Malaria kills more than 600,000 people, mostly children, each year. 1–3 No vaccine has been developed yet to fully prevent people from becoming infected with malaria parasites. 4–6 Antimalarial chemotherapy using nitrogen-containing heteroaromatic compounds like chloroquine and mefloquine has been used successfully for many years to cure malaria-infected people. 7–10 In recent years, however, widespread resistance of malaria parasites has developed to many of these heteroaromatic drugs. 11–13 Therefore, new classes of antimalarial drugs are desperately needed. A breakthrough occurred in the early 1970’s with the discovery in China that artemisinin (1), a naturally occurring endoperoxide sesquiterpene lactone, is strongly efficacious as an antimalarial. 14–15 Several short-lived, artemisinin-derived 1,2,4-trioxanes have been prepared and some, especially artemether (2) and artesunate (3), are currently used combined with long-lived, nitrogen-containing antimalarials. 16–22 Such artemisinin combination therapy (ACT) is recommended as standard chemotherapeutic protocol by the World Health Organization (WHO). 23 Typically, multidose regimens of ACT daily for several days are needed to achieve a complete cure of malaria-infected people.16–20 A major problem arises, however, when infected individuals stop taking the ACT prematurely, thereby leading often to parasite recrudescence. Therefore, a major goal of modern antimalarial chemotherapy is to develop new endoperoxides capable of single oral dose ACT cures. Toward this goal, many simple endoperoxides24–32 and artemisinin-modified trioxanes33–43 have been synthesized and evaluated for antimalarial effectiveness.
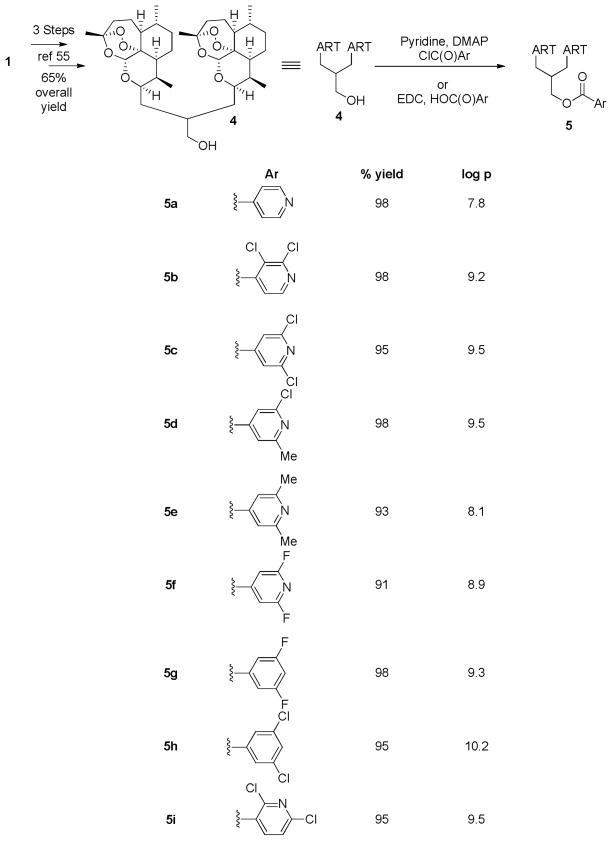
Substituted pyridines are important structural units in various pathogen-resistant agrochemicals44–50 and in some pharmaceutical drugs. 51–54 We converted artemisinin (1) into 3-carbon linked dimer primary alcohol 4 in 65% overall yield (Scheme 1). 55 Esterification of primary alcohol 4 without compromising the endoperoxide pharmacophore formed 3-carbon-linked pyridine containing dimer isonicotinate ester 5a (Scheme 1).55 Dimer isonicotinate 5a had high in vitro antimalarial potency: IC50 = 1.7 nM vs IC50 = 9 nM for artemisinin (1).55 Based on the structure of this potent antimalarial dimer isonicotinate ester 5a, we performed and report here a structure-activity (SAR) study featuring artemisinin-dervied 3-carbon-linked trioxane dimer esters 5 with diverse substituents on the pyridine ring and on the phenyl ring (Scheme 1).56
Scheme 1.
Three-carbon-linked trioxane dimer esters 5.
Antimalarial efficacy data in mice are more valuable and more demanding for preclinical drug development than in vitro potency data. Based on our experience with trioxanes we conclude that, within a family of antimalarial trioxanes, in vitro potency (IC-50) data do not precisely predict levels of in vivo efficacy. Thus, we chose to explore our antimalarial trioxane dimer esters 5 directly by ACT in malaria-infected mice.
Stock solutions were prepared by dissolving mefloquine hydrochloride (1.8 mg) in 93.9 μL of 7:3 Tween 80:ethanol. This solution was added to 0.6 mg of dimer ester 5 in a 1 dram vial. After approximately 18 h at room temperature, 906.1 μL of deionized water was added, and then 200 μL of this stock solution was administered by oral gavage one day post infection to 5-week old C57BL/6J male mice (from Jackson Laboratory) that weighed approximately 20 g, which had been infected with Plasmodium berghei ANKA strain (1.5 × 107 parasitized erythrocytes), corresponding to a dose of 6 mg/kg of trioxane dimer in combination with 18 mg/kg of mefloquine hydrochloride.
Antimalarial efficacy results are shown in Table 1. Several important SAR conclusions stand out. As a positive control, artemether (2, 6 mg/kg) plus mefloquine hydrochloride (18 mg/kg) prolonged mouse survival to an average of 20.8 days. Dimer primary alcohol 4 and all of the dimer esters 5 were more efficacious than artemether (2) plus mefloquine. Difluorobenzoate 5g prolonged two mice survival until day 30 with only one of the mice having no detectable parasitemia on day 30. Dichlorobenzoate 5h prolonged mouse survival until at least day 30, with three of the four mice having no detectable parasitemia on day 30 and looking and acting healthy. Survival until day 30 post infection with no parasitemia is widely considered to be a cure. Dichloronicotinate 5i (two cures) outperformed isomeric dichloroisonicotinate ester 5c (one cure), even though both 5i and 5c have the same log P value of 9.5. 57 Two of four mice treated with dichloronicotinate 5i had no detectable parasitemia and appeared healthy on day 30. Both esters 5h and 5i are hydrolytically stable at pH = 2 and 37 °C, in acetonitrile and water, for at least 96 hours.58 The easy synthesis of dimer esters 5 and the partial cures achieved by dichloronicotinate 5i and especially by dichlorobenzoate 5h deserve further pharmacological study, which is in progress.
Table 1.
In Vivo Antimalarial Efficacy using a Single Oral Dose of Trioxane Dimer (6 mg/kg) Combined with Mefloquine Hydrochloride (18 mg/kg) in P. berghei-Infected Mice.
| trioxane | Survival after infection (days)a | avg survival (days) | % Parasitemia Suppressionb |
|---|---|---|---|
| 4 | 29, 18, 30, 27 | 26 | >99.9 |
| 5a | 29, 18, 30, 20 | 24.3 | >99.9 |
| 5b | 24, 23, 30, 27 | 26 | >99.9 |
| 5c | 13, 30, 30, 30 | 25.8 | >99.9 |
| 5d | 29, 25, 30, 30 | 28.5 | >99.9 |
| 5e | 30, 13, 30, 30 | 25.8 | >99.9 |
| 5f | 30, 21, 13, 30 | 23.5 | >99.9 |
| 5g | 29, 27, 30, 30 | 29 | >99.9 |
| 5h | 30, 30, 30, 30 | 30 | >99.9 |
| 5i | 30, 30, 30, 30 | 30 | >99.9 |
| controls: | |||
| infected mice (no drug) | 6, 6, 6, 7 | 6.3 | 0%c |
| artemether (2) plus mefloquine | 20, 30, 13, 20 | 20.8 | >99.9 |
| mefloquine alone | 13, 29, 18, 23 | 20.8 | >99.9 |
Bold entries indicate best results.
Denotes determination on day 3 after infection.
An average of 10.3% parasitemia was determined on day 3 after infection.
Supplementary Material
Figure 1.
Artemisinin and first generation derivatives.
Acknowledgments
We thank the NIH (R37 AI 34885), the Johns Hopkins Malaria Research Institute, and the Bloomberg Family Foundation for financial support and Dr. Bryan T. Mott for early participation in this project.
Abbreviations
- SAR
structure-activity relationship
- ACT
artemisinin combination therapy
- DMAP
4-dimethylaminopyridine
- EDC
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
Footnotes
Supplementary data (experimental and tabular spectral data) associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozono R, Lopez AD. Lancet. 2012;379:413. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Gulland A. Br Med J. 2012;344:895. doi: 10.1136/bmj.e895. [DOI] [PubMed] [Google Scholar]
- 3.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. http://dx.doi.org/10.1371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The RTS,S Clinical Trials Partnership. N Engl J Med. 2011;365:1863–1875. [Google Scholar]
- 5.Schwartz L, Brown GV, Genton B, Moorthy VS. Malaria J. 2012;11:1. doi: 10.1186/1475-2875-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thera MA, Plowe CV. Annu Rev Med. 2012;63:345. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geary TG, Divo A, A’Jensen JB. Trans R Soc Trop Med Hyg. 1987;81:499–503. doi: 10.1016/0035-9203(87)90175-1. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood BM, Bojang K, Whitty CJM, Targett GAT. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 9.Boechat N, Ferreira M, de LG, Pinheiro LCS, Jesus AML, Leite MMM, CCS, Aguiar ACC, Andrade IM, de A, Krettli AU. Chem Biol Drug Des. 2014;84:325–332. doi: 10.1111/cbdd.12321. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Tanabe K, Nodiff EA. Biorg Med Chem Lett. 2014;24:4106–4109. doi: 10.1016/j.bmcl.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 11.Olliaro PL, Boland PB. In: Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Rosenthal PJ, editor. Humana Press; Totowa, NJ: 2001. p. 65. [DOI] [PubMed] [Google Scholar]
- 12.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. J Med Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlitzer M. ChemMed Chem. 2007;2:955–986. [Google Scholar]
- 14.Miller LH, Su X. Cell. 2011;146:855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woerdenbag HJ, Pras N, van Uden W, Wallaart TE, Beekman AC, Lugt CB. Pharmacy World & Science. 1994;16:169–180. doi: 10.1007/BF01872865. [DOI] [PubMed] [Google Scholar]
- 16.Yavo W, Faye B, Kuete T, Djohan V, Oga SA, Kassi RR, Diatta M, Ama MV, Tine R, Ndiaye JL, Evi JB, Same-Ekobo A, Faye O, Koné M. Malaria J. 2011;10:198–205. doi: 10.1186/1475-2875-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macedo de Oliveira A, Chavez J, Ponce de Leon G, Durand S, Arrospide N, Roberts J, Cabezas C, Marquiño W. Am J Trop Med Hyg. 2011;85:573–578. doi: 10.4269/ajtmh.2011.11-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Niambele MB, Sissoko M, Dicko A, Djimde A, Doumbo OK. Am J Trop Med Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 19.Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, Pénali LK, Valecha N, Tien NT, Abdulla S, Borghini-Fuhrer I, Duparc S, Shin CK, Fleckenstein L. N Engl J Med. 2012;366:1298–1309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- 20.Kurth F, Bélard S, Basra A, Ramharter M. Curr Opin Infect Dis. 2011;24:564–569. doi: 10.1097/QCO.0b013e32834cabdb. [DOI] [PubMed] [Google Scholar]
- 21.Welcome to Coartem.com, the Novartis Malaria Initiative Website. Coartem; http://www.coartem.com/coartem-riamet.htm. [Google Scholar]
- 22.MMV. Pyramax (pyronaridine-artesunate) http://www.mmv.org/research-development/project-portfolio/pyramax%C2%AE-pyronaridine-artesunate.
- 23.World Health Organization: Geneva, Switzerland. Guidelines for Treatment of Malaria. 2006. [Google Scholar]
- 24.Singh C, Hassam M, Verma VP, Singh AS, Naikade NK, Puri SK, Maulik PR, Kant R. J Med Chem. 2012;55:10662–10673. doi: 10.1021/jm301323k. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Chollet J, Matile H, Charman SA, Chiu FCK, Charman WN, Scorneaux B, Urwyler H, Tomas JS, Schuerer C, Snyder C, Dorn A, Wang X, Karle JM, Tang Y, Wittlin S, Brun R, Vennerstrom JL. J Med Chem. 2005;48:4953–4961. doi: 10.1021/jm049040u. [DOI] [PubMed] [Google Scholar]
- 26.Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FCK, Chollet J, Craft JC, Creek DJ, Dong Y, Matile H, Maurer M, Morizzi J, Nguyen T, Papastogiannidis P, Scheurer C, Shackleford DM, Sriraghavan K, Stingelin L, Tang Y, Urwyler H, Wang X, White KL, Wittlin S, Zhou L, Vennerstrom JL. Proc Nat Acad Sci USA. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slack RD, Jacobine AM, Posner GH. Med Chem Commun. 2012;3:281–297. [Google Scholar]
- 28.Ruiz J, Mallet-Ladeira S, Maynadier M, Vial H, André-Barrés C. Org Biomol Chem. 2014;12:5212–5221. doi: 10.1039/c4ob00787e. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons P, Verissimo E, Araujo NC, Barton V, Nixon GL, Amewu RK, Chadwick J, Stocks PA, Biagini GA, Srivastava A, Rosenthal PJ, Gut J, Guedes RC, Moreira R, Sharma R, Berry N, Cristiano MLS, Shone AE, Ward SA, O’Neill PM. J Med Chem. 2010;53:8202–8206. doi: 10.1021/jm1009567. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Maimone TJ. J Am Chem Soc. 2014;136(14):5287–5290. doi: 10.1021/ja502208z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda D, Capela R, Albuquerque IS, Meireles P, Paiva I, Nogueira F, Amewu R, Gut J, Rosenthal PJ, Oliveira R, Mota MM, Moreira R, Marti F, Prudêncio M, O’Neill PM, Lopes F. ACS Med Chem Lett. 2014;5:108–112. doi: 10.1021/ml4002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira R, Guedes RC, Meireles P, Albuquerque IS, Goncalves LM, Pires E, Bronze MR, Gut J, Rosenthal PJ, Prudêncio M, Moreira R, O’Neill PM, Lopes F. J Med Chem. 2014;57:4916–4923. doi: 10.1021/jm5004528. [DOI] [PubMed] [Google Scholar]
- 33.Haynes RK, Fugmann B, Stetter J, Rieckmann K, Hans-Dietrich H, Chan HW, Cheung MK, Lam WL, Wong HN, Croft SL, Vivas L, Rattray L, Stewart L, Peters W, Robinson BL, Edstein MD, Kotecka B, Kyle DE, Beckermann B, Gerisch M, Radtke M, Schmuck G, Steinke W, Wollborn U, Schmeer K, Romer A. Angew Chem, Int Ed. 2006;45:2082–2088. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 34.Araujo NCP, Barton V, Jones M, Stocks PA, Ward SA, Davies J, Bray PG, Shone AE, Cristiano MLS, O’Neill PM. Bioorg Med Chem Lett. 2009;19:2038–2043. doi: 10.1016/j.bmcl.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Pacorel B, Leung SC, Stachulski AV, Davies J, Vivas L, Lander H, Ward SA, Kaiser M, Brun R, O’Neill PM. J Med Chem. 2010;53:633–640. doi: 10.1021/jm901216v. [DOI] [PubMed] [Google Scholar]
- 36.Chadwick J, Jones M, Mercer AE, Stocks PA, Ward SA, Park BK, O’Neill PM. Bioorg Med Chem. 2010;18:2586–2597. doi: 10.1016/j.bmc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 37.Bégué JP, Bonnet-Delpon D. ChemMedChem. 2007;2:608–624. doi: 10.1002/cmdc.200600156. [DOI] [PubMed] [Google Scholar]
- 38.Cloete TT, de Kock C, Smith PJ, N’Da DD. Eur J Med Chem. 2014;76:470–481. doi: 10.1016/j.ejmech.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Singh C, Verma VP, Hassam M, Singh AS, Naikade NK, Puri SK. J Med Chem. 2014;57:2489–2497. doi: 10.1021/jm401774f. [DOI] [PubMed] [Google Scholar]
- 40.Singh C, Chaudhary S, Puri SK. Bioorg Med Chem Lett. 2008;18:1436–1441. doi: 10.1016/j.bmcl.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 41.Saikia B, Saikia PP, Goswami A, Barua NC, Saxena AK, Suri N. Synthesis. 2011;19:3173–3179. [Google Scholar]
- 42.Conyers RC, Mazzone JR, Siegler MA, Tripathi AK, Sullivan DJ, Mott BT, Posner GH. Bioorg Med Chem Lett. 2014;24:1285–1289. doi: 10.1016/j.bmcl.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazzone JR, Conyers RC, Tripathi AK, Sullivan DJ, Posner GH. Bioorg Med Chem Lett. 2014;24:2440–2443. doi: 10.1016/j.bmcl.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metraux JP, Goy PA, Staub T, Speich J, Steinemann A, Ryals J, Ward E. Adv Mol Genet Plant Microbe Interact. 1991;1:432–439. [Google Scholar]
- 45.Dann E, Diers B, Byrum J, Hammerschmidt R. Eur J Plant Path. 1998;104(3):271–278. [Google Scholar]
- 46.Schweizer P, Buchala A, Metraux JP. Plant Physiol. 1997;115:61–70. doi: 10.1104/pp.115.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen KK, Bojsen K, Collinge DB, Mikkelsen JD. Physiol Mol Plant Path. 1994;45(2):89–99. [Google Scholar]
- 48.Kogel KH, Beckhove U, Dreschers J, Much S, Romme Y. Plant Physiol. 1995;106(4):1269–1277. doi: 10.1104/pp.106.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colson-Hanks ES, Deverall BJ. Plant Path. 2000;49:171–178. [Google Scholar]
- 50.Vernooij B, Friedrich L, Goy PA, Staub T, Kessmann H, Ryals J. Mol Plant Microbe Interact. 1995;8:228–234. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- 51.Felding J, Sørensen MD, Poulsen TD, Larsen J, Andersson C, Refer P, Engell K, Ladefoged LG, Thormann T, Vinggaard AM, Hegardt P, Søhoel A, Nielsen SF. J Med Chem. 2014;57:5893–5903. doi: 10.1021/jm500378a. [DOI] [PubMed] [Google Scholar]
- 52.Taylor RD, MacCoss M, Lawson ADG. J Med Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 53.http://www.drugs.com/stats/top100/2013/sales.
- 54.http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM381803.pdf.
- 55.Posner GH, Paik IH, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA. J Med Chem. 2003;46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 56.Cross RM, Flanigan DL, Monastyrskyi A, LaCrue AN, Sáenz FE, Maignan JR, Mutka TS, White KL, Shackleford DM, Bathurst I, Fronczek FR, Wojtas L, Guida WC, Charman SA, Burrows JN, Kyle DE, Manetsch R. J Med Chem. 2014;57:8860–8879. doi: 10.1021/jm500942v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Log P values for artemether (2), parent dimer alcohol 4, and 3C-linked trioxane dimers 5 were calculated using MarvinSketch version 5.12.3.
- 58.Jung M, Lee S. Bioorg Med Chem Lett. 1998;8:1003–1006. doi: 10.1016/s0960-894x(98)00160-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.