Abstract
Viruses infecting the harmful bloom-causing alga Phaeocystis globosa (Prymnesiophyceae) were readily isolated from Dutch coastal waters (southern North Sea) in 2000 and 2001. Our data show a large increase in the abundance of putative P. globosa viruses during blooms of P. globosa, suggesting that viruses are an important source of mortality for this alga. In order to examine genetic relatedness among viruses infecting P. globosa and other phytoplankton, DNA polymerase gene (pol) fragments were amplified and the inferred amino acid sequences were phylogenetically analyzed. The results demonstrated that viruses infecting P. globosa formed a closely related monophyletic group within the family Phycodnaviridae, with at least 96.9% similarity to each other. The sequences grouped most closely with others from viruses that infect the prymnesiophyte algae Chrysochromulina brevifilum and Chrysochromulina strobilus. Whether the P. globosa viruses belong to the genus Prymnesiovirus or form a separate group needs further study. Our data suggest that, like their phytoplankton hosts, the Chrysochromulina and Phaeocystis viruses share a common ancestor and that these prymnesioviruses and their algal host have coevolved.
Phaeocystis globosa is a phytoplankton species with unicellular and colonial stages that can form dense blooms in temperate coastal waters of the North Sea (up to 108 liter−1 [7, 10, 11]) The ability to generate high biomass, dominate the phytoplankton community for extended periods, and produce dimethylsulfoniopropionate and dimethyl sulfide makes this alga a model species for biogeochemical studies (26, 30). Because its diverse roles in element and trophic dynamics are amplified during blooms, the wax and wane of P. globosa affects the function and structure of pelagic food webs (7). Its influence on pelagic food webs may depend on the factors responsible for bloom termination. Earlier studies have shown that the collapse of P. globosa blooms can be sudden and that cell lysis was a major source of mortality (7, 9). Through cell lysis, high concentrations of organic matter are released into the water column and remineralized by heterotrophs, thereby stimulating the microbial food web and affecting nutrient fluxes (4, 18, 19, 21, 55).
Viruses are known lytic agents of phytoplankton (6, 46). With increased awareness of the potential regulation of phytoplankton dynamics by viruses, efforts have been made to establish virus-phytoplankton model systems in culture. Although virus-like particles have been found in most classes of eukaryotic unicellular photoautotrophs (38, 52), at present, there are only approximately a dozen phytoplankton viruses in culture. Most of these infect bloom-forming species and about half infect harmful algal bloom species (20, 25, 35, 42, 47, 48). Although viruses have been implicated in the demise of phytoplankton blooms (3, 4, 12, 34), there is no evidence that viruses are responsible for the termination of P. globosa blooms.
The present work focuses on the isolation of viruses infecting P. globosa, and the phylogenetic analysis of DNA polymerase gene (pol) sequences. This gene has been shown to be a good phylogenetic marker for inferring genetic relationships among algal viruses (14). The results of the analysis showed that these P. globosa viruses (PgV) form a monophyletic group within the Phycodnaviridae that is most closely related to viruses infecting another prymnesiophyte, Chrysochromulina brevifilum.
MATERIALS AND METHODS
Sampling site and parameters measured.
Samples for the isolation of P. globosa (Prymnesiophyceae) viruses were collected from the southern part of the North Sea (Dutch coastal zone, Fig. 1), an area that experiences annual blooms of P. globosa. Sampling was performed at high tide from a permanent coastal jetty from April to July 2000, in September 2000, and from April to May 2001.
FIG. 1.
Geographical position of the sampling station (indicated by arrow). Samples were taken from a jetty at high tide.
For pigment analysis, samples ranging from 250 ml to 1 liter were filtered on Whatman GF/F filters and stored at −50°C till analysis. Within 3 months, pigments were extracted in 4 ml of 100% methanol buffered with 0.5 M ammonium acetate (resulting in a nominal concentration of 95% methanol) and homogenized for 15 s. Analysis and identification were performed according to the method of Riegman and Kraay (39).
Phytoplankton abundance (<50 μm) was determined on nonfixed, fresh samples using a Coulter XL-MCL flow cytometer equipped with an air-cooled laser at 488 nm and standard filter setup (53). Phytoplankton was identified on the basis of the chlorophyll signal (long pass 610 nm). Data were processed with Coulter software.
Specific phytoplankton lysis rates (expressed as day−1) were estimated according to the dissolved esterase activity assay described by Brussaard et al. (7) and modified by Riegman et al. (40). In short, the method is based on the detection of cytoplasmatic esterase that is released into the water column following cell lysis. Esterase activity was measured on 1.9-ml samples with the addition of 0.1 ml of Tris-EDTA (pH 8; final concentration, 0.5 mM) and 20 μl of fluorescein diacetate (in acetone; final concentration, 20 μM) during 1 h of incubation at 25°C. Fluorescence was converted into fluorescein concentration using an internal standard (fluorescein; final concentration, 5 nM) that was added directly after measurement at t = 1 h. Extracellular esterase activity was obtained from filtered (0.2-μm-pore-size Acrodisc; Gelman Sciences) samples, whereas intracellular esterase activity was deduced from total activity (nonfiltered sample) and filtered (pore size, 0.2 μm) samples. Correction for nonenzymatic hydrolysis of the substrate was obtained by removing esterases from the sample using 10K MWCO centrifuge filters (PES; Nalgene). Finally, data were corrected for the decay of esterase activity in seawater.
Samples for bacteria and viruses were fixed with glutaraldehyde (0.5% final concentration), frozen in liquid nitrogen and stored at −80°C till analysis. For analysis, samples were briefly thawed at 35°C, diluted in Tris-EDTA buffer (pH 8), and stained with SYBR Green I commercial stock (at a final concentration of 0.5 × 10−4 M) for 10 min at 80°C. Samples were analyzed using a Becton Dickinson FACScalibur flow cytometer equipped with a 15-mW, 488-nm air-cooled argon-ion laser (5, 33). The trigger was set on green fluorescence, and the samples were run for 1 min at 35 μl min−1 at an event rate of 200 to 700 events s−1. The listmode files were analyzed using the freeware CYTOWIN (available at http://www.sb-roscoff.fr/Phyto/cyto.html).
Virus isolation.
Different uni-algal strains of P. globosa were used for the isolation of viruses, of which four strains were found to be the most successful (Ph91mf, Pg-G, Pg-I, and Pg01MD-04; all originating from the North Sea and maintained in culture at the Royal Netherlands Institute of Sea Research). P. globosa cells were grown in a 1:1 mixture of f/2 medium (22) and enriched artificial seawater (24), modified by the addition of Tris-HCl and Na2SeO3 (16). Cultures were incubated at 15°C at an irradiance of 50 to 100 μmol photons m−2 s−1 and a light-dark cycle of 14 and 10 h, respectively.
Viruses were isolated from filtered (Whatman GF/F) natural seawater samples that were incubated for a week at in situ temperatures under the same lighting conditions as given above, which was found to promote the isolation of viruses infecting P. globosa (unpublished results). Enrichment of the natural samples with nitrate and orthophosphate (80 and 5 μM, respectively) enhanced the abundance of the P. globosa cells and occasionally facilitated the isolation of viruses (especially when the natural abundance of P. globosa was relatively low due to nutrient depletion). Subsamples of the incubated natural seawater were added to cultures of P. globosa (10 or 50%, vol/vol) and incubated for 10 days, during which algal growth was monitored via in vivo chlorophyll fluorescence using a fluorometer (Turner Designs). Samples from cultures in which lysis occurred were filtered through 0.2-μm-pore-size cellulose acetate filters (Schleicher and Schuell GmbH, Dassel, Germany) and added to exponentially growing P. globosa cultures. Clonal viral isolates were obtained by end point dilution. The presence and increased abundance of larger-size viruses in lysed cultures was confirmed by epifluorescence microscopy (36), flow cytometry (5, 32), and transmission electron microscopy (45). Furthermore, preliminary characterization of the isolated viruses (C. P. D. Brussaard, unpublished data) confirmed that the isolates were algal viruses, thereby discarding the potential of indirect mortality of the algal host due to bacterial lysis.
Phylogenetic analysis.
Of the 24 virus isolates obtained, 7 were used for phylogenetic analysis. Subsamples of 50 ml were concentrated by ultracentrifugation for 2 h at 14,000 × g, and the pelleted viruses were resuspended in 100 μl of Tris (pH 8). Nucleic acids were extracted from 50-ml subsamples of virus-size concentrates using an established hot-cold technique (14). A 681-bp fragment of pol was amplified from the nucleic acids using two separate PCRs. First, 3 μl of virus-size extract was added to a 47-μl PCR mixture containing Taq DNA polymerase assay buffer (50 mM KCl, 20 mM Tris-HCl [pH 8.4]), 1.5 mM MgCl2, a 0.16 mM concentration of each deoxyribonucleoside triphosphate, 10 pmol of the algal-virus-specific primer AVS1 and 30 pmol of AVS2 (13), and 0.625 U of PLATINUM Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, Calif.). The negative control contained all reagents except the template. PCR was carried out using a PCR Express thermal cycler (Hybaid Limited, Ashford, United Kingdom) with the following cycle parameters: denaturation at 95°C for 90 s; 30 cycles of denaturation at 95°C for 45 s; annealing at 45°C for 45 s; and extension at 72°C for 45 s with a final 5-min extension. PCR products were then electrophoresed in 1.8% LE agarose (FMC BioProducts, Rockland, Maine) in 1.0× TAE at 90 V for 75 min. The gels were stained with ethidium bromide and examined on a UV transilluminator. After electrophoresis, a disposable Pasteur pipette was used to excise a single plug from the 700-bp band amplified in each reaction. The plug was placed in 200 μl of sterile 1.0× TAE (40 mM Tris-base, 20 mM sodium acetate, 1 mM EDTA [pH 8.5]) and heated to 65°C for 20 min to elute DNA.
Two microliters of eluted DNA was used in a second stage of PCR to increase product yields. These reactions were identical to first-stage PCR except the number of cycles was limited to 20 to minimize the production of PCR artifacts generated from high cycle number. Second-stage negative controls were conducted using the eluant of plugs excised from the negative control lane of gels of first-stage PCR. Based on a Taq polymerase-induced error of 10−4 errors per cycle per base pair (1), the calculated sequence difference that may be due to Taq misincorporation (using a total of 50 amplification rounds) is 0.5%.
Amplified pol fragments from the second-round PCRs were cloned using a pGEM-T Easy kit (Promega, Madison, Wis.). Plasmid DNA was harvested from transformed Escherichia coli JM109 colonies using a QIAprep spin miniprep kit according to the manufacturer's recommendations (QIAGEN, Valencia, Calif.). Plasmid DNA (300 to 500 ng) and the primers M13 forward or reverse were added to sequencing reactions using AmpliTaq FS BIGDYE Terminator cycle sequencing chemistry (Applied Biosystems, Foster City, Calif.) according to the manufacturer's recommendations. Excess Dye-Terminators were removed from the completed sequencing reactions using CENTRI-SEP spin columns (Princeton Separations, Adelphia, N.J.), and reactions were run in ABI model 373 Stretch or ABI Prism 377 automated sequencers (Applied Biosystems) at the University of British Columbia sequencing facility.
Virus pol sequences were compared to each other and known virus pol genes available in GenBank (accession numbers listed below). Sequences were edited and/or translated using BioEdit (version 5.0.7) (23). Inferred amino acids of the unknown sequences were aligned with virus pol sequences from GenBank using the sequence alignment program CLUSTAL X (50) with the protein weight matrix BLOSUM. The alignment was then edited so that all sequences, with gaps included, were the same length. The alignment of the resulting 307 amino acid positions was used to construct a maximum likelihood tree using the program TREE-PUZZLE version 5.0 (44). Finally, phylogenetic trees were drawn using the program TreeView (Win32) version 1.6.1.
The accession numbers of virus pol sequences used in the phylogenetic analysis are as follows: African swine fever virus (ASFV), X73330; BSA99-1 (unidentified phycodnavirus isolate), AF405577; BSA99-2 (unidentified phycodnavirus isolate), AF405578; BSA99-5 (unidentified phycodnavirus isolate), AF405581; BSB99-2 (unidentified phycodnavirus isolate), AF405588; Chrysochromulina brevifilum virus (CbV) PW1, U32983; CbV-PW3, U32984; Paramecium bursaria Chlorella virus CVA-1, U32985; Epstein-Barr virus (EBV), V01555; Ectocarpus siliculosis virus 1 (EsV-1), AAK14511; Feldmannia sp. virus (FsV), AAB67116; herpes simplex virus 1 (HSV-1), X04771; MIB99-2 (unidentified phycodnavirus isolate), AF405592; Micromonas pusilla virus (MpV-GM1), U32977; MpV-PB6, U32978; MpV-PB7, U32979; MpV-PB8, U32980; MpV-PL1, U32982; MpV-SG1, U32981; MpV-SP1, U32975; MpV-SP1, U32976; Paramecium bursaria Chlorella virus (NY-2A), M86837; OTU2 (unidentified phycodnavirus isolate), U36932; OTU4 (unidentified phycodnavirus isolate), U36934; OTU5 (unidentified phycodnavirus isolate), U36935; Paramecium bursaria Chlorella virus (PBCV-1), M86836; PSB99-1 (unidentified phycodnavirus isolate), AF405593; PSC99-1 (unidentified phycodnavirus isolate), AF405597; PSC99-2 (unidentified phycodnavirus isolate), AF405598; SIA99-1 (unidentified phycodnavirus isolate), AF405590; SO98-1 (unidentified phycodnavirus isolate), AF405572; SO98-2 (unidentified phycodnavirus isolate), AF405573; SO98-3 (unidentified phycodnavirus isolate), AF405574; and SO98-5 (unidentified phycodnavirus isolate), AF405576.
Nucleotide sequence accession numbers.
The virus sequences obtained in this study were deposited in the GenBank database and were assigned accession numbers as follows: PgV-03T, AY345136; PgV-04T, AY345137; PgV-05T, AY345138; PgV-06T, AY345139; PgV-07T, AY345140; PgV-08T, AY345141; PgV-10T, AY345142.
RESULTS AND DISCUSSION
Virus isolation.
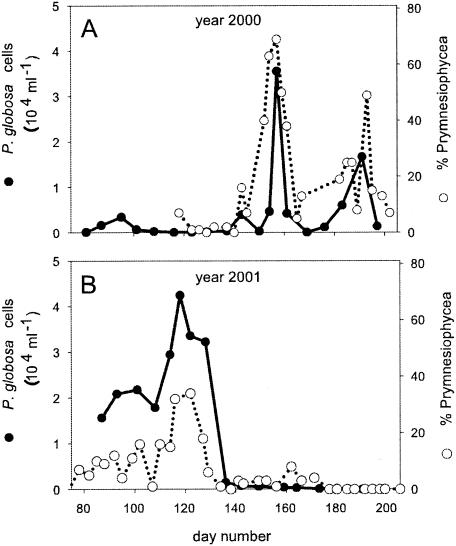
Twenty-four new isolates of viruses that infect P. globosa were obtained during this study. In the year 2000, samples were taken intensely from April until July, which included two short P. globosa blooms (Fig. 2) during which isolation of PgV was most successful. In the year 2001, virus isolates were obtained from the end of April until the beginning of May, directly following a bloom of P. globosa (Fig. 2). The isolation of viruses was not restricted to bloom periods, however, and we were able to isolate PgV during autumn 2000 and early spring 2002, when the abundance of P. globosa had been undetectable or very low (<400 cell ml−1) for months. These findings suggest that at least a significant fraction of PgV under natural conditions can retain infectivity for several months.
FIG. 2.
Dynamics of P. globosa as absolute cell abundances and as percentages of the total phytoplankton community (based on high-performance liquid chromatography pigment analysis) for the years 2000 (A) and 2001 (B). Closed circles represent P. globosa cells (milliliter−1), and open circles represent the percentage of cells belonging to the Prymnesiophyceae (which includes P. globosa) as a percentage of the total phytoplankton community.
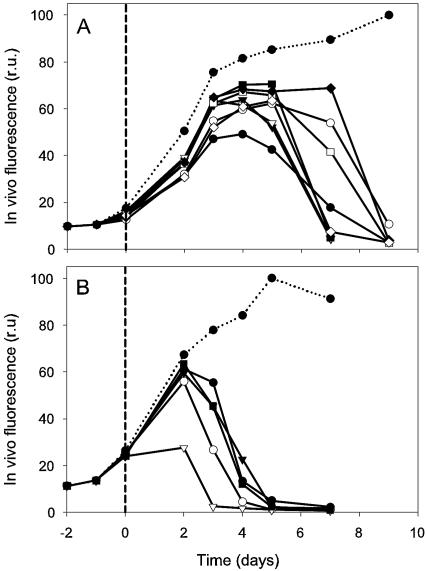
There was a difference in the time course of changes in in vivo fluorescence between cultures of P. globosa that received natural seawater collected in June and July 2000 (Fig. 3). It is likely that the more rapid decrease in in vivo fluorescence in the second experiment is the result of a higher abundance of infectious viruses during the second bloom (July) than the first (June).
FIG. 3.
In vivo chlorophyll fluorescence (relative units) of P. globosa (strain Pg-I) infected by viruses isolated in the year 2000. Viruses were isolated during June (A) and July (B) blooms of P. globosa. The dotted line represents the noninfected control culture, and the solid line represents algal cultures in which lysis occurred upon receiving equal additions of natural water. Short dashed lines indicate time of infection (t = 0).
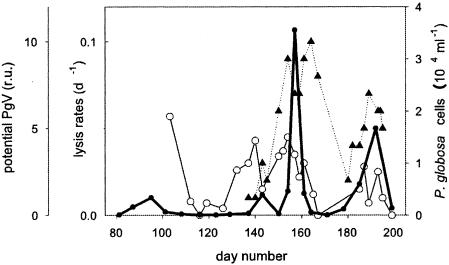
Flow cytometric estimates indicated that the abundance of putative PgV increased during blooms of P. globosa and peaked during periods of enhanced algal cell lysis (Fig. 4). The rates of phytoplankton cell lysis in 2000 were comparable to other years during relatively small blooms of P. globosa (9). An increased rate of algal lysis in conjunction with an increase in the abundance of putative PgV suggests that lysis of host cells was releasing virus particles. Estimates of the abundance of putative PgV were based on the flow cytometric characteristics of PgV isolates after staining with a nucleic acid-specific dye (C. P. D. Brussaard, unpublished results). PgV differed from the other natural viruses in their enhanced green fluorescence and scatter signals (5, 8, 32). During the entire P. globosa bloom in 2000, total virus abundance varied between 4 × 107 and 108 cells ml−1 (data not shown), of which 0.5 to 2.5% were considered to be PgV. During bloom maxima the abundance of putative PgV was about 100-fold higher than the abundance of P. globosa cells (Fig. 4), suggesting that PgV should be regarded as important agents of mortality for P. globosa. The dynamics in PgV abundance (Fig. 4), furthermore, suggests their importance as a mortality agent of P. globosa.
FIG. 4.
Development of phytoplankton cell lysis and potential PgV in relation to development of blooms of P. globosa in the year 2000. The abundance of putative PgV was determined from their flow cytometric fluorescence characteristics, matching the signatures with those of PgV isolates. Virus data were standardized to the abundance on day 140, the start of the bloom period. The thick line represents P. globosa cells, closed triangles represent the relative abundance of putative PgV (relative units [r.u.]), and open circles represent the total phytoplankton lysis rates.
The dynamics of PgV also indicates a significant loss rate of the viral particles. We speculate that the ghost colonies of P. globosa at the end of the bloom remove free virus particles from the water column by passively trapping them in the sticky colonial mucus. Blooms of P. globosa consist of colonies and single cells, but due to nutrient or light limitation colony formation is stopped and colonial cells are shed into the surrounding water (7, 9, 37), leaving ghost colonies to trap the viruses. Light became limiting for colony formation (mean water column) photosynthetically active radiation values of <100 W h m−2 day−1, (37) around day 170 in 2000 and day 130 in 2001 due to extensive cloud cover and stormy weather, respectively. The subsequent formation of empty ghost colonies probably promoted the removal of free viruses (Fig. 4).
Phylogenetic analysis.
Most characterized phytoplankton viruses are assigned to the family Phycodnaviridae (51), a family of large double-stranded (dsDNA) viruses that infect microalgae. However, relationships among algal viruses cannot be determined from morphological characteristics alone as many phytoplankton viruses look alike. Therefore, molecular analysis is required. The highly conserved DNA polymerase gene is a good genetic marker for examining evolutionary relationships among a number of large dsDNA viruses (2, 14, 54) and has also been shown to be useful for making phylogenetic inferences among algal viruses belonging to the Phycodnaviridae (13, 15, 29, 43). Evolutionary relationships among the dsDNA phytoplankton viruses and other dsDNA viruses indicated that the many algal viruses form a monophyletic group, suggesting that the Phycodnaviridae share a common ancestor (14). Furthermore, phylogenetic analysis of Phycodnaviridae based on DNA pol sequences indicated that viruses clustered according to the microalgal host infected (14, 43).
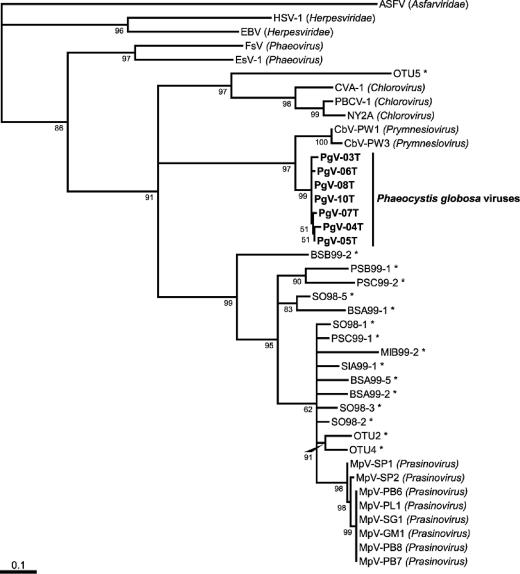
The algal virus-specific AVS1 and AVS2 primers were used to amplify the DNA pol gene from seven virus isolates infecting P. globosa (including representatives from all blooms of P. globosa for both years of isolation), coded PgV-03T to PgV-08T and PgV-10T. The virus isolates PgV-06T, PgV-08T, and PgV-10T differed by only 0.5% in their nucleotide sequences, suggesting that this may be the result of Taq polymerase misincorporation (see Materials and Methods). Although the rest of the sequences were all also very similar, they were more than 0.5% different from each other, and therefore, these differences cannot be explained by the methodological error. The molecular phylogeny of the seven virus isolates using inferred amino acid sequences of the DNA pol fragments demonstrated that the PgV clustered within a distinct monophyletic group compared to other eukaryotic algal viruses (Phycodnaviridae) (Fig. 5). No amplification products were obtained from the control host DNA.
FIG. 5.
Maximum likelihood tree of putative PgV by use of inferred amino acid sequences of dsDNA viruses with quartet puzzling support values to the lower left of corresponding nodes. Virus pol fragment sequences obtained in this study are shown in boldface type. The genera of virus isolates are shown in parentheses beside the designation, while uncultivated environmental virus sequences from previous studies are indicated with an asterisk. Virus sequences from the classes Herpesviridae and Asfarviridae are included as outgroups. The GenBank accession numbers of all sequences are provided in Materials and Methods. The scale bar is a relative measure of evolutionary distance and corresponds to a distance of 0.1 substitution per 100 nucleotide positions.
The alignment of DNA pol fragments (Fig. 5) indicates that only a few of the 226 aligned amino acids (681-bp amplified segment of the DNA polymerase gene) differed among the PgV. Two amino acid motifs (ESIDFGDG and the GIMPKILQRL) were even identical. The uniqueness of these regions compared to other eukaryotic virus pol genes may permit the development of a PgV-specific primer. Although sequences from the viruses PgV-08T and PgV-10T were identical, they were not isolated in the same time period. As PgV-08T was isolated in early summer and PgV-10T in autumn 2000, it suggests the persistence of this virus.
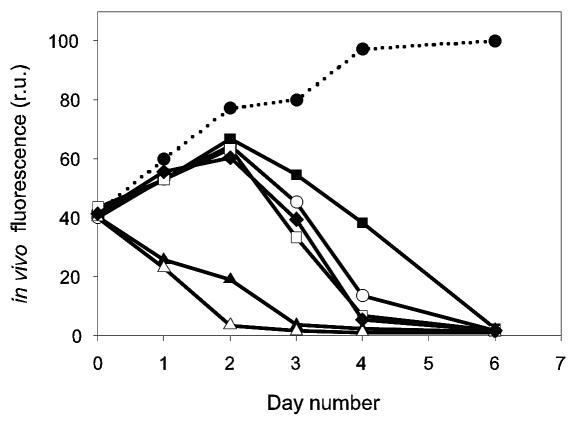
The DNA pol fragments of the viruses examined in this study are very similar, at least 96.9% identical to each other, yet the viruses differ in the onset of lysis of the algal host (Fig. 6). Using comparable virus titers, two different patterns for the in vivo fluorescence of the infected algal cultures (same strain of P. globosa used) were observed; one indicating a fast lytic cycle with lysis starting within a day and the other with a lytic cycle that is approximately twice as long. This provides further evidence for differences among the virus clones analyzed. Previous studies have demonstrated that viruses that infect the same algal species can differ in host range, length of lytic cycle, and particle size (4, 7, 10, 17, 31, 41, 49). Because DNA polymerase genes are highly conserved, discrepancies in the similarity of different viruses based on gene sequences, virion properties, and biological properties may be the norm rather than the exception.
FIG. 6.
In vivo fluorescence (relative units) of virus-infected P. globosa cultures (strain Pg-I) compared to a noninfected culture (dotted line). Open circles represent virus clone PgV-03T, filled squares represent PgV-04T, open squares represent PgV-05T, filled triangles represent PgV-06T, open triangles represent PgV-07T, and filled diamonds represent PgV-10T. Using comparable virus titers, the differences in onset of viral lysis of the P. globosa cultures indicate that (at least) two different virus types were isolated.
The closest relatives to the sequences obtained in this study were sequences from C. brevifilum-infecting viruses (CbV) belonging to the genus Prymnesiovirus (51). The alga C. brevifilum belongs, as does P. globosa, to the class Prymnesiophyceae (also referred to as Haptophytes). To judge whether the PgV isolated in this study belong to the genus Prymnesiovirus or form a separate genus needs further characterization.
Our data suggest that, like their phytoplankton hosts, Chrysochromulina and Phaeocystis viruses share a common ancestor; therefore, we can speculate that at least some phycodnaviruses and their host phytoplankton have coevolved. Phytoplankton belonging to the Prymnesiophyceae are characterized by two flagella and a haptonema (specialized thin filamentous appendage for attachment), and flagellated cells are covered by organic scales (e.g., Chrysochromulina spp. and Phaeocystis spp.) that can be calcified (e.g., Emiliania huxleyi). It is noteworthy that differences in scale types between Chrysochromulina, Phaeocystis, and Emiliania may be related to differences between the viral pathogens of these prymnesiophytes. Recently, viruses infecting E. huxleyi have been characterized and based on DNA pol sequences do not form a monophyletic group with other viruses infecting Prymnesiophyceae (43). Interestingly, the viruses isolated in this study (PgV) are closely related to CbV and the hosts of both of these types of viruses have similar scale types.
Studies of the molecular phylogeny (using the ITS1 region) of Phaeocystis show that the species P. globosa, Phaeocystis antarctica, and Phaeocystis pouchetii are monophyletic, and the cold-water species P. antarctica and P. pouchetii cluster within this clade (27, 28). This distinction between P. globosa and the cold-water species of Phaeocystis might extend to the viruses infecting these algae, as attempts to amplify DNA pol fragments using AVS1 and AVS2 from viruses that infect P. pouchetii have not succeeded (G. Bratbak, personal communication; this study). It appears that P. globosa and P. pouchetii and the viruses that infect them differ in their biogeographical and evolutionary history.
We have demonstrated that viruses infecting the bloom-forming alga P. globosa can be readily isolated from waters in which the alga occurs. Based on the increase in putative PgV during bloom events, viral lysis appears to be a significant source of mortality. Despite differences among virus isolates in the rates at which they caused cell lysis in infected cultures, the viruses were very similar based on sequence analysis of DNA polymerase genes. Identical sequences from virus clones isolated one year apart and isolation of PgV during periods with very low host abundance suggest stability of some virus populations. Phylogenetic analysis of DNA pol sequences indicates that PgV form a tight monophyletic group, with the closest relative being CbV.
Acknowledgments
This work was supported in part by a grant from the European Commission Research Programs Environment & Sustainable Development to C.P.D.B (under contract EVK3-CT-1999-00015 BIOHAB) and a Natural Science and Engineering Research Council (NSERC) research grant to C.A.S.
Special thanks go to Gerhard Cadee and Marcel Veldhuis for making available the Phaeocystis counts and the Secchi data. We thank Louis Peperzak for providing information on irradiance; Amy Chan for confirmation of the presence of the algal virus particles by transmission electron microscopy; and Arjen Kop, Anna Noordeloos, and Anne-Claire Baudoux for their assistance. The strains of P. globosa used in this study were kindly provided by Louis Peperzak (Ph91mf) and Jacqueline Stefels (Pg-G and Pg-I) or were isolated by C.P.D.B. from the Dutch coastal waters during early spring 2001 (Pg01MD-04).
REFERENCES
- 1.Bracho, M. A., A. Moya, and E. Barrio. 1998. Contribution of Taq-polymerase-induced errors to the estimation of RNA virus diversity. J. Gen. Virol. 79:2921-2928. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite, D. K., and J. Ito. 1993. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 21:787-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratbak, G., J. K. Egge, and M. Heldal. 1993. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 93:39-48. [Google Scholar]
- 4.Brussaard, C. P., R. S. Kempers, A. J. Kop, R. Riegman, and M. Heldal. 1996. Virus-like particles in a summer bloom of Emiliania huxleyi in the North Sea. Aquat. Microb. Ecol. 10:105-113. [Google Scholar]
- 5.Brussaard, C. P., D. Marie, and G. Bratbak. 2000. Flow cytometric detection of viruses. J. Virol. Methods 85:175-182. [DOI] [PubMed] [Google Scholar]
- 6.Brussaard, C. P. D. 2004. Viral control of phytoplankton populations: a review. J. Eukaryot. Microbiol. 125-138. 51: [DOI] [PubMed]
- 7.Brussaard, C. P. D., G. J. Gast, F. C. Van Duyl, and R. Riegman. 1996. Impact of phytoplankton bloom magnitude on a pelagic microbial food web. Mar. Ecol. Prog. Ser. 144:211-221. [Google Scholar]
- 8.Brussaard, C. P. D., D. Marie, R. Thyrhaug, and G. Bratbak. 2001. Flow cytometric analysis of phytoplankton viability following viral infection. Aquat. Microb. Ecol. 26:157-166. [Google Scholar]
- 9.Brussaard, C. P. D., R. Riegman, A. A. M. Noordeloos, G. C. Cadée, H. J. Witte, A. J. Kop, G. Nieuwland, F. C. Van Duyl, and R. P. M. Bak. 1995. Effects of grazing, sedimentation and phytoplankton cell lysis on the structure of a coastal pelagic food web. Mar. Ecol. Prog. Ser. 123:259-271. [Google Scholar]
- 10.Cadée, G. C. 1992. Trends in Marsdiep phytoplankton. Neth. Inst. Sea Res. Pub. Ser. 20:143-149. [Google Scholar]
- 11.Cadée, G. C., and J. Hegeman. 1986. Seasonal and annual variation in Phaeocystis pouchetii (Haptophyceae) in the westernmost inlet of the Wadden Sea during the 1973 to 1985 period. Neth. J. Sea Res. 20:29-36. [Google Scholar]
- 12.Castberg, T., A. Larsen, R.-A. Sandaa, C. P. D. Brussaard, J. K. Egge, M. Heldal, R. Thyrhaug, E. J. Van Hannen, and G. Bratbak. 2001. Microbial population dynamics and diversity during a bloom of the marine coccolithophorid Emiliania huxleyi (Haptophyta). Mar. Ecol. Prog. Ser. 221:39-46. [Google Scholar]
- 13.Chen, F., and C. A. Suttle. 1995. Amplification of DNA polymerase gene fragments from viruses infecting microalgae. Appl. Environ. Microbiol. 61:1274-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, F., and C. A. Suttle. 1996. Evolutionary relationships among large double-stranded DNA viruses that infect microalgae and other organisms as inferred from DNA polymerase genes. Virology 219:170-178. [DOI] [PubMed] [Google Scholar]
- 15.Chen, F., C. A. Suttle, and S. M. Short. 1996. Genetic diversity in marine algal virus communities as revealed by sequence analysis of DNA polymerase genes. Appl. Environ. Microbiol. 62:2869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottrell, M. T., and C. A. Suttle. 1991. Wide-spread occurrence and clonal variation in viruses which cause lysis of a cosmopolitan, eukaryotic marine phytoplankter, Micromonas pusilla. Mar. Ecol. Prog. Ser. 78:1-9. [Google Scholar]
- 17.Cottrell, M. T., and C. A. Suttle. 1995. Genetic diversity of algal viruses which lyse the photosynthetic picoflagellate Micromonas pusilla (Prasinophyceae). Appl. Environ. Microbiol. 61:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman, J. A., and C. A. Suttle. 1993. Viruses in marine planktonic systems. Oceanography 6:51-63. [Google Scholar]
- 20.Garry, R. T., P. Hearing, and E. M. Cosper. 1998. Characterization of a lytic virus infectious to the bloom-forming microalga Aureococcus anophagefferens (Pelagophyceae). J. Phycol. 34:616-621. [Google Scholar]
- 21.Gobler, C. J., D. A. Hutchins, N. S. Fisher, E. M. Cosper, and S. A. SanudoWilhelmy. 1997. Release and bioavailability of C, N, P, Se, and Fe following viral lysis of a marine chrysophyte. Limnol. Oceanogr. 42:1492-1504. [Google Scholar]
- 22.Guillard, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29-60. In W. L. Smith and M. H. Chanley (ed.), Culture of marine invertebrate animals. Plenum Press, New York, N.Y.
- 23.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 24.Harrison, P. J., R. E. Waters, and F. J. R. Taylor. 1980. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 16:28-35. [Google Scholar]
- 25.Jacobsen, A., G. Bratbak, and M. Heldal. 1996. Isolation and characterization of a virus infecting Phaeocystis pouchetii (Prymnesiophyceae). J. Phycol. 32:923-927. [Google Scholar]
- 26.Lancelot, C., P. Wassmann, and H. Barth. 1994. Ecology of Phaeocystis-dominated ecosystems. J. Mar. Syst. 5:1-4. [Google Scholar]
- 27.Lange, M., Y.-Q. Chen, and L. K. Medlin. 2002. Molecular genetic delineation of Phaeocystis species (Prymnesiophyceae) using coding and non-coding regions of nuclear and plastid genomes. Eur. J. Phycol. 37:77-92. [Google Scholar]
- 28.Lange, M., and L. K. Medlin. 2002. Design and testing of ITS probes for distinguishing Phaeocystis species. Protist 153:275-282. [DOI] [PubMed] [Google Scholar]
- 29.Lee, A. M., R. G. Ivey, and R. H. Meints. 1998. The DNA polymerase gene of a brown algal virus: structure and phylogeny. J. Phycol. 34:608-615. [Google Scholar]
- 30.Liss, P. S., G. Malin, S. M. Turner, and P. M. Holligan. 1994. Dimethyl sulphide and Phaeocystis: a review. J. Mar. Syst. 5:41-53. [Google Scholar]
- 31.Lu, J.-R., F. Chen, and R. E. Hodson. 2001. Distribution, isolation, host specificity, and diversity of cyanophages infecting marine Synechococcus spp. in river estuaries. Appl. Environ. Microbiol. 67:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marie, D., C. P. D. Brussaard, R. Thyrhaug, G. Bratbak, and D. Vaulot. 1999. Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl. Environ. Microbiol. 65:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marie, D., F. Partensky, D. Vaulot, and C. P. Brussaard. 1999. Enumeration of phytoplankton, bacteria, and viruses in marine samples, p. 11.11.1-11.11.15. In J. P. Robinson et al. (ed.), Current protocols in cytometry, suppl. 10. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 34.Nagasaki, K., M. Ando, I. Imai, S. Itakura, and Y. Ishida. 1994. Virus-like particles in Heterosigma akashiwo (Raphidophyceae): a possible red tide disintegration mechanism. Mar. Biol. 119:307-312. [Google Scholar]
- 35.Nagasaki, K., and M. Yamaguchi. 1997. Isolation of a virus infectious to the harmful bloom causing microalga Heterosigma akashiwo (Raphidophyceae). Aquat. Microb. Ecol. 13:135-140. [Google Scholar]
- 36.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 37.Peperzak, L. 1993. Daily irradiance governs growth rate and colony formation of Phaeocystis (Prymnesiophyceae). J. Plankton Res. 15:809-821. [Google Scholar]
- 38.Proctor, L. M. 1997. Advances in the study of marine viruses. Microsc. Res. Tech. 37:136-161. [DOI] [PubMed] [Google Scholar]
- 39.Riegman, R., and G. W. Kraay. 2001. Phytoplankton community structure derived from HPLC analysis of pigments in the Faroe-Shetland Channel during summer 1999: the distribution of taxonomic groups in relation to physical/chemical conditions in the photic zone. J. Plankton Res. 23:191-205. [Google Scholar]
- 40.Riegman, R., J. D. L. Van Bleijswijk, and C. P. D. Brussaard. 2002. The use of dissolved esterase activity as a tracer of phytoplankton lysis. Limnol. Oceanogr. 47:916-920. [Google Scholar]
- 41.Sahlsten, E. 1998. Seasonal abundance in Skagerrak-Kattegat coastal waters and host specificity of viruses infecting the marine photosynthetic flagellate Micromonas pusilla. Aquat. Microb. Ecol. 16:103-108. [Google Scholar]
- 42.Sandaa, R.-A., M. Heldal, T. Castberg, R. Thyrhaug, and G. Bratbak. 2001. Isolation and characterization of two viruses with large genome size infecting Chrysochromulina ericina (Prymnesiophyceae) and Pyramimonas orientalis (Prasinophyceae). Virology 290:272-280. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder, D. C., J. Oke, G. Malin, and W. H. Wilson. 2002. Coccolithovirus (Phycodnaviridae): characterization of a new large dsDNA algal virus that infects Emiliania huxleyi. Arch. Virol. 147:1685-1698. [DOI] [PubMed] [Google Scholar]
- 44.Strimmer, K., and A. Von Haeseler. 1996. Quartet puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 45.Suttle, C. A. 1993. Enumeration and isolation of viruses, p. 121-134. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. cole (ed.), Handbook of methods in aquatic ecology. Lewis Publishers, Inc., Boca Raton, Fla.
- 46.Suttle, C. A. 2000. Ecological, evolutionary, and geochemical consequences of viral infection of cyanobacteria and eukaryotic algae, p. 247-296. In C. J. Hurst (ed.), Viral ecology, 1st ed., vol. 1. Academic Press, San Diego, Calif. [Google Scholar]
- 47.Suttle, C. A., and A. M. Chan. 1995. Viruses infecting the marine prymnesiophyte Chrysochromulina spp.: isolation, preliminary characterization and natural abundance. Mar. Ecol. Prog. Ser. 118:275-282. [Google Scholar]
- 48.Tarutani, K., K. Nagasaki, S. Itakura, and M. Yamaguchi. 2001. Isolation of a virus infecting the novel shellfish-killing dinoflagellate Heterocapsa circularisquama. Aquat. Microb. Ecol. 23:103-111. [Google Scholar]
- 49.Tarutani, K., K. Nagasaki, and M. Yamaguchi. 2000. Viral impacts on total abundance and clonal composition of the harmful bloom-forming phytoplankton Heterosigma akashiwo. Appl. Environ. Microbiol. 66:4916-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequences alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Etten, J. L. 2000. Family Phycodnaviridae, p. 183-192. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 52.Van Etten, J. L., L. C. Lane, and R. H. Meints. 1991. Viruses and viruslike particles of eukaryotic algae. Microbiol. Rev. 55:586-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldhuis, M. J. W., and G. W. Kraay. 2000. Application of flow cytometry in marine phytoplankton research: current applications and future perspectives. Sci. Mar. 64:121-134. [Google Scholar]
- 54.Villarreal, L. P., and V. R. DeFilippis. 2000. A hypothesis for DNA viruses as the origin of eukaryotic replication proteins. J. Virol. 74:7079-7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelm, S. W., and C. A. Suttle. 1999. Viruses and nutrient cycles in the sea. BioScience 49:781-788. [Google Scholar]