Abstract
Background and Objective
Pancreatic resection is the standard therapy for patients with stage I/II pancreatic ductal adenocarcinoma (PDA), yet many studies demonstrate low rates of resection. The objective of this study is to evaluate whether increasing resection rates would result in an increase in average survival in patients with stage I/II PDA.
Methods
SEER data were analyzed for patients with stage I/II pancreatic head cancers treated from 2004–2009. Pancreatectomy rates were examined within Health Service Areas (HSA) across 18 SEER regions. An instrumental variables (IV) analysis was performed, using HSA rates as an instrument, to determine the impact of increasing resection rates on survival.
Results
Pancreatectomy was performed in 4,322 of the 8,323 patients evaluated with stage I/II PDA (overall resection rate=51.9%). The resection rate across HSAs ranged from an average of 38.6% in the lowest quintile to 67.3% in the highest quintile. Median survival was improved in HSAs with higher resection rates. IV analysis revealed that, for patients whose treatment choices were influenced by the rates of resection in their geographic region, pancreatectomy was associated with a statistically significant increase in overall survival.
Conclusions
When controlling for confounders using IV analysis, pancreatectomy is associated with a statistically significant increase in survival for patients with resectable PDA. Based on these results, if resection rates were to increase in select patients, then average survival would also be expected to increase. It is important that this information be provided to physicians and patients so they can properly weigh the risks and advantages of pancreatectomy as treatment for PDA.
Keywords: pancreatic cancer, pancreatectomy, SEER, instrumental variable analysis, epidemiology, Health Services Area
INTRODUCTION
Pancreatectomy is the only known curative treatment option for patients with pancreatic ductal adenocarcinoma (PDA),1,2 and numerous studies have demonstrated that resection is associated with longer survival.3–7 To date, only one prospective randomized study has been performed comparing pancreatic resection to chemoradiation, and this study showed a small but statistically significant increase in survival in resected patients.8 Based on the data published to date, pancreatic resection is the treatment of choice for patients with American Joint Committee on Cancer9 (AJCC) stage I and II (localized and resectable) PDA as outlined by the National Comprehensive Cancer Network.10 Given the clear relationship with improved survival, it is surprising that resection rates are low for patients who, according to clinical disease stage, should be appropriate operative candidates. Published resection rates for patients with localized and/or locoregional PDA range from 27% to 36%.3,11,12 From these data, it is tempting to conclude that surgery is underutilized for PDA patients with resectable disease. The reason for this apparent underutilization is unclear. It may stem from 1) the uniformly poor outcome associated with the biology of the disease (i.e., nihilism) and 2) the magnitude of the operation and the expertise required to perform a pancreatectomy.13
Bilimoria and colleagues first reported that 40% of patients with stage I PDA were ‘not offered surgery’, and less than 30% actually had a pancreatectomy.11 Many explanations for the seemingly low rate of resection have been proposed including advanced age, prohibitive comorbidities, pancreatic head tumors, and the hospital where the patient is being treated.4,11 This low percentage may reflect that pancreatectomies are already being performed on those patients who have the greatest potential to benefit from the procedure. If the best candidates for resection are already identified, then raising pancreatectomy rates will not necessarily improve average survival rates—it will simply lead to increased operative morbidity and mortality without a demonstrable impact on overall survival.
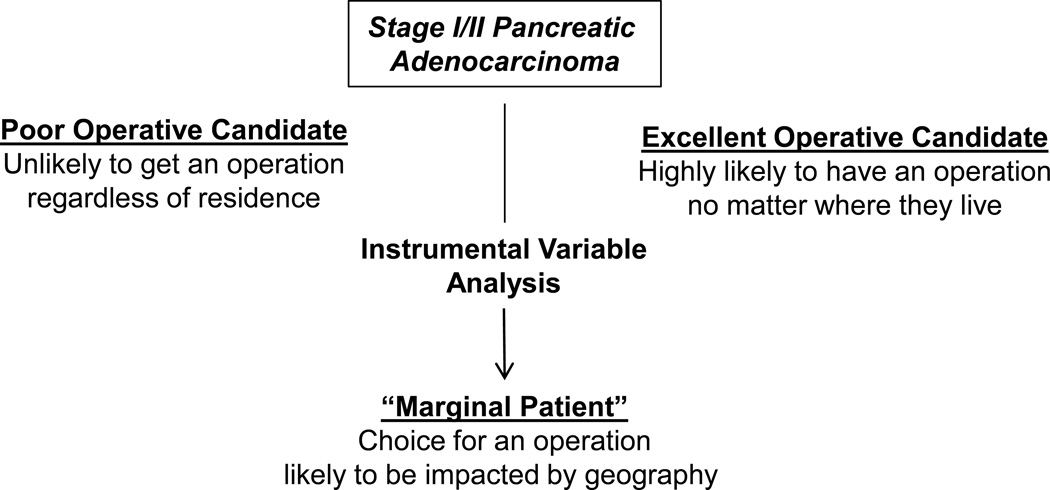
The objective of this study was to examine whether increasing pancreatectomy rates would impact overall survival for patients with pancreatic head tumors (i.e., patients who would require pancreaticoduodenectomy). To do this, we employed an instrumental variable (IV) analysis to examine variation in outcomes across geographical areas that differ in resection rates.14–18 This analysis technique is theorized to control for potential unmeasured confounders in treatment decision making by estimating treatment effects using only the variation in treatment choices determined by a “natural experiment” related to the instrument.16,19–21 For the purposes of this study, we utilized Health Service Area (HSA) rates as our instrument. This study is designed to test the hypothesis increasing pancreatectomy rates would be associated with an increase in survival in patients with stage I/II PDA. The inferences made from this analysis pertain to “marginal” patients: those patients for whom the decision to have an operation is affected by the resection rate in their area of residence.18,19 There are likely patients who are “always treated” and those that are “never treated” and the remaining marginal patients reflect those whose treatment choice for pancreatectomy is less certain and may be subject to beliefs, preferences, or practice styles of physicians in their HSA—these are the patients to whom our IV estimates apply.
PATIENTS AND METHODS
The study cohort was developed from the Surveillance, Epidemiology, and End Results (SEER) program’s 2012 Research Data release.10 The SEER program comprises 18 surveillance programs across the United States that identify, document, and follow-up cancer cases in their respective geographic areas and cover 28% of the population.10 SEER programs document clinical tumor characteristics, demographic variables, and first course of treatment including pancreatic resection.
Included patients were diagnosed between 2004 and 2009 at age 40 years or older with stage I/II adenocarcinoma of the pancreatic head (ICD-O-3 Code: C25.0, malignant neoplasm of the head of the pancreas; histology codes included were M8140, M8500, M8010, M8000, M8480, M8481, M8490, M8255, M8021, M8020, M8521, M8141, M8022, M8144).10 Tumor, node, and metastasis stage was derived by SEER using collaborative staging fields in accordance with AJCC stage version 6.9 In order to focus on patients who were likely to be candidates fit for resection, only patients that had survived at least 3 months follow-up after their date of diagnosis were included. Those diagnosed at autopsy or by death certificate were excluded. Known race and resection status were required. Due to tumor location being in the pancreatic head, this data set is meant to include patients who would require pancreaticoduodenectomy.
SEER data were linked to county-level data from the 2000 United States Census to obtain group-level measures of education and other socioeconomic indicators.10 HSA definitions were also linked to the SEER data by county of residence, allowing the patients to be organized into these contiguous clusters of counties that correspond to geographical markets for health care. There are 3,217 HSAs in the United States. We used the National Cancer Institute modification which defines the Health Services Areas within specific SEER catchment areas.10
Pancreatectomy rates were divided into quintiles based on the percentage of patients residing within each Health Service Area (HSA) who underwent pancreatectomy (increasing from Quintile 1, the lowest rate of resection, to Quintile 5, the highest rate of resection). Associations between HSA pancreatectomy rates and clinicopathologic and treatment-related variables were analyzed. Tests of association were performed with Pearson chi-square tests. Survival probabilities were estimated and plotted with the methods of Kaplan-Meier.22
Instrumental Variable Analysis
The primary analysis employed an instrumental variable (IV) approach. IV techniques generate an estimate of treatment effectiveness for marginal patients, the subset of the population whose choice of treatment was influenced by a specified instrumental variable – in this case, geographic variation in resection rates.14,23–25 The minimal assumptions of the analysis are that the IV is related to treatment choice but unrelated to survival or other unmeasured variables related to survival, independent of treatment.18,19 To the extent that these conditions are satisfied, this comparison balances potential confounders and simulates a design in which patients are essentially randomized to treatment/no treatment conditions.26
For the purposes of this study, variation in treatment rates across geography was employed as the IV, a variable that has been used successfully by other researchers.21,27 Specifically, overall survival (i.e., time between cancer diagnosis and death from any cause) was examined as a function of resection receipt, using HSA resection rates as an instrument. This was performed using two methods: (1) a traditional linear two-stage least squares (2SLS) method which is commonly used in IV analyses,17,18,24,28–30 and (2) a nonlinear two-stage residual inclusion (2SRI) method. The 2SRI method has been shown to have positive properties when estimating treatment effects using nonlinear regression methods.31,32 In this study, the first-stage treatment choice model of the 2SRI approach was estimated using a linear specification, and the outcome survival model was estimated by Cox proportional hazards regression. In each IV method, the effect of pancreatic resection on survival time was predicted using continuous HSA resection rates as the IV27 and controlling for other covariates (age at diagnosis, gender, race, marital status, AJCC stage, and county-level measures of poverty and education in both first- and second-stage models.
These IV methods yield a measure of treatment effectiveness for marginal patients, those patients for whom the decision to have a resection is affected by the location in which they live.18,23 These may be the patients for whom the benefits of resection are less certain where physicians may be “sorting” patients into treatments based on expected gains or effectiveness of the given treatment. This is based on two observations: 1) excellent operative candidates for pancreatic resection will usually have an operation irrespective of where they live, and 2) poor operative candidates will rarely have an operation no matter where they live (Figure 1). The decision to treat these two subgroups is generally not sensitive to changes in HSA rates of resection. Marginal patients, however, are those patients who are more likely to have their treatment choice affected by local area preferences or beliefs and therefore more likely perhaps to have an operation if they live in an area with a high rate of resection. These are the patients whom are likely to be most affected if efforts to increase pancreatectomy rates are successful. The IV analysis thus provides an estimate of treatment effectiveness that applies to this group of patients whose treatment choice was determined by the resection rates of the area, which makes it an ideal tool to evaluate the hypothesis.23
Figure 1.
Instrumental Variable Analysis Controls for Unmeasured Confounding to help Determine the Impact of Raising Pancreatectomy Rates on the Marginal Patient with Pancreatic Cancer.
All statistical tests were two-sided and assessed for significance at the 5% level. STATA (StataCorp, LP, College Station, TX, USA) was used for the IV analysis, and SAS version 9.3 was used for all other analyses (SAS Institute Inc., Cary NC, USA).
RESULTS
There were 8,323 patients who met criteria for analysis. Summary statistics for the demographics and clinicopathologic variables of patients treated with and without pancreatectomy are shown in Table 1. Pancreatectomy was performed in 4,322 patients (overall resection rate = 51.9%). This included 476/1,454 (32.7%) Stage IA/IB patients and 3,846/6,869 (56.0%) stage IIA/B patients.
Table 1.
Comparison of 8,323 Patients with Pancreatic Adenocarcinoma Treated with or without Pancreatectomy
| Pancreatectomy N (%) |
|||
|---|---|---|---|
| Variable | No | Yes | p-value |
| Sex | |||
| Female | 2153 (50.30) | 2127 (49.70) | 0.6911 |
| Male | 1848 (45.71) | 2195 (54.29) | <.0001 |
| Race | |||
| White | 3292 (47.36) | 3659 (52.64) | <.0001 |
| Black | 441 (53.00) | 391 (47.00) | 0.0830 |
| Other* | 268 (49.63) | 272 (50.37) | 0.8633 |
| Age at Diagnosis | |||
| 40–49 years | 137 (33.33) | 274 (66.67) | <.0001 |
| 50–59 years | 544 (37.60) | 903 (62.40) | <.0001 |
| 60–69 years | 902 (38.95) | 1414 (61.05) | <.0001 |
| 70–79 years | 1215 (47.59) | 1338 (52.41) | 0.0149 |
| 80+ years | 1203 (75.38) | 393 (24.62) | <.0001 |
| Marital Status | |||
| Married | 2180 (44.10) | 2763 (55.90) | <.0001 |
| Single (never married) | 414 (49.17) | 428 (50.83) | 0.6295 |
| Widowed | 934 (62.10) | 570 (37.90) | <.0001 |
| Divorced | 306 (41.63) | 429 (58.37) | <.0001 |
| Separated | 43 (58.11) | 31 (41.89) | 0.1630 |
| Unknown | 124 (55.11) | 101 (44.89) | 0.1252 |
| AJCC Stage | |||
| Stage IA | 176 (50.14) | 175 (49.86) | 0.9574 |
| Stage IB | 802 (72.71) | 301 (27.29) | <.0001 |
| Stage II NOS | 207 (98.10) | 4 (1.90) | <.0001 |
| Stage IIA | 1795 (64.02) | 1009 (35.98) | <.0001 |
| Stage IIB | 1021 (26.49) | 2833 (73.51) | <.0001 |
| Radiation | |||
| No | 2641 (52.32) | 2407 (47.68) | 0.0010 |
| Yes | 1307 (41.47) | 1845 (58.53) | <.0001 |
American Indian, Alaska Native, Asian/Pacific Islander
Pancreatectomy rates vary considerably over several demographic variables including gender, race, age, and marital status. There was also a difference when patients were analyzed by AJCC stage, where patients treated with resection appear to have more precise subgroup staging based on pathologic tumor size and nodal status. Patients who had a pancreatectomy also more often received radiation as part of their treatment. These findings highlight that there are notable patient-specific differences between patients who did and did not receive a pancreatectomy.
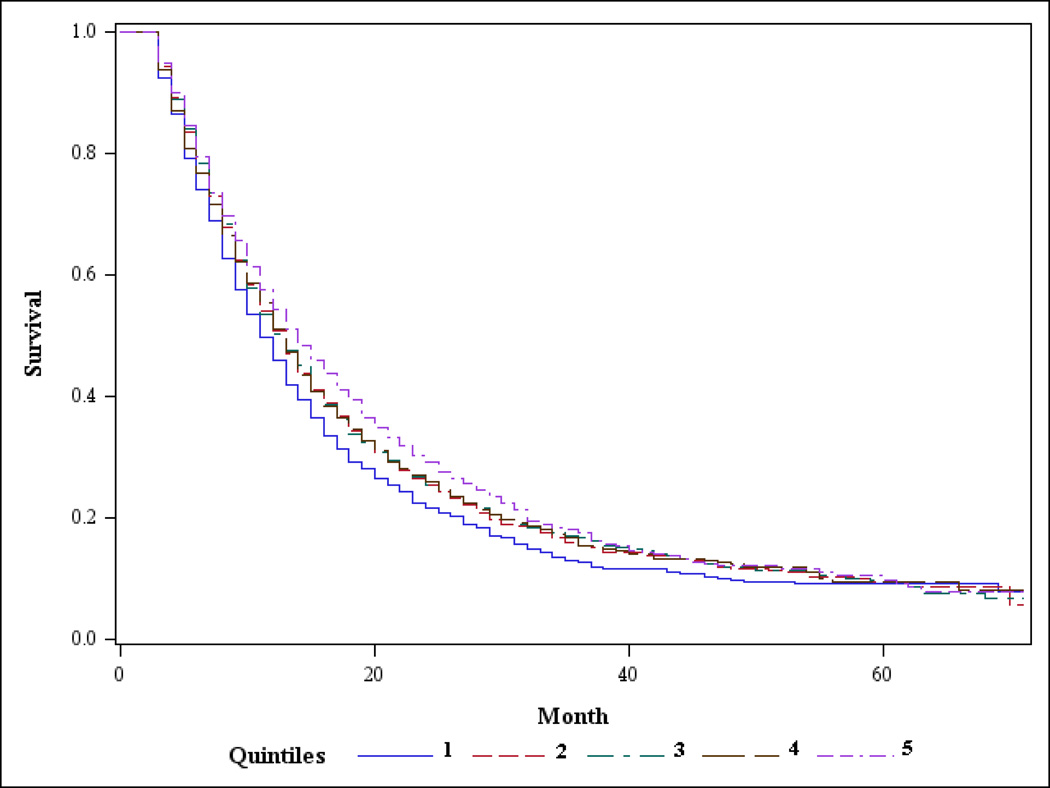
Patients were divided into quintiles based on the percentage of patients within each Health Service Area (HSA) who underwent pancreatectomy (Figure 2). The corresponding average HSA pancreatectomy rates for the five groups increased from an average of 38.6% resected (Quintile 1) to an average of 67.3% resected (Quintile 5), Table 2. Also shown in Table 2 are the clinicopathologic variables across quintiles. Median survival significantly increased across quintiles as resection rates increased (11 months in Quintile 1 to 14 months in Quintile 5 (Table 2 and Figure 3). Age, one of the most important factors in determining whether patients are offered or receive a pancreatectomy,4 was balanced across quintiles as was gender. Race was significantly related to area resection rate (p<0.001), but did not vary systematically as resection rates increased across the quintiles. AJCC stage also varied across quintiles, reflecting that most patients who undergo resection are found to have node positive disease and T2/T3 tumors. Radiation therapy was also more likely to have been administered in areas with high resection rates.
Figure 2.
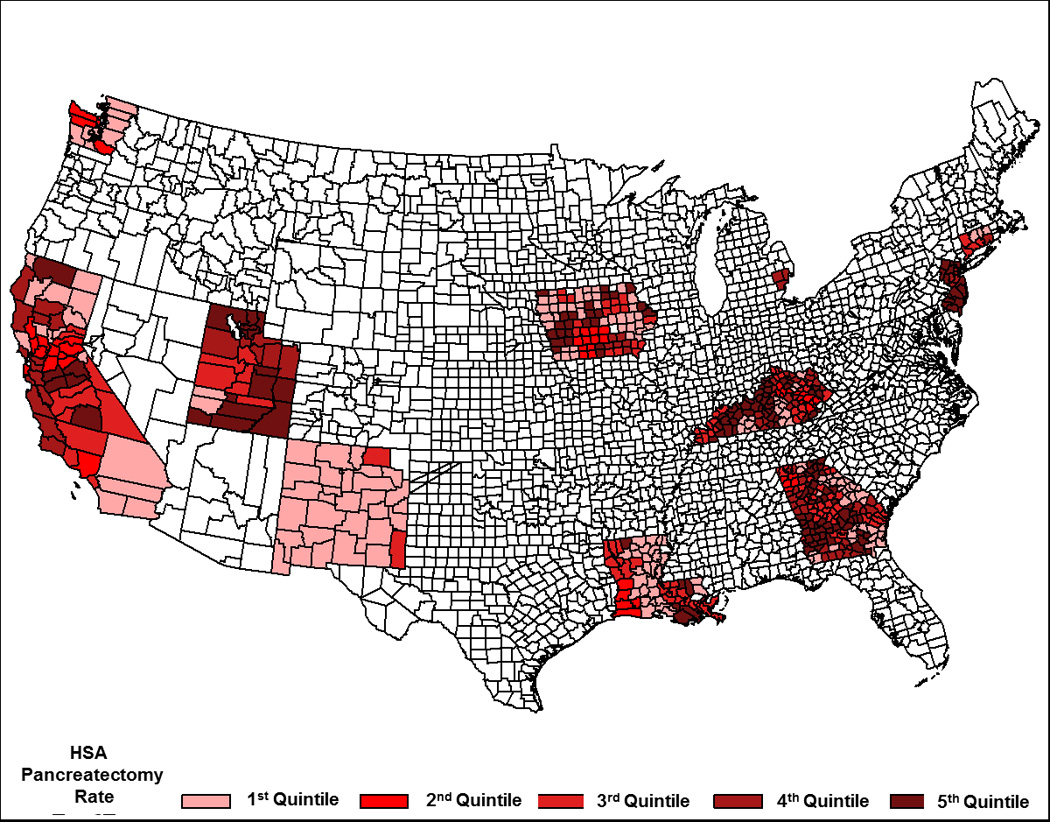
Map of the United States Showing Geographic Distribution of Health Service Area Quintiles. (Quintile 1 = Lowest Rate of Pancreatectomy and Increases to Quintile 5 = Highest Rate of pancreatectomy)
Table 2.
Characteristics of Patients by Quintile of Health Services Area Pancreatectomy Rates
| Area Resection Rate Quintile | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p-value* | |
| Number of patients | 1,532 | 1,741 | 1,698 | 1,708 | 1,644 | |
| Rate of Resection (average) | 38.6 | 47.2 | 50.1 | 55.6 | 67.3 | |
| Median survival (months) | 11 | 13 | 13 | 13 | 14 | 0.0001 |
| Sex | ||||||
| Female | 49.4 | 52.3 | 51.1 | 51.2 | 53.0 | 0.300 |
| Male | 50.7 | 47.7 | 48.9 | 48.8 | 47.0 | |
| Race | ||||||
| White | 89.4 | 78.4 | 86.6 | 80.0 | 83.9 | <0.0001 |
| Black | 7.4 | 10.3 | 9.4 | 13.2 | 9.4 | |
| Other | 3.1 | 11.3 | 4.1 | 6.8 | 6.7 | |
| Age at Diagnosis | ||||||
| 40–49 years | 5.4 | 4.6 | 4.5 | 5.0 | 5.2 | 0.720 |
| 50–59 years | 16.3 | 17.1 | 16.8 | 19.4 | 17.2 | |
| 60–69 years | 27.4 | 27.3 | 29.6 | 26.8 | 27.9 | |
| 70–79 years | 31.5 | 31.4 | 30.0 | 29.5 | 31.0 | |
| 80+ years | 19.4 | 19.6 | 19.0 | 19.2 | 18.7 | |
| Marital Status | ||||||
| Married | 58.2 | 57.7 | 60.9 | 58.8 | 61.4 | 0.051 |
| Single (never married) | 10.9 | 11.7 | 9.3 | 9.8 | 8.8 | |
| Widowed | 19.1 | 17.4 | 17.6 | 17.5 | 19.0 | |
| Divorced | 8.2 | 9.3 | 9.1 | 9.3 | 8.2 | |
| Separated | 0.9 | 1.1 | 0.8 | 0.9 | 0.8 | |
| Unknown | 2.7 | 2.9 | 2.3 | 3.8 | 1.8 | |
| AJCC Stage | ||||||
| Stage IA | 4.11 | 3.22 | 4.65 | 4.33 | 4.81 | <0.0001 |
| Stage IB | 15.86 | 12.81 | 12.72 | 13.17 | 11.92 | |
| Stage II NOS | 2.94 | 4.31 | 1.88 | 2.46 | 1.03 | |
| Stage IIA | 37.4 | 35.67 | 35.34 | 31.15 | 29.08 | |
| Stage IIB | 39.69 | 44 | 45.41 | 48.89 | 53.16 | |
| Radiation | ||||||
| No | 63.27 | 68.25 | 31.37 | 58.16 | 56.61 | <.0001 |
| Yes | 36.73 | 31.75 | 38.63 | 41.84 | 43.39 | |
Chi-square test of independence;
Cutoff values rounded to nearest tenth for presentation.
Figure 3.
Kaplan-Meier Curve Showing Overall Survival by Health Services Area Quintiles for Pancreatic Head Adenocarcinoma. (Quintile 1 = Lowest Rate of Pancreatectomy and Increases to Quintile 5 = Highest Rate of pancreatectomy)
Results from nonlinear 2SRI and linear 2SLS regressions are shown in Table 3. Estimates from Cox proportional hazards models suggest that resection has a statistically significant protective effect for marginal patients; the hazard ratio associated with receipt of resection is 0.54 (p < 0.001). This relative effect is consistent with absolute treatment effect estimates using 2SLS. For marginal patients, 2SLS estimates indicate that having an operation was associated with an average 5.54 month increase (p < 0.001) in overall survival. Age at diagnosis, sex, marital status, and tumor stage were also associated with survival, along with two county-level measures of poverty and education. The assumption for whether our instrument was significantly and non-arbitrarily related to treatment choice was tested using a Chow test. A Chow test for whether our instrument was significantly related to treatment choice produced an F-Stat of 274, well above the traditionally accepted threshold of 10.33
Table 3.
Instrumental Variable Analysis of the Impact of HSA Resection Rate on Survival for Patients with Pancreatic Ductal Adenocarcinoma
| 2SRI IV Model (Cox) | 2SLS IV Model | |||
|---|---|---|---|---|
| Variable | Hazard Ratio |
95% Confidence Interval |
Parameter Estimate (change in months survived) |
95% Confidence Interval |
| Pancreatectomy | 0.54*** | 0.39, 0.75 | 5.54*** | 2.34, 8.74 |
| Residual Term | 0.68* | 0.49, 0.94 | N/A | N/A |
| Age | 1.02*** | 1.01, 1.02 | −0.13*** | −0.17, −0.10 |
|
Sex (ref = Female) |
||||
| Male | 1.05 | 1.00, 1.11 | −0.70* | −1.23, −0.17 |
|
Race (ref = White) |
||||
| Black | 1.09 | 1.00, 1.19 | −0.49 | −1.37, 0.39 |
| Other | 0.99 | 0.89, 1.10 | −0.36 | −1.40, 0.69 |
|
Marital Status (ref = Married) |
||||
| Divorced | 1.18*** | 1.08, 1.30 | −1.35** | −2.27, −0.42 |
| Separated | 1.29 | 0.99, 1.69 | −1.77 | −4.53, 1.00 |
| Single | 1.13** | 1.04, 1.24 | −1.16* | −2.05, −0.26 |
| Widowed | 1.21*** | 1.12, 1.30 | −1.28** | −2.05, −0.50 |
| Unknown | 1.08 | 0.92, 1.27 | −0.79 | −2.38, 0.80 |
|
Tumor Stage (ref = Stage IA) |
||||
| Stage IB | 1.42*** | 1.20, 1.68 | −2.41** | −4.00, −0.83 |
| Stage II NOS | 1.80*** | 1.40, 2.32 | −3.71** | −6.27, −1.14 |
| Stage IIA | 1.48*** | 1.27, 1.72 | −2.56*** | −3.97, −1.15 |
| Stage IIB | 1.58*** | 1.36, 1.84 | −3.84*** | −5.25, −2.43 |
| County % Below Poverty | 1.00 | 1.00, 1.01 | −0.09** | −0.15, −0.03 |
| County % Bachelor’s Degree | 0.99*** | 0.99, 1.00 | 0.03* | 0.0003, 0.07 |
| Model Intercept | N/A | N/A | 24.93*** | 20.33,29.53 |
indicate parameter estimate associated p-value of <0.05, <0.01, <0.001 respectively.
DISCUSSION
Although pancreatic resection is associated with better outcomes for PDA,5,12 resection rates are much lower than expected for patients with localized disease.11 This has been evaluated using national data and interpreted as an underuse of the therapy, but another possibility is that resection is simply being performed only on well-selected patients.4,30,34,35 It is known from large retrospective studies that patients with PDA who undergo pancreatectomy have significantly improved median survival times (14–24 months) compared to those who are locally advanced and unresectable (11 months).5 Prior studies have reported a median survival of 8 months in patients with stage I PDA who do not undergo pancreatic resection.11 In this study, we have demonstrated that having an operation is associated with a 5.5 month increase in survival (Hazard Ratio for death = 0.54) for patients whose decision whether to have surgery is influenced by their area resection rate. These are the patients who are most likely to be affected by efforts to raise resection rates.19,23 By establishing that patients with stage I/II PDA have longer average adjusted survival times when treated with pancreatectomy, we are able to suggest with greater confidence that efforts to increase resection rates could result in increased survival time for these patients.
It is important to emphasize that the predicted increase in survival of 5.5 months and predicted hazard ratio of 0.54 reflect the benefit of resection only for marginal patients – those for whom receipt of resection was affected by the rates of use in their geographic area. The effect of resection for the best surgical candidates who are always treated irrespective of where they live is possibly higher than what we measured for the marginal patient. Similarly, our results do not indicate that resection is appropriate for all patients; some patients are clearly poor candidates for pancreaticoduodenectomy and should perhaps never be treated with this operation.
Our analysis employs an IV approach that has been useful in addressing similar questions about treatment effectiveness.16,19,20,26,27 The principle assumptions of these methods are that the IVs used are (1) significantly related to treatment choice, which we confirmed for our instrument; and (2) unrelated to outcomes or other unmeasured variables related to outcomes, independent of treatment (which is not directly testable). To the extent that these assumptions are satisfied, this comparison simulates a design in which patients are randomized to treatment/no treatment conditions.26 The advantage to this approach is that potential confounders tend to be controlled by focusing on the marginal patients for whom treatment choice was, in this case, associated with where they lived. Indeed, we showed that age at diagnosis, sex, and marital status were balanced across area resection rate quintiles which supports the validity of our IV. Racial proportions were different across the quintiles, but the proportions did not change in a systematic way as area resection rate increased. Although clinical variables such as AJCC stage changed with area resection rates, we hypothesize that since most patients with PDA who undergo resection have T2/T3 tumors and node positive disease, that stage migration occurs following resection. This makes it appear that more patients with stage II disease have a resection when compared to patients with stage I disease.36
We also found that radiation treatment was not balanced across area resection rates; patients were more likely to receive radiation treatment if they lived in areas with high resection rates. This is not surprising since radiation therapy is often part of the multidisciplinary approach following resection for PDA.37 Nonetheless, the close association of the two treatment modalities makes it difficult to clearly attribute all of the observed survival improvement to pancreatectomy alone. Alternatively, it may also be interpreted as an effect of the overall multidisciplinary approach provided to resected patients including adjuvant chemotherapy and radiation in addition to the operation itself.38
One potential threat to the validity of IV results is if observed variation in rates of resection across geographic regions is due to characteristics of physicians or health care systems that may related to quality. For example, if areas with higher resection rates also have higher quality physicians and hospitals that may affect outcomes for patients, independent of treatment, then our instrument may not be valid. This assumption is not directly testable.
Nihilism is sometimes mentioned as reason for the perceived barrier to resection for patients with PDA, and it is important to acknowledge the advances that have been made in recent years.36,39 We observed an overall resection rate of 51.9%, which is higher than some previous studies.11 One of the reasons that we observed higher resection rates may be that we required patients to survive at least 3 months post-diagnosis to be part of the cohort to exclude very poor surgical candidates. These inclusion criteria resulted in the resection rates in this study to be comparable to resection rates reported by Bilimoria and colleagues when they considered only the “best” candidates for resection in their analysis of data from the National Cancer Data Base in patients with stage I PDA.11
Notably, the Bilimoria study evaluated patients with stage I PDA from 1995 to 2004 using the National Cancer Data Base and reported an overall resection rate of 28.6%. The current study evaluated patients from 2004 to 2009 using SEER data and found a resection rate of 32.7% (476/1,454) for Stage IA/IB patients. Therefore it appears that there have not been significant changes in utilization of pancreatectomy despite there being an increase in the awareness of this problem. In fact, the large range in average area resection rates in our study (from an average of 38.6% in the lowest quintile to an average of 67.3% in the highest) may reflect considerable uncertainty across the United States regarding the benefits of resection for patients with PDA.
There are limitations to this study. Although indications are that patient-level prognostic characteristics are well-balanced across areas that differ in resection rates (e.g., age), specific data on all possible confounders are lacking. The SEER database does not have information such comorbidities and performance status that are important considerations for patient selection for pancreatectomy. The database also does not include where patients were treated (e.g., specialty cancer center) or information on receipt of chemotherapy. The time from diagnosis to time of treatment is also not available in SEER. If any of these variables are related to HSA resection rate, then this could impact the interpretation of the data. As mentioned above, a 3-month cutoff was utilized as inclusion criteria to enrich for strong surgical candidates. This cutoff may slightly alter our survival results since some immediate postoperative deaths following pancreatectomy may have occurred prior to three months. With postoperative mortality ranging as high as 5% from some centers, survival in the resected patients group may be slightly higher due to excluding patients who did not survive at least 3 months.
Our results indicate that pancreatectomy provides a survival benefit for marginal patients with stage I and II PDA. These data suggest that if resection rates were to increase in the future, average survival may also be expected to increase. It is important that this information be provided to physicians and patients so they can properly weigh the risks and advantages of pancreatectomy as treatment for PDA.
Acknowledgments
Funding and Support: This work was made possible by the Holden Comprehensive Cancer Center Population Research Core and Biostatistics Core (supported in part by P30 CA086862).
Abbreviations
- PDA
Pancreatic Ductal Adenocarcinoma
- HSA
Health Services Area
- SEER
Surveillance Epidemiology End Results
- AJCC
American Joint Commission on Cancer
- IV
instrumental variable
Footnotes
The authors have no financial disclosures or conflicts of interest.
Previous Communication: Presented in part at the Society of Surgical Oncology Annual Symposium, March 2013 and the American Society of Clinical Oncology Gastrointestinal Symposium, February 2013.
REFERENCES
- 1.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004 Mar 27;363(9414):1049. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Annals of surgery. 1996 Mar;223(3):273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann.Surg.Oncol. 2007 Apr;14(4):1320. doi: 10.1245/s10434-006-9249-8. [DOI] [PubMed] [Google Scholar]
- 4.Riall TS, Sheffield KM, Kuo YF, et al. Resection benefits older adults with locoregional pancreatic cancer despite greater short-term morbidity and mortality. J.Am.Geriatr.Soc. 2011;59(4):647. doi: 10.1111/j.1532-5415.2011.03353.x. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Annals of surgery. 2013 Apr;257(4):731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 6.Shaib Y, Davila J, Naumann C, et al. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. The American journal of gastroenterology. 2007 Jul;102(7):1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 7.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. The British journal of surgery. 2004 May;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 8.Imamura M, Doi R, Imaizumi T, et al. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery. 2004 Nov;136(5):1003. doi: 10.1016/j.surg.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 9.AJCC Cancer Staging Manual. 6th ed. Chicago, IL: Springer; 2002. [Google Scholar]
- 10. www.nccn.org.
- 11.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Annals of surgery. 2007 Aug;246(2):173. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J.Gastrointest.Surg. 2006 Nov;10(9):1212. doi: 10.1016/j.gassur.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Riall TS, Lillemoe KD. Underutilization of surgical resection in patients with localized pancreatic cancer. Annals of surgery. 2007 Aug;246(2):181–182. doi: 10.1097/SLA.0b013e31811eaa2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. Jama. 1994 Sep 21;272(11):859–866. [PubMed] [Google Scholar]
- 15.Fang G, Brooks JM, Chrischilles EA. Apples and oranges? Interpretations of risk adjustment and instrumental variable estimates of intended treatment effects using observational data. American journal of epidemiology. 2012 Jan 1;175(1):60–65. doi: 10.1093/aje/kwr283. [DOI] [PubMed] [Google Scholar]
- 16.Brooks JM, Chrischilles EA, Landrum MB, et al. Survival implications associated with variation in mastectomy rates for early-staged breast cancer. International journal of surgical oncology. 2012;2012:127854. doi: 10.1155/2012/127854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angrist JD, Pischke JS. Mostly Harmless Econometrics: An Empiricists Companion. Mostly Harmless Econometrics: An Empiricists Companion. 2009:1–373. [Google Scholar]
- 18.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996 Jun;91(434):444–455. [Google Scholar]
- 19.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. Jama. 1994 Sep 21;272(11):859. [PubMed] [Google Scholar]
- 20.Sheffield KM, Riall TS, Han Y, et al. Association between cholecystectomy with vs without intraoperative cholangiography and risk of common duct injury. Jama. 2013 Aug 28;310(8):812–820. doi: 10.1001/jama.2013.276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmar AD, Sheffield KM, Han Y, et al. Evaluating comparative effectiveness with observational data: Endoscopic ultrasound and survival in pancreatic cancer. Cancer. 2013 Nov 1;119(21):3861–3869. doi: 10.1002/cncr.28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 23.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health services research. 1998 Dec;33(5 Pt 1):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 24.Imbens GW, Angrist JD. Identification and Estimation of Local Average Treatment Effects. Econometrica. 1994 Mar;62(2):467–475. [Google Scholar]
- 25.Brooks JM, Fang G. Interpreting treatment-effect estimates with heterogeneity and choice: simulation model results. Clinical therapeutics. 2009 Apr;31(4):902–919. doi: 10.1016/j.clinthera.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J.Clin.Oncol. 2004;22(2):315. doi: 10.1200/JCO.2004.08.136. 01/15. [DOI] [PubMed] [Google Scholar]
- 27.Gray SW, Landrum MB, Lamont EB, et al. Improved outcomes associated with higher surgery rates for older patients with early stage nonsmall cell lung cancer. Cancer. 2012 Mar 1;118(5):1404. doi: 10.1002/cncr.26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks JM, Chrischilles EA, Scott SD, et al. Was breast conserving surgery underutilized for early stage breast cancer? Instrumental variables evidence for stage II patients from Iowa. Health services research. 2003 Dec;38(6 Pt 1):1385–1402. doi: 10.1111/j.1475-6773.2003.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadley J, Polsky D, Mandelblatt JS, et al. An exploratory instrumental variable analysis of the outcomes of localized breast cancer treatments in a medicare population. Health economics. 2003 Mar;12(3):171–186. doi: 10.1002/hec.710. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Heckman JJ, Navarro-Lozano S, et al. Use of instrumental variables in the presence of heterogeneity and self-selection: an application to treatments of breast cancer patients. Health economics. 2007 Nov;16(11):1133–1157. doi: 10.1002/hec.1291. [DOI] [PubMed] [Google Scholar]
- 31.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. Journal of health economics. 2008 May;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health services research. 2008 Jun;43(3):1102–1120. doi: 10.1111/j.1475-6773.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow G. Tests of equality between sets of coefficients in two linear models. Econometrica. 1960;28(3):591–605. [Google Scholar]
- 34.Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J.Am.Coll.Surg. 2012;214(1):33. doi: 10.1016/j.jamcollsurg.2011.09.022. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J.Clin.Oncol. 2003;21(18):3409. doi: 10.1200/JCO.2003.03.007. 09/15. [DOI] [PubMed] [Google Scholar]
- 36.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012 Jan;19(1):169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 37.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. Jama. 2008 Mar 5;299(9):1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 38.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. Jama. 2013 Oct 9;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 39.Mezhir JJ, McDowell BD. Pancreaticobiliary surgery for the treatment of malignancy: spread the word. J.Surg.Res. 2012 Sep 27; doi: 10.1016/j.jss.2012.09.018. [DOI] [PubMed] [Google Scholar]