Abstract
Objective
Parenteral nutrition associated liver disease (PNALD) is a deadly complication of long term parenteral nutrition (PN) use in infants. Fish oil-based lipid emulsion has been shown in recent years to effectively treat PNALD. Alternative fat sources free of essential fatty acids have recently been investigated for health benefits related to decreased inflammatory response. We hypothesized that the addition of medium-chain triglycerides (MCT) to a purified fish oil-based diet would decrease the response to inflammatory challenge in mice, while allowing for sufficient growth and development.
Materials/Methods
Six groups of ten adult male C57/Bl6 mice were pair-fed different dietary treatments for a period of twelve weeks, varying only in fat source (percent calories by weight): 10.84% soybean oil (SOY), 10% coconut oil (HCO), 10% medium-chain triglycerides (MCT), 3% purified fish oil (PFO), 3% purified fish oil with 3% medium-chain triglycerides (50:50 MCT:PFO) and 3% purified fish oil with 7.59% medium-chain triglycerides (70:30 MCT:PFO). An endotoxin challenge was administered to half of the animals in each group at the completion of dietary treatment.
Results
All groups demonstrated normal growth throughout the study period. Groups fed MCT and HCO diets demonstrated biochemical essential fatty acid deficiency and decreased IL-6 and TNF-α response to endotoxin challenge. Groups containing PFO had increased inflammatory response to endotoxin challenge, and the addition of MCT to PFO mitigated this inflammatory response.
Conclusion
These results suggest that the addition of MCT to PFO formulations may decrease the host response to inflammatory challenge, which may pose potential for optimized PN formulations. Inclusion of MCT in lipid emulsions given with PN formulations may be of use in therapeutic interventions for disease states resulting from chronic inflammation.
Keywords: medium-chain triglycerides, fish oil, parenteral nutrition, inflammation
Introduction
Essential fatty acids (EFAs), necessary for growth, development, and a variety of biological functions, must be consumed in the diet. Historically, alpha linolenic acid (ALA, n-3 PUFA) and linoleic acid (LA, n-6 PUFA) have been considered the two essential fatty acids. However, recent studies have shown that the downstream metabolic products of ALA and LA, docosahexaenoic acid (DHA) and arachidonic acid (ARA), respectively, are sufficient to sustain growth, development and reproductive function1–3
For individuals dependent on parenteral nutrition (PN), EFAs must be provided intravenously as a lipid emulsion. Commercially available lipid formulations in the United States have been exclusively soybean oil-based and contain high levels of LA and lower levels of ALA. Soybean oil-based lipid emulsions are implicated in the development of parenteral nutrition-associated liver disease (PNALD), a progressive and often lethal complication affecting up to 74% of patients dependent on long-term PN4–7.
Alternative lipid sources have been investigated as a potential strategy to prevent liver disease in PN-dependent individuals. A fish oil-based lipid emulsion, provided at 1g/kg/day, has been shown to reverse the progression toward liver failure in patients with PNALD8–10. However, PN formulations provided with low doses of lipids necessitate higher calories from carbohydrates (dextrose) in order to meet the daily caloric needs of the PN-dependent individual, a consideration that is especially important in developing infants and children. PN formulations high in dextrose predispose patients to hyperglycemia and increased central venous catheter infections, hepatic steatosis, and glycosuria, complications that can lead to significant morbidity and mortality in an already-fragile population11–13.
Recently, attention has been given to non-essential fatty acids, so called “EFA-free” lipids, which may be utilized as additives to lipid emulsions in order to augment the total fat calories provided and decrease the requirement for additional dextrose in PN. Ling et al. examined the metabolic effects of combinations of EFAs with hydrogenated coconut oil (HCO), an EFA-free lipid source. Rats fed a diet with HCO as the sole source of calories and then given an endotoxin challenge demonstrated a lower inflammatory response, as measured by serum IL-6 and C-reactive protein, when compared to rats fed HCO supplemented with DHA and ARA14. These findings are in keeping with Cook et al., who also demonstrated a decreased systemic inflammatory response in states of essential fatty acid deficiency in rats15.
Medium chain triglycerides (MCT) are an alternative source of EFA-free lipids. Studies have shown benefits of MCT oil in the prevention of alcohol-induced liver injury in animal models by a mechanism that is not fully understood but may be related to decreased inflammation16–18. Others have demonstrated that dietary supplementation of MCT reduces the degree of endotoxin-induced intestinal and hepatic injury in rats19,20. In 2012, the American Society of Parenteral and Enteral Nutrition identified MCT oil as a potentially beneficial additive to lipid emulsions containing soybean and/or fish oil and recommended further research in identifying an optimal ratio of n-3 fatty acids, n-6 fatty acids, and MCT.21
The creation of an optimized lipid formulation, which provides sufficient EFAs to sustain growth and development, while modifying the intensity of inflammation, is of high value to the PN-dependent population. Purified fish oil (PFO) is a rich source of DHA and ARA that meets essential fatty acid requirements in rodent models2,22 and in humans.23,24 Herein, we utilize a murine model to study several dietary lipids including soybean oil, PFO, HCO and MCT to determine the effects of these lipid sources on growth, serum EFA levels, liver histology, and inflammatory profiles with and without an endotoxin challenge.
Methods
Animals and diets
Adult male C57/Bl6 mice (Jackson Laboratories, Bar Harbor ME) were housed five to a cage and maintained in a climate-controlled facility with a 12:12-hour light-dark cycle for a period of four days of acclimation prior to experimentation. During this period, animals had free access to water and a standard rodent chow diet. Experimental protocols were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee. Animals were then assigned by cage to one of the following six dietary treatment groups (10 animals per group) based on fat content by weight: 10.84% soybean oil (SOY), 10% coconut oil (HCO), 10% medium-chain triglycerides (MCT), 3% purified fish oil (PFO), 3% purified fish oil with 3% medium-chain triglycerides (50:50 MCT:PFO) and 3% purified fish oil with 7.59% medium-chain triglycerides (70:30 MCT:PFO). Purified fish oil was derived primarily from sardine oil from which all saturated fat and fats with carbon lengths less than 18 were removed, donated by Pronova BioPharma / BASF (Lysaker, Norway). Medium-chain triglyceride (MCT) oil was purchased from Health and Sport, LLC (Amston, CT). All diets were formulated by or purchased from Dyets, Inc. (Bethlehem, PA) and provided in pelleted form. Equivalent amounts of casein, L-cystine, sucrose, dyetrose (a version of dextrose used by the manufacturer which allows the diet to be easily pelleted), and vitamin and mineral mixtures were included in all diets. Slight variations in corn starch and cellulose were necessary to make the diets as nearly isocaloric as possible. Total kcal/kg was slightly lower in the PFO and 50:50 MCT:PFO diets. Table 1 lists the composition of the six different diets.
Table 1.
Dietary compositions (grams per kg)
| MCT | HCO | SOY | PFO | 50:50 MCT:PFO |
70:30 MCT:PFO |
|
|---|---|---|---|---|---|---|
| Casein | 140 | 140 | 140 | 140 | 140 | 140 |
| L-Cystine | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 |
| Cornstarch | 405.68 | 405.68 | 405.68 | 481.58 | 451.58 | 405.68 |
| Dyetrose | 155 | 155 | 155 | 155 | 155 | 155 |
| Medium Chain Triglycerides |
108.4 | 0 | 0 | 0 | 30 | 75.9 |
| Purified Fish Oil | 0 | 0 | 0 | 30 | 30 | 30 |
| Soybean Oil | 0 | 0 | 100 | 0 | 0 | 0 |
| Hydrogenated Coconut Oil |
0 | 100 | 0 | 0 | 0 | 0 |
| t-Butylhydroquinone | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Cellulose | 41.6 | 50 | 50 | 44.1 | 44.1 | 44.1 |
| Mineral Mix #210050 | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin Mix #310025 | 10 | 10 | 10 | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Total kcal per kg | 3925.7 | 3925.9 | 3925.9 | 3569.2 | 3710.2 | 3925.9 |
Dietary compositions (grams per kg). Equivalent amounts of casein, L-]cystine, sucrose, dyetrose (a version of dextrose used by the manufacturer which allows the diet to be easily pelleted), and vitamin and mineral mixtures were included in all diets. Slight variations in corn starch and cellulose were necessary to make the diets as nearly isocaloric as possible. Total kcal/kg was slightly lower in the PFO and 50:50 MCT:PFO diets.
Experimental design
Animals were pair-fed the experimental diets in groups of 5 for a period of 12 weeks. Individual body weight and total cage food intake were recorded every three days for the duration of the study period. Food intake was controlled by the lowest food intake among the six groups at each time to ensure that there were no differences in food intake across the six dietary groups. Blood samples were obtained from each animal prior to initiation of dietary treatment and again at 2, 4, 6, 9, and 12 weeks after diet initiation. At the end of 12 weeks, animals in each dietary group were divided into two subgroups: one group received an intraperitoneal (IP) injection of lipopolysacchardie (LPS) 150 µg in saline ( /µL) and the second group received an IP saline injection of equivalent volume. Four hours after receiving LPS or saline, blood samples were obtained via retro-orbital venous access and animals were euthanized. Livers were immediately harvested and lobes were divided into three sections: one was prepared for H&E staining as described by Lillie25, a second for oil red O staining as described by Bancroft26, and a third was snap frozen and stored at −80C for later analysis. Liver slides were reviewed by two independent blinded board-certified pathologists for qualitative analysis, and there were no discrepancies in interpretation between the two pathologists.
Analytical methods
Total lipid from plasma was extracted into chloroform / methanol (2:1) by the method of Folch et al.27 Before the extraction, 30 µL of a 1mg/mL solution of diheptadecanoyl phospatidylcholine and 30µL of a 1mg/ml solution of triheptadecanoyl glycerol (Nu-Check Prep, Elysian, NY) in chloroform-methanol (1:1, vol/vol) were added as an internal standard to all samples. Plasma phospholipids and triglycerides were separated and transmethylated, and fatty acid methyl ester profiles were acquired by gas chromatography as previously described.2
Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels were determined using commercially available ELISA kits from R&D Systems (Minneapolis, MN) and Invitrogen (Camarillo, CA), respectively, according to the manufacturers’ instructions. All samples were prepared and analyzed in duplicate.
Statistical analyses
Results are presented as mean± SEM for each outcome (all fatty acid profiles and inflammatory markers). A generalized linear mixed model was used to assess the statistical significance of differences in mean outcome after adjusting for baseline levels among the different dietary groups over time.28 The covariance matrix of the parameter estimates was computed using a sandwich estimator, and further adjusted for small sample size by the method of Morel, Bokossa and Neerchal29. Significance for all analysis was defined as P≤0.05. The type I error rate was controlled by limiting within-group comparisons to those specified a priori, and only when the diet-by-time interaction was significant. All analysis was performed with GraphPad Prism® v6, La Jolla, CA and SAS® v9, Cary, NC.
Results
Food intake and body weight
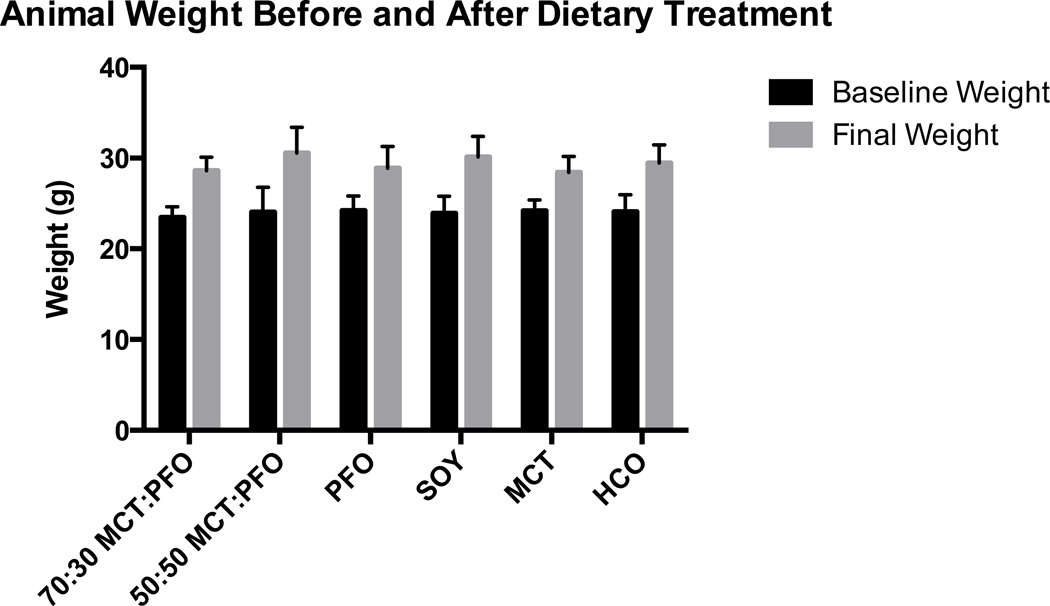
There were no differences in food intake among the six groups for the duration of the study period. Average food intake per group remained consistent at approximately 3–4 g/mouse/day for 12 weeks. Animals in all dietary groups gained weight throughout the study. Baseline weights ranged from 23 to 24 g, and at the end of 12 weeks final weights ranged from 29 to 31 g (Figure 1). No significant differences in weight were noted among the six groups. No animal demonstrated physical signs of EFA deficiency (EFAD), such as scaly dermatitis, hair loss, or skin atrophy.
Figure 1.
Animal weight by treatment group. All groups gained weight during the study period. There were no significant weight differences between treatment groups at the beginning or the end of treatment.
Fatty acid profiles
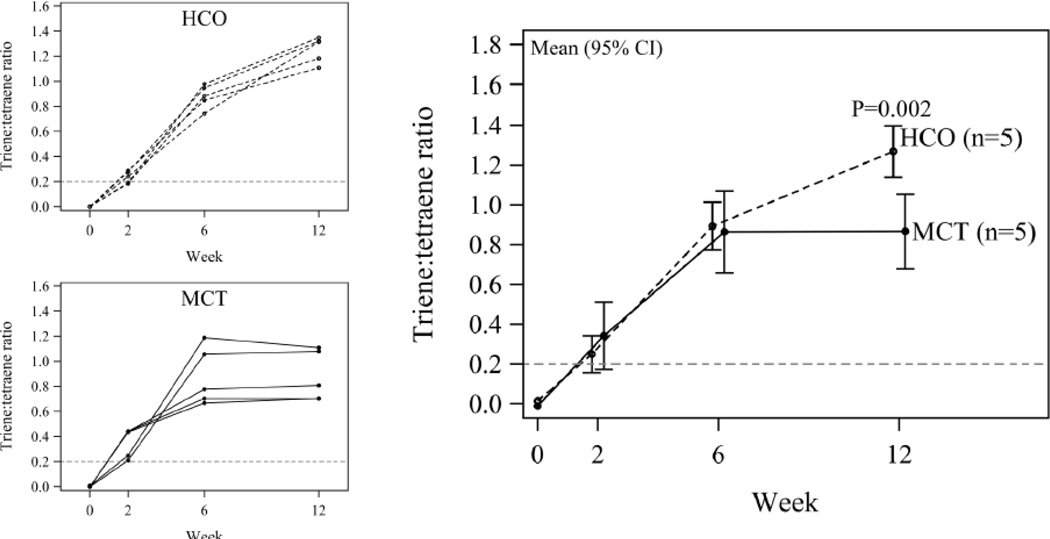
Animals in both MCT and HCO groups had biochemical EFAD, as defined by triene:tetraene ratios >0.2, by 2 weeks of dietary treatment, and these ratios continued to significantly increase for both groups over the remaining 10 weeks of treatment (HCO P<0.0001; MCT P=0.003). None of the groups fed diets containing PFO or SOY demonstrated biochemical EFAD at any point. At 12 weeks, HCO-fed animals showed increased triene:tetraene ratios relative to MCT-fed animals (mean (95% CI) 0.40 (0.17, 0.63)) indicating a slightly greater degree of EFAD in the HCO group (Figure 2).
Figure 2.
Triene:tetraene ratio at weeks 0, 2, 6 and 12. Shown is the trajectory for each animal over time for HCO and MCT separately (left), as well as aggregated by diet (right; mean and 95% confidence interval from a generalized linear model). The week-by-diet interaction is statistically significant (P=0.003). At 12 weeks, HCO-fed animals showed increased triene:tetraene ratios relative to MCT-fed animals, indicating a slightly greater degree of EFAD in the HCO group.
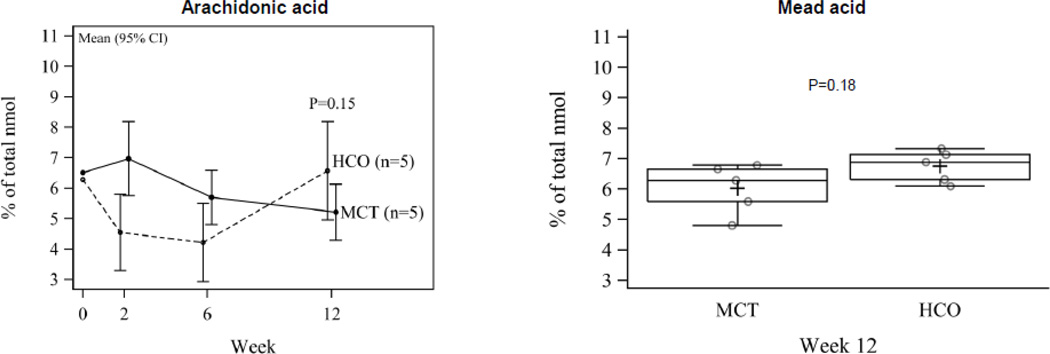
The increased triene:tetraene ratio in HCO-fed animals is due to higher mead acid (20:3n9) in the HCO group despite despite lower ARA in the MCT group compared to the HCO group at 12 weeks (5.21±0.45 and 6.57±0.78, respectively, P=0.15) (Figure 3). This slight increase in Mead acid is presumably attributable in part to the presence of substantial stearic acid (18:0), a Mead acid precursor found in HCO and not in MCT oil.
Figure 3.
Arachidonic acid over time (mean, 95% CI) and mead acid at week 12 (median, IQR). ARA is lower and mead acid is higher in the MCT group compared to the HCO group at 12 weeks.
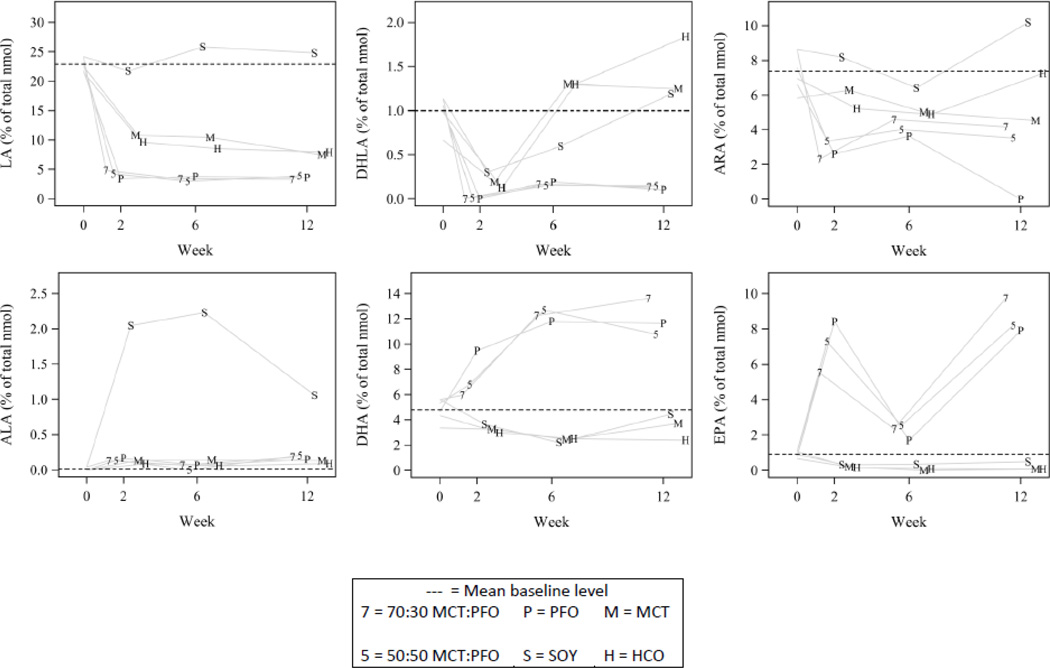
At 2 weeks of dietary treatment, significant differences among treatment groups were observed in plasma levels of various n-6 and n-3 fatty acids. SOY animals had only modest decreases in linoleic acid (LA, 18:2n6) from baseline (−2.4±2.9; P=0.40), compared to decreases on the order of 11% of total nmol in HCO and MCT groups (P<0.0001 for each), and even greater decreases on the order of 19% of total nmol in all groups containing PFO (P<0.0001 for each). Dihomo-gamma-linolenic acid (20:3n6) was similar among SOY, HCO and MCT, and present in very low levels in all groups containing both MCT and PFO. Arachidonic acid (ARA, 20:4n6) decreased by week 2 in all groups containing PFO (P<0.001), while unchanged from baseline for both SOY (P=0.81) and MCT (P=0.60). HCO experienced a modest decrease from baseline (P=0.04). No significant changes were observed from baseline to week 2 for alpha linolenic acid (ALA, 18:3n3). Docosahexaenoic acid (DHA, 22:6n3) increased by week 2 to 9.50±1.27% of total nmol in animals on the PFO diet (P=0.008) while remaining constant in all other groups. Eicosapentaenoic acid (EPA, 20:5n3) increased in all groups containing PFO (P<0.05) while being barely detectable in the non-PFO containing groups (Figure 4).
Figure 4.
Omega-3 and omega-6 fatty acid concentrations. Shown are unadjusted means at weeks 0, 2, 6 and 12. The horizontal dashed reference line indicates the baseline mean. Data are shifted left or right of the week label to spread the data to enhance readability
At 12 weeks, mice fed HCO and MCT still had detectable levels of LA, dihomo-gammalinolenic acid, and ARA, despite continued treatment with a diet deplete of these essential fatty acids. In fact, ARA levels in HCO and MCT groups were higher than in any PFO containing group. PFO, 50:50 MCT:PFO, and 70:30 MCT:PFO groups demonstrated low levels of all n-6 fatty acids measured at 12 weeks. ARA was higher in SOY than all other groups at 12 weeks, significantly so when compared to the PFO groups (P<0.05). Although DHA and EPA were lower in all non-PFO containing groups, DHA levels were preserved in SOY and MCT, with only a modest decrease from baseline in HCO (−1.96±0.93; P=0.04), despite the lack of n-3 fatty acids in MCT and HCO and low n-3 source in SOY. Figures 3 and 4 show n-3 and n-6 fatty acid levels, respectively.
IL-6 and TNF-α
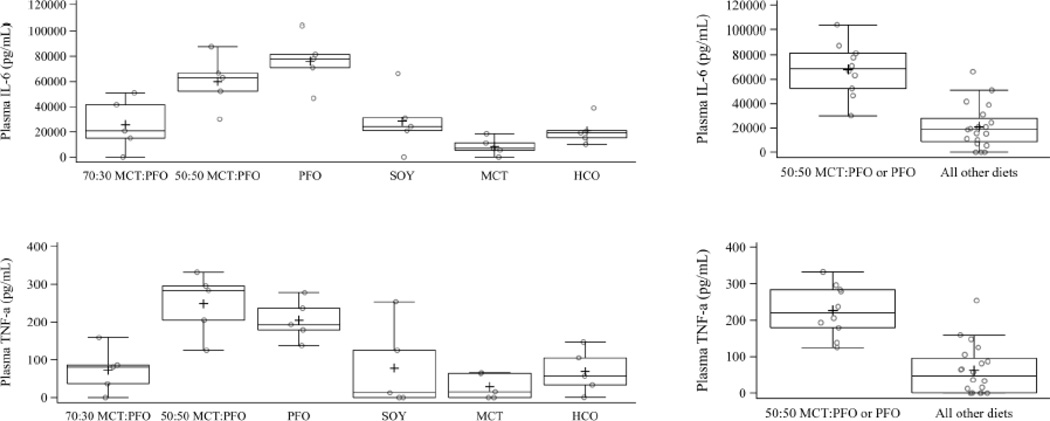
Measurement of IL-6 and TNF-α was performed on serum samples from all animals after 12 weeks of dietary treatment to establish a baseline. Baseline levels of IL-6 and TNF-α were found to be just at the detection threshold with no differences between dietary groups. After saline treatment, no differences were seen in either IL-6 or TNF-α from baseline in any dietary group (data not shown). After LPS treatment, IL-6 and TNF-α levels increased significantly in all groups. Animals fed PFO or 50:50 MCT:PFO diets were found to have the highest mean level of IL-6 when compared to all other dietary groups. Animals in the 50:50 MCT:PFO or PFO groups had highest levels of TNF-α relative to the other groups. IL-6 and TNF-α were lowest in MCT, though not significantly different than HCO, 70:30 MCT:PFO or SOY. Figure 5 shows the IL-6 and TNF-α levels, respectively, in all animals treated with LPS.
Figure 5.
IL-6 and TNF-α at week 12. Diets 70:30 MCT:PFO, soy, MCT, and HCO are not different from one another (IL-6 P=0.30; TNF-α P=0.69). The 50:50 MCT:PFO and PFO diets are higher in both IL-6 and TNF-α than all other diets combined (P<0.0001 for each). Results are based on analysis of variance.
Liver analysis
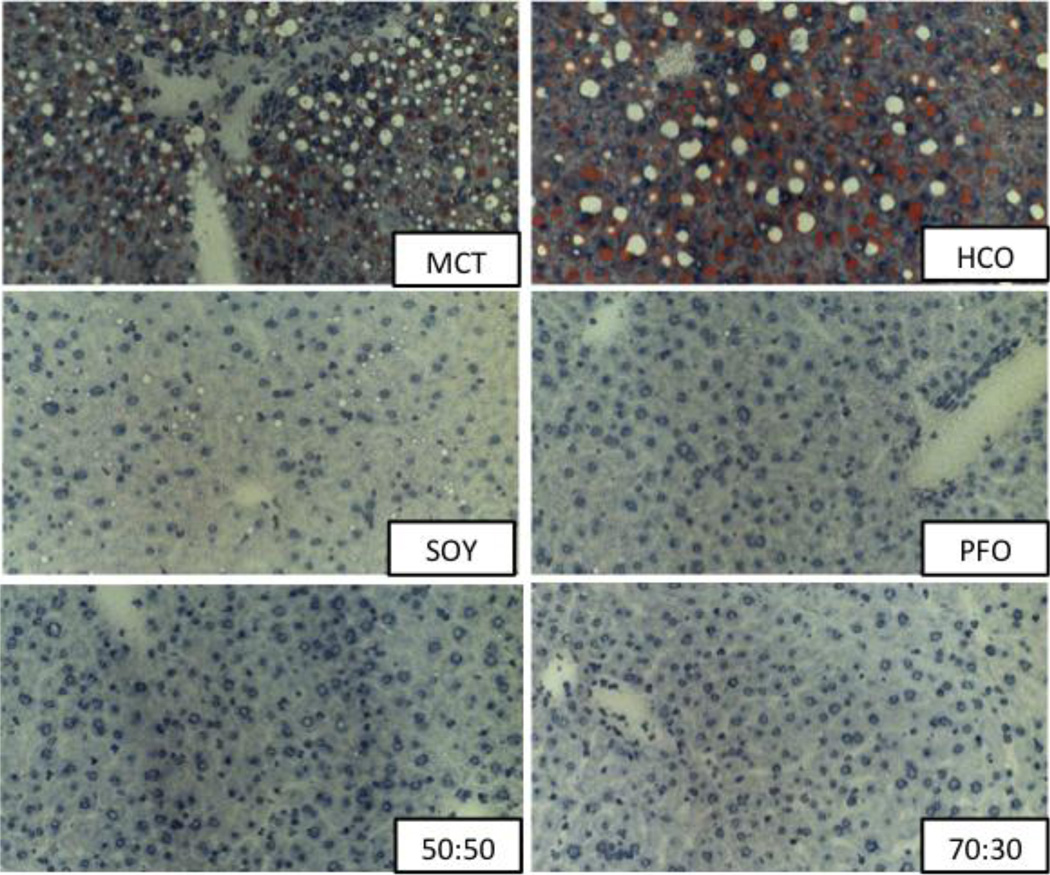
All liver specimens were stained for H&E and oil red O and analyzed. All MCT and HCO livers demonstrated significant peri-portal and mid-zonal micro- and macrosteatosis, representing the increased de novo lipogenesis of essential fatty acid deficiency. SOY livers showed rare to mild mid-zonal microsteatosis to a much lesser degree than MCT or HCO livers. PFO, 50:50 MCT:PFO and 70:30 MCT:PFO livers appeared grossly normal without evidence of hepatosteatosis. Among all groups, livers of animals treated with saline injection had glycogen deposits throughout the tissue, which were notably absent in livers of animals treated with LPS. Administration of LPS was not noted to have an effect on degree of steatosis or presence of inflammatory cells in any group. Representative histological sections are presented in Figure 6.
Figure 6.
Liver samples stained for Oil Red O. All MCT and HCO livers demonstrated significant peri-portal and mid-zonal micro- and macrosteatosis. SOY livers showed rare to mild mid-zonal microsteatosis. PFO, 50:50 MCT:PFO and 70:30 MCT:PFO livers appeared normal without evidence of hepatosteatosis.
Discussion
The incorporation of fish oil into PN formulations has emerged as a viable approach to treat parenteral nutrition-associated liver injury. However, critics of the use of fish oil monotherapy have speculated that fish oil alone may be insufficient to support growth and prevent EFAD. Short-term animal studies have shown appropriate growth and prevention of EFAD after dietary treatment with native fish oil in mice when given as at least 20% of total calories, which supplies sufficient ARA to complement the very limited amount of linoleic acid found in fish oil.2 Nehra et al. demonstrated that animals fed a diet providing DHA and ARA as the sole source of fat in at a 20:1 ratio (similar to that which is found in native fish oil) at 2.1% of total calories is sufficient to sustain reproduction over several generations in mice.3 To our knowledge, this present study is the first study to demonstrate that dietary treatment with PFO alone long-term (over 12 weeks) sustains growth and prevents EFAD in mice.
Our study shows an increase in IL-6 and TNF-α following treatment with PFO-based diets and subsequent endotoxin challenge compared to HCO- and soy-based diets. This increased inflammatory response is mitigated by the addition of MCT at ≥ 50% of fat calories. These findings are similar to those published by Ling et al., who demonstrated that the addition of DHA and ARA to an HCO-based diet leads to increased IL-6 and C-reactive protein (CRP) after endotoxin administration. However, this is the first study to demonstrate that the inflammatory response is mitigated by the addition of MCT to PFO. The increase in the systemic inflammatory response noted when using either PFO or only two of the active components (DHA and ARA) is unexpected given the multitude of evidence that standard fish oil preparations have been shown to be anti-inflammatory.30,31 Other studies have shown a decrease in pro-inflammatory markers with fish oil treatment. Endres et al. demonstrated that the production of IL-1 and TNF-α are decreased after endotoxin administration in healthy adults whose diet was supplemented with 18g of fish oil for a period of six weeks.32 It is important to note that previous studies have often utilized native fish oil as a supplement in much smaller amounts than studied by Endres et al. and that the supplements are taken with a normal diet containing substantial amounts of other fats.32–34 In native or unpurified fish oil, EPA and DHA are present at 25–30% of total fatty acids and ARA at approximately 0.5–1% with the remaining majority of fatty acids as saturated fatty acids.35 A potential mechanism for the changes seen in inflammatory potential in the present study relates to the principal fatty acids that underlie inflammation. ARA is presumed to be pro-inflammatory through its production of 2-series eicosanoids like prostaglandin and prostacyclin and 4 series leukotrienes.15,36 In contrast, EPA and DHA are anti-inflammatory in part due to the production of 3-series prostanoids and 5-series leukotrienes, and DHLA is presumed to be anti-inflammatory from its production of 1-series prostanoids37. In this study, while ARA levels are high and EPA levels low in the SOY group, DHLA levels are also higher in PFO-containing groups compared to those not containing PFO. Moreover a state of essential fatty acid deficiency in itself, as with HCO and MCT, has been shown to lead to increased mortality in humans due to infection, presumably because of the underlying decreased response to endotoxin challenge.38 In contrast, groups containing PFO have the highest EPA and DHA and lowest ARA, which should severely down regulate inflammation; notably, these groups have essentially no DHLA. Whether the effect of DHLA would be enough to change the degree of inflammation vis-à-vis PFO or whether EPA and DHA alone in these amounts might have opposite effects cannot be determined in this study. Furthermore the increase in systemic inflammatory response also reflects an increased immune response, which, under certain circumstances, could be beneficial as in the correction of essential fatty acid deficiency. Whatever the ultimate explanation, these results should give pause to the potential effects of purifying fish oil without consideration of the potential fatty acid interactions that this might entail. At least provisionally the dilution of PFO by 50% MCT seems to provide the optimal liver histology with the best inflammatory profile in response to endotoxin and would clinically allow the provision of substantial fat calories to reduce the carbohydrate burden.
In conclusion, we have shown that long-term treatment with PFO as the sole source of fat calories sustains growth and prevents EFAD in a murine model. In addition, these results suggest that the addition of MCT to PFO formulations may decrease the host response to inflammatory challenge, though further investigation is warranted. Inclusion of MCT in lipid emulsions given with PN formulations may be of use in therapeutic interventions for disease states resulting from chronic inflammation.
Acknowledgments
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number F532 HD 071715-02. Further research support was also given by the National Research Service Award Institutional Training Grant of the National Institutes of Health under award number 2T32DK007754-14, 5 T32 HD007466-16 and 5 T32 HD007466-17, the Joshua Ryan Rappaport Research Fellowship, and the Boston Children’s Hospital Department of Surgery and the Vascular Biology Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- EFA
essential fatty acid
- PUFA
polyunsaturated fatty acid
- ALA
alpha linolenic acid
- LA
linoleic acid
- DHA
docosahexaenoic acid
- ARA
arachidonic acid
- PN
parenteral nutrition
- PNALD
parenteral nutrition associated liver disease
- HCO
hydrogenated coconut oil
- MCT
medium-chain tryglycerides
- PFO
purified fish oil
- IP
intraperitoneal
- LPS
lipopolysaccharide
- IL-6
interleukin 6
- TNF-α
tumor necrosis factor alpha
- EFAD
essential fatty acid deficiency
- DHLA
dihomo-gamma-linolenic acid
- CRP
C-reactive protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no disclosures.
Author contributions: SC, BB, KG, and MP designed the study. SC, AO, PN, VN, MC and EC performed the animal study and tissue preparation / analysis. PM performed statistical analyses. All authors interpreted the data. SC prepared the manuscript. All authors reviewed and approved the final manuscript.
References
- 1.Le HD, Meisel JA, de Meijer VE, Gura KM, Puder M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins, leukotrienes, and essential fatty acids. 2009 Aug-Sep;81(2–3):165–170. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling PR, Puder M, Bistrian BR. Purified fish oil eliminating linoleic and alpha linolenic acid meets essential fatty acid requirements in rats. Metabolism: clinical and experimental. 2012 Oct;61(10):1443–1451. doi: 10.1016/j.metabol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Nehra D, Le HD, Fallon EM, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging cell. 2012 Dec;11(6):1046–1054. doi: 10.1111/acel.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN. Journal of parenteral and enteral nutrition. 2000 Nov-Dec;24(6):345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 5.Kurvinen A, Nissinen MJ, Andersson S, et al. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. Journal of pediatric gastroenterology and nutrition. 2012 Jun;54(6):803–811. doi: 10.1097/MPG.0b013e3182474118. [DOI] [PubMed] [Google Scholar]
- 6.Merritt RJ. Cholestasis associated with total parenteral nutrition. Journal of pediatric gastroenterology and nutrition. 1986 Jan;5(1):9–22. doi: 10.1097/00005176-198601000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. The Journal of pediatrics. 2012 Oct;161(4):723–728. e722. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calkins KL, Dunn JC, Shew SB, et al. Pediatric Intestinal Failure-Associated Liver Disease Is Reversed With 6 Months of Intravenous Fish Oil. JPEN. Journal of parenteral and enteral nutrition. 2013 Jul 26; doi: 10.1177/0148607113495416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Premkumar MH, Carter BA, Hawthorne KM, King K, Abrams SA. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. The Journal of pediatrics. 2013 Apr;162(4):793–798. e791. doi: 10.1016/j.jpeds.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Annals of surgery. 2009 Sep;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guglielmi FW, Regano N, Mazzuoli S, et al. Catheter-related complications in long-term home parenteral nutrition patients with chronic intestinal failure. The journal of vascular access. 2012 Oct-Dec;13(4):490–497. doi: 10.5301/jva.5000133. [DOI] [PubMed] [Google Scholar]
- 12.Pironi L, Joly F, Forbes A, et al. Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut. 2011 Jan;60(1):17–25. doi: 10.1136/gut.2010.223255. [DOI] [PubMed] [Google Scholar]
- 13.Sanders RA, Sheldon GF. Septic complications of total parenteral nutrition. A five year experience. American journal of surgery. 1976 Aug;132(2):214–220. doi: 10.1016/0002-9610(76)90050-7. [DOI] [PubMed] [Google Scholar]
- 14.Ling PR, Malkan A, Le HD, Puder M, Bistrian BR. Arachidonic acid and docosahexaenoic acid supplemented to an essential fatty acid-deficient diet alters the response to endotoxin in rats. Metabolism: clinical and experimental. 2012 Mar;61(3):395–406. doi: 10.1016/j.metabol.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cook JA, Wise WC, Halushka PV. Elevated thromboxane levels in the rat during endotoxic shock: protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. The Journal of clinical investigation. 1980 Jan;65(1):227–230. doi: 10.1172/JCI109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kono H, Enomoto N, Connor HD, et al. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. American journal of physiology. Gastrointestinal and liver physiology. 2000 Mar;278(3):G467–G476. doi: 10.1152/ajpgi.2000.278.3.G467. [DOI] [PubMed] [Google Scholar]
- 17.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. The Journal of pharmacology and experimental therapeutics. 2001 Nov;299(2):638–644. [PubMed] [Google Scholar]
- 18.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. The Journal of nutrition. 2004 Apr;134(4):904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 19.Kono H, Fujii H, Asakawa M, et al. Medium-chain triglycerides enhance secretory IgA expression in rat intestine after administration of endotoxin. American journal of physiology. Gastrointestinal and liver physiology. 2004 Jun;286(6):G1081–G1089. doi: 10.1152/ajpgi.00457.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kono H, Fujii H, Asakawa M, et al. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Annals of surgery. 2003 Feb;237(2):246–255. doi: 10.1097/01.SLA.0000048450.44868.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanek VW, Seidner DL, Allen P, et al. A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2012 Apr;27(2):150–192. doi: 10.1177/0884533612439896. [DOI] [PubMed] [Google Scholar]
- 22.Le HD, Meisel JA, de Meijer VE, et al. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. JPEN. Journal of parenteral and enteral nutrition. 2012 Jul;36(4):431–441. doi: 10.1177/0148607111414580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le HD, de Meijer VE, Zurakowski D, Meisel JA, Gura KM, Puder M. Parenteral fish oil as monotherapy improves lipid profiles in children with parenteral nutrition-associated liver disease. JPEN. Journal of parenteral and enteral nutrition. 2010 Sep-Oct;34(5):477–484. doi: 10.1177/0148607110371806. [DOI] [PubMed] [Google Scholar]
- 24.Nehra D, Fallon EM, Potemkin AK, et al. A Comparison of 2 Intravenous Lipid Emulsions: Interim Analysis of a Randomized Controlled Trial. JPEN. Journal of parenteral and enteral nutrition. 2013 Jun 14; doi: 10.1177/0148607113492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillie RD. Histopathologic Technic and Practical Histochemistry. 3rd ed. New York: McGraw-Hill Book Co; 1965. [Google Scholar]
- 26.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Edinburgh: Churchill Livingston; 2007. [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- 28.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Morel J, Bokossa M, Neerchal N. Small sample correction for the variance of GEE estimators. Biometrical Journal. 2003;4:395–409. [Google Scholar]
- 30.Mascioli E, Leader L, Flores E, Trimbo S, Bistrian B, Blackburn G. Enhanced survival to endotoxin in guinea pigs fed IV fish oil emulsion. Lipids. 1988 Jun;23(6):623–625. doi: 10.1007/BF02535609. [DOI] [PubMed] [Google Scholar]
- 31.Pomposelli JJ, Mascioli EA, Bistrian BR, Lopes SM, Blackburn GL. Attenuation of the febrile response in guinea pigs by fish oil enriched diets. JPEN. Journal of parenteral and enteral nutrition. 1989 Mar-Apr;13(2):136–140. doi: 10.1177/0148607189013002136. [DOI] [PubMed] [Google Scholar]
- 32.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. The New England journal of medicine. 1989 Feb 2;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KJ, Galvin K, Kiely M, Morrissey PA, Mann NJ, Sinclair AJ. Low dose supplementation with two different marine oils does not reduce proinflammatory eicosanoids and cytokines in vivo. Asia Pacific journal of clinical nutrition. 2006;15(3):418–424. [PubMed] [Google Scholar]
- 34.Zulyniak MA, Perreault M, Gerling C, Spriet LL, Mutch DM. Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism: clinical and experimental. 2013 Aug;62(8):1107–1113. doi: 10.1016/j.metabol.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Sierra P, Ling PR, Istfan NW, Bistrian BR. Fish oil feeding improves muscle glucose uptake in tumor necrosis factor-treated rats. Metabolism: clinical and experimental. 1995 Oct;44(10):1365–1370. doi: 10.1016/0026-0495(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 36.Ling PR, Boyce P, Bistrian BR. Role of arachidonic acid in the regulation of the inflammatory response in TNF-alpha-treated rats. JPEN. Journal of parenteral and enteral nutrition. 1998 Sep-Oct;22(5):268–275. doi: 10.1177/0148607198022005268. [DOI] [PubMed] [Google Scholar]
- 37.Palombo JD, DeMichele SJ, Boyce PJ, et al. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Critical care medicine. 1999 Sep;27(9):1908–1915. doi: 10.1097/00003246-199909000-00032. [DOI] [PubMed] [Google Scholar]
- 38.Hansen A, Wiese H, Boelsche A, Haggard M, Adam D, Davis H. Role of linoleic acid in infant nutrition. Pediatrics. 1963;31(suppl):171–192. [Google Scholar]