solubility in sodium phosphate buffer (0.1 M pH 7.4) containing 1% of DMSO was determined by turbidity measurement at 600 nm, as described.24 Compared to AUDA, compounds 1471, 1749, 2119 and 2225 are markely more soluble, suggesting they will be easier to use. Solubility seems to be enhanced by the presence of an adamantyl (2225 vs 2228) and an acid function (1471 vs 2225). The stability of the seven inhibitors (1 μM) in both chicken liver microsomes and human liver microsomes (1 mg/mL) was measured in the presence or absence of NADPH as described.25 After a 30 minute incubation at 37°C, the amount of remaining inhibitor was quantified using mass spectrometry.25 Of the seven compounds tested, four of them, AUDA, 1471, 2119 and 2225, display similar stability in both human and chicken liver microsomes. Interstingly, 1749 shows a great disparity between its stability in human and chicken liver microsomes. While it very stable in the chicken microsomes, it was completely metabolized in 30 min by the human microsomes. Because this occurs in the presence and absence of the NADPH regenerating system, it suggests that 1749 metabolism in human microsomes is not P450-dependent. Because 1749 contains an ester, its rapid metabolism probably resulted from the action of esterases, such as carboxylesterases that are well known to be present in liver microsomes.26 Our results suggest that the selectivity of these human esterases is quite different than the chicken esterases. In the presence of NADPH, 1138 was rapidly metabolized in the chicken liver microsomes (2% left after 30 min), while half of it remain after 30 min in contact with the human liver microsomes (Table 1). Similarly, with NADPH, 2228 is rapidly metabolized by the human liver microsomes, but not by the chicken liver microsomes, underlying the difference in selectivity of the human and chicken microsomal enzymes.
Table 1.
cLogP, solubility and stability of selected compounds.
| Structure | EHI# | cLogP | Solubility (μM)b | Stability (%)c | |||
|---|---|---|---|---|---|---|---|
| Human liver mic. | Chicken liver mic. | ||||||
|
| |||||||
| NADPH | − | + | − | + | |||
|
|
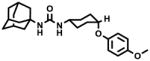
AUDA | 5.98 −1.14a |
37.5–50 | 98 ± 14 | 69 ± 5 | 106 ± 7 | 70 ± 1 |
|
1138 | 4.97 | 10–12.5 | 98 ± 3 | 57 ± 21 | 69 ± 29 | 2.1 ± 0.5 |
|
|
1471 | 4.84 0.62a |
> 100 | 97 ± 8 | 69 ± 2 | 77 ± 2 | 60 ± 4 |
|
|
1749 | 2.90 | 75–100 | 1.2 ± 0.5 | 2.7 ± 0.8 | 105 ± 9 | 94 ± 4 |
|
|
2119 | 3.84 | > 100 | 91 ± 6 | 53 ± 2 | 73 ± 1 | 24 ± 2 |
|
|
2225 | 3.88 | 50–75 | 101 ± 4 | 94 ± 2 | 96 ± 5 | 96 ± 3 |
|
|
2228 | 4.08 | 10–12.5 | 101 ± 5 | 5.4 ± 0.1 | 119 ± 15 | 89 ± 15 |
Base form.
In sodium phosphate buffer (0.1 M pH 7.4)
Percent of compound (1 μM) remaining after 30 minutes incubation with human or chicken liver microsomes (1 mg/mL) at 37°C with or without NADPH generating system. Results are average ± standard deviation (n = 3).
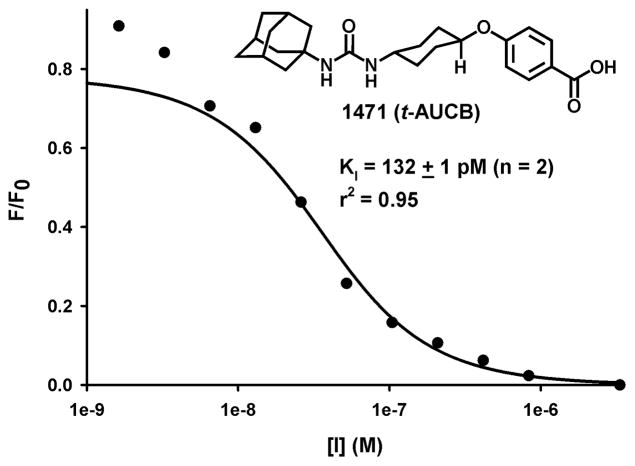
Put together, the combined results from Table 1 suggest that compounds 1471, 2119 and 2225 have the best set of properties making them excellent inhibitors of chxEH for use in chick embryo or in chicken. To confirm the high inhibitory potency of these compounds, their dissociation constants (KI) for chxEH were determined using a Förster resonance energy transfer (FRET)-based competitive displacement assay.27 The reporter ligand used is this assay is 1-(adamantan-1-yl)-3-(1-(2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetyl) piperidin-4-yl) urea (ACPU). Using our recombinant purified (> 95% pure) chxEH,14 a KD of 283 pM for ACPU was found. Using this value, the KIs were calculated by non-linear regression from the displacement curve of chxEH–ACPU complex by increasing amount of the tested inhibitor (Figure 4).27 A KI of 132 ± 1 pM was obtained for 1471, while KIs of less than 20 pM (the limit of quantification) were obtained for 2119 and 2225, supporting the inhibtory potency of the selected compounds.
Figure 4.
Displacement curve of chxEH–ACPU complex with EH inhibitor #1471. [I] are shown in log scale.
In conclusion, our results show that while the substrate selectivity for the human sEH and chxEH are similar, the enzymes’ inhibitor selectivities are quite different. In addition, the microsomal metabolic stabilities of a few inhibitors were quite different between the human and chicken liver. Nevertheless, we identified three chxEH inhbitors, 1471, 2119 and 2225, that are very potent, have suitable physical properties, and are metabolically stable in chicken liver microsomes, making them suitable for future use in chicken models of disease.
Supplementary Material
Acknowledgments
This work was supported in part by NIEHS grant ES02710 and NIEHS Superfund grant P42 ES04699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors want to thank Dr Huaijun Zhou for the gift of chicken liver.
Footnotes
Structures of the 40 potent chxEH inhibitors obtained are given in Supplementary material, as well as the details of the mass spectrometric method used to determine microsomal stability.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Spector AA. J Lipid Res. 2009;50:S52. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G, Kodani S, Hammock BD. Prog Lipid Res. 2014;53:108. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imig JD. Physiol Rev. 2012;92:101. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morisseau C, Hammock BD. Annu Rev Pharmacol Toxicol. 2005;45:311. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 5.Morisseau C, Hammock BD. Annu Rev Pharmacol Toxicol. 2013;53:37. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wang JN, Liu ZJ, Wei X, Wang DW. Zhonghua Xin Xue Guan Bing Za Zhi. 2005;33:1122. [PubMed] [Google Scholar]
- 7.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Lee SKS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Proc Natl Acad Sci USA. 2013;110:6530. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HK, De Vito V, Giorgi M. Isr J Vet Med. 2014;69:55. [Google Scholar]
- 9.Guedes AG, Morisseau C, Sole A, Soares JH, Ulu A, Dong H, Hammock BD. Vet Annaesth Analg. 2013;40:440. doi: 10.1111/vaa.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannaccini M, Cuschieri A, Dente L, Raffa V. Nanomedicine. 2013;10:703. doi: 10.1016/j.nano.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Stern CD. Dev Cell. 2005;8:9. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Kain KH, Miller JW, Jones-Paris CR, Thomason RT, Lewis JK, Bader DM, Barnett JV, Zijlstra A. Dev Dyn. 2014;243:216. doi: 10.1002/dvdy.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn LK, Gruenloh SK, Dunn BE, Reddy DS, Falck JR, Jacobs ER, Medhora M. Anat Rec A Discov Mol Cell Evol Biol. 2005;285:771. doi: 10.1002/ar.a.20212. [DOI] [PubMed] [Google Scholar]
- 14.Harris TR, Morisseau C, Walzem RL, Ma SJ, Hammock BD. Poult Sci. 2006;85:278. doi: 10.1093/ps/85.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisseau C, Wecksler AT, Deng C, Dong H, Yang J, Lee KS, Kodani SD, Hammock BD. J Lipid Res. 2014;55:1131. doi: 10.1194/jlr.M049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. J Lipid Res. 2010;51:3481. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherman MS, North EJ, Jones V, Hess TN, Grzegorzewicz AE, Kasagami T, Kim IH, Merzlikin O, Lenaerts AJ, Lee RE, Jackson M, Morisseau C, McNeil MR. Bioorg Med Chem. 2012;20:3255. doi: 10.1016/j.bmc.2012.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf NM, Morisseau C, Jones PD, Hock B, Hammock BD. Anal Biochem. 2006;355:71. doi: 10.1016/j.ab.2006.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morisseau C, Hammock BD. Curr Protoc Toxicol. 2007;4(23):1. doi: 10.1002/0471140856.tx0423s33. [DOI] [PubMed] [Google Scholar]
- 20.Shen HC, Hammock BD. J Med Chem. 2012;55:1789. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. J Med Chem. 2007;50:3825. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang SH, Chiamvimonvat N, Hammock BD. Eur J Pharm Sci. 2013;48:619. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Deliv Rev. 2001;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 24.Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Biochem Pharmacol. 2002;63:1599. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 25.Burmistrov V, Morisseau C, Lee KS, Shihadih DS, Harris T, Butov G, Hammock BD. Bioorg Med Chem Lett. 2014;24:2193. doi: 10.1016/j.bmcl.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laizure SC, Herring V, Hu Z, Witbrodt K, Parker RB. Pharmacotherapy. 2013;33:210. doi: 10.1002/phar.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KS, Morisseau C, Yang J, Wang P, Hwang SH, Hammock BD. Anal Biochem. 2013;434:259. doi: 10.1016/j.ab.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.