Abstract
A new cross-bridged cyclam chelator, CB-TE1K1P, was developed for copper-based radiopharmaceuticals, and this chelator can be labelled with 64Cu under mild conditions in high specific activity. DBCO–PEG4–CB-TE1K1P was synthesized for conjugation to proteins, while Dde–CB-TE1K1P(tBu2)–OH was synthesized for solid-phase peptide synthesis. Examples of the conjugation chemistry, radiolabelling and serum stability of each are presented.
The copper radionuclides Cu-60, Cu-61, Cu-62, Cu-64 and Cu-67 have applications in diagnostic imaging, radiotherapy, and theranostics.1–3 There are two principle oxidation states (I and II), and the coordination and redox chemistry of copper is well documented. Additionally, the biochemistry and metabolism of copper are also well known, since there are many mammalian and bacterial copper-containing enzymes and proteins.4 The redox and kinetic properties of Cu(II) present a challenge for the development of kinetically inert copper complexes for diagnostic imaging and radiotherapy.
To prepare copper-based radiopharmaceuticals, the copper radio-nuclides are incorporated into a biomolecule through a bifunctional chelator (BFC). An optimal BFC should bind with copper rapidly under mild conditions in high specific activity (SA). The resulting radiometal complex should exhibit high thermodynamic stability,5,6 and more importantly, also sufficient stability in vivo to withstand a challenge from copper-containing proteins, such as ceruloplasmin and metallothio-nein.7 Considerable efforts have been focused on the development of novel BFCs for copper radionuclides, and a number of chelators, such as DOTA, TETA, NOTA, TE2A, CB-TE2A and their analogues, have been intensively studied as BFCs for this purpose (Fig. 1).8–12
Fig. 1.
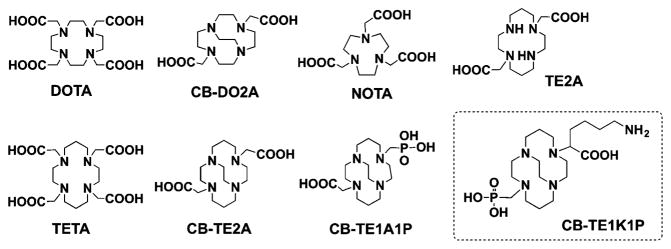
Chelators for copper radionuclides.
Among these BFCs, CB-TE2A demonstrated excellent kinetic inertness and in vivo stability, and has been recognized as one of the “gold standards” in recent years. However, harsh radiolabelling conditions are required (95 °C for 1 h), which hinder applications with temperature-sensitive molecules, such as proteins and heat-sensitive peptides. Some of the newly developed chelators (Fig. S1, ESI†), such as p-SCN-Bn-PCTA, p-SCN-Bn-Oxo,13 SarAr,14 CB-TE2A,15 were labelled with Cu-64 under mild conditions in high specific activity; however, none of these chelators demonstrated improved in vivo behavior compared to CB-TE2A. Furthermore, these chelators cannot be directly used as N-protected amino acid surrogates for SPPS (solid phase peptide synthesis), and usually they are conjugated to peptides through either an N-terminus or primary amine in the side chain.
We recently reported on CB-TE1A1P (Fig. 1), which can be labelled at room temperature in high SA. The resulting 64Cu-complex exhibited comparable or even better in vivo stability with more rapid clearance through blood, kidneys and liver compared to 64Cu labelled NOTA, Diamsar and CB-TE2A.10 CB-TE1A1P can be conjugated to peptides using a primary amine; however, the conversion of the carboxylate group to an amide linkage potentially decreases its in vivo stability. Therefore, an improved chelator that can be rapidly labelled under mild conditions (e.g. short labelling times at physiological temperature), and also maintains high stability of the cross-bridge cyclam, would be highly attractive for the design of copper-based radiopharmaceuticals.
CB-TE1K1P contains a carboxylate and a methanephosphonate pendant group for ease of labelling at room temperature with maintained in vivo stability. Furthermore, in order to facilitate its conjugation with biomolecules, two derivatives (7 and 9) were prepared for facile conjugation to either proteins or peptides.
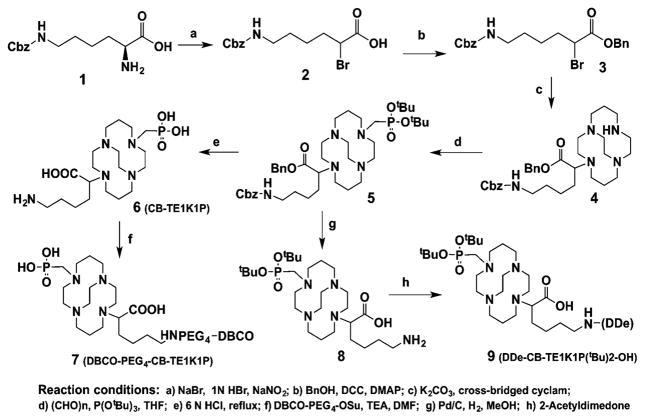
As illustrated in Scheme 1, the α-amino group in H-Lys(Cbz)-OH was converted to the corresponding bromide, and 2 was obtained after silica gel purification in 60.5% isolation yield. In the second step, the carboxylic acid group of 2 was protected as a benzyl ester by coupling with benzyl alcohol. The resulting compound 3 underwent mono-N-alkylation of cross-bridged cyclam, yielding pure 4 after removing excess cross-bridged cyclam. Compound 4 was then converted to the mono-di-tert-butyl-phosphonate intermediate 5 via three-component Kabachnik–Fields reaction. Intermediate 5 was hydrolyzed using 6 M HCl by refluxing overnight and gave the product CB-TE1K1P (6) after purification by ion exchange chromatography. 6 was functionalized with DBCO (dibenzo-cyclooctyne) by forming a peptide bond, and after HPLC purification yielded pure DBCO–PEG4–CB-TE1K1P (7) for conjugation to biomolecules via metal-free click chemistry. Compound 5 was readily converted to compound 8 after stirring with Pd/C under H2, and then crude 8 was treated with DDe and yielded 9 that was purified by silica gel chromatography.
Scheme 1.
Synthesis of CB-TE1K1P and its two derivatives.
In order to demonstrate the utility of CB-TE1K1P, we prepared two bioconjugates and labelled them with 64Cu. Compound 7 was conjugated to the anti-EGFR monoclonal antibody, cetuximab. Compound 9 was attached to the cFlFlF peptide, which binds the N-formyl peptide receptor 1 (FPR-1).
The conjugation and radiolabelling of CB-TE1K1P–PEG4-click- cetuximab were chosen since there have been no successful studies of 64Cu-labelling of a cross-bridged macrocycle–antibody conjugate. This was compared to direct conjugation of CB-TE1A1P with cetux-imab, which resulted in an average of 2 chelators per antibody measured using a published method.16 The low chelator/antibody ratio in the CB-TE1A1P–cetuximab conjugate is likely due to the poor efficiency of the NHS-aided coupling reaction between CB-TE1A1P and the primary amines of the antibody, possibly due to the effect of the methanephosphonate group in CB-TE1A1P.17 Inspired by previous reports on the conjugation of BFCs to nanoparticles via metal-free click chemistry (also called strain-promoted alkyne–azide cycloaddition, SPAAC),18,19 we designed and synthesized 7 for conjugation to azido functionalized cetuximab (Scheme 2a). The number of chelators per antibody can be easily modified in the cetuximab azidolation step by adjusting the amount of azide-NHS ester. The cetuximab conjugate with ~9 CB-TE1K1P chelators showed high binding affinity to the EGFR receptor (Kd = 1.4 ± 0.4 nM) and was used for further evaluation. Two other cetuximab conjugates were also synthesized, including CB-TE1A1P-click-cetuximab prepared using DBCO–CB-TE1A1P, and non-click CB-TE1A1P–cetuximab prepared via a standard peptide coupling reaction. A comparison of the 64Cu–CB-TE1K1P–cetuximab with other 64Cu-labeled conjugates is summarized in Fig. 2.
Scheme 2.
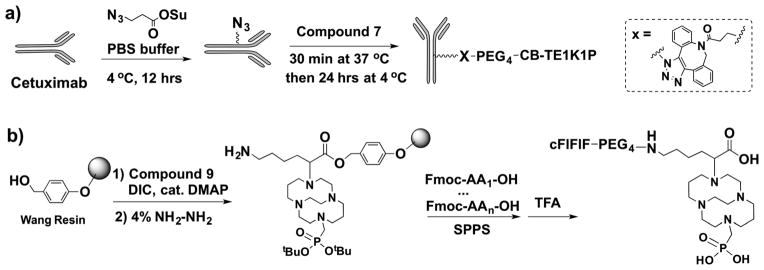
Synthesis of two CB-TE1K1P conjugates: (a) cetuximab-click-PEG4–CB-TE1K1P; (b) cFlFlF–PEG4–CB-TE1K1P.
Fig. 2.
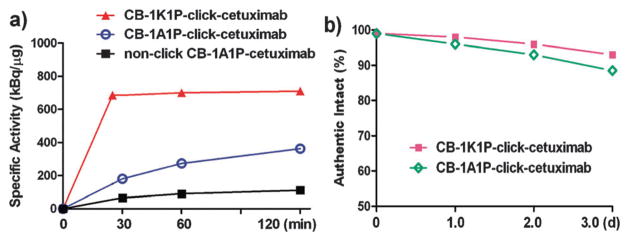
(a) 64Cu-labellings of three cetuximab–chelate conjugates at 40 °C; (b) serum stability of two cetuximab–chelate conjugates.
64Cu-labeled CB-TE1K1P-click-cetuximab with 9 chelates per antibody had a specific activity of 685 kBq μg−1 after incubation at 37 °C for 30 min (or 40 °C for 25 min). In contrast, after incubation at 40 °C for 2 h, the 64Cu-labeled CB-TE1A1P-click-cetuximab with the same number of chelates per antibody had a SA of 334 kBq μg−1, which may be attributed to the loss of the carboxylate group that facilitates the copper ion complexation. The SA of 64Cu-labelled NHS conjugated CB-TE1A1P–cetuximab with 2 chelates per antibody was only 111 kBq μg−1 when labelled for 2 h at 40 °C. These data demonstrate that CB-TE1K1P is rapidly labelled with Cu-64 under mild conditions in high SA (see Fig. 2a for details). Furthermore, both the resulting 64Cu–CB-TE1K1P-click-cetuximab and 64Cu–CB-TE1A1P-click-cetuximab exhibited good serum stability (Fig. 2b).
Besides macromolecules, we also explored the application of CB-TE1K1P in preparing 64Cu-labelled peptides. We designed and synthesized Dde–CB-TE1K1P(tBu2)–OH (9) that is suitable for SPPS. Dde–CB-TE1K1P(tBu2)–OH was first loaded onto a Wang resin by forming an ester bond, and then the primary amine in CB-TE1K1P was released after deprotection with 4% hydrazine. The resulting resin pre-loaded with CB-TE1K1P can be readily used for preparation of any peptide using standard Fmoc chemistry based SPPS, and then the CB-TE1K1P attached peptide can be subsequently cleaved using trifluoroacetic acid (Scheme 2b). By using this strategy, a neutrophil-targeted cFlFlF–PEG4–CB-TE1K1P conjugate was successfully synthesized and compared with the analogous cFlFlF–PEG4–CB-TE1A1P conjugate. The CB-TE1K1P–peptide was radiolabelled under much milder conditions in a shorter time (Fig. S2a, ESI†) compared to the CB-TE1A1P–peptide. Greater than 95% 64Cu-labelling yield of the CB-TE1K1P–peptide was achieved after 5 min at 37 °C or 1 h at room temperature; in contrast, at the same specific activity of 37 GBq μmol−1, ≥95% radiolabelling yield of the CB-TE1A1P–peptide could only be achieved after 30 min at 37 °C. It is postulated that the carboxylate group in CB-TE1K1P might form an additional coordinate bond to the Cu2+ ion and facilitate the 64Cu labelling. The 64Cu–CB-TE1K1P–peptide also exhibited better serum stability when compared with that of the 64Cu–CB-TE1A1P peptide (Fig. S2b, ESI†). The log P values of these two 64Cu radiotracers were also measured, and the log P value of the 64Cu–CB-TE1K1P peptide is −0.370 ± 0.010, whereas the log P value of the 64Cu–CB-TE1A1P peptide is significantly higher (−0.253 ± 0.005; P = 0.0005), which is likely due to the extra negative charge of the carboxylate group in the CB-TE1K1P chelate.
In the absence of crystal structures of Cu(II)–CB-TE1K1P– and Cu(II)–CB-TE1A1P–biomolecule conjugates, density functional theory (DFT) calculations were performed to address whether the carboxylate in the CB-TE1K1P chelator is capable of forming a coordinate bond with Cu(II) (see ESI† for computational details).20 Fig. 3 shows the DFT [uB3LYP/6-31+G(d,p)] optimized geometry of two model Cu(II)–chelates. The optimized metal-to-ligand bond lengths reveal that the Cu–CB-TE1K1P complex is highly distorted and its r(Cu–O3) and (Cu–O5) bond lengths are 1.945 Å and 1.941 Å, respectively, which are consistent with the expected Jahn–Teller effects of the d9 electron configuration. On the other hand, the Cu–CB-TE1A1P complex has a similar configuration, but there is only weak interaction between N5 of the amide and Cu, demonstrated by the 3.741 Å bond length. These calculations suggest that the carboxylate in the CB-TE1K1P chelator may promote the formation of a more stable complex with Cu2+.
Fig. 3.
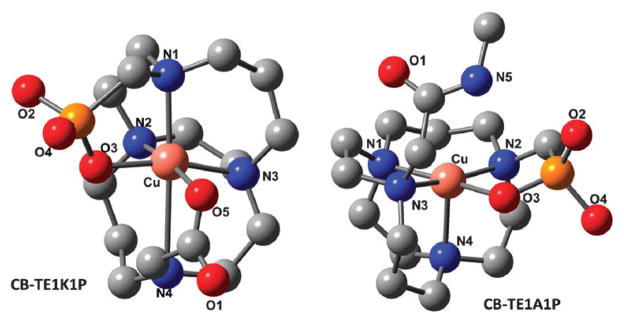
DFT optimized structure of the hypothetical model complex of 64 Cu–CB-TE1K1P and 64Cu–CB-TE1A1P.
It should be noted that in the CB-TE1K1P chelator, there is one chiral center in the pendent arm and two enantiomers (R,R and S,S) associated with the cross-bridge cyclam, statistically resulting in four diastereomers. Synthesis of pure enantiomers is in progress and the stereochemistry effects will be investigated as part of a future study.
In summary, here we reported the design and synthesis of a new chelator, CB-TE1K1P, containing one methanephosphonate and one carboxylate pendant group, and its use in preparing bioconjugates through either traditional SPPS or SPAAC. Rapid and efficient conjugations with the CB-TE1K1P were demonstrated using cetuximab, and a neutrophil-targeted peptide, cFlFlF. The resulting conjugates were rapidly radiolabelled with 64Cu under mild conditions in high SA, and the resulting radiotracers showed improved serum stability when compared with previously reported 64Cu-labelled cross-bridged chelates, such as CB-TE1A1P. Therefore, CB-TE1K1P can serve as an improved BFC for conjugation to small molecules, peptide and protein-based biomolecules, for labelling with 64Cu or other copper radionuclides with applications in diagnostic imaging, radiotherapy, and/or theranostics.
Supplementary Material
Acknowledgments
Supported by NCI R01CA093375.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc45928d
Notes and references
- 1.Blower PJ, Lewis JS, Zweit J. Nucl Med Biol. 1996;23:957–980. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- 2.Chervu LR, Sternlieb I. J Nucl Med. 1974;15:1011–1013. [PubMed] [Google Scholar]
- 3.Anderson CJ, Green MA, Fujibayashi Y. Handb Radiopharm. 2003:401–422. [Google Scholar]
- 4.Wheeler TD, Zeng D, Desai AV, Önal B, Reichert DE, Kenis PJ. Lab Chip. 2010;10:3387–3396. doi: 10.1039/c0lc00162g. [DOI] [PubMed] [Google Scholar]
- 5.Linder MC, Hazegh-Azam M. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- 6.Sarko D, Eisenhut M, Haberkorn U, Mier W. Curr Med Chem. 2012;19:2667–2688. doi: 10.2174/092986712800609751. [DOI] [PubMed] [Google Scholar]
- 7.Robinson NJ, Winge DR. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebedev AY, Holland JP, Lewis JS. Chem Commun. 2010;46:1706–1708. doi: 10.1039/b924784j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandya DN, Kim JY, Park JC, Lee H, Phapale PB, Kwak W, Choi TH, Cheon GJ, Yoon YR, Yoo J. Chem Commun. 2010;46:3517–3519. doi: 10.1039/b925703a. [DOI] [PubMed] [Google Scholar]
- 10.Ferdani R, Stigers DJ, Fiamengo AL, Wei L, Li BT, Golen JA, Rheingold AL, Weisman GR, Wong EH, Anderson CJ. Dalton Trans. 2012;41:1938–1950. doi: 10.1039/c1dt11743b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong EH, Weisman GR, Hill DC, Reed DP, Rogers ME, Condon JS, Fagan MA, Calabrese JC, Lam KC, Guzei IA. J Am Chem Soc. 2000;122:10561–10572. [Google Scholar]
- 12.Liu W, Hao G, Long MA, Anthony T, Hsieh JT, Sun X. Angew Chem, Int Ed. 2009;48:7346–7349. doi: 10.1002/anie.200903556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira CL, Yapp DT, Lamsa E, Gleave M, Bensimon C, Jurek P, Kiefer GE. Nucl Med Biol. 2008;35:875–882. doi: 10.1016/j.nucmedbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Di Bartolo N, Sargeson AM, Smith SV. Org Biomol Chem. 2006;4:3350–3357. doi: 10.1039/b605615f. [DOI] [PubMed] [Google Scholar]
- 15.Pandya DN, Dale AV, Kim JY, Lee H, Ha YS, An GI, Yoo J. Bioconjugate Chem. 2012;23:330–335. doi: 10.1021/bc200539t. [DOI] [PubMed] [Google Scholar]
- 16.Zeng D, Desai AV, Ranganathan D, Wheeler TD, Kenis PJ, Reichert DE. Nucl Med Biol. 2013;40:42–51. doi: 10.1016/j.nucmedbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Ferdani R, Anderson CJ. Bioconjugate Chem. 2012;23:1470–1477. doi: 10.1021/bc300092n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D, Zeglis BM, Lewis JS, Anderson CJ. J Nucl Med. 2013;54:829–832. doi: 10.2967/jnumed.112.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng D, Lee NS, Liu Y, Zhou D, Dence CS, Wooley KL, Katzenellenbogen JA, Welch MJ. ACS Nano. 2012;6:5209–5219. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, et al. Gaussian 03, version C.02. Wallingford, CT: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.