Abstract
Bacterial contamination of gelatin is of great concern. Indeed, this animal colloid has many industrial applications, mainly in food and pharmaceutical products. In a previous study (E. De Clerck and P. De Vos, Syst. Appl. Microbiol. 25:611-618), contamination of a gelatin production process with a variety of gram-positive and gram-negative bacteria was demonstrated. In this study, bacterial contamination of semifinal gelatin extracts from several production plants was examined. Since these extracts are subjected to harsh conditions during production and a final ultrahigh-temperature treatment, the bacterial load at this stage is expected to be greatly reduced. In total, 1,129 isolates were obtained from a total of 73 gelatin batches originating from six different production plants. Each of these batches was suspected of having bacterial contamination based on quality control testing at the production plant from which it originated. For characterization and identification of the 1,129 bacterial isolates, repetitive-element PCR was used to obtain manageable groups. Representative strains were identified by means of 16S rRNA genesequencing, species-specific gyrB PCR, and gyrA and rpoB sequencing and were tested for gelatinase activity. The majority of isolates belonged to members of Bacillus or related endospore-forming genera. Representative strains were identified as Bacillus cereus, Bacillus coagulans, Bacillus fumarioli, Bacillus amyloliquefaciens, Bacillus licheniformis, Bacillus pumilus, Bacillus sonorensis, Bacillus subtilis, Bacillus gelatini, Bacillus thermoamylovorans, Anoxybacillus contaminans, Anoxybacillus flavithermus, Brevibacillus agri, Brevibacillus borstelensis, and Geobacillus stearothermophilus. The majority of these species include strains exhibiting gelatinase activity. Moreover, some of these species have known pathogenic properties. These findings are of great concern with regard to the safety and quality of gelatin and its applications.
The bacteriological quality of gelatin is of great importance, as it is applied for its gelling and stabilizing properties in the food industry (confectionery products, dairy products, etc.) and the pharmaceutical industry (hard and soft capsules, tablets, etc.) and in the production of photographic films, matches, glues, etc. Gelatin is a proteinaceous colloid and is extracted from animal connective tissue during a multistage process which involves, besides the actual extraction, a chemical treatment, purification, and drying of the extracts. Skin and bones mainly of bovine or porcine origin are used for this purpose. These raw materials are collected from slaughterhouses, butcher shops, or other plants dealing with animal raw materials.
In a previous study, contamination of a gelatin production process with a variety of gram-positive and gram-negative bacteria was reported (6). However, extreme temperature and pH conditions during the manufacture, ultrahigh-temperature (UHT) treatment, and drying of the gelatin extracts should guarantee the microbial sterility of the end product. Nevertheless, quality control testing at gelatin-producing factories has indicated that thermotolerant, aerobic, endospore-forming bacteria may persist in the final product (Paul Stevens, personal communication).
In general, contamination of industrial plants and products with aerobe endosporeformers is a widespread problem. The ubiquitous occurrence of these bacteria in combination with their wide nutritional versatility and wide pH and temperature ranges for the growth and formation of endospores, which are much more resistant to heat, chemicals, irradiation, and desiccation than vegetative forms (22), makes this group of bacteria an ever-present problem in different industries (see, e.g., references 3 and 10). Bacillus licheniformis, members of the Bacillus cereus group, Bacillus coagulans, Bacillus fumarioli, Bacillus badius, Bacillus subtilis, Brevibacillus agri, Alicyclobacillus acidocaldarius, and Paenibacillus cookii were found to be contaminants in a Belgian gelatin production process (6).
The bacterial load is expected to be greatly reduced in semifinal gelatin extracts. These extracts are subjected to harsh conditions during production and a final UHT treatment. Different semifinal extracts are mixed according to their individual physicochemical and microbiological characteristics to a final product according to the requirements of the consumer. Since there are no further procedures that diminish the bacterial contamination after the semifinal-extract stage, the bacterial load at this stage is of great concern. Indeed, some of these contaminants may be pathogenic for humans and could be a threat to human health in food and pharmaceutical applications. Furthermore, contaminants may exhibit gelatinase activity. Enzymatic degradation of gelatin would affect the viscosity and therefore the quality of the product itself and its applications. Further processing of contaminated gelatin batches in food and other industries could lead to the enrichment of contaminants to unacceptable levels and thus products of low quality and safety. In order to preserve the technical properties of gelatin, the UHT treatment step cannot be extended and decontamination temperatures cannot be increased to completely prevent the survival of bacterial spores.
To address the problem of end product contamination, the present study aimed to isolate, characterize, and identify bacterial contaminants in semifinal gelatin extracts. A better insight into the diversity and identities of these contaminants may provide information on the actual hazards of this contamination to the quality and/or safety of gelatin and lay a foundation for more targeted detection methods. Contaminants were isolated from gelatin batches from six production plants: three in France, one in Belgium, one in North America, and one in Argentina. These batches were suspected of being contaminated with thermotolerant bacteria based on the results of quality control testing at the producing plants involving plating on a broad-range medium and growth at elevated temperatures. Gelatin batches of bovine and porcine origins that were extracted from bones and skin and produced by different extraction approaches were selected for isolation studies. Repetitive-element PCR (rep-PCR) was used for the first screening of isolates. Representatives of the different rep-PCR fingerprint types were selected for further identification by 16S ribosomal RNA gene (rDNA) sequencing. Although the 16S rRNA gene is generally used as a framework for modern bacterial classification, it often shows limited variation for the discrimination of closely related taxa (7). Protein-coding genes exhibit higher genetic variation, which can be used for the classification and identification of closely related taxa (see, e.g., references 4 and 33). Sequencing of the gyrA and rpoB genes has been found to be useful in discriminating species of the Bacillus subtilis group (4, 17), while the gyrB gene has been studied for the discrimination of members of the Bacillus cereus group (33). Therefore, based on a first identification obtained with 16S rDNA sequencing, the affiliation of selected groups was further studied by gyrA and rpoB sequencing or by species-specific gyrB-targeted PCR. In addition, the gelatinase activities of selected isolates were examined.
MATERIALS AND METHODS
Sample origins and isolation procedure.
Samples of 73 gelatin batches from six production plants, suspected of being contaminated with thermotolerant, aerobic, endospore-forming bacteria based on quality control tests conducted in the production plants, were selected. Details on the origins of and extraction processes used for the gelatin batches are shown in Table 1. A 30-g sample of each gelatin batch was dissolved in 70 ml of Trypticase soy broth (Oxoid) in duplicate. One portion of broth was incubated overnight at 45°C, while the other was enriched at 55°C. One milliliter of the enriched sample was plated on Trypticase soy agar (Oxoid), brain heart infusion (BBL) supplemented with 1 mg of vitamin B12 ml−1, or nutrient agar (Oxoid) supplemented with 1.2% gelatin and incubated at the appropriate enrichment temperature (45 or 55°C). Incubation for each sample-medium-temperature combination was performed in duplicate. As many different colony types as were visually distinguishable were picked up, purified, and stored in Microbank tubes (PRO-LAB Diagnostics) at −80°C.
TABLE 1.
Details on the origins of and extraction process used for gelatin batches included in this study
| Production plant | Location | Raw material | Extraction procedure | Gelatin batch(es) |
|---|---|---|---|---|
| A | Belgium | Porcine skin | Acid | A(1-23) |
| B | France | Bovine bones | Alkaline | B(1-20) |
| C | France | Porcine bones | Alkaline | C(1-7) |
| D | France | Bovine skin | Alkaline | D(1, 2) |
| Porcine skin | Acid | D(3, 4) | ||
| E | Argentina | Bovine skin | Alkaline | E(1-6) |
| Bovine skin | Acid | E(7-15) | ||
| Bovine skin | Alkaline/acid | E(16) | ||
| F | United States | Porcine skin | Acid | F(1-5) |
Repetitive-element genomic fingerprinting (rep-PCR).
Template DNA was prepared by using a slight modification of the method of Pitcher et al. (20) as previously described (11). The (GTG)5 primer (5′-GTGGTGGTGGTGGTG-3′) was used as previously described (32). PCR amplifications were performed as previously described (32) by using a DNA thermocycler (Perkin-Elmer 9600) and Goldstar DNA polymerase (Eurogentec, Seraing, Belgium). The PCR products were electrophoresed in a 1.5% agarose gel (15 by 20 cm) for 16 h at 1.9 V cm−1 in 1× TAE (40 mM Tris-acetate, 1 mM EDTA [pH 8.0]) at 4°C. The rep-PCR profiles were visualized after staining with ethidium bromide under UV light, followed by digital image capturing with a charge-coupled device camera. The resulting fingerprints were analyzed with the BioNumerics V3.0 software package (Applied Maths, St.-Martens-Latem, Belgium). Similarities between digitized profiles were calculated by using the Pearson correlation, and an average linkage (unweighted pair group method with arithmetic average) dendrogram was obtained.
16S rDNA sequencing.
Amplification and sequencing reactions were performed as described previously (12). The sequencing primers used were 5′-CTCCTACGGGAGGCAGCACT-3′ (forward primer, corresponding to positions 339 to 358 according to Escherichia coli numbering), 5′-AACTCAAAGGAATTGACGG-3′ (forward, positions 908 to 926), 5′-AGTCCCGCAACGAGCGCAAC-3′ (forward, positions 1093 to 1112), 5′-ACTGCTGCCTCCCGTAGGAG-3′ (reverse, positions 358 to 339), 5′-GTATTACCGCGGCTGCTG-3′ (reverse, positions 536 to 519), and 5′-GTTGCGCTCGTTGCGGGACT-3′ (reverse, positions 1112 to 1093). An ABI 3100 automated DNA sequencer (Applied BioSystems) was used according to the manufacturer's instructions. The FASTA program (18) was applied to find the most similar sequences from the EMBL database.
PCR with species-specific gyrB primers.
PCR with primers targeting the gyrB gene specific for Bacillus cereus (BC1 [5′-ATTGGTGACACCGATCAAACA-3′, positions 490 to 510] and BC2r [5′-TCATACGTATGGATGTTATTC-3′, positions 834 to 854]), Bacillus anthracis (BA1 [5′-AATCGTAATATTAAACTGACG-3′, positions 607 to 627] and BA2r [5′-CCTTCATACGTGTGAATGTTG-3′, positions 831 to 851]), or Bacillus thuringiensis (BT1 [5′-ATCGGTGATACAGATAAGACT-3′, positions 175 to 195] and BT2r [5′-CCTTCATACGTATGAATATTATTT-3′, positions 519 to 542]) was performed. Primer positions were determined by using Bacillus cereus ATCC 14579 (accession no. AE016877), Bacillus anthracis strain Ames (accession no. AE016879), or Bacillus thuringiensis IAM 12077 (accession no. AF090331) gyrB numbering. The reactions were carried out with a 25-μl reaction mixture containing 25 pmol of each primer, 5 nmol of each deoxynucleoside triphosphate, 2.5 μl of 10× PCR buffer (Applied BioSystems), 0.5 U of Taq polymerase (Applied BioSystems), and 50 ng of template DNA. The PCR profile consisted of 30 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 90 s, and extension at 72°C for 150 s and a final extension at 72°C for 7 min. The resulting amplicons were examined by electrophoresis on a 1% agarose gel.
Partial sequencing of the rpoB and gyrA genes.
An rpoB fragment corresponding to Bacillus subtilis rpoB positions 6 to 585 was PCR amplified by using primers rpoB-f (5′-AGGTCAACTAGTTCAGTATGGAC-3′) and rpoB-r (5′-AAGAACCGTAACCGGCAACTT-3′). A gyrA fragment corresponding to Bacillus subtilis gyrA positions 43 to 1070 was PCR amplified by using primers gyrA-f (5′-CAGTCAGGAAATGCGTACGTCCTT-3′) and gyrA-r (5′-CAAGGTAATGCTCCAGGCATTGCT-3′). The reactions were carried out with a 50-μl reaction mixture containing 20 pmol of each primer, 10 nmol of each deoxynucleoside triphosphate, 5 μl of 10× PCR-buffer (Applied BioSystems), 1 U of Taq polymerase (Applied BioSystems), and 50 ng of template DNA. The PCR profile consisted of denaturation at 94°C for 2 min; 40 cycles of denaturation at 94°C for 30 s, annealing at 51°C for 45 s, and extension at 68°C for 50 s (or 60 s for the gyrA gene); and a final extension at 68°C for 90 s (or 10 min for the gyrA gene). The resultant amplicons were purified with the NucleoFast 96 PCR system (Millipore) and sequenced in both directions by using the same primers. Sequencing was performed with an ABI 3100 automated DNA sequencer (Applied BioSystems) according to the manufacturer's instructions. Phylogenetic analysis was performed with CLUSTAL W (28) and TREECON software (31).
Gelatinase tests.
The gelatinase activity of the isolates was investigated by two different tests. In the first test, performed with tubes, a small amount of cells of a pure culture was inoculated into 5 ml of medium consisting of (wt/vol) 0.25% yeast extract, 0.5% Bacto Peptone, 0.5% glucose, 0.1% MgSO4•7H2O, and 12% gelatin suspended in 0.02 M phosphate buffer (pH 7) (0.3% KH2PO4 and 1% Na2HPO4•12H2O). After incubation for 1 week at 37°C, gelatinase activity was revealed as liquefaction of the medium after an extra 24 h of incubation at room temperature. The second test, performed with plates, was based on the method described by Smibert and Krieg (23). Bacterial cells were streaked as a single line across the center of a plate with nutrient agar supplemented with 1.2% gelatin. After incubation for 1 week at the optimal growth temperature, the medium was overlaid with a 10% HCl-15% HgCl2 solution. A clear zone around the growth of the bacteria indicated gelatinase activity.
RESULTS
A set of 1,129 isolates was obtained from the analyzed gelatin batches. Microscopic analysis revealed that the majority of the isolates were endospore-forming rods, and therefore these isolates were expected to belong to Bacillus or related genera.
Repetitive-element genomic fingerprinting.
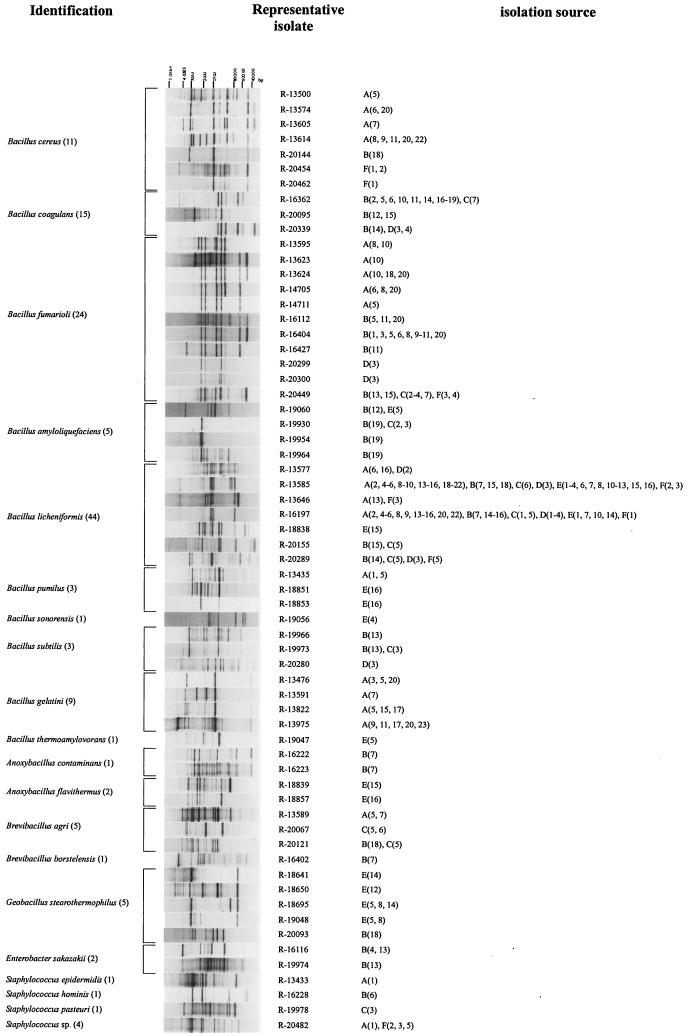
To cope with this large set of isolates, rep-PCR was used as a first screening method. Rep-PCR is a relatively rapid DNA fingerprinting technique that is known to discriminate bacterial isolates at the intraspecific level and potentially up to the strain level (32). Moreover, rep-PCR has been shown to be a useful technique in the subtyping of Bacillus species (9). Therefore, we assumed that isolates displaying the same banding pattern in terms of the presence or absence of bands, and thus showing the same fingerprint type, are highly related. The (GTG)5 primer was applied as in a previous study (6); the use of this primer generated sufficiently complex banding patterns attributable to Bacillus or related genera for all analyzed strains. Hence, as could be expected, a banding pattern was also obtained for all 1,129 isolates studied here. Since the main goal of this study was to characterize and identify gelatin isolates at the species level and since rep-PCR is expected to discriminate at least at this level, we selected representative strains for each of the fingerprint types to unravel species affiliation. In total, 63 representative isolates were selected, and their rep-PCR banding patterns are shown in Fig. 1. Gelatin batches from which strains displaying this banding pattern were isolated are indicated. Some fingerprint types were found in several plants.
FIG.1.
Normalized rep-PCR patterns of representative gelatin isolates and their identification and isolation sources. 16S rDNA sequencing was performed for all representative isolates as a first identification tool. Members of the Bacillus cereus group and the Bacillus subtilis group were further identified based on protein-coding genes. The identification of Bacillus cereus was based on the use of species-specific gyrB-targeting primers. For Bacillus amyloliquefaciens and Bacillus subtilis, identification was based on partial gyrA sequencing. The identification of Bacillus licheniformis was based on gyrA and rpoB sequencing. The identification of Bacillus sonorensis was based on rpoB sequencing. For each identification, the number of different batches in which the species was found is given in parentheses next to the species. A representative isolate is an isolate representative of a specific banding pattern. The isolation source is the gelatin batch from which strains displaying the corresponding rep-PCR banding pattern were isolated.
16S rDNA sequencing.
Sequences of the 16S rRNA gene are generally used as a framework for bacterial classification. Therefore, sequencing of this gene was used as a first identification tool. According to Stackebrandt and Goebel (26), organisms showing less than 97% 16S rDNA sequence similarity will have less than 70% DNA-DNA relatedness, and, according to the recommendations for species delineation (25), these strains should be considered to belong to different species. A 5′-end hypervariable region of the 16S rDNA cistron (positions 70 to 344 according to E. coli numbering) has been shown to be most informative for the rapid identification of Bacillus species (8). Since the majority of the isolates are expected to belong to Bacillus or related genera, sequencing of the 5′-end region of the 16S rDNA cistron was performed initially, allowing a first tentative species assignment. For strains attributed to related endospore-forming genera on the basis of this partial sequence analysis and a selection of strains attributed to Bacillus, more complete 16S rDNA sequences were generated. Results of FASTA analysis of the generated sequences are shown in Table 2. All strains show a first match with similarity above 99%.
TABLE 2.
16S rDNA sequences determined in this study
| Strain no. (other designation)b | Sequence length (bp) | Accession no. | Best match | Similarity (%) |
|---|---|---|---|---|
| R-13433 | 454 | AJ586379 | Staphylococcus epidermidis (AE016751) | 100 |
| R-13435 | 474 | AJ586336 | Bacillus pumilus (AF288735) | 100 |
| R-13476a | 1,508 | AJ586337 | Bacillus gelatini | 100 |
| R-13500 | 477 | AJ586338 | Bacillus cereus (AF176322) | 100 |
| Bacillus thuringiensis (AF155955) | 100 | |||
| Bacillus anthracis (AF176321) | 100 | |||
| R-13574 | 489 | AJ586339 | Bacillus cereus (AF176322) | 99.8 |
| Bacillus athracis (AF176321) | 99.8 | |||
| Bacillus thuringiensis (AF155955) | 99.8 | |||
| R-13577 | 1,511 | AJ586340 | Bacillus licheniformis (AB039328) | 99.9 |
| R-13585 | 1,512 | AJ586341 | Bacillus licheniformis (AB039328) | 99.9 |
| R-13589 | 1,492 | AJ586380 | Brevibacillus agri (AB039334) | 99.9 |
| R-13591a | 450 | AJ586342 | Bacillus gelatini | 100 |
| R-13595 | 1,515 | AJ581124 | Bacillus fumarioli (AJ250059) | 99.9 |
| R-13605 | 482 | AJ586343 | Bacillus cereus (AF176322) | 99.8 |
| Bacillus anthracis (AF176321) | 99.8 | |||
| Bacillus thuringiensis (AF155955) | 99.8 | |||
| R-13614 | 482 | AJ586344 | Bacillus cereus (AF176322) | 99.8 |
| Bacillus anthracis (AF176321) | 99.8 | |||
| Bacillus thuringiensis (AF155955) | 99.8 | |||
| R-13623 | 480 | AJ586345 | Bacillus fumarioli (AJ250058) | 99.8 |
| R-13624 | 1,513 | AJ587725 | Bacillus fumarioli (AJ250058) | 99.8 |
| R-13646 | 1,505 | AJ586346 | Bacillus licheniformis (AF276309) | 100 |
| R-13822a | 1,508 | AJ586347 | Bacillus gelatini | 100 |
| R-13975a | 467 | AJ586348 | Bacillus gelatini | 100 |
| R-14705 | 1,514 | AJ581126 | Bacillus fumarioli (AJ250059) | 99.9 |
| R-14711 | 451 | AJ586349 | Bacillus fumarioli (AJ250058) | 99.8 |
| R-16112 | 486 | AJ586350 | Bacillus fumarioli (AJ250058) | 99.6 |
| R-16116 | 475 | AJ586351 | Enterobacter sakazakii (AB004746) | 99.9 |
| R-16197 | 322 | AJ586352 | Bacillus licheniformis (AB055006) | 99.7 |
| R-16222a (LMG 21881T) | 1,541 | AJ551330 | Anoxybacillus contaminans | 100 |
| R-16223a | 480 | AJ586381 | Anoxybacillus contaminans | 100 |
| R-16228 | 476 | AJ586353 | Staphylococcus hominis (AY030318) | 100 |
| R-16362 (LMG 21801) | 484 | AJ563374 | Bacillus coagulans (D16267) | 99.6 |
| R-16402 | 1,499 | AJ586382 | Brevibacillus borstelensis (AF378230) | 100 |
| R-16404 | 484 | AJ586354 | Bacillus fumarioli (AJ250058) | 99.6 |
| R-16427 | 438 | AJ586355 | Bacillus fumarioli (AJ250058) | 100 |
| R-18641 | 1,518 | AJ586383 | Geobacillus stearothermophilus (AY044053) | 99.7 |
| R-18650 | 1,073 | AJ586384 | Geobacillus stearothermophilus (AY044052) | 99.9 |
| R-18695 | 1,141 | AJ586385 | Geobacillus stearothermophilus (AY044052) | 99.9 |
| R-18838 | 494 | AJ586356 | Bacillus licheniformis (AB055006) | 99.8 |
| R-18839 | 1,517 | AJ586357 | Anoxybacillus flavithermus (Z26932) | 99.5 |
| R-18851 | 485 | AJ586358 | Bacillus pumilus (AY030327) | 100 |
| R-18853 | 492 | AJ586359 | Bacillus pumilus (AY030327) | 99.8 |
| R-18857 | 1,515 | AJ586360 | Anoxybacillus flavithermus (Z26932) | 99.5 |
| R-19047 | 1,513 | AJ586361 | Bacillus thermoamylovorans (L27478) | 99.0 |
| R-19048 | 1,521 | AJ586362 | Geobacillus stearothermophilus (AY044053) | 99.8 |
| R-19056 | 1,531 | AJ586363 | Bacillus licheniformis (AF397062) | 99.7 |
| R-19060 | 493 | AJ586364 | Bacillus subtilis (AB018487) | 99.8 |
| R-19930 | 463 | AJ586365 | Bacillus subtilis (AB018487) | 99.8 |
| R-19954 | 491 | AJ586366 | Bacillus subtilis (AB018487) | 99.4 |
| R-19964 | 461 | AJ586367 | Bacillus subtilis (AB018487) | 99.8 |
| R-19966 | 459 | AJ586368 | Bacillus subtilis (Z99104) | 100 |
| R-19973 | 484 | AJ586369 | Bacillus subtilis (Z99104) | 99.8 |
| R-19974 | 297 | AJ586370 | Enterobacter sakazakii (AB004746) | 99.7 |
| R-19978 | 454 | AJ586371 | Staphylococcus pasteuri (AY126212) | 100 |
| R-20067 | 1,437 | AJ586386 | Brevibacillus agri (AY319301) | 99.4 |
| R-20093 | 1,516 | AJ586387 | Geobacillus stearothermophilus (AJ294817) | 99.3 |
| R-20095 | 493 | AJ586372 | Bacillus coagulans (AF466695) | 99.8 |
| R-20121 | 1,496 | AJ586388 | Brevibacillus agri (AB039334) | 99.9 |
| R-20144 | 463 | AJ586389 | Bacillus cereus (AE016998) | 100 |
| Bacillus anthracis (AE017024) | 100 | |||
| R-20155 | 461 | AJ586390 | Bacillus licheniformis (X68416) | 100 |
| R-20280 | 461 | AJ586391 | Bacillus subtilis (Z99104) | 99.8 |
| R-20289 | 458 | AJ586392 | Bacillus licheniformis (AF372616) | 99.8 |
| R-20299 | 464 | AJ586373 | Bacillus fumarioli (AJ250058) | 99.8 |
| R-20300 | 464 | AJ586374 | Bacillus fumarioli (AJ250058) | 99.8 |
| R-20339 | 458 | AJ586393 | Bacillus coagulans (D16267) | 99.6 |
| R-20449 | 699 | AJ586375 | Bacillus fumarioli (AJ250058) | 99.9 |
| R-20454 | 631 | AJ586376 | Bacillus cereus (AY138279) | 100 |
| Bacillus anthracis (AE017025) | 100 | |||
| R-20462 | 594 | AJ586377 | Bacillus cereus (AY138276) | 100 |
| Bacillus anthracis (AE017024) | 100 | |||
| R-20482 | 495 | AJ586378 | Staphylococcus lungdunensis (AB009941) | 100 |
| Staphylococcus warneri (L37603) | 100 |
The sequence of this strain was determined as part of the description of new species Bacillus gelatini sp. nov. and Anoxybacillus contaminans sp. nov. (De Clerck et al., unpublished).
LMG, BCCM/LMG Bacteria Collection, Laboratorium voor Microbiologie Gent, Universiteit Gent, Ghent, Belgium.
Strains identified as Bacillus coagulans, Bacillus fumarioli, Bacillus pumilus, Bacillus gelatini, Bacillus thermoamylovorans, and Anoxybacillus, Brevibacillus, and Geobacillus species had as a second match another species with similarity significantly lower than that to the first match. This result is a strong indicator of correct species allocation. A minority of isolates were identified as species of non-endospore-forming genera, Staphylococcus and Enterobacter. Based on partial 16S rDNA sequencing and with the exception of strain R-20482, a first match for these strains with significantly higher similarity than that for the other matches was obtained. Since endospore-forming contaminants are the major concern in gelatin contamination, we did not complete the identification of these non-endospore-forming bacteria and identification results for them should be considered tentative. Strains R-13500, R-13574, R-13605, R-13614, R-20144, R-20454, and R-20462 show the same 16S rDNA sequence similarity with Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. It is well known that 16S rDNA sequence data do not permit one to distinguish these species (1, 29). They show a high degree of DNA reassociation (13) and are therefore called members of the Bacillus cereus group. Likewise, strains identified as Bacillus licheniformis and Bacillus subtilis also show high 16S rDNA sequence similarities to other species. Strains identified as Bacillus licheniformis also show high similarities to Bacillus sonorensis, while strains identified as Bacillus subtilis also show high similarities to Bacillus amyloliquefaciens, Bacillus vallismortis, Bacillus mojavensis, Bacillus atrophaeus, and Bacillus licheniformis strains. These species are regarded as members of the Bacillus subtilis group, and their discrimination on the basis of 16S rDNA sequence analysis has been questioned (4).
Since strains identified as Bacillus licheniformis, Bacillus subtilis, and members of the Bacillus cereus group on the basis of 16S rDNA sequencing (Table 2) are frequent contaminants in gelatin (Fig. 1) and their identification on the basis of 16S rDNA sequence analysis is not satisfactory, we applied other methods for these groups of isolates to obtain more reliable species identification.
Identification of Bacillus cereus group members.
Yamada et al. (33) designed species-specific primer sets for the selective amplification of Bacillus cereus, Bacillus thuringiensis, and Bacillus anthracis gyrB sequences. We applied these species-specific primer sets for further identification of gelatin isolates preliminarily identified as a members of the Bacillus cereus group on the basis of 16S rDNA sequencing (Table 2). For each of these strains, a typical 365-bp PCR product was generated with the Bacillus cereus-specific primers, while with Bacillus thuringiensis- and Bacillus anthracis-specific primers no typical species-specific products like those described by Yamada et al. (33) were obtained. Based on these data, all gelatin isolates attributed to the Bacillus cereus group based on 16S rDNA sequencing may now be regarded as Bacillus cereus strains.
Identification of Bacillus subtilis group members.
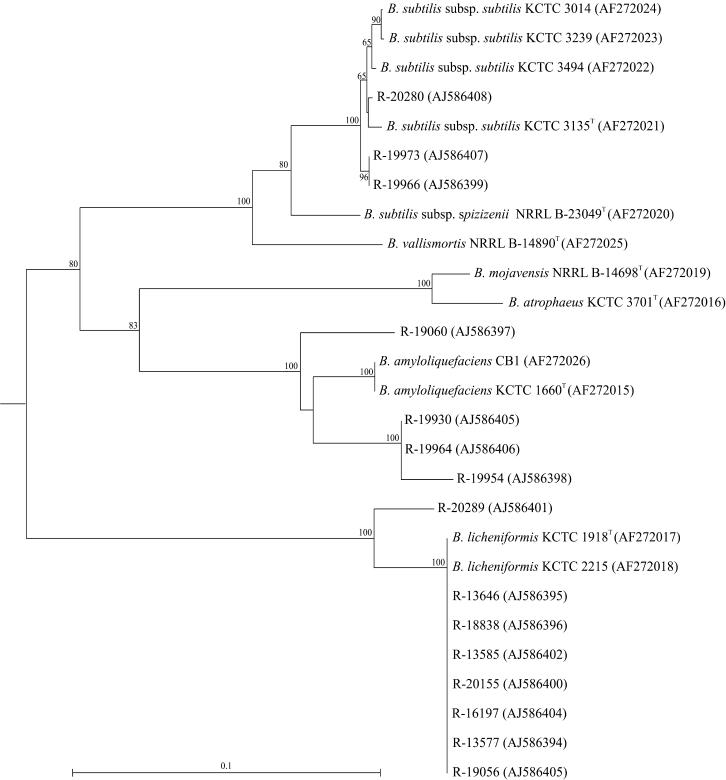
Chun and Bae (4) demonstrated the use of gyrA sequences for accurate classification of Bacillus subtilis and related taxa, including Bacillus amyloliquefaciens, Bacillus vallismortis, Bacillus mojavensis, Bacillus atrophaeus, and Bacillus licheniformis. Therefore, we performed gyrA sequencing of gelatin isolates preliminarily identified as Bacillus licheniformis or Bacillus subtilis on the basis of 16S rDNA sequencing (Table 2). Cluster analysis of these gyrA sequences with those from the study of Chun and Bae (4) is shown in Fig. 2. All isolates identified as Bacillus licheniformis on the basis of 16S rDNA sequencing grouped with Bacillus licheniformis strains when gyrA sequences were used. Three strains (R-20280, R-19973, and R-19966) that were identified as Bacillus subtilis based on 16S rDNA sequencing clustered with Bacillus subtilis subsp. subtilis strains, while others (R-19060, R-19930, R-19964, and R-19954) grouped most closely with Bacillus amyloliquefaciens strains.
FIG. 2.
Rooted neighbor-joining tree constructed by using partial gyrA sequences of gelatin isolates (indicated with an “R-” number) attributed to species of the Bacillus subtilis group based on 16S rDNA sequencing among strains from the study of Chun and Bae (4). Bootstrap values (expressed as percentages of 1,000 replications) of >60% are shown at branch points. Accession numbers are given in parentheses. The tree was rooted by using the gyrA sequence of Bacillus sp. strain C125 (AB010081) as an outgroup. The scale bar indicates 0.1% nucleotide substitutions. B., Bacillus.
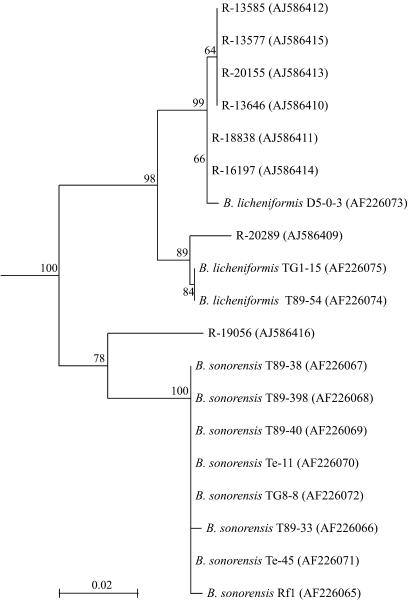
Palmisano et al. (17) described the use of the rpoB sequence to discriminate between Bacillus licheniformis and the closely related species Bacillus sonorensis. Therefore, we performed rpoB sequencing of the strains identified as Bacillus licheniformis on the basis of 16S rDNA (Table 2) and gyrA (Fig. 2) sequence analysis and the sequences were compared with those from Bacillus sonorensis and Bacillus licheniformis strains of the study of Palmisano et al. (17). Cluster analysis (Fig. 3) reveals a close relationship of most strains with Bacillus licheniformis. Only strain R-19056 groups more closely with Bacillus sonorensis.
FIG. 3.
Rooted neighbor-joining tree constructed by using partial rpoB sequences of gelatin isolates (indicated with an “R-” number) attributed to Bacillus licheniformis based on 16S rDNA and gyrA sequencing among strains from the study of Palmisano et al. (17). Bootstrap values (expressed as percentages of 1,000 replications) of >60% are shown at branch points. Accession numbers are given in parentheses. The tree was rooted by using the rpoB sequence of Bacillus subtilis LMG 7135T (AJ586566) as an outgroup. The scale bar indicates 0.1% nucleotide substitutions. B., Bacillus.
Results of consensus identification of gelatin isolates representative of a specific fingerprint type based on the 16S rDNA, gyrB, gyrA, and rpoB genes are shown in Fig. 1.
Gelatinase tests.
At least one representative strain of each rep-PCR fingerprint type was examined for its gelatinase activity. With the exception of Bacillus thermoamylovorans strain R-19047 and all tested Geobacillus stearothermophilus strains, all strains expressed gelatinase activity.
DISCUSSION
In this study, we isolated, characterized, and identified bacterial contaminants in semifinal gelatin batches from six gelatin production plants. Rep-PCR fingerprinting was used to select representative strains at the subspecies level. 16S rDNA sequence analysis of these representative strains allowed a first tentative species identification. However, members of the Bacillus cereus group and some members of the Bacillus subtilis group could not be discriminated. Indeed, despite the general use of the 16S rRNA gene as a framework for modern bacterial classification, it often shows limited variation for the discrimination of closely related taxa (7, 27). On the other hand, protein-coding genes exhibit higher genetic variation, which can be used for the classification and identification of closely related taxa. Therefore, species-specific primer sets targeting the gyrB gene (33) were used to carry out further species allocation of gelatin isolates identified as members of the Bacillus cereus group on the basis of 16S rDNA sequencing, while gyrA (4) and rpoB sequence analysis (17) allowed the clarification of species assignment for strains identified as members of the Bacillus subtilis group on the basis of 16S rDNA sequencing.
As expected, the majority of isolates were identified as members of Bacillus or related endospore-forming genera. These strains were attributed to Bacillus cereus, Bacillus coagulans, Bacillus fumarioli, Bacillus amyloliquefaciens, Bacillus licheniformis, Bacillus pumilus, Bacillus sonorensis, Bacillus subtilis, Bacillus gelatini, Bacillus thermoamylovorans, Anoxybacillus contaminans, Anoxybacillus flavithermus, Brevibacillus agri, Brevibacillus borstelensis, or Geobacillus stearothermophilus.
Except for Bacillus sonorensis, Bacillus thermoamylovorans, Bacillus gelatini, Brevibacillus borstelensis, and the Anoxybacillus species, which were isolated with only one type of extraction process at only one gelatin production plant, all species were found to be contaminants in different types of extraction processes at more than one production plant. Bacillus licheniformis was found in all types of gelatin extracts included and at all production plants. Also, Bacillus fumarioli was found to be a frequent contaminant, as it was isolated from gelatin batches from all production plants except the Argentinian plant. The frequent isolation of Bacillus fumarioli from gelatin is remarkable, as the only other habitat known for Bacillus fumarioli is geothermal soil (16). Non-endospore-forming species, such as Enterobacter and Staphylococcus species, were found in only a very limited number of samples. Non-endospore-forming bacteria are not expected to survive the gelatin production process, and contamination may be a result of the handling of UHT-treated batches.
Some of the species found (e.g., Bacillus cereus and Bacillus licheniformis) are known to exhibit pathogenic properties, which are of great concern to human health, especially in food and pharmaceutical applications of gelatin. Bacillus cereus has been shown to contaminate food-processing plants and, because of its pathogenic potential, constitutes a public health hazard (2, 14). Bacillus licheniformis has been shown to be a frequent contaminant of industrial processes (see, e.g., references 19, 24, and 30). Although this organism is exploited industrially for the large-scale production of enzymes, its generally-recognized-as-safe status is a subject of debate (21). Processing of contaminated gelatin batches in the food and pharmaceutical industries may lead to unacceptable levels of these species in the corresponding end products.
Bacillus coagulans has frequently been reported to be a contaminant and spoiling organism of milk products, vegetables, and fruits (see, e.g., references 5 and 15), and processing of contaminated gelatin in foods may thus affect shelf lives.
All species isolated from semifinal gelatin extracts except Bacillus thermoamylovorans and Geobacillus stearothermophilus were found to encompass strains exhibiting gelatinase activity. Enzymatic degradation of gelatin affects the viscosity and therefore the quality of the product itself and its applications. Moreover, because of this degradation, essential nutrients may become available for gelatinase-negative contaminants, promoting their growth.
In this study, we have shown that thermotolerant, aerobic, endospore-forming bacteria assigned to Bacillus, Anoxybacillus, Brevibacillus, and Geobacillus species contaminate the semifinal product of a gelatin extraction process. Some of these species have known pathogenic traits, and the majority of these species include strains exhibiting gelatinase activity. These findings clearly raise concerns about the safety and quality of gelatin and its applications, especially since more effective sterilization conditions, such as an extension of the UHT treatment or an elevation of the sterilization temperature would also affect the technical properties of gelatin. Currently, quality control tests used in gelatin production plants rely on classical bacteriological methods to assess bacterial contamination in the end product. Although these methods are standardized and often incorporated in a well-founded quality management system and hence are generally accepted among consumers, they show limitations. First, because these methods rely on bacterial growth, they are time-consuming and may cause prolonged delivery terms. Second, when limited numbers of selective growth media and phenotypic tests are used, these methods yield inadequate information concerning species identity. Consequently, the actual hazards of observed contaminations and suitable remediation procedures to be taken are not straightforward. A fast and sensitive detection method for the most important contaminants in terms of prevalence and/or pathogenicity, as indicated by this study, would help gelatin producers in the distribution of a safe and high-quality product.
Acknowledgments
E. De Clerck was supported by a fellowship of the IWT (Institution for the Promotion of Innovation by Science and Technology in Flanders). P. De Vos is indebted to the FWO Vlaanderen for research grant G.0156.02.
We thank Renata Coopman, Stefanie Hubeau, and Katrien De Ridder for excellent technical assistance.
REFERENCES
- 1.Ash, C., J. A. E. Farrow, M. Dorsch, E. Stackebrandt, and M. D. Collins. 1991. Comparative analysis of Bacillus anthracis, Bacillus cereus and related species on the basis of reverse transcriptase sequencing of 16S ribosomal RNA. Int. J. Syst. Bacteriol. 41:343-346. [DOI] [PubMed] [Google Scholar]
- 2.Borge, G., M. Skeie, T. Sorhaug, T. Langsrud, and P. Granum. 2001. Growth and toxin profiles of Bacillus cereus isolated from different food sources. Int. J. Food Microbiol. 69:237-246. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K. L. 2000. Control of bacterial spores. Br. Med. Bull. 56:158-171. [DOI] [PubMed] [Google Scholar]
- 4.Chun, J., and K. S. Bae. 2000. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Leeuwenhoek 78:123-127. [DOI] [PubMed]
- 5.Cosentino, S., A. F. Mulargia, B. Pisano, P. Tuveri, and F. Palmas. 1997. Incidence and biochemical characteristics of Bacillus flora in Sardinian dairy products. Int. J. Food Microbiol. 38:235-238. [DOI] [PubMed] [Google Scholar]
- 6.De Clerck, E., and P. De Vos. 2002. Study of the bacterial load in a gelatine production process focussed on Bacillus and related endosporeforming genera. Syst. Appl. Microbiol. 25:611-618. [DOI] [PubMed] [Google Scholar]
- 7.Fox, G. E., J. D. Wisotzkey, and P. Jurtshuk. 1992. How close is close—16S ribosomal RNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 8.Goto, K., T. Omura, Y. Hara, and Y. Sadaie. 2000. Application of the partial 16S rDNA sequence as an index for rapid identification of species in the genus Bacillus. J. Gen. Appl. Microbiol. 46:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Herman, L., and M. Heyndrickx. 2000. The presence of intragenically located REP-like elements in Bacillus sporothermodurans is sufficient for REP-PCR typing. Res. Microbiol. 151:255-261. [DOI] [PubMed] [Google Scholar]
- 10.Heyndrickx, M., and P. Scheldeman. 2002. Bacilli associated with spoilage in dairy products and other food, p. 64-82. In R. Berkeley, M. Heyndrickx, N. A. Logan, and P. De Vos (ed.), Applications and systematics of Bacillus and relatives. Blackwell Publishing, Oxford, United Kingdom.
- 11.Heyndrickx, M., L. Vauterin, P. Vandamme, K. Kersters, and P. De Vos. 1996. Applicability of combined amplified ribosomal DNA restriction analysis (ARDRA) patterns in bacterial phylogeny and taxonomy. J. Microbiol. Methods 26:247-259. [Google Scholar]
- 12.Heyrman, J., and J. Swings. 2001. 16S rDNA sequence analysis of bacterial isolates from biodeteriorated mural paintings in the Servilia tomb (necropolis of Carmona, Seville, Spain). Syst. Appl. Microbiol. 24:417-422. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, T., R. Nozaki, and K. Aizawa. 1978. Deoxyribonucleic-acid relatedness between Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis. Microbiol. Immunol. 22:639-641. [DOI] [PubMed] [Google Scholar]
- 14.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 15.Kunene, N. F., J. W. Hastings, and A. von Holy. 1999. Bacterial populations associated with a sorghum-based fermented weaning cereal. Int. J. Food Microbiol. 49:75-83. [DOI] [PubMed] [Google Scholar]
- 16.Logan, N. A., L. Lebbe, B. Hoste, J. Goris, G. Forsyth, M. Heyndrickx, B. L. Murray, N. Syme, D. D. Wynn-Williams, and P. De Vos. 2000. Aerobic endospore forming bacteria from geothermal environments in Northern Victoria Land, Antarctica, and Candlemas Island, South Sandwich Archipelago, with the proposal of Bacillus fumarioli sp. nov. Int. J. Syst. Evol. Microbiol. 50:1741-1753. [DOI] [PubMed] [Google Scholar]
- 17.Palmisano, M. M., L. K. Nakamura, K. E. Duncan, C. A. Istock, and F. M. Cohan. 2001. Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. Int. J. Syst. Evol. Microbiol. 51:1671-1679. [DOI] [PubMed] [Google Scholar]
- 18.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirttijärvi, T. S. M., M. A. Andersson, and M. S. Salkinoja-Salonen. 2000. Properties of Bacillus cereus and other bacilli contaminating biomaterial based industrial processes. Int. J. Food Microbiol. 60:231-239. [DOI] [PubMed] [Google Scholar]
- 20.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 21.Salkinoja-Salonen, M. S., R. Vuorio, M. A. Andersson, P. Kampfer, M. C. Andersson, T. Honkanen-Buzalski, and A. C. Scoging. 1999. Toxigenic strains of Bacillus licheniformis related to food poisoning. Appl. Environ. Microbiol. 65:4637-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setlow, P. 1994. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 76:S49-S60. [DOI] [PubMed] [Google Scholar]
- 23.Smibert, R. M., and N. R. Krieg. 1994. Phenotypic characterization, p. 607-654. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 24.Sorokulova, I. B., O. N. Reva, V. V. Smirnov, I. V. Pinchuk, S. V. Lapa, and M. C. Urdac. 2003. Genetic diversity and involvement in bread spoilage of Bacillus strains from flour and ropy bread. Lett. Appl. Microbiol. 37:169-173. [DOI] [PubMed] [Google Scholar]
- 25.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. D. Grimont, P. Kämpfer, M. C. J. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Trüper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the ad hoc committee for the reevaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 26.Stackebrandt, E., and B. M. Goebel. 1994. A place for DNA-DNA reassociation and 16S ribosomal RNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 27.Stackebrandt, E., and J. Swiderski. 2002. From phylogeny to systematics: the dissection of the genus Bacillus. In R. Berkeley, M. Heyndrickx, N. A. Logan, and P. De Vos (ed.), Applications and systematics of Bacillus and relatives. Blackwell Publishing, Oxford, United Kingdom.
- 28.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbull, P. C. B., P. J. Jackson, K. K. Hill, P. Keim, A. Kolsto, and D. J. Beecher. 2002. Longstanding taxonomic enigmas within the ‘Bacillus cereus group' are on the verge of being resolved by far-reaching molecular developments: forecasts on the possible outcome by an ad-hoc team, p. 23-36. In R. Berkeley, M. Heyndrickx, N. Logan, and P. De Vos (ed.), Applications and systematics of Bacillus and relatives. Blackwell Publishing, Oxford, United Kingdom.
- 30.Vaerewijck, M. J. M., P. De Vos, L. Lebbe, P. Scheldeman, B. Hoste, and M. Heyndrickx. 2001. Occurrence of Bacillus sporothermodurans and other aerobic spore forming species in feed concentrate for dairy cattle. J. Appl. Microbiol. 91:1074-1084. [DOI] [PubMed] [Google Scholar]
- 31.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 32.Versalovic, J., M. Schneider, F. J. De Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 33.Yamada, S., E. Ohashi, N. Agata, and K. Venkateswaran. 1999. Cloning and nucleotide sequence analysis of gyrB of Bacillus cereus, B. thuringiensis, B. mycoides, and B. anthracis and their application to the detection of B. cereus in rice. Appl. Environ. Microbiol. 65:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]