Summary
Spironolactone and eplerenone are widely used as mineralocorticoid antagonists. Spironolactone has several nonspecific actions including inhibition of androgen receptor and steroid hormone biosynthesis. While studies have shown that eplerenone does not exhibit nonspecific actions on androgen receptor, its effects on steroid hormone production have not been reported. Herein, the effects of eplerenone (0.1 to 30 µM) and spironolactone (0.1 to 30 µM) on steroid production were examined in human adrenocortical H295R cells. Spironolactone inhibited basal production of cortisol (91%) and aldosterone (53%). Treatment of H295R cells with angiotensin II (Ang II) for 24 h increased aldosterone production by 11-fold. Spironolactone inhibited Ang II stimulation of aldosterone production by 80%. Addition of pregnenolone increased aldosterone (9-fold) and cortisol (3-fold) production. Spironolactone inhibited pregnenolone metabolism to aldosterone (67%) and cortisol (74%). The inhibitory effects of spironolactone occurred at concentrations far higher than those needed to block mineralocorticoid receptor, suggesting an action directly on the enzymes involved in steroid production. In contrast, eplerenone did not inhibit basal, Ang II, forskolin, pregnenolone-stimulated cortisol or aldosterone production. Together, these data demonstrate that opposed to spironolactone, pharmacologic concentrations of eplerenone do not inhibit adrenal cell aldosterone or cortisol production.
Keywords: aldosterone, cortisol, angiotensin, mineralocorticoid receptor blocker, steroid
Introduction
There is increasing evidence that aldosterone can have an effect on vascular remodeling, collagen formation, and endothelial function in addition to its effect on blood pressure [1–5]. A role for myocardial mineralocorticoid receptor (MR) in cardiac function is also highlighted by the improvement of heart failure and cardiac fibrosis when the expression of MR is down-regulated [6] and the susceptibility to arrhythmias is over-expression [7]. Similarly, heterologous expression of 11β-hydroxysteroid dehydrogenase-2 which allows aldosterone to occupy cardiac MR in the myocardium of transgenic mice, also leads to dilated cardiomyopathy [8]. These actions of the MR contribute substantively to the pathophysiology of congestive heart failure, and progressive renal dysfunction [9]. This insight has spurred interest in new treatment strategies to block the MR and the deleterious effects of aldosterone, not just because of its diuretic effect, but primarily because of its potential cardiovascular and renal protective effects [10–12].
Several important clinical trials support the cardio-protective effects of aldosterone blockade in patients with heart failure, such as the Randomized Aldactone Evaluation Study (RALES) [13] and the Eplerenone Post-AMI Heart Failure Efficacy and Survival Study (EPHESUS) [14]. Although spironolactone (Aldactone) is an effective anti-aldosterone agent, its widespread use in humans is limited by its tendency to produce undesirable side-effects. Doses of spironolactone above 100 mg per day are a significant cause of impotence and gynecomastia in men [15, 16]. These adverse effects are primarily due to the binding of spironolactone to androgen receptors and are a substantial cause of drug discontinuation [17]. In prolonged spironolactone-treated patients, spironolactone bodies were detected in the zona glomerulosa of human and the adrenal cortex of rat, although its functional significance remains to be elucidated [18, 19]. There is, however considerable evidence that spironolactone inhibits adrenal cell aldosterone production [20–23]. It is also well documented that spironolactone binds and blocks cytochrome P450 enzymes involved in steroidogenesis [24–26].
Eplerenone is the only clinically available agent in a new class of selective MR blockers. Its chemical structure differs from spironolactone by replacement of the 17α-thioacetyl group with a carbomethoxy group. Eplerenone is a competitive antagonist of the MR that effectively blocks aldosterone action [27]. Like spironolactone, eplerenone has been shown to be effective in antihypertensive therapy in controlling blood pressure similar to inhibitors of angiotensin converting enzyme (ACE) [14, 28], angiotensin type I receptor (AT1R) [29], and calcium-channels [30]. While previous studies suggest that eplerenone has less non-target interaction with progesterone or androgen receptor, the effects of eplerenone on adrenal steroid production have not been reported. In the current study, we compared the effects of spironolactone and eplerenone on steroidogenesis in the cultured human adrenal cell model, H295R cells [31]. Consistent with previous studies, spironolactone inhibited adrenal cell steroid production. In contrast, eplerenone had no effect on adrenal cell production of aldosterone or cortisol.
Material & Methods
Cell Culture
H295R human adrenocortical tumor cells were cultured in Dulbecco's modified Eagles/Ham's F12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% cosmic calf serum (Hyclone, Logan, UT) and antibiotics. Quantification of cell number was accomplished using a Z1 series Coulter counter (Beckman, CA). Spironolactone, Ang II, aldosterone and forskolin were obtained from Sigma. Eplerenone was generously provided by Pfizer (New York, NY).
Steroid Assays
Media concentrations of cortisol and aldosterone, were determined by enzyme immunoassay kits (Diagnostic Systems Laboratories Inc, TX). The well washing was accomplished using an ELX50 automatic microplate washer (Bio-Tek Instruments Inc, VT). The absorbance of the solution was read using an ELX800 microplate reader (Bio-Tek Instruments Inc, VT).
MR transactivation assay
MR transactivation activity was examined using human embryonic kidney 293 cells, 293T/17 (American Type Culture Collection CRL no. 1573), which were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% (v/v) fetal bovine serum with antibiotics. The MR/MMTV-luciferase receptor reporter system was composed of pXM4-MR plasmid which specifies the full-length human MR with a C-terminal nanopeptide hemagglutinin epitope tag (provided by Stefan Andersson) [32, 33], and a MR responsive mouse mammary tumor virus luciferase reporter vector (pMMTV-luc), and a control β-galactosidase plasmid (pSV-β-galactosidase) (Promega, WI). On day 0, 100,000 HEK-293 cells/well were plated onto 24-well dishes. On day 1, cells were transfected with a mixture of plasmid DNA (1 µg/well) consisting of pXM4-MR (180 ng), pMMTV-luc (720 ng), and pSV-β-galactosidase (50 ng), for 6 h using the transfection reagent Transfast (Promega, Madison, WI) according to the manufacturer’s directions. Forty-eight hours after transfection, spironolactone or eplerenone were added at concentrations ranging from 0.03 to 30 µM. After an additional 6 h incubation, the cells were lysed and assayed for luciferase activity (Promega, Madison, WI) and β-galactosidase activity (Tropix, Bedford, MA) according to the manufacturer’s instructions using a Fluostar Optima Microplate Luminometer (BMG Labtech, Inc, NC). Relative level of transactivation was calculated by dividing luciferase units by β-galactosidase units.
Statistical Analysis
Each experiment was repeated a minimum of three times with three replicates within each experiment. Mean values from each experiment were analyzed and compared to basal control values using the ANOVA test with the SigmaStat 3.1 software package (SPSS, Chicago, IL). Results were considered significantly different when p value was 0.05. The concentration of ligand that resulted in 50% of maximal activation of MR (EC50) was calculated using GraphPad Prism 4 software package (GraphPad Software, Inc, CA).
Results
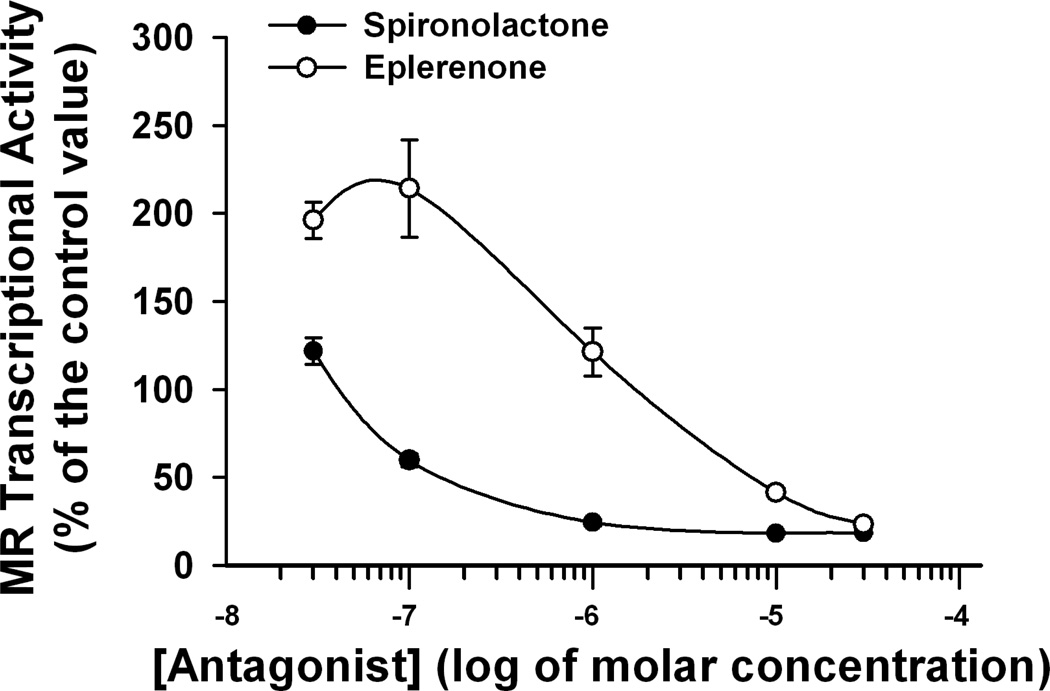
The anti-MR efficiency of spironolactone and eplerenone was tested using a cell culture model expressing MR and a MR-luciferase reporter. Both antagonists successfully suppressed aldosterone-stimulated MR reporter activity; however, spironolactone was a more potent MR blocker than eplerenone (Fig 1). The EC50 values of spironolactone and eplerenone were 0.04 µM and 2 µM, respectively.
Figure 1.
The effects of eplerenone (open circles) and spironolactone (solid circles) on MR transactivation by aldosterone (1 nM) in HEK-293T/17 cells. Expression plasmids for human MR and -galactosidase were transfected into HEK-293T/17 cells together with a mineralocorticoid responsive MMTV-luciferase reporter plasmid. Luciferase and -galactosidase enzyme activities were measured in cell lysates after incubation with aldosterone (1 nM) for 6 h. Basal luciferase activity was 19 % of that seen with aldosterone treatment. Mean values are based on data from three independent experiments.
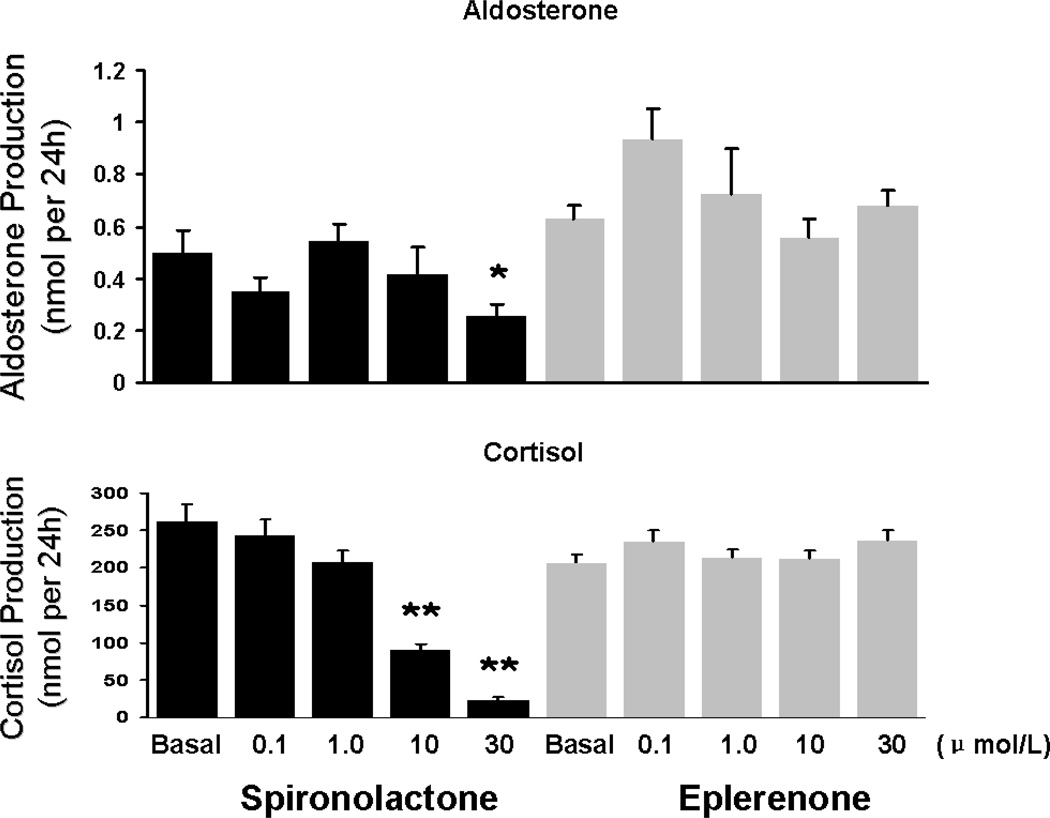
To examine the effects of sprironolactone and eplerenone on adrenal cell steroid production, H295R cells were incubated for 24 h with and without the MR antagonists. Spironolactone (0.1–30 µM) caused a concentration-dependent inhibition of the basal production of both cortisol (91% at 30 µM) and aldosterone (53% at 30 µM) (Fig 2). On the other hand, eplerenone (0.1–30 µM) did not significantly affect basal cortisol (200 nmol/24 h) or basal aldosterone (0.6 nmol/24 h) production (Fig 2).
Figure 2.
The effects of eplerenone and spironolactone on basal aldosterone and cortisol production. H295R adrenal cells were incubated with eplerenone or spironolactone at the indicated concentrations for 24 h. Media concentrations of cortisol and aldosterone were measured by EIA. Values represent data from three independent experiments each ran in triplicate. *: p<0.05 **: p<0.01 vs. basal.
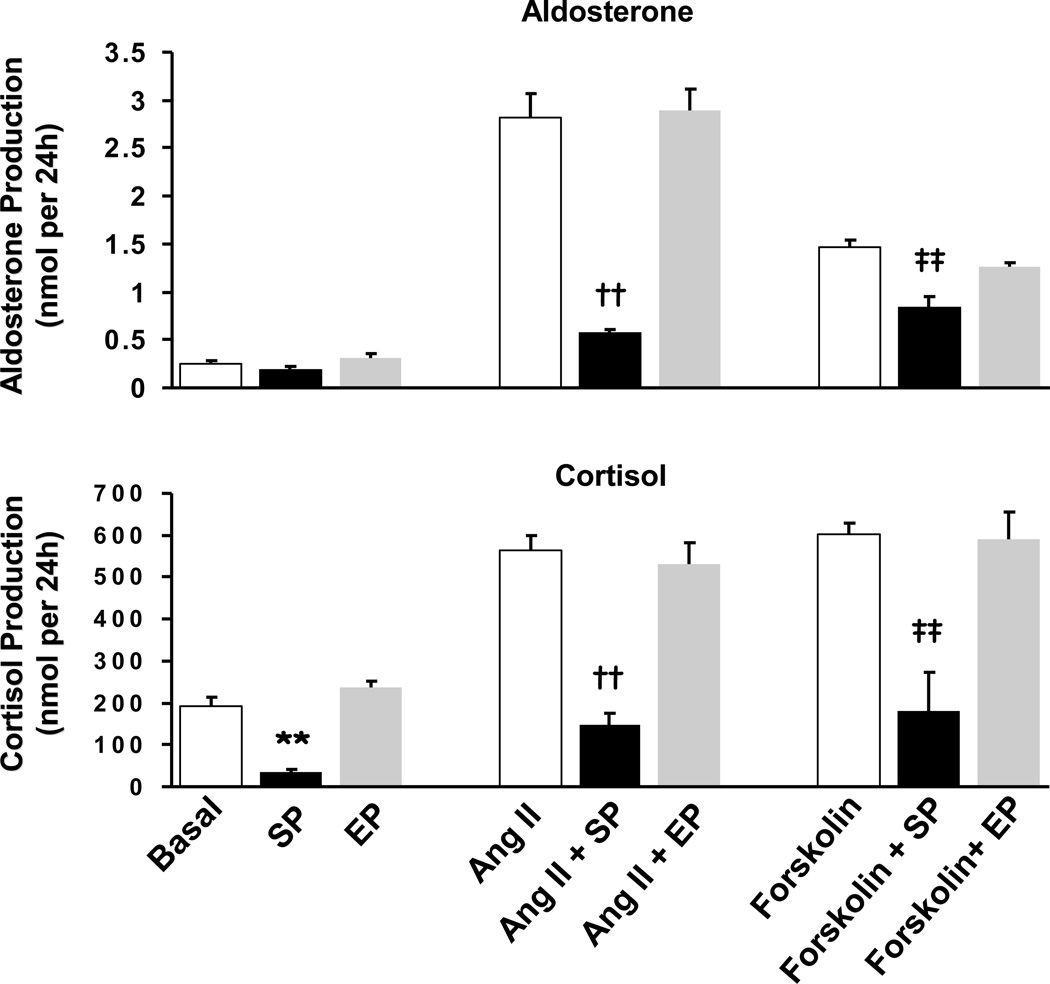
To test the effects of spironolactone and eplerenone on agonist-stimulated adrenal cell steroidogenesis, H295R cells were treated with Ang II or forskolin for 24 h. Ang II (100 nM) treatment stimulated aldosterone production by 11-fold and cortisol production by 3-fold (Fig 3). Spironolactone (30 µM) inhibited Ang II-stimulated aldosterone production by 80% and Ang II-stimulated cortisol production by 74% (Fig 3). Treatment with forskolin (10 µM) for 24 h stimulated cortisol production by 3-fold and aldosterone production by 6-fold (Fig 3). Spironolactone blocked the forskolin-stimulated cortisol production by 70% (Fig 3). On the other hand, eplerenone (30 µM) had no influence on basal, Ang II or forskolin stimulated aldosterone or cortisol production (Fig 3).
Figure 3.
The effects of spironolactone and eplerenone on Ang II and forskolin stimulated aldosterone and cortisol production. H295R adrenal cells were stimulated with Ang II (100 nM) or forskolin (10 µM) with or without eplerenone (30 µM) or spironolactone (30 µM) for 24 h. Media concentrations of cortisol and aldosterone were measured by EIA. Values represent data from three independent experiments each ran in triplicate. SP: spironolactone, EP: eplerenone, *: p<0.05 **: p<0.01 vs. basal, ††: p<0.01 vs. Ang II, ‡‡: p<0.01 vs. forskolin.
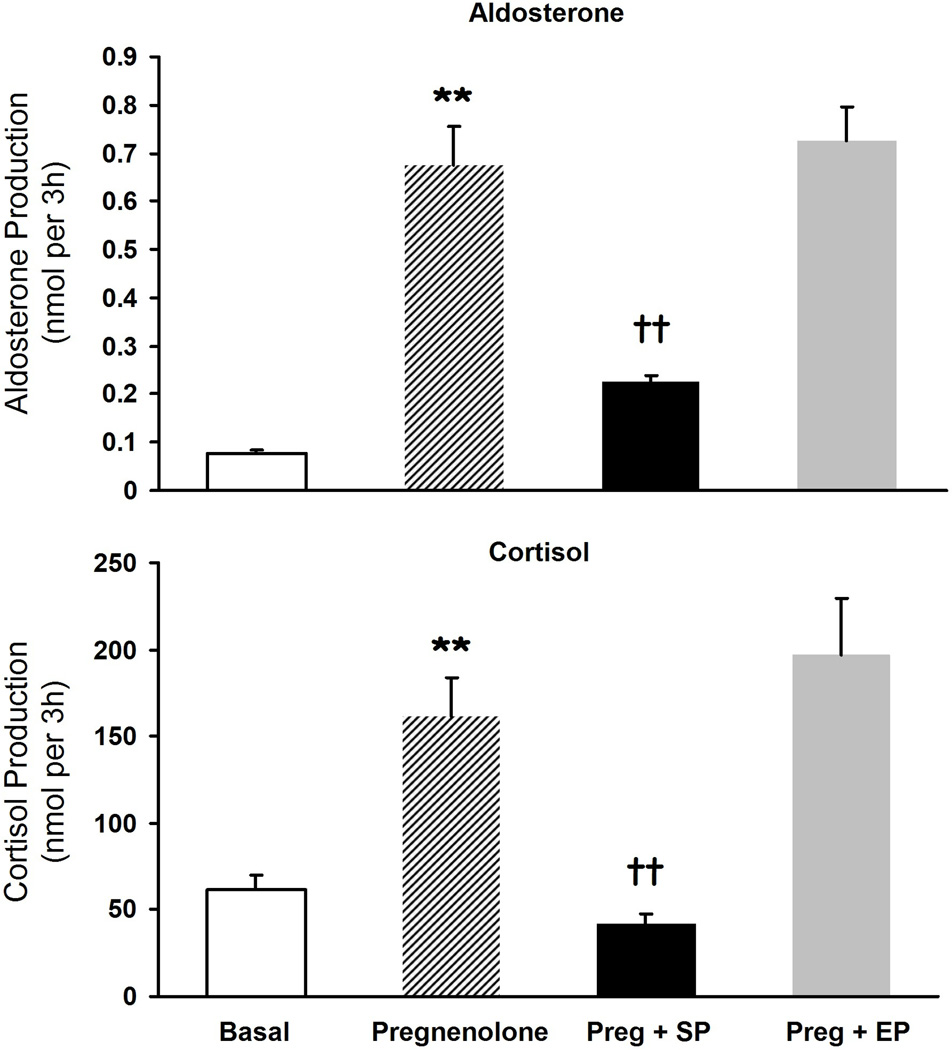
The rate-limiting step in adrenal steroid hormone production is the conversion of cholesterol to pregnenolone. Bypassing the rate-limiting step of steroidogenesis by supplying adrenal cells with exogenous substrate (10 µM pregnenolone) increased the production of aldosterone (8.7-fold) (Fig 4) and cortisol (2.6-fold) (Fig 4). Spironolactone (30 µM) inhibited pregnenolone metabolism to both aldosterone (67%) and cortisol (74%) (Fig 4). Eplerenone (30 µM) did not inhibit pregnenolone-stimulated cortisol or aldosterone production (Fig 4).
Figure 4.
The effects of spironolactone and eplerenone on pregnenolone metabolism to aldosterone and cortisol. H295R adrenal cells were incubated with pregnenolone (10 µM) with or without eplerenone (30 µM) or spironolactone (30 µM) for 24 h. Media concentrations of cortisol and aldosterone were measured by EIA. Values represent data from three independent experiments each ran in triplicate. Preg: pregnenolone, SP: spironolactone, EP: eplerenone. **: p<0.01 vs. basal, ††: p<0.01 vs. pregnenolone.
Discussion & Conclusions
The renin-angiotensin-aldosterone system (RAAS) plays an integral role in cardiovascular homeostasis through its effects on vascular tone and plasma volume. Activation of the RAAS is associated with an increased risk of ischemic cardiovascular events, independent of effects on blood pressure [34], whereas interruption of the RAAS by ACE and AT1R blockade reduces cardiovascular mortality [35, 36], and slows the progression of renal disease [37, 38]. Although ACE inhibitors and Ang II receptor antagonists suppress aldosterone production, plasma aldosterone levels often rise after chronic therapy in a phenomenon called “aldosterone breakthrough” [39]. Thus, many patients with hypertension and congestive heart failure treated with these agents remain unprotected from the effects of inappropriate levels of circulating aldosterone.
The RALES and EPHESUS studies have shown that this “aldosterone breakthrough” is an important factor because it is a determinant of outcome in heart failure patients. Therefore, it may be beneficial if the therapies employed were downstream of this system and acted specifically at the MR. Moreover, in some forms of resistant hypertension, especially in cases of low-renin hypertension, such as in aldosterone-producing adenoma (Conn’s syndrome) patients, the antihypertensive action of the MR antagonists is unequalled. To normalize blood pressure in these patients, high doses of spironolactone (up to 400 mg daily) are sometimes required [40]. Unfortunately, spironolactone lacks specificity for the MR and possesses an affinity for androgen and progesterone receptors [41]. The RALES trial reported a 10% incidence of gynecomastia and breast pain in its male subjects (patients in this trial received 25–50 mg/day spironolactone) [42]. This incidence was significantly higher than placebo (10% vs. 1%) and caused significantly more patients to discontinue treatment. Eplerenone on the other hand, is more selective for the MR. The critical feature of the eplerenone molecule conferring selectivity for the mineralocorticoid receptor is the presence of the 9, 11-epoxy ring [43]. In vitro studies have shown that this structural difference produces an approximate 10 to 20-fold lower affinity for the MR compared with spironolactone [44, 45]. Also, the epoxy ring significantly decreases eplerenone’s binding affinity [44]. As a result, the incidences of progesterone- and androgen-related adverse events are reported to be less frequent with eplerenone.
As early as the 1970s, the inhibitory effects of spironolactone on the steroidogenic cytochrome P-450 enzymes were reported in several models, including steroidogenic tissues from bovine, guinea pig, and human [46–49]. This effect mainly resulted from the covalent binding of 7α-thiospironolactone, a metabolite of spironolactone, to microsomal proteins, including cytochrome P-450 enzymes, with subsequent inhibition and/or degradation [50–52]. Using concentrations of spironolactone similar to those studied previously, we showed that spironolactone (10 µM) suppressed steroid production in the human adrenal cortical H295R cell line. In addition, our experiments indicate that spironolactone decreases the ability of Ang II and forskolin to stimulate cortisol and aldosterone production. The inhibitory effects of spironolactone on aldosterone production have been observed in a variety of in vitro cell culture and in vivo animal model systems [21–23, 53]. These observations can be explained by previous studies that described the ability of spironolactone to bind and inhibit steroidogenic cytochrome P-450 enzymes (including 18, 17- and 21-hydroxylase activities) [24, 50, 54]. There are no studies that suggest that spironolactone inhibition of steroidogenesis is due its effects on MR. As noted above, the concentrations needed to effectively inhibit steroid production (10 and 30 µM spironolactone) were far greater than the amounts needed to inhibit MR activation. This indicates that the inhibition of steroid production by sprironolactone is likely due to a non-genomic action, which is related to direct inhibitory actions on the enzymes involved in steroid hormone biosynthesis. Currently, it is less clear if treatment with high doses of spironolactone inhibit production of human adrenal steroid in vivo. However, treatment of men with 400 mg doses of spironolactone has been shown to cause a significant rise in plasma progesterone and 17-hydroxyprogesterone levels, suggesting an effect on adrenal 21-hydroxylase activity leading to the release of precursor steroids [55].
Interestingly, similar concentrations of eplerenone did not inhibit steroidogenesis in the adrenal cells. Pharmacokinetic studies suggest that when eplerenone is administrated at a dose of 100 mg per person, it is well absorbed, with a mean Cmax of 1.72 µg/ml; this is achieved in 1.2 h post-dosing [56]. Thus, the concentrations used in the current study (30 µM) are well above the levels seen in vivo, suggesting that this compound is unlikely to inhibit adrenal steroid production in vivo. It is interesting that the spironolactone off target inhibition of steroid production (particularly that of aldosterone) has been suggested to be part of the beneficial effect in mineralocorticoid blockade. Further studies will be needed to determine if the lack of eplerenone inhibition of adrenal steroid production affects its overall efficacy as an anti-mineralocorticoid.
Primary aldosteronism is currently considered to represent 8% of the hypertensive population. Treatment of this group of patients with MR blockade is increasing, and there are currently two main MR blockers: spironolactone and eplerenone. Previous studies have shown that spironolactone and its metabolites are more potent inhibitors of MR than eplerenone. However, higher doses of spironolactone have several non-specific actions, including its effects on androgen receptors, progesterone receptors, and steroid hormone production. Herein, we demonstrate that eplerenone, unlike spironolactone, does not inhibit adrenal steroid production.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK43140 to WER).
References
- 1.Duprez D, De Buyzere M, Rietzschel ER, Clement DL. Aldosterone and vascular damage. Curr Hypertens Rep. 2000;2:327–334. doi: 10.1007/s11906-000-0017-z. [DOI] [PubMed] [Google Scholar]
- 2.Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93:2578–2583. doi: 10.1172/JCI117269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornel L. Colocalization of 11 beta-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. Am J Hypertens. 1994;7:100–103. doi: 10.1093/ajh/7.1.100. [DOI] [PubMed] [Google Scholar]
- 4.Bonvalet JP, Alfaidy N, Farman N, Lombes M. Aldosterone: intracellular receptors in human heart. Eur Heart J. 1995;16(Suppl N):92–97. doi: 10.1093/eurheartj/16.suppl_n.92. [DOI] [PubMed] [Google Scholar]
- 5.Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res. 1992;71:503–510. doi: 10.1161/01.res.71.3.503. [DOI] [PubMed] [Google Scholar]
- 6.Beggah AT, Escoubet B, Puttini S, Cailmail S, Delage V, Ouvrard-Pascaud A, Bocchi B, Peuchmaur M, Delcayre C, Farman N, Jaisser F. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mRNA of the mineralocorticoid receptor in cardiomyocytes. Proc Natl Acad Sci U S A. 2002;99:7160–7165. doi: 10.1073/pnas.102673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, Perrier R, Soukaseum C, Cat AN, Royer A, Le Quang K, Charpentier F, Demolombe S, Mechta-Grigoriou F, Beggah AT, Maison-Blanche P, Oblin ME, Delcayre C, Fishman GI, Farman N, Escoubet B, Jaisser F. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–3033. doi: 10.1161/CIRCULATIONAHA.104.503706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW, McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 9.Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 2007;113:267–278. doi: 10.1042/CS20070123. [DOI] [PubMed] [Google Scholar]
- 10.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 11.Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. 2005;14:235–241. doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- 12.Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19:88–90. doi: 10.1016/j.tem.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Pierard LA, Bilge A, Bourassa G, White M, Lepage S, Castaigne A, Aumont MC, Charbonnier B, Pacouret G, Just H, Heitzer T, Riegger GA, Kramer B, Pilz M, Alcocer L, Avila L, Lie KI, Girbes A, Remme WJ, vanderEnt M, CerqueiraGomes M, Brandao F, Cardoso JS, Polonia J, SolerSoler J, Galve EB, LopezSendon JL, Kappenberger L, Beuret P, Hess OMU, Meyer I, Tan LB, Hamid A, Hubner PJB, Nicklas J, Blumenfeld J, Laragh JH, Cody RJ, Julian D, Boissel JP, Furberg C, Kulbertus H, Pocock S, Hall C, Cody R, Riegger G. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15:79–87. doi: 10.1023/a:1011119003788. [DOI] [PubMed] [Google Scholar]
- 15.Zarren HS, Black PM. Unilateral gynecomastia and impotence during low-dose spironolactone administration in men. Mil Med. 1975;140:417–419. [PubMed] [Google Scholar]
- 16.Greenblatt DJ, Koch-Weser J. Gynecomastia and impotence: complications of spironolactone therapy. Jama. 1973;223:82. [PubMed] [Google Scholar]
- 17.Whitebread S, Mele M, Kamber B, de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- 18.Shrago SS, Waisman J, Cooper PH. Spironolactone bodies in an adrenal adenoma. Arch Pathol. 1975;99:416–420. [PubMed] [Google Scholar]
- 19.Aiba M, Suzuki H, Kageyama K, Murai M, Tazaki H, Abe O, Saruta T. Spironolactone bodies in aldosteronomas and in the attached adrenals. Enzyme histochemical study of 19 cases of primary aldosteronism and a case of aldosteronism due to bilateral diffuse hyperplasia of the zona glomerulosa. Am J Pathol. 1981;103:404–410. [PMC free article] [PubMed] [Google Scholar]
- 20.Perroteau I, Netchitailo P, Delarue C, Leboulenger F, Philibert D, Deraedt R, Vaudry H. The effect of the antimineralocorticoid RU 28318 on aldosterone biosynthesis in vitro. J Steroid Biochem. 1984;20:853–856. doi: 10.1016/0022-4731(84)90395-9. [DOI] [PubMed] [Google Scholar]
- 21.Netchitailo P, Perroteau I, Delarue C, Leboulenger F, Capron MH, Vaudry H. [Direct action of mineralocorticoid antagonists on biosynthesis of aldosterone: comparative activities of several new compounds] Can J Physiol Pharmacol. 1983;61:23–28. [PubMed] [Google Scholar]
- 22.Netchitailo P, Delarue C, Perroteau I, Jegou S, Tonon MC, Leroux P, Leboulenger F, Kusmierek MC, Capron MH, Vaudry H. Effect of aldosterone antagonists on mineralocorticoid synthesis in vitro. Inhibition of aldosterone production by prorenoate-K. Eur J Pharmacol. 1982;77:243–249. doi: 10.1016/0014-2999(82)90125-x. [DOI] [PubMed] [Google Scholar]
- 23.Netchitailo P, Delarue C, Perroteau I, Leboulenger F, Capron MH, Vaudry H. Relative inhibitory potency of five mineralocorticoid antagonists on aldosterone biosynthesis in vitro. Biochem Pharmacol. 1985;34:189–194. doi: 10.1016/0006-2952(85)90123-6. [DOI] [PubMed] [Google Scholar]
- 24.Colby HD. Chemical suppression of steroidogenesis. Environ Health Perspect. 1981;38:119–127. doi: 10.1289/ehp.8138119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penhoat A, Darbeida H, Bernier M, Saez JM, Durand P. Inhibition of hormonal-induced cAMP and steroid production by inhibitors of pregnenolone metabolism in adrenal and Leydig cells. Mol Cell Endocrinol. 1988;60:55–60. doi: 10.1016/0303-7207(88)90119-0. [DOI] [PubMed] [Google Scholar]
- 26.Walsh PC, Siiteri PK. Suppression of plasma androgens by spironolactone in castrated men with carcinoma of the prostate. J Urol. 1975;114:254–256. doi: 10.1016/s0022-5347(17)67001-0. [DOI] [PubMed] [Google Scholar]
- 27.Eplerenone: new drug. Recent myocardial infarction with heart failure: a spironolactone me too. Prescrire Int. 2006;15:46–49. [PubMed] [Google Scholar]
- 28.Williams GH, Burgess E, Kolloch RE, Ruilope LM, Niegowska J, Kipnes MS, Roniker B, Patrick JL, Krause SL. Efficacy of eplerenone versus enalapril as monotherapy in systemic hypertension. Am J Cardiol. 2004;93:990–996. doi: 10.1016/j.amjcard.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger MH, White WB, Ruilope LM, MacDonald TM, Davidson RC, Roniker B, Patrick JL, Krause SL. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005;150:426–433. doi: 10.1016/j.ahj.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021–1026. doi: 10.1161/01.HYP.0000067463.13172.EA. [DOI] [PubMed] [Google Scholar]
- 31.Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 32.Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11beta-hydroxysteroid dehydrogenase type 2. J Biol Chem. 2001;276:28484–28492. doi: 10.1074/jbc.M100374200. [DOI] [PubMed] [Google Scholar]
- 33.Sharma KK, Lindqvist A, Zhou XJ, Auchus RJ, Penning TM, Andersson S. Deoxycorticosterone inactivation by AKR1C3 in human mineralocorticoid target tissues. Mol Cell Endocrinol. 2006;248:79–86. doi: 10.1016/j.mce.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 35.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 37.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 38.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 39.Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res. 2006;29:211–216. doi: 10.1291/hypres.29.211. [DOI] [PubMed] [Google Scholar]
- 40.Karagiannis A, Tziomalos K, Kakafika A, Florentin M, Athyros VG. Eplerenone relieves spironolactone-induced painful gynaecomastia in a patient with primary aldosteronism. Nephrol Dial Transplant. 2007;22:293. doi: 10.1093/ndt/gfl500. [DOI] [PubMed] [Google Scholar]
- 41.Funder JW, Mercer JE, Ulick S, Marver D, Adam WR. Toward more specific aldosterone antagonists. A radioreceptor assay approach. Circ Res. 1980;46:I101–I102. [PubMed] [Google Scholar]
- 42.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 43.Keating GM, Plosker GL. Eplerenone : a review of its use in left ventricular systolic dysfunction and heart failure after acute myocardial infarction. Drugs. 2004;64:2689–2707. doi: 10.2165/00003495-200464230-00005. [DOI] [PubMed] [Google Scholar]
- 44.Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27–31. doi: 10.1016/j.mce.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Menard J. The 45-year story of the development of an anti-aldosterone more specific than spironolactone. Mol Cell Endocrinol. 2004;217:45–52. doi: 10.1016/j.mce.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Menard RH, Bartter FC, Gillette JR. Spironolactone and cytochrome P-450: impairment of steroid 21-hydroxylation in the adrenal cortex. Arch Biochem Biophys. 1976;173:395–402. doi: 10.1016/0003-9861(76)90277-0. [DOI] [PubMed] [Google Scholar]
- 47.Greiner JW, Kramer RE, Jarrell J, Colby HD. Mechanism of action of spironolactone on adrenocortical function in guinea pigs. J Pharmacol Exp Ther. 1976;198:709–715. [PubMed] [Google Scholar]
- 48.Rourke KA, Bergstrom JM, Larson IW, Colby HD. Mechanism of action of spironolactone on cortisol production by guinea pig adrenocortical cells. Mol Cell Endocrinol. 1991;81:127–134. doi: 10.1016/0303-7207(91)90211-a. [DOI] [PubMed] [Google Scholar]
- 49.Menard RH, Guenthner TM, Kon H, Gillette JR. Studies on the destruction of adrenal and testicular cytochrome P-450 by spironolactone. Requirement for the 7alpha-thio group and evidence for the loss of the heme and apoproteins of cytochrome P-450. J Biol Chem. 1979;254:1726–1733. [PubMed] [Google Scholar]
- 50.Kossor DC, Kominami S, Takemori S, Colby HD. Role of the steroid 17 alpha-hydroxylase in spironolactone-mediated destruction of adrenal cytochrome P-450. Mol Pharmacol. 1991;40:321–325. [PubMed] [Google Scholar]
- 51.Colby HD, O'Donnell JP, Flowers NL, Kossor DC, Johnson PB, Levitt M. Relationship between covalent binding to microsomal protein and the destruction of adrenal cytochrome P-450 by spironolactone. Toxicology. 1991;67:143–154. doi: 10.1016/0300-483x(91)90138-q. [DOI] [PubMed] [Google Scholar]
- 52.Takamura N, Maruyama T, Ahmed S, Suenaga A, Otagiri M. Interactions of aldosterone antagonist diuretics with human serum proteins. Pharm Res. 1997;14:522–526. doi: 10.1023/a:1012168020545. [DOI] [PubMed] [Google Scholar]
- 53.Lasaridis AN, Tourkantonis A, Spanos P, Apostolopoulou K, Pharmakiotis A. The effects of canrenoate K on corticosteroid biosynthesis in nephrectomized dogs. J Steroid Biochem. 1984;20:923–929. doi: 10.1016/0022-4731(84)90407-2. [DOI] [PubMed] [Google Scholar]
- 54.Cheng SC, Suzuki K, Sadee W, Harding BW. Effects of spironolactone, canrenone and canrenoate-K on cytochrome P450, and 11beta- and 18-hydroxylation in bovine and human adrenal cortical mitochondria. Endocrinology. 1976;99:1097–1106. doi: 10.1210/endo-99-4-1097. [DOI] [PubMed] [Google Scholar]
- 55.Stripp B, Taylor AA, Bartter FC, Gillette JR, Loriaux DL, Easley R, Menard RH. Effect of spironolactone on sex hormones in man. J Clin Endocrinol Metab. 1975;41:777–781. doi: 10.1210/jcem-41-4-777. [DOI] [PubMed] [Google Scholar]
- 56.Cook CS, Berry LM, Bible RH, Hribar JD, Hajdu E, Liu NW. Pharmacokinetics and metabolism of [14C]eplerenone after oral administration to humans. Drug Metab Dispos. 2003;31:1448–1455. doi: 10.1124/dmd.31.11.1448. [DOI] [PubMed] [Google Scholar]