Abstract
A rapid and simple approach to the small-subunit (SSU) rRNA-based quantitative detection of a specific group of microorganisms in complex ecosystems has been developed. The method employs sequence-specific cleavage of rRNA molecules with oligonucleotides and RNase H. Defined mixtures of SSU rRNAs were mixed with an oligonucleotide (referred to as a “scissor probe”) that was specifically designed to hybridize with a particular site of targeted rRNA and were subsequently digested with RNase H to proceed to sequence-dependent rRNA scission at the hybridization site. Under appropriate reaction conditions, the targeted rRNAs were correctly cut into two fragments, whereas nontargeted rRNAs remained intact under the same conditions. The specificity of the cleavage could be properly adjusted by controlling the hybridization stringency between the rRNA and the oligonucleotides, i.e., by controlling either the temperature of the reaction or the formamide concentration in the hybridization-digestion buffer used for the reaction. This enabled the reliable discrimination of completely matched rRNA sequences from single-base mismatched sequences. For the detection of targeted rRNAs, the resulting RNA fragment patterns were analyzed by gel electrophoresis with nucleotide-staining fluorescent dyes in order to separate cleaved and intact rRNA molecules. The relative abundance of the targeted SSU rRNA fragments in the total SSU rRNA could easily be calculated without the use of an external standard by determining the signal intensity of individual SSU rRNA bands in the electropherogram. This approach provides a fast and easy means of identification, detection, and quantification of a particular group of microbes in clinical and environmental specimens based on rRNA.
Microorganisms are an essential component of the earth's biota, playing integral roles in ecosystems in terms of function and sustainability. To unambiguously understand these roles, extensive studies of the microbial ecology of systems such as aquatic environments, soils, subsurfaces, and animals have been carried out. Due to such studies, there is now a much better understanding of microbial diversity as well as the functions of each microbial constituent and of the nature of the interactions among individual members (and environments or hosts) in various ecosystems. The recent accumulation of such knowledge in the field of microbial ecology can be attributed to a great extent to the development and application of molecular techniques in environmental microbiology, particularly those based on small-subunit (SSU) rRNA and the rRNA gene (6). Among rRNA-rRNA gene-based techniques developed to date, the use of group-specific DNA probes complementary to SSU rRNA provides the most powerful tool to precisely identify different populations in complex systems (3, 6). For example, whole-cell in situ hybridization based on SSU rRNA is now commonly used to detect specific groups of microbes and to quantify populations of interest in environments by direct counting (2). Another example of a method based on SSU rRNA is the quantitative membrane hybridization of labeled DNA probes to community rRNAs (43, 50). This method has also been applied to various environmental rRNAs for the quantitative detection of specific groups of microbes present in complex communities (21, 37, 38, 44, 45). More recently, an oligonucleotide-based DNA microchip format targeting multiple rRNA molecules is being developed, providing a powerful framework for the parallel hybridization of different rRNA fragments to a matrix array of DNA probes (15, 18, 30, 32, 52, 53). However, in spite of the potential advantages of these techniques, all of them are often laborious and require time-consuming procedures in general, particularly when they are employed for the quantitative detection of specific microbial groups in natural populations (5, 10, 55). To precisely and rapidly evaluate the abundance and activity of selected groups of microbes in complex ecosystems, more direct, rapid, simple, quantitative, and cost-effective tools which can be applied to various types of heterogeneous environments should be developed.
Here, we report the concept of sequence-specific cleavage of rRNA fragments using oligonucleotides and RNase H as a rapid and easy means of rRNA-based microbial identification, detection, and quantification (Fig. 1). RNase H is known to specifically degrade the RNA strand of RNA-DNA hybrid duplexes (9, 22, 24). By using this method, total RNAs from complex microbial communities are mixed with oligonucleotides that bind in a sequence-specific manner to a particular site of targeted SSU rRNAs and are subsequently digested with RNase H to proceed to sequence-dependent rRNA scission at the hybridization site. Upon the digestion, the targeted rRNAs are specifically cut into two fragments, whereas nontargeted rRNAs remain intact under the same conditions. For the detection of the cleaved rRNAs, the resulting RNA fragment patterns can be resolved by gel electrophoresis with RNA-staining dyes. The relative abundance of SSU rRNA fragments in the total SSU rRNA of the targeted species can also be quantified without the use of external standards by determining the signal intensity of individual SSU rRNA bands in an electropherogram. Since a large collection of SSU rRNA-targeted DNA probes has already been developed for studies in the fields of clinical, pharmaceutical, and environmental microbiology, such probes may directly be used as “scissor” oligonucleotides (here, we refer to them as scissor probes) in the digestion process.
FIG. 1.
Flow diagram showing the concept of sequence-specific digestion of SSU rRNA with oligonucleotides (scissor probes) and RNase H.
In this report, we tested the feasibility of this rationale by using a model microbial community, and we optimized the digestion procedure to clearly discriminate single-base mismatches in the hybridization between rRNA fragments and the scissor probes. The method was then applied to environmental samples of RNAs extracted from several complex ecosystems.
MATERIALS AND METHODS
Microorganisms and environmental samples.
The following organisms were used in this study. Escherichia coli (DSM5717), Thiothrix disciformis (JCM11364), Methanosaeta concilii (DSM3671), and Methanosarcina barkeri (DSM800) were obtained from either the Japan Collection of Microorganisms (Wako, Japan) or the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). Genomic nucleic acids of Legionella pneumophila (ATCC 33152) were kindly provided by Yoshikazu Ishii at the Toho University School of Medicine, Tokyo, Japan. Environmental samples were collected from (i) two types of activated sludge processes used for treating sewage at different locations in Japan, (ii) mesophilic anaerobic digesters (operated at 35 to 40°C) decomposing sewage sludge, and (iii) a mesophilic (37°C) upflow anaerobic sludge blanket (UASB) reactor treating high-strength wastewater from a food-processing plant. In addition, cow feces were collected from healthy Holstein dairy cattle bred at the National Institute of Livestock and Grassland Science, Tsukuba, Japan. Solid fractions of the samples were collected by centrifuging them at 15,000 × g for 5 min, and the samples were kept at −80°C until they were used for RNA extraction.
RNA extraction.
RNA extraction from pure cultures, as well as from environmental samples, was performed according to the method of Stahl et al. (50) with slight modifications. Briefly, pellets of cells (or samples; approximately 0.1 to 0.2 g [wet weight]) were harvested in conical 2.2-ml screw-cap tubes; each tube contained 1 g of baked glass beads (0.1 mm in diameter) and 1 ml of pH 5.1 buffer (10 mM EDTA, 50 mM sodium acetate [pH 5.1]). The remaining volume in the tubes was filled with phenol equilibrated with pH 5.1 buffer. The tubes were then subjected to mechanical disruption for 1 min on a bead-beating device (FastPrep machine; Bio101, Holbrook, N.Y.). Additional extractions with an equal volume of pH 5.1 buffer-equilibrated phenol (once), an equal volume of pH 5.1 buffer-equilibrated phenol-chloroform-isoamyl alcohol (50:49:1) (twice), and an equal volume of chloroform (once) were performed in order to purify the RNA molecules. Total nucleic acids (mainly composed of RNA) were ethanol precipitated and were subjected to further purification of the RNA with RNase-free DNase (Promega) in Tris-EDTA buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). The RNA samples were further extracted with an equal volume of phenol-chloroform-isoamyl alcohol (once) and an equal volume of chloroform (once) and were recovered by ethanol precipitation. The recovered nucleic acids were suspended in water to give final concentrations of 100 to 500 ng/μl. Shorter RNA fragments of approximately 500 nucleotides (nt) or less were removed with a purification column (MicroSpin column S-400; Amersham Pharmacia Biotech) when the RNAs were extracted from complex communities (environmental, industrial, and fecal samples).
PCR, cloning, sequencing, and in vitro transcription.
For all reference bacterial and archaeal strains, plasmids containing each SSU rRNA gene of the strains and a T7 promoter were constructed for in vitro transcription of the respective SSU rRNAs. DNA extraction from pure cultures was performed by using the method of Hiraishi (23). SSU rRNA genes were amplified by PCR using the following primer pairs: bacterial primer 8f (5′-AGAGTTTGATCCTGGCTCAG-3′ [positions 8 to 27 in the E. coli system]) (56) or archaeal primer 7f (5′-TTCCGGTTGATCCYGCCGGA-3′ [positions 7 to 26 in the E. coli system]) (12) and universal primer 1492r (5′-TACGGYTACCTTGTTACGACTT-3′ [positions 1492 to 1471; Y represents T or C]) (31). PCR and subsequent TA cloning with a pT7 Blue vector (containing a T7 promoter; Novagen) were performed as described previously (48). Clones containing the SSU rRNA gene of the reference strains were partially sequenced with a T7 promoter primer (Novagen) in order to determine whether the gene was inserted in the correct direction for subsequent in vitro transcription (36, 40). Partial sequencing was performed with the CEQ2000 DNA analysis system (Beckman) and a DTCS quick start kit (Beckman). Each of the T7 promoter-conjugated SSU rRNA genes was amplified by PCR, and the SSU rRNA transcripts were generated in vitro with the PCR-amplified fragments and T7 RNA polymerase from a T7 RiboMAX express kit (Promega). The concentration of SSU rRNA transcripts was measured with a spectrofluorophotometer (RF-1500; Shimadzu) and a RiboGreen RNA quantification kit (Molecular Probes).
Sequence-dependent scission of rRNA with RNase H.
Protocols for the sequence-specific cleavage of rRNA fragments developed for this study were primarily based on a previously reported method (13). The following steps were carried out for the optimized digestion of rRNA fragments. Cleavage reactions were carried out at 40 to 60°C for 15 min with hybridization-digestion buffer (25 mM Tris-HCl [pH 7.5], 1 mM EDTA, 25 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol [DTT], 30 μg of bovine serum albumin [BSA]/ml, 5 U of RNase H/ml) containing the extracted RNAs (10 ng/μl) and each of the oligonucleotide scissor probes (0.2 pmol/μl). The hybridization stringency was adjusted by adding formamide to the hybridization-digestion buffer (see Table 2). Briefly, to create a 100-μl hybridization-digestion mixture, an aliquot (10 μl) of RNA suspension (containing approximately 100 ng of RNA/μl) was mixed with 5 μl of 15× hybridization buffer (375 mM Tris-HCl [pH 7.5], 15 mM EDTA, 375 mM NaCl), 2 μl of each of the oligonucleotide solutions (10 pmol/μl), and a defined amount of formamide. Then, diethyl pyrocarbonate-treated water was added to make a 75-μl solution. The mixture was subsequently heated at 95°C for 1 min to unfold the RNA fragments, and then the mixture was kept at an appropriate hybridization and digestion temperature (40 to 60°C). To initiate the cleavage reaction, 25 μl of preheated 4× enzyme solution (25 mM Tris-HCl [pH 7.5], 40 mM MgCl2, 25 mM NaCl, 4 mM DTT, 120 μg of BSA/ml, 20 U of RNase H [TaKaRa]/ml) was added to the mixture. After incubation at the hybridization and digestion temperature for 15 min, 50 μl of 3× stop solution (30 mM EDTA, 0.9 M sodium acetate [pH 7.0]) was added to the mixture to terminate the reaction. The RNA in the mixture was then precipitated with 380 μl of ethanol and centrifuged at 15,000 rpm for 20 min at 4°C, dissolved in diethyl pyrocarbonate-treated water, and subjected to further analysis. The oligonucleotides used as scissor probes in this study (Tables 1 and 2) were commercially synthesized. The specificity of newly designed probes (ARC915m and MX825m) was evaluated in silico by using the ARB program (33) as well as public nucleotide databases.
TABLE 2.
DNA scissor probes used for quantitative detection of specific microorganisms in complex microbial communities
| Probe name | Target group | Probe sequence (5′-3′) | Probe length (mer) | Target site (E. coli position) | Optimal cleavage conditions
|
|
|---|---|---|---|---|---|---|
| Formamide (%) | Cleavage coefficienta | |||||
| UNI530 | Virtually all organisms | CCGCGGC(G/T)GCTGGCAC | 16 | 530-545 | 15 | 1.00 |
| EUB338 | Domain Bacteria | GCTGCCTCCCGTAGGAGT | 18 | 338-355 | 20 | 0.96 |
| ARC915mb | Domain Archaea | CCCCCGCCAATTCCTTTA | 18 | 912-929 | 30 | 0.80 |
| MX825mc | Family Methanosaetaceae | TGGCCGACACCTAGCGAG | 18 | 821-838 | 10 | 0.94 |
| G123T | Genus Thiothrix | CCTTCCGATCTCTATGCA | 18 | 697-714 | 30 | 0.55 |
The cleavage coefficient represents the proportion of fragmented SSU rRNA to the total targeted SSU rRNA at the optimal conditions. A cleavage coefficient of 0.5 indicates that 50% of the targeted SSU rRNA can be cleaved at the defined conditions in the RNase H digestion.
The scissor probe (ARC915m) is a slightly modified version of the probe ARC915 primarily reported by Stahl et al. (50).
The scissor probe (MX825m) is a modified version of the probe MX825 previously described by Raskin et al. (43).
TABLE 1.
DNA scissor probes used for the evaluation of sequence-dependent cleavage of 16S rRNA with E. coli total RNA
| Probe name | Sequence (5′-3′)a | Target position | PM or MM | Probe length (mer) | G + C content (%) | Reaction conditions for 50% digestionb
|
|
|---|---|---|---|---|---|---|---|
| Td (°C) | Fd (% at 50°C) | ||||||
| 530 probes (high G + C content)c | |||||||
| 530-12 | CCGCGGCTGCTG | 519-530 | PM | 12 | 83 | 59.5 ± 0.5 | |
| 530-16 | CCGCGGCTGCTGGCAC | 515-530 | PM | 16 | 81 | 50 | 24.9 ± 0.8 |
| 530-20 | CCGCGGCTGCTGGCACGGAG | 511-530 | PM | 20 | 80 | 50 | 30.9 ± 0.3 |
| 327 probes (around 50% G + C content) | |||||||
| 327-12 | TCAGTTCCAGTG | 314-325 | PM | 12 | 50 | 52.8 ± 2.5 | |
| 327-14 | CTCAGTTCCAGTGT | 313-326 | PM | 14 | 50 | 59.5 ± 1.1 | |
| 327-16 | TCTCAGTTCCAGTGTG | 312-327 | PM | 16 | 50 | 50 | 23.2 ± 0.7 |
| 327-16M8 | TCTCAGTACCAGTGTG | 312-327 | MM | 16 | 50 | 50 | 10.6 ± 0.7 |
| 327-16M16 | TCTCAGTTCCAGTGTC | 312-327 | MM | 16 | 50 | 50 | 11.3 ± 0.2 |
| 327-18 | TGTCTCAGTTCCAGTGTG | 312-329 | PM | 18 | 50 | 50 | 28.8 ± 0.4 |
| 327-18M9a | TGTCTCAGTACCAGTGTG | 312-329 | MM | 18 | 50 | 50 | 15.7 ± 0.5 |
| 327-18M9c | TGTCTCAGTCCCAGTGTG | 312-329 | MM | 18 | 56 | 50 | 12.0 ± 0.1 |
| 327-18M13 | TGTCTCAGTTCCGCTGTG | 312-329 | MM | 18 | 56 | 50 | 23.9 ± 0.7 |
| 327-18M14 | TGTCTCAGTTCCACTGTG | 312-329 | MM | 18 | 50 | 50 | 9.0 ± 1.6 |
| 327-18M6 | TGTCTGAGTTCCAGTGTG | 312-329 | MM | 18 | 50 | 50 | 12.9 ± 0.1 |
| 327-18M1 | AGTCTCAGTTCCAGTGTG | 312-329 | MM | 18 | 50 | 50 | 25.9 ± 0.1 |
| 327-18M18 | TGTCTCAGTTCCAGTGTC | 312-329 | MM | 18 | 50 | 50 | 11.2 ± 0.2 |
| 327-20 | GTGTCTCAGTTCCAGTGTGG | 311-330 | PM | 20 | 55 | 50 | 29.8 ± 0.5 |
| 327-20M10 | GTGTCTCAGTACCAGTGTGG | 311-330 | MM | 20 | 55 | 50 | 21.9 ± 0.6 |
| 327-20M20 | GTGTCTCAGTTCCAGTGTGC | 311-330 | MM | 20 | 55 | 50 | 27.5 ± 0.9 |
| 907 probes (low G + C content) | |||||||
| 907-12 | CGTCAATTCATT | 912-923 | PM | 12 | 33 | 44.0 ± 2.4 | |
| 907-16 | GTCAATTCATTTGAGT | 908-923 | PM | 16 | 31 | 58.0 ± 0.6 | |
| 907-20 | CGTCAATTCATTTGAGTTTT | 904-923 | PM | 20 | 30 | 61.2 ± 0.4 | |
Underlined bases represent MM sites with targeted rRNA sequences.
Under the conditions shown here, 50% of the targeted SSU rRNA molecules can be cleaved. These values were determined from the probe dissociation curves in cleavage experiments with E. coli RNA. Td, hybridization or digestion temperature for 50% digestion; Fd, formamide concentration for 50% digestion at 50°C hybridization or digestion temperature.
Based on the probe UNI 530 (28).
Electrophoresis of RNA fragments.
The resultant RNA fragments were electrophoresed by either 1.5% agarose gels (Nusieve 3:1 agarose; BioWhittaker Molecular Applications) or the Agilent 2100 bioanalyzer with the RNA 6000 nano kit (Agilent). For electrophoresis with agarose gels, digested RNA fragments were denatured at 70°C for 2 min, rapidly cooled on ice, and subjected to electrophoresis. Nucleic acids in the gels were stained by CYBER GOLD nucleotide-staining dye (Molecular Probes) to make the RNA bands visible. For electrophoresis with the Agilent 2100 bioanalyzer, the RNA samples and electrophoresis medium (microchips) were prepared according to the manufacturer's instructions. The signal intensities of individual bands in the electropherograms were determined with the software associated with the 2100 bioanalyzer (Bio Sizing; Agilent). Signals were calculated by duplicate determinations in different digestion experiments with different electrophoresis media (the ranges of values obtained from replicate measurements are shown as standard deviations in Fig. 3 to 6 and Table 3).
FIG. 3.
Effect of oligonucleotide type (G+C% and nucleotide length) on the 16S rRNA cleavage reaction. (A) Electropherogram of E. coli RNA digested with the 907-16 probe at 41°C, as resolved by an Agilent 2100 bioanalyzer with an RNA 6000 nano kit. Numbers with arrows indicate approximate estimates of the molecular weight of each peak (unit, nt). A gel-like image of the electropherogram is also shown in the graph; lane 1, RNA 6000 ladder marker (TaKaRa); lane 2, digested E. coli RNA fragments. (B) Temperature dependence of the rRNA cleavage reaction with the 907 probes. Percentages of cleaved 16S rRNA in the total 16S rRNA were directly estimated based on the peak areas of intact and cleaved 16S rRNA fragments in the electro- pherograms, and the percentages were plotted with the hybridization and digestion temperatures at which the respective reactions were performed. Error bars indicate the standard deviation of duplicate determinations. (C) Temperature dependence of the rRNA cleavage reaction with the 530 probes. Percentages of cleaved 16S rRNA in the total 16S rRNA were calculated in the same manner used for the graph in panel B and were plotted along with the hybridization and digestion temperatures used. Error bars indicate the standard deviation of duplicate determinations.
FIG. 6.
Probe dissociation curves of scissor probes under increasingly stringent hybridization and digestion conditions for the cleavage reactions. For each graph, data points indicate percentages of cleaved 16S rRNA in the total 16S rRNA estimated from electropherograms of RNA fragments with duplicate determinations (error bars indicate standard deviations). (A) Probe EUB338, specific for the domain Bacteria; (B) probe ARC915m, specific for the domain Archaea; (C) probe MX825m, specific for the genus Methanosaeta; (D) probe G123T, specific for the genus Thiothrix. In all of the experiments, in vitro-transcribed 16S rRNAs of each representative microbe were used for the digestion. For each probe, the probe sequences and the corresponding target sequences of the 16S rRNA of the tested organisms are indicated; dashes in the nontargeted rRNA sequences represent nucleotides identical to those of the targeted rRNA sequences. The vertical dotted lines indicate the optimum formamide concentrations for individual probes.
TABLE 3.
Comparison of abundances of respective microbial populationsa
| Sample | Quantitative method used | % of total SSU rRNA (mean ± SD) for:b
|
||||
|---|---|---|---|---|---|---|
| Prokaryotes | Bacteria | Archaea | Methanosaetaceae | Thiothrix | ||
| Activated sludge (I) | RNase H method | 92.4 ± 2.6 | 91.2 ± 2.4 | ND | ND | 2.7 ± 0.2 |
| Membrane hybridization | − | − | 0.5 ± 0.1 | 0.4 ± 0.2 | 4.7 ± 1.2 | |
| Activated sludge (II) | RNase H method | 92.6 ± 1.5 | 90.2 ± 2.5 | ND | ND | ND |
| Membrane hybridization | − | − | 0.4 ± 0.1 | ND | 1.3 ± 0.3 | |
| Cow feces (I) | RNase H method | 93.1 ± 3.8 | 89.4 ± 1.8 | ND | ND | ND |
| Membrane hybridization | − | − | ND | ND | ND | |
| Cow feces (II) | RNase H method | 96.4 ± 2.2 | 75.9 ± 3.6 | ND | ND | ND |
| Membrane hybridization | − | − | ND | ND | ND | |
| Digested sewage sludge | RNase H method | 62.6 ± 0.5 | 46.5 ± 2.5 | 15.6 ± 1.7 | 13.8 ± 1.7 | ND |
| Membrane hybridization | − | − | 15.5 ± 2.6 | 11.2 ± 0.6 | 1.2 ± 0.3 | |
| UASB granular sludge | RNase H method | 83.5 ± 0.9 | 62.5 ± 5.4 | 20.9 ± 1.7 | 4.9 ± 0.5 | ND |
| Membrane hybridization | − | − | 20.5 ± 3.2 | 9.3 ± 1.0 | 0.5 ± 0.1 | |
As determined by the RNase H and membrane hybridization methods.
ND, not detected; −, not determined.
Fluorescence in situ hybridization.
The fixation of community samples was performed as described previously (48). Whole-cell in situ hybridization was performed according to a method described previously elsewhere (47, 48). The following Cy-3-labeled, 16S rRNA-targeted oligonucleotide probes were used in this study: ARC915 for the domain Archaea (50), MX825 for the genus Methanosaeta (43), and G123T for the genus Thiothrix (27). For in situ hybridization, we adjusted the stringency of hybridization by adding formamide to the hybridization buffer (20% [vol/vol] for MX825, 35% [vol/vol] for ARC915, and 40% [vol/vol] for G123T). Cells immobilized and hybridized on glass slides were viewed with a fluorescence microscope (BX50F; Olympus).
Quantitative membrane hybridization.
For quantitative membrane hybridization, the following digoxigenin-labeled, 16S (and 18S) rRNA-targeted oligonucleotide probes were used: UNI1390 for all organisms (57), ARC915 for the domain Archaea (50), MX825 for the genus Methanosaeta (43), and G123T for the genus Thiothrix (27). Denatured RNA samples (50 to 500 ng) were applied by slot blotting to Hybond-N+ membranes (Amersham) and were hybridized according to the methods described in previous reports (43, 57). In vitro-transcribed 16S rRNA of E. coli, M. concilii, and T. disciformis was also applied to membranes as standard RNA samples for quantification. After hybridization, the membranes were washed with washing solution (0.9 M NaCl, 1% sodium dodecyl sulfate, 1% blocking reagent [Roche], and formamide) at 46°C for 15 min; this washing step was repeated twice. For washing, we adjusted the stringency of hybridization by adding formamide to the hybridization buffer (0% [vol/vol] for UNIV1390, 20% [vol/vol] for MX825, 35% [vol/vol] for ARC915, and 40% [vol/vol] for G123T). digoxigenin-labeled probes on the membranes were subsequently detected with an ECF chemifluorescence signal amplification kit (Amersham) according to the manufacturer's instructions. Fluorescence signals on the membranes were detected with a confocal laser scanning device (molecular imager FX; Bio-Rad) and were quantified with the software equipped with the device. Each experiment was performed in triplicate.
RESULTS AND DISCUSSION
RNase H has been detected in multiple forms in all organisms that have been studied to date, and it is thought to be involved in DNA replication as well as in cDNA synthesis from retroviral RNA (9, 22, 24). In addition to these roles, RNase H has attracted increased interest regarding the two following issues (24): (i) the presumed role of endogenous RNase H activity in the mechanisms of action of antisense oligonucleotides in vivo and (ii) the potential importance of the enzyme as an antiviral agent. Due to these concerns, the physiological functions and structure of this enzyme derived, in particular, from human immunodeficiency virus type 1 (11), E. coli (28, 29), and Thermus thermophilus (25) have been intensively studied in detail, and the enzyme is now commonly used in molecular biological research, such as in reverse transcription-PCR analyses and in in vitro studies involving the sequence-specific digestion of particular RNA strands (9). In addition, rRNA has been cleaved by using similar methods to create partial fragments of rRNA for in vitro reconstitution studies (1, 7). However, to our knowledge, there has been no report on the use of this enzyme for rRNA-based ecological studies.
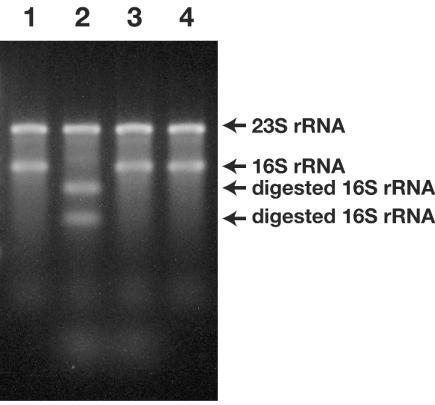
To verify the feasibility of sequence-dependent SSU rRNA scission with RNase H, we first used the total RNA extracted from E. coli cells. Highly purified RNA samples were prepared from exponentially grown cells by use of acid phenol extraction procedures, and the samples were then subjected to digestion with RNase H and an oligonucleotide scissor probe (probe 530-16) (Table 1). Initially, we partially optimized the reaction conditions (e.g., buffer constituents, etc.) for the digestion based on a previous report concerned with the sequence-dependent digestion of RNA with RNase H (13). The cleavage procedures developed here were primarily composed of the following five steps: (i) denaturing rRNA at 95°C for 1 min in a hybridization buffer containing a scissor probe, (ii) maintaining the reaction mixture at a hybridization temperature (normally 50°C) and then subsequently adding a digestion buffer containing RNase H, (iii) digesting rRNA at the hybridization temperature (typically 50°C for 15 min), (iv) stopping the digestion reaction and then the carrying out the subsequent purification of RNA, and (v) performing electrophoresis of the resultant RNA fragments (Fig. 1). Under the appropriate reaction conditions, we confirmed that the site-specific scission of 16S rRNA could occur in the presence of RNase H and the oligonucleotide probe, and we demonstrated that E. coli 16S rRNA was cut into two fragments (Fig. 2). The molecular sizes of the cleaved RNA molecules were approximately 500 and 1,000 nt, suggesting that E. coli 16S rRNA was correctly fragmented at around position 500 of the 16S rRNA when the 530-16 probe was used. No fragmentation of the rRNA was observed when either the RNase H or the oligonucleotide was removed from the digestion buffer (Fig. 2).
FIG. 2.
Cleavage of the 16S rRNA of E. coli with oligonucleotides (probe 530-16) and RNase H, as resolved in 1.5% agarose gel. Lanes: 1, whole E. coli RNA before digestion with RNase H; 2, digested RNA in the presence of oligonucleotides and RNase H; 3, digested RNA in hybridization-digestion buffer from which only RNase H was eliminated; 4, digested RNA in the buffer from which only the oligonucleotides had been removed. Lanes 3 and 4 demonstrate that the rRNA cleavage reaction does not occur in the absence of either the oligonucleotides or the RNase H. The digestion reactions were performed at 55°C for 15 min under the conditions described in Materials and Methods.
Optimization of the reaction conditions for the sequence-specific digestion of rRNA.
To further optimize the reaction conditions, we examined the effects of the respective concentrations of scissor probe, NaCl, and RNase H in the hybridization-digestion buffer, as well as the digestion time and temperature on the specific cleavage of 16S rRNA by using E. coli total RNA.
(i) Sequence-specific digestion effects of the respective concentrations of DNA probe, NaCl, and RNase H in the hybridization-digestion buffer.
Under the defined conditions (500 ng of E. coli RNA and 0.25 U of RNase H in 50 μl of hybridization-digestion buffer [hybridization and digestion at 50°C for 5 to 90 min]), specific but partial cleavage of 16S rRNA was observed within 5 to 30 min of incubation in hybridization-digestion buffer (50 μl) containing 2.5 pmol of the scissor probe 530-16 (data not shown). However, specific and complete cleavage of 16S rRNA was observed in 1 h under these conditions. The optimum cleavage was observed with 50 μl of hybridization-digestion buffer containing 10 to 200 pmol of the scissor probe; this range of probe concentrations resulted in the specific and rapid cleavage of 16S rRNA fragments within 5 min of incubation, and no nonspecific fragmentation was observed, even under conditions of prolonged digestion (>1 h). In contrast, higher concentrations (>250 pmol) of the probe in 50 μl of hybridization-digestion buffer resulted in the slight but nonspecific digestion of the RNA within 1 h of incubation. Under the defined conditions (500 ng of E. coli RNA, 10 pmol of scissor probe, and 0.25 U of RNase H in 50 μl of hybridization-digestion buffer [hybridization and digestion at 50°C for 15 min]), no significant differences in banding patterns of digested RNA were observed with buffer containing 0 to 100 mM NaCl. In addition, no significant changes in banding patterns were found with different RNase H concentrations (0.01 to 5 U/50 μl of hybridization-digestion buffer) under the defined conditions (500 ng of E. coli RNA, 10 pmol of 530-16 probe [hybridization and digestion at 50°C for 15 min], 25 mM NaCl). These data demonstrated that the concentrations of NaCl and RNase H are not crucial factors affecting sequence-specific digestion.
(ii) Reaction temperature for hybridization and digestion.
The temperature used for hybridization and subsequent digestion was found to be an important factor affecting the cleavage reaction. In our procedure, hybridization and digestion were simultaneously performed at the same temperature. Sequence-specific cleavage was observed at a wide range of temperatures, i.e., from 40 up to 70°C, under the defined conditions (500 ng of E. coli RNA, 10 pmol of 530-16 scissor probe, 25 mM NaCl in 50 μl of hybridization-digestion buffer [hybridization and digestion, 15 min]) (data not shown). However, nonspecific fragmentation of the RNAs was observed at hybridization and digestion temperatures above 70°C; moreover, the nonspecific digestion occurred even in the absence of RNase H and the oligonucleotides, suggesting that the nonspecific digestion might be due to physicochemical degradation at high temperatures (possibly due to the presence of MgCl2 in the buffer). To avoid this phenomenon, for further study, we carried out sequence-specific rRNA cleavage reactions at 40 to 60°C for 15 min with a hybridization-digestion buffer (25 mM Tris-HCl [pH 7.5], 1 mM EDTA, 25 mM NaCl, 10 mM MgCl2, 1 mM DTT, 30 ng of BSA/μl, 0.025 U of RNase H/μl) containing the extracted RNAs (10 ng/μl) and each of the oligonucleotide scissor probes (0.2 pmol/μl; total volume, 50 μl).
Effects of the length and the G+C content of scissor probes on the efficiency of specific digestion.
We then examined the effects of the length and the G+C content of scissor probes on the efficiency of specific digestion. To precisely evaluate the effects of oligonucleotide length and the G+C content of the scissor probes in the cleavage reactions, three different sites (around positions 327, 530, and 907) were targeted in E. coli 16S rRNA; the G+C content of these individual sites differs from each other (ranging from approximately 30 to 80%). Position 327 has a G+C content of around 50%, and five scissor probes (12-, 14-, 16-, 18-, and 20-mer oligonucleotides, designated as 327-12, 327-14, 327-16, 327-18, and 327-20 probes, respectively) complementary to this site were made (Table 1). For positions 530 and 907, which possess higher G+C content (approximately 80%) and lower G+C content (approximately 30%), respectively, three scissor probes (12-, 16-, and 20-mer oligonucleotides, designated as 530-12, 530-16, and 530-20 probes for position 530, and 907-12, 907-16, 907-20 probes for site 907, respectively) complementary to each of the sites were employed (Table 1). All probes were used in the cleavage reaction with E. coli RNA at different hybridization temperatures ranging from 40 to 60°C.
When low-percentage G+C probes (i.e., a G+C percentage [G+C%] of 30%, targeting position 907) were used, E. coli 16S rRNA was specifically cleaved at hybridization and digestion temperatures ranging from 40 to 60°C (Fig. 3A and B). A sample electropherogram of the resultant RNA fragments is shown in Fig. 3A. As shown in Fig. 3A, E. coli 16S rRNA was specifically but partially cleaved with scissor probe 907-16 at a hybridization and digestion temperature of 41°C; the resulting RNA banding pattern suggested that 16S rRNA (approximately 1,550 nt) was cut into two fragments (approximately 910 and 610 nt), demonstrating that the cleavage occurred at the correct position (position 907). Based on the electropherogram, the percentage of digested SSU rRNA in the total SSU rRNA was calculated by the following equation: percentage of digested SSU rRNA in the total SSU rRNA = [(peak area of cleaved SSU rRNA band A, i.e., the peak at 612 nt in Fig. 3A) + (peak area of cleaved SSU rRNA band B, i.e., the peak at 907 nt)]/[peak area of all bands derived from SSU rRNA (= the sum of peak areas of band A, band B, and the intact SSU rRNA band, i.e., the peak at 1548 nt)] × 100. According to this equation, we calculated the digestion efficiencies (percentage of digested SSU rRNA) at different hybridization and digestion temperatures. As shown in Fig. 3B, when scissor probe 907-20 was used, the plotted line (i.e., the “cleavage curve”) was similar to typical probe dissociation (melting) curves, as has often been indicated in DNA probe evaluations (17, 27); in other words, E. coli 16S rRNA was completely digested at hybridization and digestion temperatures ranging from 40 to 50°C, whereas it was only partially digested at hybridization and digestion temperatures higher than 50°C (Fig. 3B). Complete, sequence-specific cleavage of rRNA occurred at 50 to 60°C with other oligonucleotides such as the 530-16 probe (as mentioned above). Thus, it was thought that the decrease in digestion efficiency at high temperatures (i.e., the decrease in the percentage of fragmented rRNA in the total 16S rRNA with increases in the reaction temperature) in the case of the 907-20 probe was not due to a loss of enzyme activity (RNase H) at high temperatures but instead was due to the dissociation of oligonucleotide-rRNA duplexes. This finding also indicated that the stringency of hybridization between oligonucleotides and rRNA can be controlled by controlling the reaction temperature (hybridization and subsequent digestion temperature) during the digestion. However, reactions with probes 907-12 and 907-16 resulted in only the partial cleavage of 16S rRNA at all tested temperatures (40 to 60°C) (Fig. 3B). As will be discussed in more detail below, this result was probably due to incomplete heteroduplex formation between the 16S rRNA and relatively short oligonucleotides.
When neutral G+C% probes (327 probes; G+C% = 50%) were used, specific and complete cleavage of E. coli 16S rRNA molecules was found at hybridization and digestion temperatures ranging from 45 to 60°C with 327-16, 327-18, and 327-20 probes, whereas probes 327-12 and 327-14 allowed only the partial cleavage of 16S rRNA at all temperatures tested (40 to 60°C) (data not shown). Temperatures lower than 45°C showed insufficient cleavage of 16S rRNA with scissor probes 327-16, 327-18, and 327-20 (cleaved rRNA was 80 to 90% of the 16S rRNA).
When high G+C% probes (530 probes; G+C% = 80%) were used, all E. coli 16S rRNA was specifically digested at hybridization and digestion temperatures ranging from 50 to 60°C (with probe 530-16) and from 55 to 60°C (with probe 530-20) (Fig. 3C). As was seen with the probes for position 327, temperatures lower than the above values resulted in the incomplete digestion of 16S rRNA. In addition, a short-length scissor probe (i.e., a 12-mer probe) was unable to completely cleave 16S rRNA at any of the temperatures examined (40 to 60°C) (Fig. 3C); these findings may imply that the heteroduplex formation that occurred between the scissor probes and the rRNA under these conditions was unstable.
These examinations demonstrated that both the length and the G+C content of a scissor probe were significant factors affecting the rRNA cleavage reaction. The effects of these factors can be clearly accounted for by consideration of the commonly known characteristics of DNA-DNA and DNA-RNA duplex formation (49); hence, it was concluded that the reaction stringency could be controlled in a similar manner to that used for several rRNA-based molecular techniques, including fluorescence in situ hybridization and membrane hybridization (3, 4, 43, 50).
As indicated by some of the oligonucleotide scissor probes tested, hybridization and digestion of rRNA at low temperatures (40 to 50°C) often exhibited lower rates of rRNA fragmentation than those exhibited at higher temperatures (50 to 60°C). A similar phenomenon was also reported in the site-specific cleavage of MS2 RNA with RNase H, the tertiary structure of which is known to be stable (8). This fact may imply that DNA-RNA heteroduplex formation was hindered by the secondary and tertiary structural conformations of rRNA under low-temperature conditions. In fact, similar findings have been reported in whole-cell hybridization studies showing that probe accessibility is often limited at lower hybridization temperatures (17).
Effect of formamide in the hybridization-digestion buffer on the efficiency of specific digestion.
To accurately cleave targeted rRNA in mixed RNA extracts, single-base-mismatch discrimination between the rRNA and the scissor probe is a crucial goal of this method. In the case of some scissor probes possessing shorter nucleotides or lower G+C content, we were able to optimize the conditions by simply increasing the reaction temperature after determining the dissociation temperature (Td) (°C) for each probe, at which 50% of the targeted rRNA was cleaved (Table 1). However, since the specific and complete digestion of the targeted 16S rRNA was obtained at 60°C with several probes (such as probes 530-16, 530-18, and 530-20) (Fig. 3C), a further increase in the hybridization stringency was impossible due to the upper limit (60°C) of the reaction temperature in the RNase H digestion procedures (see above). To overcome this obstacle, we attempted to modify the cleavage reaction conditions and eventually found that the use of formamide as a denaturing agent provided a simple solution to this problem. As shown in Fig. 4, the addition of formamide in the hybridization-digestion buffer enabled a further increase in hybridization stringency without any loss of RNase H activity; i.e., the cleavage curves with scissor probes 327-16, 327-18, and 327-20 showed typical probe dissociation curves. We defined the formamide concentration in the buffer as Fd (percentage at a hybridization and digestion temperature of 50°C), at which 50% of the sequence-specific cleavage of 16S rRNA occurs, and we then determined the Fd values for each probe. We obtained highly reproducible Fd values for each probe, and different Fd values were clearly found among different probes (Table 1). In all cases, shorter oligonucleotides exhibited lower Fd values than those of long oligonucleotides (Fig. 4 and Table 1). In addition, high G+C probes exhibited higher Fd values in general (Table 1); all of these Fd values suggested that the cleavage curves occurred in a sequence-dependent hybridization manner, even in the presence of formamide. The present results therefore suggest that the addition of formamide can effectively be used to adjust the stringency of hybridization in the case of RNase H digestion as well, instead of increasing the digestion temperature to higher than 60°C.
FIG. 4.
Effect of formamide concentration in hybridization-digestion buffer on rRNA scission. E. coli total RNA was cleaved with 327 scissor probes by using hybridization-digestion buffer containing different concentrations of formamide (%) (hybridization-digestion temperature, 50°C). Top panel, gel-like images of electropherograms of E. coli RNA cleaved with the 327-18 probe with different formamide concentrations. Bottom panel, formamide dependence of the rRNA cleavage reaction with the 327 probes. The percentages of cleaved 16S rRNA of the total 16S rRNA were estimated as described in the legend of Fig. 3 and were plotted together with the formamide concentrations used for creating the hybridization-digestion buffer. Error bars indicate the standard deviation of duplicate determinations.
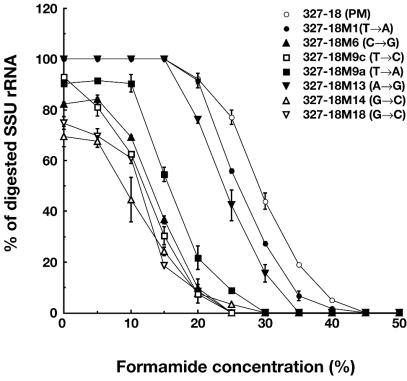
Effect of mismatch bases in scissor probe-rRNA heteroduplexes on the efficiency of specific digestion.
We then evaluated the effects of mismatched bases in oligonucleotide-rRNA heteroduplexes. For resolving perfect-match (PM) and single-base-mismatch (MM) complexes, we constructed 11 different probes containing MM at various sites with different forms based on 327-16, 327-18, and 327-20 probes (Table 1). Examples of the 16S rRNA cleavage curves, along with those obtained with the addition of formamide, are shown in Fig. 5, which indicates that almost all of the MM probes were clearly distinguished from the PM probe (327-18). Almost all MM probes exhibited >0% lower Fds than that of the PM probe. However, two MM probes (327-M13 and 327-M1 probes) showed similar cleavage curves and Fds close to those of the PM probe. The probe 327-M13 contained a single-base mismatch (i.e., an A→G change in the base) at position 13 in the oligonucleotide (Table 1); the change resulted in the formation of a G:U pair instead of an A:U pair at position 13 of the oligonucleotide-rRNA duplex. It has been well recognized that the G:U pair can form a nearly isomorphic bond between nucleotides to Watson-Crick base pairs and that this pair often plays a role as an alternative base pairing in tRNA and rRNA structures (19, 54). Therefore, the imprecise discrimination between the PM probe and 327-M13 in RNase H digestion can be explained by the nature of the weak bond between 327-M13 and 16S rRNA at the MM base. Another example that demonstrated unclear discrimination from a PM probe was the 327-M1 probe. The probe contained a single-base mismatch at position 1 (5′ terminal) of the oligonucleotide (i.e., a T→A change in the base). In this case, we could not clearly identify the reasons why such imprecise discrimination occurred. However, this finding suggested that the single-base mismatch at the 5′ terminus of oligonucleotides with nontargeted species of rRNA should be avoided in the development of scissor probes to be used for RNase H digestion.
FIG. 5.
Effect of single-base mismatches between oligonucleotides and E. coli 16S rRNA on the rRNA scission reaction. A PM probe (327-18) and MM probes containing a single-base MM at different positions and of different types were used for the cleavage of E. coli whole RNA at different formamide concentrations.
Regarding the length of scissor probes, we found that 18-mer probes showed better discrimination between the PM probe and MM probes compared to longer scissor probes (i.e., 20-mer probes) (Table 1). This result was likely due to the relatively stable heteroduplex formation between the longer oligonucleotides and rRNA. This finding suggested that scissor probes should be shorter than 20 mer for the precise detection of targeted rRNA in the present method.
Evaluation of scissor probes for different microbial groups.
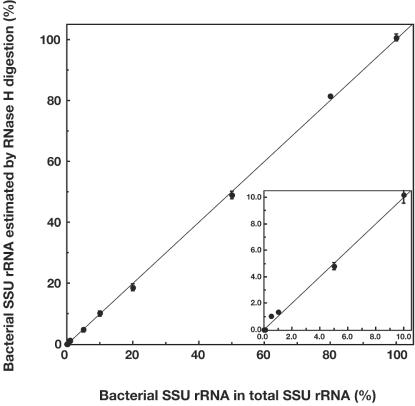
We then evaluated oligonucleotide scissor probes which target 16S rRNA sequences of important groups of microbes, i.e., the prokaryotes (including Bacteria and Archaea) (31), the domain Bacteria (5), the domain Archaea (50), the family Methanosaetaceae (43), and the genus Thiothrix (27) (Table 2) for use in our sequence-dependent cleavage method. The nucleotide sequences of the UNI530 probe for virtually all prokaryotes, the EUB338 probe for Bacteria, and the G123T probe for Thiothrix were obtained from previous studies and were directly used as scissor probes. The ARC915m probe for Archaea and the MX825m for Methanosaeta were developed in this study, with slight modifications of the previously published oligonucleotide probes ARC915 and MX825, respectively; the original probes were shortened to 18-mer probes according to the guidelines for creating scissor probes used in this study (see above). The 16S rRNAs of E. coli, T. disciformis, M. concilii, Methanosarcina barkeri, and L. pneumophila were synthesized in vitro with T7 RNA polymerase and were subjected to cleavage reaction with the scissor probes at different concentrations of formamide in hybridization-digestion buffer. The probe dissociation curves of individual probes are shown in Fig. 6; using these curves, we determined the optimum formamide concentration at which each scissor probe would specifically cleave only the targeted 16S rRNA. For example, the EUB338 probe could cleave only bacterial 16S rRNA at a formamide concentration of 20% (Fig. 6A). Under these conditions, 96% of the total E. coli 16S rRNA was digested reproducibly; this value remained constant throughout the range of concentrations of E. coli rRNA that was tested (50 to 500 ng in a 50-μl reaction mixture; data not shown). Therefore, we defined the optimum formamide concentration to be 20%, and a coefficient value (i.e., a “cleavage coefficient”) of 0.96 was employed for estimating the amount of targeted rRNA in complex rRNA mixtures. To judge whether or not this approach (i.e., the use of formamide and a cleavage coefficient) would be feasible for mixed rRNA samples containing different rRNA molecules, we then applied this protocol to model rRNA mixtures. For the quantitative detection of targeted species of rRNA molecules in mixed 16S rRNA transcripts, the known amounts of transcribed 16S rRNA of E. coli and M. concilii were mixed to give a range of different ratios of E. coli 16S rRNA to M. concilii 16S rRNA (0 to 100% of E. coli rRNA in the total rRNA) (Fig. 7). The mixed rRNAs were subjected to a cleavage reaction with the EUB338 scissor probe in hybridization-digestion buffer containing 20% formamide. During digestion, the amount of fragmented rRNA was directly measured by an electropherogram, and the results were subsequently corrected with a coefficient value of 0.96 (see above) to estimate the actual values of the proportions; i.e., the measured values were divided by the coefficient to give the actual estimates. The data in Fig. 7 clearly demonstrated that this method could be used for the quantitative determination of specific 16S rRNA molecules; the experimentally estimated ratios correlated well with the actual ratios (at least within a range of 0.5% to 100%), and the estimated values were highly reproducible upon duplicate examination.
FIG. 7.
Quantitative detection of bacterial (E. coli) 16S rRNA molecules with the sequence-dependent rRNA cleavage method in artificially mixed 16S rRNA transcripts containing E. coli 16S rRNA and M. concilii 16S rRNA. Probe EUB338 was used as the scissor probe with a formamide concentration of 20% for the hybridization-digestion buffer. Defined (actual) percentages of E. coli rRNA in the total rRNA are plotted along the x axis, whereas the measured values of the percentages obtained by the present methods are shown along the y axis. The values on the y axis were estimated from the electropherograms of digested RNA, with corrections made with a cleavage coefficient of 0.96.
These examinations led us to conclude that the RNase H cleavage method developed here is applicable for the quantitative measurement of specific 16S rRNA fragments in complex rRNA samples. More importantly, it was clearly demonstrated that the stringency of the cleavage reactions could be properly controlled by using formamide and a cleavage coefficient for the precise and quantitative discrimination of different species of rRNA. The findings also revealed that previously published oligonucleotide probes could be employed directly, or with slight modifications, when used in this method. We optimized the conditions for the specific cleavage of rRNA of prokaryotes, Bacteria, Archaea, Methanosaeta, and Thiothrix, as summarized in Table 2, and we then used the probes for further analysis of actual community samples.
Quantitative detection of various microbial groups in complex ecosystems.
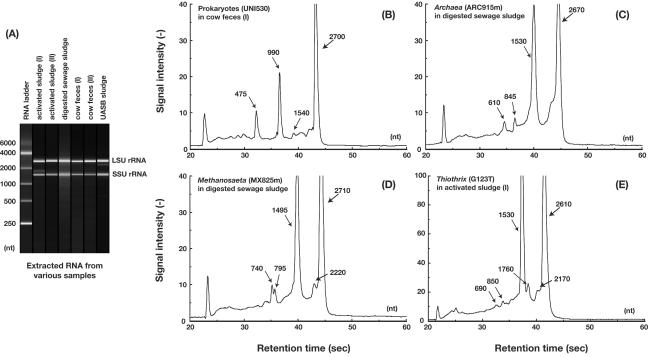
By using acid phenol extraction and DNase treatment procedures, we prepared highly intact total RNAs from activated sludge treating sewage, from anaerobic sludges treating waste or wastewater, and from cow feces. In all cases, sharp RNA bands corresponding to 16S rRNA and 23S rRNA were visualized upon electrophoresis. In some samples, low-molecular-weight RNA-like bands were observed upon gel electrophoresis (data not shown), but such substances could be removed by using an RNA or DNA purification column (see Materials and Methods). Consequently, highly purified RNAs were successfully obtained from all samples (Fig. 8A). Similarly, the direct ribosome extraction method developed by Felske et al. (16) may also be used for this purpose.
FIG. 8.
Application of the sequence-specific rRNA cleavage method to quantify microbes in actual community samples. (A) Gel-like images of total RNA extracted from various community samples showing virtually intact rRNA peaks, as resolved by an Agilent 2100 bioanalyzer. (B to E) Electropherograms of community RNAs digested with group-specific scissor probes and RNase H. Digestion of total RNA from cow feces (I) with the UNI530 probe specific for virtually all prokaryotes (B), from digested sewage sludge with the ARC915m probe specific for Archaea (C), from digested sewage sludge with the MX825m probe specific for the genus Methanosaeta (D), and from activated sludge (I) with the G123T probe specific for the genus Thiothrix (E) are shown. Numbers with arrows indicate approximate estimates of the molecular size (in nucleotides) of each peak.
By using the total extracted RNA, we measured the abundance of populations of prokaryotes, Bacteria, Archaea, Methanosaeta, and Thiothrix with this 16S rRNA-based RNase H digestion method (Fig. 8 and Table 3). Figure 8B to E shows examples of the resultant RNA fragment patterns with various oligonucleotides and actual RNA samples.
(i) Quantitative detection of prokaryotic populations.
By using the UNI530 probe specific for the 16S rRNA of virtually all prokaryotes, almost all of the SSU rRNA was cleaved into two fragments (Fig. 8B). The resulting fragments (corresponding to 500 and 1,000 nt of the RNA fragments) were in good accordance with the predicted fragments generated with the probe, suggesting that the targeted rRNA was precisely cut into two segments. By this cleavage method, the total prokaryote populations were estimated to account for 63 to 96% of the total organisms present in all of the samples, based on SSU rRNA. Interestingly, all of the samples had certain fractions of 16S rRNA which were not cleaved with the UNI530 probe, even if the correction with cleavage coefficients was taken into consideration; this finding may indicate that all of the samples contained some 16S rRNA molecules that do not hybridize with the prokaryote-universal probe. It may also be possible that certain rRNA molecules hinder the access of an oligonucleotide and/or RNase H to the targeted site, depending on the respective tertiary structure of the rRNA, even though the targeted site is complementary to the oligonucleotide used. Surprisingly, only 63% of the total SSU rRNA from anaerobically digested sewage sludge could be digested with the universal probe.
(ii) Detection of bacterial and archaeal populations.
The abundance of bacterial 16S rRNA was estimated to be 47 to 91% of the total SSU rRNA in all RNA samples with the EUB338 probe (Table 3). In contrast, archaeal rRNAs were detected only in digested sewage sludge (16% of total SSU rRNA) (Fig. 8C) and anaerobic sludge (UASB granular sludge; 21% of the total SSU rRNA) (Table 3). To compare the data with the estimation by a more standard hybridization method, quantitative membrane hybridization was performed for the same RNA samples (Table 3). The abundance of archaeal populations estimated by the membrane hybridization method are in good agreement with the data obtained by the RNase H cleavage method (Table 3), suggesting that the present method is comparable with other established methods in terms of the quantitativeness of a particular population in actual samples. In addition, fluorescence in situ hybridization with the ARC915 probe, specific for Archaea, showed similar population structures within the samples, as measured by the present cleavage method (data not shown). The data obtained by the present method were also in good agreement with findings from other reports on community analyses of similar samples (20, 42, 44, 48).
(iii) Detection of Methanosaeta populations.
Members of Methanosaeta, which are well-recognized aceticlastic methanogens (26, 39), were also examined by using the MX825m probe for all samples; peaks at the predicted positions of the rRNA fragments in the electropherogram were detected only in the digested sewage sludge and anaerobic wastewater sludge samples (Fig. 8D and Table 3). The abundance of these populations was calculated to be 5 to 14% of the total populations, based on rRNA. These estimates were in good agreement with the data obtained by the membrane hybridization method (Table 3). In addition, the two anaerobic samples showed a number of MX825-positive rods when they were examined with fluorescence in situ hybridization (data not shown). Methanosaeta species were frequently detected in molecular ecological surveys of anaerobic sludge such as UASB granular sludge and digested sewage sludge (20, 42, 44, 46, 47, 51). Therefore, these values were within the range of the known abundances of Methanosaeta reported from previous observations, and these results very likely reflect the actual population (activity) sizes in the present samples.
(iv) Detection of Thiothrix populations.
Members of the genus Thiothrix are recognized as potential bulking agents in activated sludge systems, and this genus is known for its unique multicellular filamentous morphology, previously identified as Eikelboom type 021N (14, 27). The oligonucleotide probe (G123T) targeting the 16S rRNA of the Thiothrix species was used for the quantitative detection of Thiothrix populations in all of the RNA samples. Since several other microorganisms such as L. pneumophila were found to have similar 16S rRNA sequences within the targeted site (single-base MM) (27), a high-stringency condition was employed for resolving the targeted rRNA from the MM sequences. As shown in Fig. 6D, we determined the optimum formamide concentration (30%) required to specifically digest the target rRNA, although we had to sacrifice the complete digestion of the targeted rRNA (cleavage coefficient, 0.55) (Table 2). We applied this scissor probe to activated sludge samples under the optimized conditions. As shown in Fig. 8E, two peaks corresponding to approximately 690- and 850-nt RNA fragments were detected in the activated sludge (I), whereas these peaks were not seen in the RNase H digestion of the same RNA sample without the addition of a scissor probe (data not shown). Since the scissor probe G123T was expected to hybridize at around position 700 of the 16S rRNA (697 to 714 in the E. coli 16S rRNA numbering system), the two peaks were highly likely to be the rRNA fragments formed by the correct cleavage of the targeted rRNA. According to our cleavage analyses, the abundance of the targeted rRNA of Thiothrix species in activated sludge (I) was estimated to be approximately 3% of the total SSU rRNA (Table 3; the value was estimated by correction with a cleavage coefficient of 0.55). In fact, fluorescence in situ hybridization with the Cy-3-labeled G123T probe (27) revealed that a number of Thiothrix-like G123T-positive filaments were present in activated sludge (I) (Table 3). In contrast, G123T-reactive filaments were rarely found upon fluorescence in situ hybridization of the activated sludge sample (II), which was consistent with the data obtained from the sequence-specific cleavage of the rRNA. In addition, few G123T probe-positive cells were detected upon whole-cell hybridization in the case of the other actual community samples (data not shown). Similarly, membrane hybridization data suggested the same magnitudes of the abundance of these populations in respective samples (Table 3). The activated sludge (I) did not show a severe bulking phenomenon, but it did have had good settling properties; thus, the data indicated that Thiothrix populations are present in small amounts in typical activated sludges (<3%, based on rRNA).
All of these examinations suggested that the present method was applicable to actual complex ecosystems. Because this method does not require an external RNA standard for each experiment, as is required for membrane hybridization, and because the present method is relatively easy to perform within a short period of time (i.e., within 2 h), this technique may provide direct, rapid, and easy means of quantitatively detecting particular groups of microbes based on their rRNA. RNA-dependent community analysis is known to indicate the in situ activity of individual members in ecosystems for the following reasons: (i) RNA synthesis is known to reflect the in situ growth rates of organisms (6, 41), and (ii) the turnover of RNA is thought to be much higher than that of DNA. Therefore, rRNA-dependent molecular techniques like the present one provide precise information about the dynamic nature of individual microbes in systems. The rRNA cleavage method developed here has the potential to facilitate rapid and easy evaluation of individual rRNAs for real-time measurements. Therefore, a wide range of applications of this method will contribute to a better understanding of the active and dynamic populations in particular environments. In addition, the present method can also be applied for separating particular rRNA molecules of interest (e.g., isolation of rRNA molecules that are cleaved by scissor probes targeting specific groups or isolation of molecules that are not cleaved by scissor probes targeting general groups). Potential fields of study that may benefit from this technology might be those involving stable isotope probing based on rRNA (34, 35); such studies would aim to explore the functions of uncultured organisms.
Drawbacks of the present cleavage method.
The method we developed here has two major drawbacks: (i) intact and high-purity RNA samples, in which sharp rRNA bands can be seen, must be prepared from fresh samples, and (ii) rRNAs from low-abundance microbial populations (<1 to 2% of the total SSU rRNA) cannot be clearly detected. The quality of the RNA samples is the most critical factor when using this method. For example, degradation of rRNA in samples prior to cleavage led to a higher background of RNA banding patterns in the electropherograms, which caused problems in distinguishing digested rRNA fragments from background signals. Therefore, the quality of the RNA should be very carefully taken into consideration when using the present method. In addition, the detection of rRNA from low-abundance microbial populations may be, to some extent, limited for the following reasons: (i) short fragments of RNA (digested rRNA fragments) are relatively difficult to detect when compared to the same moles of large RNA molecules (intact 16S rRNA) stained with nucleotide-binding fluorescence dyes, and (ii) from nt 200 to 1500, several RNA fragments such as mRNAs are usually contained in certain amounts, and these give a smear background in the RNA banding pattern. Some of these obstacles might be overcome by improving the experimental procedures, for example, by enriching only SSU rRNA fragments from whole RNA samples or by labeling either of the termini of SSU rRNA with fluorescence dyes. Further improvements should be incorporated into the present method to achieve a more clear and sensitive detection of rRNA fragments. Methods for preserving RNA molecules in preserved specimens should be also developed.
Conclusions.
In this study, we have developed a novel method for the detection and quantification of microorganisms based on the sequence-specific digestion of microbial SSU rRNA using DNA probes (scissor probes) and RNase H. Sequence-specific SSU rRNA cleavage of particular groups at a range of phylogenetic levels as well as the quantitative detection of targeted rRNA molecules were successfully achieved using these experimental protocols. The molecular method proposed here was fully applicable to actual ecosystems containing complex microbial communities, providing reproducible data regarding quantitative measurements of specific microbial populations based on their rRNA. Of particular interest in this context are the following advantages of this method: (i) the experimental procedures are very simple to carry out and can be completed within less than 3 h, (ii) the present method requires only a thermal controller and an electrophoresis unit, and (iii) the method does not require an external RNA standard in each experiment for the quantitative estimation of targeted RNA (as is required for membrane hybridization) and can easily accomplish the calculation of the proportion of targeted SSU rRNA relative to the total SSU rRNA. In this regard, this approach has the potential to provide a fast and easy means for the quantitative detection and identification of microbes of interest as is required in clinical, pharmaceutical, and environmental microbiology.
REFERENCES
- 1.Afonina, E. I., N. V. Chichkova, and A. A. Bogdanov. 1991. RNA-RNA and RNA-protein interactions in 30S ribosomal subunits: association of 16S rRNA fragments in the presence of ribosomal proteins. FEBS Lett. 283:251-254. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R., B. M. Fuchs, and S. Behrens. 2001. The identification of microorganisms by fluorescence in situ hybridization. Curr. Opin. Biotechnol. 12:231-236. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R., and W. Ludwig. 2000. Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol. Rev. 24:555-565. [DOI] [PubMed] [Google Scholar]
- 4.Amann, R. I. 1995. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes, p. 1-15. In A. D. L. Akkermans and J. D. van Elass (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, London, United Kingdom.
- 5.Amann, R. I., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdanov, A. A., N. V. Chichkova, A. M. Kopylov, A. S. Markin, and E. A. Skripkin. 1988. Surface topography of ribosomal RNA. Methods Enzymol. 164:440-456. [DOI] [PubMed] [Google Scholar]
- 8.Chon, H., Y. Tsunaka, M. Haruki, M. Morikawa, and S. Kanaya. 2002. Site-specific cleavage of MS2 RNA by a thermostable DNA-linked RNase H. Protein Eng. 15:683-688. [DOI] [PubMed] [Google Scholar]
- 9.Crouch, R. J., and J. J. Toulmé. 1998. Ribonucleases H. Les Editions INSERM, Paris, France.
- 10.Daims, H., N. B. Ramsing, K. Schleifer, and M. Wagner. 2001. Cultivation-independent, semiautomatic determination of absolute bacterial cell numbers in environmental samples by fluorescence in situ hybridization. Appl. Environ. Microbiol. 67:5810-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, J. F., Z. Hostomska, Z. Hostomsky, S. Jordan, and D. A. Matthews. 1991. Crystal structure of the RNase H domain of HIV-1 reverse transcriptase. Science 252:88-95. [DOI] [PubMed] [Google Scholar]
- 12.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donis-Keller, H. 1979. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 7:179-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikelboom, D. H. 1975. Filamentous organisms observed in activated sludge. Water Res. 9:365-388. [Google Scholar]
- 15.Fantroussi, S. E., H. Urakawa, A. E. Bernhard, J. J. Kelly, P. A. Noble, H. Smidt, G. M. Yershov, and D. A. Stahl. 2003. Direct profiling of environmental microbial populations by thermal dissociation analysis of native rRNAs hybridized to oligonucleotide microarrays. Appl. Environ. Microbiol. 69:2377-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felske, A., B. Engelen, U. Nübel, and H. Backhaus. 1996. Direct ribosome isolation from soil to extract bacterial rRNA for community analysis. Appl. Environ. Microbiol. 62:4162-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guschin, D. Y., B. K. Mobarry, D. Proudnikov, D. A. Stahl, B. E. Rittmann, and A. D. Mirzabekov. 1997. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl. Environ. Microbiol. 63:2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutell, R. R., N. Larsen, and C. R. Woese. 1994. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol. Rev. 58:10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmsen, H. J. M., K. M. P. Kengen, A. D. L. Akkermans, A. J. M. Stams, and W. M. de Vos. 1996. Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl. Environ. Microbiol. 62:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen, H. J. M., D. Prieur, and C. Jeanthon. 1997. Group-specific 16S rRNA-targeted oligonucleotide probes to identify thermophilic bacteria in marine hydrothermal vents. Appl. Environ. Microbiol. 63:4061-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausen, P., and H. Stein. 1970. Ribonuclease H: an enzyme degrading the RNA moiety of DNA-RNA hybrids. Eur. J. Biochem. 14:278-283. [DOI] [PubMed] [Google Scholar]
- 23.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15:210-213. [DOI] [PubMed] [Google Scholar]
- 24.Hostomsky, Z., Z. Hostomska, and D. A. Matthews. 1993. Ribonucleases H. In S. M. Linn, R. S. Lloyd, and R. J. Roberts (ed.), Nucleases, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Ishikawa, K., M. Okumura, K. Katayanagi, S. Kimura, S. Kanaya, H. Nakamura, and K. Morikawa. 1993. Crystal structure of ribonuclease H from Thermus thermophilus HB8 at 2.8 Å resolution. J. Mol. Biol. 230:529-542. [DOI] [PubMed] [Google Scholar]
- 26.Kamagata, Y., H. Kawasaki, H. Oyaizu, K. Nakamura, E. Mikami, G. Endo, Y. Koga, and K. Yamasato. 1992. Characterization of three thermophilic strains of Methanothrix (“Methanosaeta”) thermophila sp. nov. and rejection of Methanothrix (“Methanosaeta”) thermoacetophila. Int. J. Syst. Bacteriol. 42:463-468. [DOI] [PubMed] [Google Scholar]
- 27.Kanagawa, T., Y. Kamagata, S. Aruga, T. Kohno, M. Horn, and M. Wagner. 2000. Phylogenetic analysis of and oligonucleotide probe development for Eikelboom type 021N filamentous bacteria isolated from bulking activated sludge. Appl. Environ. Microbiol. 66:5043-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanaya, S., A. Kohara, M. Miyagawa, T. Matsuzaki, K. Morikawa, and M. Ikehara. 1989. Over-production and preliminary crystallographic study of ribonuclease H from Escherichia coli. J. Biol. Chem. 264:11546-11549. [PubMed] [Google Scholar]
- 29.Katayanagi, K., M. Miyagawa, M. Matsushima, M. Ishikawa, S. Kanaya, H. Nakamura, M. Ikehara, T. Matsuzaki, and K. Morikawa. 1992. Structural details of ribonuclease H from Escherichia coli as refined to an atomic resolution. J. Mol. Biol. 223:1029-1052. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi, Y., J. J. Kelly, T. Nakagawa, H. Urakawa, S. El-Fantroussi, S. Al-Muzaini, M. Fukui, Y. Urushigawa, and D. A. Stahl. 2002. Parallel characterization of anaerobic toluene- and ethylbenzene-degrading microbial consortia by PCR-denaturing gradient gel electrophoresis, RNA-DNA membrane hybridization, and DNA microarray technology. Appl. Environ. Microbiol. 68:3215-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 32.Liu, W., A. D. Mizabekov, and D. A. Stahl. 2001. Optimization of an oligonucleotide microchip for microbial identification studies: a non-equilibrium dissociation approach. Environ. Microbiol. 3:619-629. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüßmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. P. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K.-H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacGregor, B. J., V. Brüchert, S. Fleischer, and R. Amann. 2002. Isolation of small-subunit rRNA for stable isotopic characterization. Environ. Microbiol. 4:451-464. [DOI] [PubMed] [Google Scholar]
- 35.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA of stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon, K. D., D. A. Stahl, and L. Raskin. 1998. A comparison of the use of in vitro-transcribed and native rRNA for the quantification of microorganisms in the environment. Microb. Ecol. 36:362-371. [DOI] [PubMed] [Google Scholar]
- 37.Moran, M., V. L. Torsvik, T. Torsvik, and R. E. Hodsen. 1993. Direct extraction and purification of rRNA for ecological studies. Appl. Environ. Microbiol. 59:915-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogram, A., W. Sun, F. J. Brockman, and R. E. Fredrickson. 1995. Isolation and characterization of RNA from low-biomass deep-subsurface sediments. Appl. Environ. Microbiol. 61:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel, G. B., and G. D. Sprott. 1990. Methanosaeta concilii gen. nov., sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., comb. nov. Int. J. Syst. Bacteriol. 40:79-82. [Google Scholar]
- 40.Polz, M. F., and C. M. Cavanaugh. 1997. A simple method for quantification of uncultured microorganisms in the environment based on in vitro transcription of 16S rRNA. Appl. Environ. Microbiol. 63:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulsen, L., G. Ballard, and D. A. Stahl. 1993. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl. Environ. Microbiol. 29:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raskin, L., L. Poulsen, D. R. Noguera, B. E. Rittmann, and D. A. Stahl. 1994. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 60:1241-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raskin, L., J. M. Stromley, B. E. Rittmann, and D. A. Stahl. 1994. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 60:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raskin, L., D. Zhang, M. E. Griffin, P. G. Stroot, and P. Misra. 1995. Characterization of microbial communities in anaerobic bioreactors using molecular probes. Antonie Leeuwenhoek 68:297-308. [DOI] [PubMed] [Google Scholar]
- 45.Risatti, J., W. C. Capman, and D. A. Stahl. 1994. Community structure of a microbial mat: the phylogenetic dimension. Proc. Natl. Acad. Sci. USA 91:10173-10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocheleau, S., C. W. Greer, J. R. Lawrence, C. Cantin, L. Laramee, and S. R. Guiot. 1999. Differentiation of Methanosaeta concilii and Methanosarcina barkeri in anaerobic mesophilic granular sludge by fluorescent in situ hybridization and confocal scanning laser microscopy. Appl. Environ. Microbiol. 65:2222-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekiguchi, Y., Y. Kamagata, K. Nakamura, A. Ohashi, and H. Harada. 1999. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl. Environ. Microbiol. 65:1280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekiguchi, Y., Y. Kamagata, K. Syutsubo, A. Ohashi, H. Harada, and K. Nakamura. 1998. Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 144:2655-2665. [DOI] [PubMed] [Google Scholar]
- 49.Stahl, D. A., and R. I. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 50.Stahl, D. A., B. Flesher, H. R. Mansfield, and L. Montgomery. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tagawa, T., K. Syutsubo, Y. Sekiguchi, A. Ohashi, and H. Harada. 2000. Quantification of methanogen cell density in anaerobic granular sludge consortia by FISH. Water Sci. Technol. 42:77-82. [Google Scholar]
- 52.Urakawa, H., S. E. Fantroussi, H. Smidt, J. C. Smoot, E. H. Tribou, J. J. Kelly, P. A. Noble, and D. A. Stahl. 2003. Optimization of single-base-pair mismatch discrimination in oligonucleotide microarrays. Appl. Environ. Microbiol. 69:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urakawa, H., P. A. Noble, S. E. Fantroussi, J. J. Kelly, and D. A. Stahl. 2002. Single-base-pair discrimination of terminal mismatches by using oligonucleotide microarrays and neural network analysis. Appl. Environ. Microbiol. 68:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varani, G., and W. H. McClain. 2000. The G•U wobble base pair. EMBO Rep. 1:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallner, G., R. Erhart, and R. Amann. 1995. Flow cytometric analysis of activated sludge with rRNA-targeted probes. Appl. Environ. Microbiol. 61:1859-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng, D., E. W. Alm, D. A. Stahl, and L. Raskin. 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 62:4504-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]