SUMMARY
Glioma are the most common supra-tentorial brain tumor in the USA with an estimated annual incidence of 17,000 new cases per year. Dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion MRI noninvasively characterizes tumor biology allowing for the diagnosis and therapeutic monitoring of glioma. This MRI technique utilizes the rapid changes in signal intensity caused by a rapid intravascular bolus of paramagnetic contrast agent to calculate physiologic perfusion metrics. DSC perfusion MRI has increasingly become an integrated part of glioma imaging. The specific aim of this article is to review the benefits of DSC perfusion MRI in the therapy of glioma.
KEYWORDS : cerebral blood volume, dynamic susceptibility-weighted contrast-enhanced perfusion MRI, glioblastoma, glioma, imaging genomics, metastasis, molecular imaging, peak height, percentage of signal intensity recovery
Practice points.
Dynamic susceptibility-weighted contrast-enhanced perfusion MRI utilizes the rapid changes of signal intensity induced by the intravascular administration of paramagnetic contrast agents to calculate cerebral hemodynamic parameters including cerebral blood volume, peak height and percentage of signal intensity recovery.
The inherent limitations of histopathologic analysis to actively assess tumor angiogenesis has led to the development of imaging based methods for the noninvasive quantification of glioma biological processes.
Contrast enhancing regions of intra-axial metastatic disease typically demonstrate significantly reduced percentage of signal intensity recovery when compared with treatment-naive high-grade glioma.
Among high-grade gliomas, grade IV glioblastoma tend to demonstrate significantly elevated cerebral blood volume when compared with grade III anaplastic astrocytomas.
Low-grade glioma exhibit significantly lower cerebral blood volume when compared with higher-grade glioma.
Oligodendroglioma, regardless of grade, tend to demonstrate elevated cerebral blood volume when compared with low-grade astrocytomas.
Oligodendroglioma with allelic loss of chromosome 1p and 19q demonstrate improved clinical outcomes; however, they tend to demonstrate elevated cerebral blood volume when compared to those with intact genomic status.
EGFR mutation is a potential indicator of poor clinical outcomes in glioblastoma. Tumors with this genomic mutation tend to demonstrate elevated cerebral blood volume.
Relative cerebral blood volume and peak height tend to be significantly elevated within contrast-enhancing recurrent glioma when compared with similar appearing regions of treatment effect following temozolomide-based medical therapy.
Glioma are the most common supra-tentorial brain tumor affecting approximately 138,000 individuals in the USA in 2010 with an annual incidence of 17,000 new cases [1]. Despite significant advances in surgical, radiation and medical therapies the prognosis of this disease remains dismal with a median survival of less than 2 years for the most malignant form; glioblastoma [2]. A number of biological, clinical and diagnostic factors account for the poor prognostic outcomes for patients with glioma. The development of dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion MRI has allowed for the noninvasive assessment of biological properties that influence the clinical prognosis of patients with glioma. Specifically, metrics obtained from this sequence allow for the quantification of biologically aggressive cellular features; tumor induced angiogenesis and microvascular proliferation.
The specific aim of this article is to review the benefits of DSC perfusion MRI in the therapy of glioma. First, we will introduce technical considerations for performing DSC perfusion MRI. Next, we will review how the inherent biology of glioma influences DSC perfusion metrics. Finally, we will discuss how DSC perfusion MRI provides added diagnostic capabilities to standard morphological T1- and T2-weighted sequences when evaluating glioma prior to, concurrent with, and following therapeutic intervention.
Technical considerations
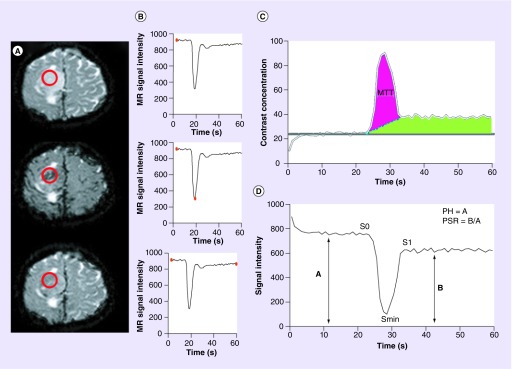
DSC perfusion MRI has increasingly become an integrated part of glioma imaging. This is due to the added value of a fast and easy to analyze sequence that provides physiologic maps of regional variations in cerebral microvasculature pathology as contributed to by a number of biological parameters of the tumor cytoarchitecture [3–9]. DSC perfusion MRI utilizes the rapid changes in signal intensity caused by a rapid intravascular bolus of paramagnetic contrast agents to calculate physiologic metrics; cerebral blood volume (CBV), mean transit time (MTT), cerebral blood flow (CBF), peak height (PH) and percentage of signal intensity (PSR) recovery (Figure 1) [8,9].
Figure 1. . Calculation of dynamic susceptibility-weighted contrast-enhanced perfusion MRI parameters; peak height, percentage of signal intensity recovery and cerebral blood volume.
(A & B) Precontrast bolus (top), peak bolus (middle), and post-contrast bolus (bottom) T2* axial images from a patient with right convexity glioma. (A) Demonstrates the changes in signal intensity (darkened vasculature) caused by the rapid intravascular bolus of paramagnetic contrast agents. Placement of a region of interest (red circle) over the lesion allows for the measurement of signal intensity (B) at any given time point during dynamic imaging sequence (red dot on graph bar). Generation of (C) a contrast concentration–time and (D) signal intensity–time curves allows for the quantification of perfusion metrics. (C) Idealized illustration of cerebral blood volume quantification in the setting of a disrupted blood–brain barrier. Two quantification methods are depicted. Baseline subtraction method (blue dashed line) can be applied allowing for the calculation of cerebral blood volume which is proportional to the area under the concentration–time curve (purple shaded area). Alternatively, the negative enhancement integral method utilizes the contrast concentration prior to the bolus phase of imaging (gray bar) to estimate the end bolus contrast concentration and calculates the area within the first pass bolus of contrast without consideration of the recirculation phase (green shaded area). Mean transit time is calculated as the half width maximum of the contrast concentration-time curve (gray bars). (D) PH is calculated as S0–Smin, where S0 is the precontrast bolus baseline signal intensity and Smin is the minimum signal intensity obtained during the first pass bolus phase of contrast (double-headed arrow A). PSR is calculated as (S1–Smin/S0–Smin), where S1 is the average post-bolus signal intensity (double-headed arrow B).
For color images please see online http://www.futuremedicine.com/doi/full/10.2217/cns.14.44
MR: Magnetic resonance; PH: Peak height; PSR: Percentage of signal intensity recovery.
DSC perfusion MRI is a relatively straight forward imaging technique that can be completed within 2 min. The successfully clinical implementation of this imaging technique requires consideration of several critical components. First, a fast-imaging technique, such as multishot echo-planar imaging, must be utilized to acquire images with a temporal resolution of 1 or 2 s before, during, and after the contrast agent bolus injection (Figure 1A). Various forms of gadolinium-based intravenous agents can used to provide contrast enhancement of CNS tissues for MRI. At out institution, we currently utilize 0.2 ml/kg gadopentetate dimeglumine (Magnevist®, Bayer HealthCare Pharmaceuticals, Leverkusen, Germany) for research purposes and 0.1 ml/kg gadobutrol (Gadavist®, Bayer HealthCare Pharmaceuticals) for clinical purposes. We aim to provide a contrast concentration of 0.1 mmol/kg. Second, consideration should be given to the repetition time (TR) and echo time. We utilize a TR of 1400 ms, echo time of 25 ms, slice thickness of 3 mm, skip 0 mm. Five to seven axial sections are obtained to cover the entire lesion volume. Finally, consideration should be given to the flip angle and predosing of contrast. We utilize a flip angle of 30º that in combination with the TR ensures that T1 effects are minimized. We do not administer a prebolus dosage of contrast agent since we utilize repetition time and flip angle to minimize T1 relaxivity effects.
The calculation of DSC perfusion MRI metrics assumes that the data acquisition occurred during the first pass of bolus contrast agent through the cerebral vasculature with an intact blood–brain barrier. Under these conditions a signal intensity–time curve can be accurately measured (Figure 1B). Meier and Zierler have demonstrated that CBV is proportional to the area under the contrast agent concentration–time curve, therefore, the conversion of signal intensity into intravascular contrast concentration is necessary (Figure 1C) [10]. MTT can be estimated from the concentration–time curve utilizing a standardized measurement such as the width of the perfusion curve at half of the maximum height. CBF is easily calculated given its relationship to the product of CBV and MTT.
While CBV remains the most widely used DSC perfusion parameter in neuro-oncologic imaging, many assumptions, including an intact blood–brain barrier, must be fulfilled to ensure accurate measurement. Other quantitative DSC perfusion MRI parameters, which do not rely on these assumptions first proposed by Meier and Zierler, have been characterized from the signal intensity–time curve and show promise in characterizing glioma. Maximum susceptibility-weighted signal intensity change (PH) and PSR at the end of the first pass of contrast material bolus can be easily calculated without complex modeling or utilization of sophisticated leakage correction algorithms (Figure 1D). These DSC perfusion parameters facilitate regional tumor quantification and correlation with morphologic MRI sequences.
Tumor biology influences DSC perfusion MRI
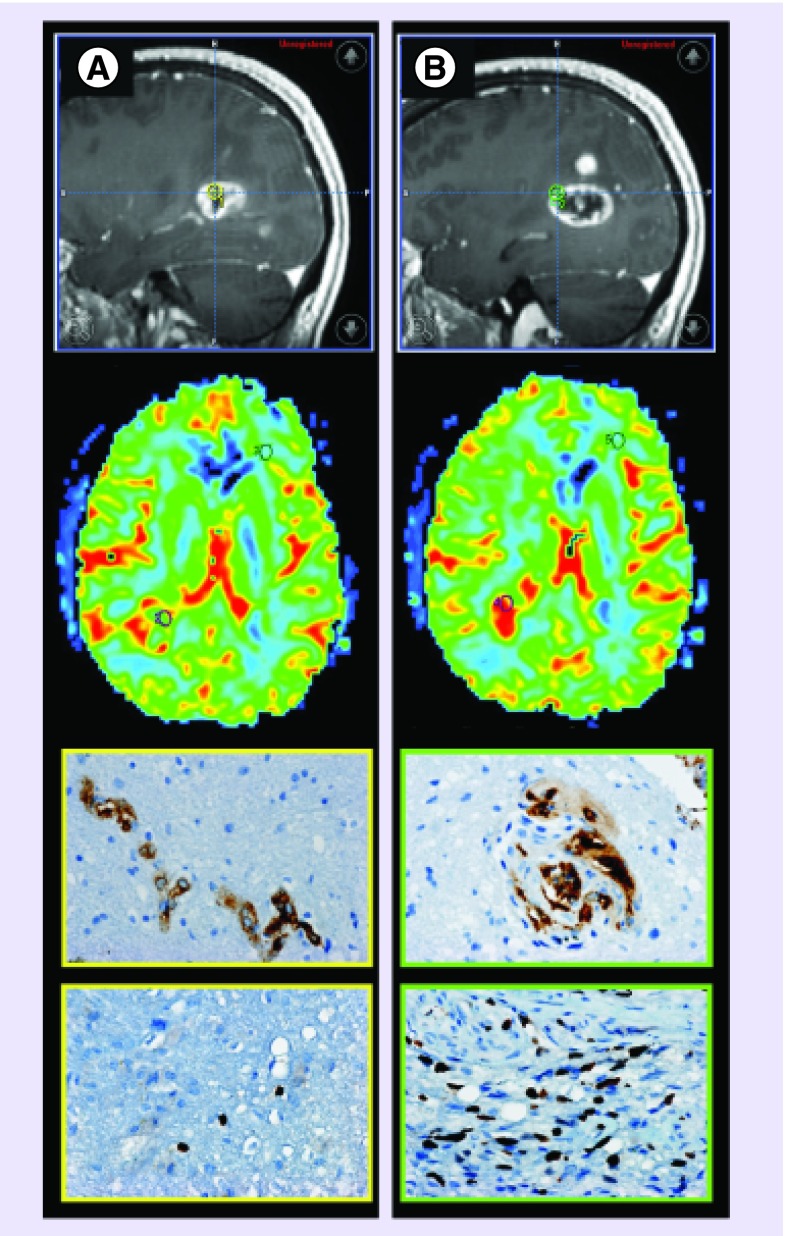
The most widely used classification system for glioma is that of the WHO [11]. The WHO classification grades glioma based on histological features including cellularity, mitotic activity, nuclear atypia, necrosis and vascularity. Examination of tumor microvasculature is a primary feature utilized in the diagnosis and grading of glioma. Low-grade glioma (grade 2) demonstrate vascularity that is more numerous than the normal brain; however, vascular hyperplasia is absent and blood–brain barrier remains mostly intact. Conversely, malignant glioma (grade 3 and 4) transformation is manifested by hypoxia-induced neovascularity, endothelial hyperplasia and disorganized tortuous microvasculature with disrupted blood–brain barrier. The intra-tumoral expression of these aggressive biological features is often nonuniform leading to spatial heterogeneity that can be difficult to assess without image-guided tissue sampling (Figure 2) [12,13].
Figure 2. . MRI-guided tissue sampling demonstrates glioblastoma biological heterogeneity.
(A & B) Similar appearing contrast-enhancing foci (first row, yellow circle, [A]; green circle, [B]) demonstrates dissimilar biological features of glioblastoma aggressiveness. (A) Enhancing tissue sampling site with mildly elevated cerebral blood volume (CBV) and peak height (second row, axial CBV map reformation, purple regions of interest corresponds to tissue sampling site) demonstrates simple microvascular hyperplasia (third row, factor VIII brown stained cells) without elevation of cellular proliferation (fourth row, Ki-67 proliferative index less than 1%). (B) Similar appearing enhancing foci demonstrates markedly increased CBV and peak height with complex glomeruloid microvascular hyperplasia (factor VIII brown stained cells) with elevated cellular proliferation (Ki-67 proliferative index of 20%). This case demonstrates the nonspecificity of contrast enhancement and the additional value of dynamic susceptibility-weighted contrast-enhanced perfusion MRI metrics for the selection of biologically aggressive tumor sites.
Pathologic images courtesy of Joanna J Phillips (Departments of Pathology and Neurological Surgery, University of California, San Francisco, CA, USA).
Glioma are biologically heterogeneous tumors; however, the microvasculature characteristics utilized to grade glioma can be noninvasively quantified by DSC perfusion MRI metrics. Prior investigations have demonstrated that CBV, PH and PSR measurements correlate with histologic vascular morphology and microvascular proliferation in glioblastoma (Figure 2) [14–16]. It has been suggested that these correlations between CBV, PH and neoplastic angiogenesis are likely due to regional variation in microvascular morphology [15–19]. We have demonstrated that complex and simple microvascular hyperplasia are significantly overexpressed within contrast-enhancing regions that correspond to a significantly increased CBV and PH measurements [15].
Clinical applications of DSC perfusion MRI
DSC perfusion MRI noninvasively provides in vivo metrics that depicts overall tumor vascularity and thereby permits the indirect assessment of tumor angiogenesis. This additional physiologic information provides added diagnostic capabilities to standard morphological T1- and T2-weighted sequences when differentiating solitary metastatic brain lesion from high-grade glioma, preoperative grading of glioma, predicting prognostic molecular markers and noninvasive therapeutic monitoring.
• Differentiating high-grade glioma from solitary metastatic tumors
Distinguishing high-grade glioma from intracranial metastases is usually straightforward since metastatic lesions tend to be multiple, well-circumscribed and favor the gray–white matter junction. However, a diagnostic dilemma can arise when a patient presents with a solitary mass with no history of systemic cancer. It is clinically important to distinguish between these two disease etiologies because therapeutic considerations are drastically different. In this situation, conventional contrast-enhanced T1- and T2-weighted MRI characteristics of both diseases are nonspecific and cannot be confidently utilized to narrow the differential diagnosis.
Despite the similar morphologic imaging appearance between high-grade glioma and metastasis, the capillary ultrastructure of these two disease etiologies is markedly different. Metastatic tumors spread to the CNS via hematogenous routes inducing intratumoral neovascularization as they noninvasively expand. Metastatic disease capillaries resemble those of the primary systemic tumor, with gap junctions, fenestrated membranes and open endothelial junctions, all of which are significantly different from normal brain capillaries. This unique intracerebral capillary morphology results in greatly increased capillary permeability uniformly throughout the tumor microvasculature resulting in peritumoral vasogenic edema. Conversely, the capillaries of glioma have various degrees of blood–brain barrier disruption that is, when taken in its entirety, less severe than those of metastatic tumors.
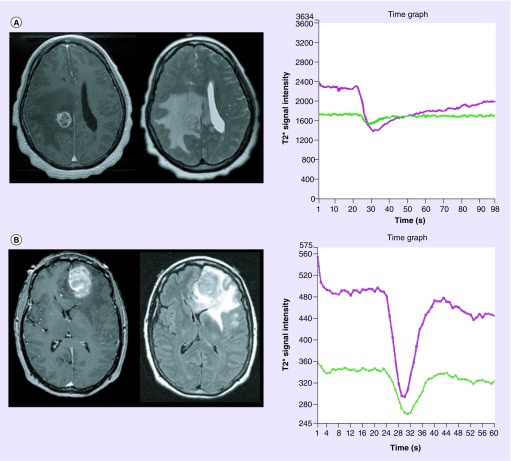
The inherent differences in histologic capillary features between high-grade glioma and metastatic tumors form the basis for differentiating disease etiology using DSC perfusion MRI. We have previously demonstrated CBV and PH measurements obtained from nonenhancing regions and tumor-wide PSR measurements can be helpful in differentiating glioblastoma from solitary brain metastasis (Figure 3) [20]. CBV and PH tend to be significantly elevated within nonenhancing T2 hyperintense regions of glioblastoma when compared with metastatic lesions. Furthermore, tumor-wide PSR values tend to be significantly reduced within metastatic lesions when compared with glioblastoma. The observed differences in regional perfusion metrics can, in part, be explained by differences in histologically defined pathophysiology. In metastatic tumors, peritumoral edema represents pure vasogenic edema caused by increased interstitial water due to leaky capillaries without evidence of infiltrative tumor growth or elevated microvascular expression. Conversely, the significant reduction in PSR within brain metastasis is likely due to the profound differences in capillary permeability between the tumor types.
Figure 3. . Dynamic susceptibility-weighted contrast-enhanced perfusion metrics differentiate intracranial metastatic disease from gliomblastoma.
Axial T1 postcontrast-enhanced (left) and T2 (top middle) fluid-attenuated inversion recovery (bottom middle) images demonstrate similar appearing lesions. Signal intensity–time curves (right) generated from lesion wide region of interest (not shown) in a patient with (A) metastatic lung cancer and (B) glioblastoma differentiates disease etiology utilizing perfusion metrics. Metastatic lesion demonstrates markedly reduced percentage of signal intensity ([A] purple curve) when compared with normal appearing white matter ([A] green curve) and glioblastoma ([B] purple curve). Additionally, cerebral blood volume and peak height measurements are elevated within glioblastoma when compared with metastatic lesions.
For color images please see online at http://www.futuremedicine.com/doi/full/10.2217/cns.14.44
• Pre-therapeutic glioma grading
Grade III (anaplastic astrocytoma) and grade IV (glioblastoma) glioma are biologically aggressive tumors that histologically demonstrate significantly elevated angiogenic features. The ability to recruit and synthesize vascular networks to facilitate tumor growth is an important biological feature of tumor aggressiveness. The degree of vascular proliferation is an essential component in discriminating glioma grade.
Differentiating high- and low-grade glioma prior to surgical intervention is critically important in the clinical management of the patient as the therapeutic approach to low- and high-grade tumors are drastically different. T1-weighted contrast-enhanced morphologic MRI, the current standard of care in this patient population, is markedly limited in its ability to accurately grade glioma due to its nonspecificity for delineating regions of elevated microvascular proliferation [21–24]. Conversely, DSC perfusion MRI have shown promise in tumor grading. Since DSC perfusion MRI has been shown to directly correlate with histological features of glioma angiogenesis there is a growing consensus that perfusion metrics, in conjunction with conventional T1- and T2-weighted morphologic MRI sequences, may serve as a noninvasive marker of tumor biological aggressiveness that may be useful in the preoperative differentiation of glioma grades [3,4,14–16]. The observation that DSC perfusion metrics increase with increasing biological aggressiveness is consistent with histologic studies demonstrating that microvascular proliferation and the presence of deranged vascular morphology become more prominent in higher-grade tumors.
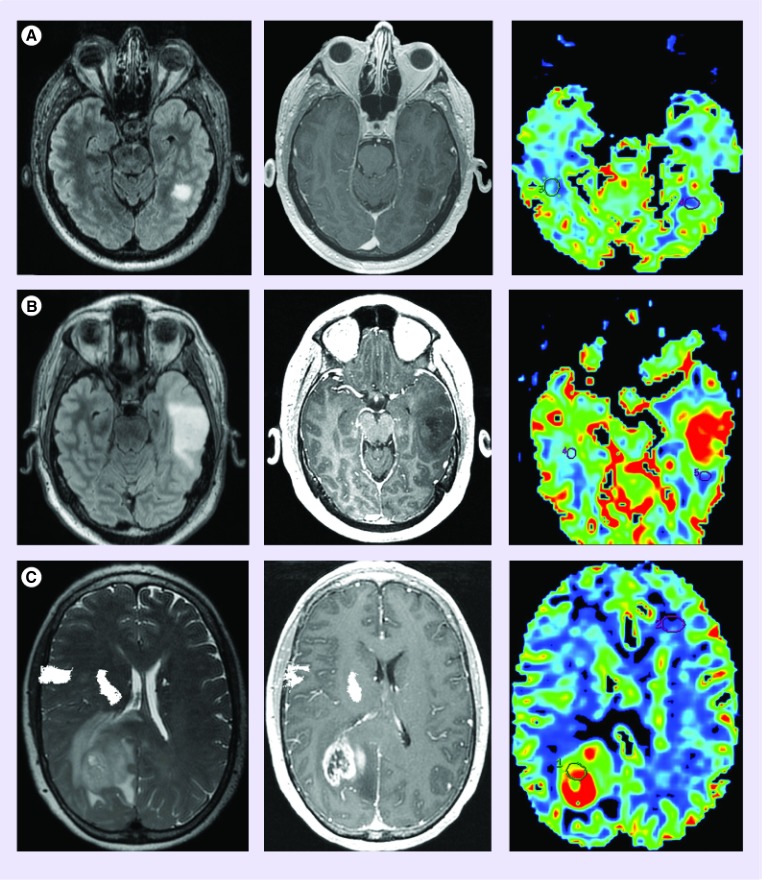
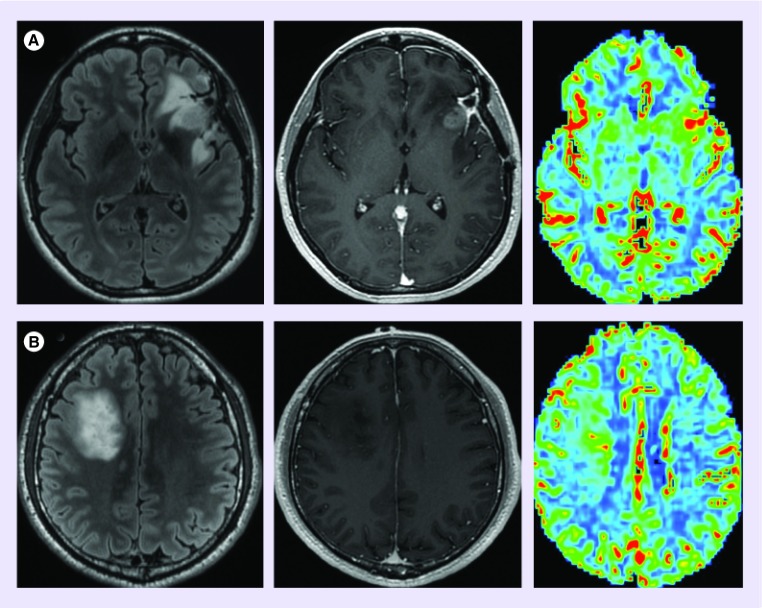
Low-grade glioma (grade II, diffuse astrocytoma) demonstrate significantly lower mean CBV when compared with higher-grade tumors. In general, low-grade tumors tend to demonstrate little, if any, elevation of CBV within the T2 hyperintense lesion when compared with the contralateral normal appearing brain (Figure 4A) [3,4]. Among high-grade glioma, grade III anaplastic astrocytomas tend to demonstrate lower CBV measurements when compared with grade IV glioblastoma (Figure 4B & C).
Figure 4. . Cerebral blood volume differentiates high from low-grade glioma.
(A) Diffuse astrocytoma (WHO grade II) is morphologically manifested as a fluid-attenuated inversion recovery hyperintense (FLAIR; left) nonenhancing (middle) mass with cerebral blood volume (right) measurements similar to normal appearing white matter. (B) Anaplastic astrocytoma (WHO grade 3) typically presents as a FLAIR hyperintense mass with minimal if any contrast enhancement; however, unlike low-grade glioma, demonstrates elevated cerebral blood volume. The presence of increased perfusion metrics within a nonenhancing glioma suggests the presence of aggressive biological features that portend a high-grade diagnosis. (C) glioblastoma (WHO grade 4) can present as a rim enhancing T2/FLAIR hyperintense mass with markedly elevated cerebral blood volume.
Oligodendroglial and oligoastrocytic tumors, when matched for grade, demonstrate increased CBV when compared with astrocytic tumors (Figure 5). The evaluation of DSC perfusion metrics in oligodendroglial and oligoastrocytic tumors in isolation can result in misinterpretation and classification as a higher-grade astrocytic tumor [3,4,6,7,14,25] Histologically, the elevated perfusion metrics within oligodendroglial tumors is due to the increased expression of microvasculature with delicate ‘chicken wire’ morphology [25]. In addition to elevated CBV, oligodendroglial tumors have characteristic morphologic features on contrast-enhanced T1- and T2-weighted MRI that include cortical involvement with frontal lobe predominance, intratumoral cysts and susceptibility changes due to intratumoral calcification. The combination of elevated CBV and characteristic morphological features help to improve the diagnostic accuracy for differentiating oligodendroglial from astrocytic tumors.
Figure 5. . Oligodenroglial tumors demonstrate elevated cerebral blood volume.
(A & B) Fluid-attenuated inversion recovery hyperintense (left) enhancing 1p19q codeleted ([A] middle) and nonenhancing 1p19q intact ([B] middle) grade 2 oligodendroglioma demonstrates mildly elevated cerebral blood volume when compared with diffuse astrocytoma (Figure 4). Also demonstrated is the tendency for oligodenroglial tumors with the prognostically favorable 1p19q codeleted (A) molecular marker status to express higher cerebral blood volume when compared with tumors with intact 1p19q status. Prior studies have suggested that dynamic susceptibility-weighted contrast-enhanced perfusion MRI may be a noninvasive biomarker of 1p19q genotype.
• Predicting prognostic molecular markers using DSC perfusion MRI
Advances in cytogenetic analysis of primary glial neoplasms have resulted in the identification of prognostically relevant molecular classifications. Currently, the two most commonly referenced molecular genetic markers are 1p19q genotype in oligodendroglial tumors and EGF receptor (EGFR) amplification status in primary glioblastoma.
Oligodendroglial 1p19q genotype
The most common cytogenetic abnormality observed within oligodendroglial and oligoastrocytic tumors is loss of chromosomes 1p and 19q [26]. This genetic alteration arises through an unbalanced centromeric translocation between chromosomes 1p and 19q resulting in their complete loss (codeletion). This molecular marker is associated with chemoradiosensitivity, prolonged progression-free survival and improved overall clinical outcomes [26–28]. Genomic classification has been found to better correlate with clinical outcome when compared with the WHO classification system [29].
Currently, the diagnosis of 1p19q codeletion is made using tissues specimens obtained during primary surgical resection or selected tumor sampling. The potential to identify prognostic molecular subtypes of oligodendroglial tumors has prompted investigators to explore the use of DSC perfusion MRI as a noninvasive biomarker of 1p19q genotype. Prior investigators have demonstrated that CBV measurements are consistently higher within Oligodendroglial tumors with this co-deletion (Figure 5) [30–32]. When stratified by WHO grade, the elevation in CBV tends to be observed within grade II tumors. Grade III tumors demonstrate a greater degree of CBV heterogeneity and overlap of values [33,34]. Taken together these observations suggest that, unlike astrocytic tumors, elevated CBV within oligodendroglial tumors with 1p19q codeletion may not be indicative of aggressive biological characteristics.
EGFR mutational status in primary glioblastoma
Recent advances in primary glial neoplasm molecular classification have identified EGFR mutation as a potential indicator of poor clinical outcomes in glioblastoma. Up to 50% of primary glioblastoma with this molecular classification have a mutated form of EGFR variant III (EGFRvIII) [35]. EGFRvIII mutation encodes an in-frame deletion resulting in pro-oncogenic effects such as elevated proliferation, angiogenesis and tumor invasiveness. Multiple prior investigations have not definitively demonstrated a link between EGFRvIII mutation status and clinical outcomes in patients with primary glioblastoma [36–39]. However, identification of EGFRvIII mutational status remains clinical relevant as it has been demonstrated to convey resistance to chemotherapies [40–45].
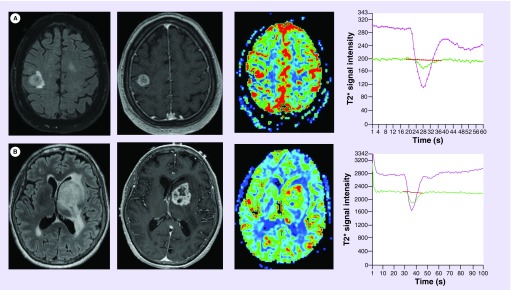
Currently, the EGFR molecular classification of glioblastoma relies on genomic or immunohistochemical analysis of tissue specimens obtained during surgical resection or stereotactic tissue sampling. The ability to noninvasively define molecular classification of primary glioblastoma could allow for earlier targeted therapeutic intervention and serial monitoring of therapeutic efficacy. To this end, prior investigators have identified morphologic and physiologic DSC perfusion MRI metrics that are differentially expressed between primary glioblastoma with and without EGFR mutations [46–48]. Aghi et al. has demonstrated that tumors with indistinct T2 hyperintensity borders and elevated T2 hyperintensity to enhancing volume ratio were more likely to demonstrate EGFRvIII mutation. Tykocinski et al. have demonstrated an association between EGFRvIII mutational status and DSC perfusion metrics. EGFRvIII mutants demonstrated significantly elevated relative CBV when compared with those primary glioblastoma without the mutation (Figure 6). Gupta et al. have also demonstrated that glioblastoma EGFR mutational status was positively associated with PH and inversely associated with PSR metrics.
Figure 6. . EGFR-mutated primary glioblastoma demonstrates differential dynamic susceptibility-weighted contrast-enhanced perfusion metrics.
(A) Fluid-attenuated inversion recovery (left) and T1-weighted postcontrast (middle left) images demonstrate a rim enhancing mass found to represent an EGFR-mutated primary glioblastoma. Cerebral blood volume map (middle right) and signal intensity–time curve (right) demonstrates markedly elevated relative cerebral blood volume (8.2) and relative peak height (9.2) about the enhancing foci. (B) Fluid-attenuated inversion recovery and T1-weighted post contrast images demonstrate a similar appearing rim enhancing mass found to represent a primary glioblastoma without EGFR mutation. Cerebral blood volume map and signal intensity–time curve demonstrates moderately elevated relative cerebral blood volume (3.7) and relative peak height (2.7) about the enhancing foci. While both tumors demonstrates elevated dynamic susceptibility-weighted contrast-enhanced perfusion metrics, the EGFR-mutated tumor does so to a significantly higher degree.
• Monitoring temozolomide-based chemoradiotherapy using DSC perfusion MRI
The choice of optimal chemotherapeutic management for newly diagnosed glioma is a multifactorial decision that integrates WHO tumor grade, extent of surgical resection, genomic phenotype and molecular classification; among other factors [49]. Patients diagnosed with high-grade glioma often undergo concomitant radiotherapy and oral administration of a DNA alkylating agent such as temozolomide. This treatment regimen has been shown to improve progression-free and overall survival when compared with radiotherapy alone [50–53].
Therapeutic monitoring is achieved with serial contrast-enhanced MRI. However, the interpretation of MRI can be difficult given that changes in T1- and T2-weighted sequences are highly nonspecific for distinguishing recurrent tumor from treatment-related changes. To this end, several standardized methods for the interpretation of MRI in patients being monitored for tumor recurrence have been developed; Macdonald Criteria, RECIST (Response Evaluation Criteria in Solid Tumors), RANO (Response Assessment in Neuro-Oncology working group) [54–56]. Despite these standardized approaches the assessment of disease progression following standard therapy remains a commonly encountered clinical conundrum.
Some advocate that noninvasive MRI evaluation of surrogate markers of disease progression, such as neovascularization, can provide additional diagnostic information that aids in the interpretation of standard morphologic MRI sequences. Since prior investigators have demonstrated a direct correlation between DSC perfusion metrics and the histophatologic features of glioma angiogenesis, these metrics have been proposed for treatment monitoring. Vöglein et al. performed serial DSC perfusion MRI prior to and following surgical resection, radiotherapy and temozolomide based medical therapy in 37 patients histologically diagnosed with glioma (20 low and 17 high grade) throughout the course of the disease process [57]. Patients demonstrating recurrent disease have significant increases in CBV from the postsurgical pre-chemoradiothearpy baseline. Conversely, patients without evidence of disease recurrence did not demonstrate increased CBV. DSC perfusion metrics have also been utilized to prognosticate clinical outcome in patients undergoing temozolomide-based chemoradiothearpy. Mangla et al. in their retrospective study of 36 patients with glioblastoma treated with temozolomide and radiotherapy has demonstrated that the percentage change in CBV one month following therapy significantly correlated with overall survival [58]. Additionally, this study demonstrated similar findings to Vöglein et al. in that patients with disease recurrence were found to have a progressive elevation of CBV on serial follow-up DSC MRI. Taken together these studies, and others, suggest that DSC perfusion metrics could be useful in the treatment monitoring of gliomas [57–60].
Current clinical limitations
As we have highlighted, the use of DSC perfusion MRI has great potential in directly contributing to the care of patients with primary and metastatic brain tumors. Given this potential, we must highlight the current limitations in the integration of this imaging technique into day-to-day clinical practice. Standardization of DSC perfusion technique, postprocessing parameters and clinical interpretation across institutions remains a major impediment to the sequence's clinical implementation and determination of prognostic meaning. To this end, working groups from the National Cancer Institute Quantitative Imaging Network and American Society of Neurological Radiology (among many others) are currently advocating for the standardization and validation of imaging biomarkers. It is also important to note that DSC perfusion MRI is not a replacement for histological assessment obtained at surgery. To the contrary, analysis of MRI and histopathology play a complementary role in assessing tumor grade, patient prognosis, and treatment response.
Conclusion & future perspective
DSC perfusion MRI is a valuable noninvasive tool that complements traditional morphological MRI. Prior investigations demonstrating the utility of DSC perfusion MRI in differentiating between disease etiologies, identifying prognostic glioma molecular markers, and as a prognostic biomarker during glioma therapy have made perfusion metrics an integral role in the clinical practice in neuro-oncology. As the utility of perfusion MRI becomes increasingly recognized its wide-spread clinical implementation will lead to further sequence refinement, technique standardization and integration into clinical scanners that may result in improved patient outcomes by noninvasively characterizing tumor pathophysiology, quantifying tumor angiogenesis and serving as an arbiter of treatment response.
Acknowledgements
RF Barajas thanks Bethany Barajas for her helpful comments regarding this manuscript.
Footnotes
Financial & competing interests disclosure
This work was supported by 5T32EB001631-07 from the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omuro A1, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 4.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999;211:791–798. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 5.Cha S, Knopp EA, Johnson G, et al. Dynamic contrast-enhanced T2-weighted MR imaging of recurrent malignant gliomas treated with thalidomide and carboplatin. Am. J. Neuroradiol. 2000;21:881–890. [PMC free article] [PubMed] [Google Scholar]
- 6.Sugahara T, Korogi Y, Shigematsu Y, et al. Perfusion-sensitive MRI of cerebral lymphomas: a preliminary report. J. Comput. Assist. Tomogr. 1999;23:232–237. doi: 10.1097/00004728-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Sugahara T, Korogi Y, Shigematsu Y, et al. Value of dynamic susceptibility contrast magnetic resonance imaging in the evaluation of intracranial tumors. Top. Magn. Reson. Imaging. 1999;10:114–124. doi: 10.1097/00002142-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Villringer A, Rosen BR, Belliveau JW, et al. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn. Reson. Med. 1988;6:164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- 9.Rosen BR, Belliveau JW, Vevea JM, et al. Perfusion imaging with NMR contrast agents. Magn. Reson. Med. 1990;14:249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 10.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J. Appl. Physiol. 1954;6(12):731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- 11.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. IARC; Lyon: 2008. WHO Classification of tumours of the central nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prayson RA, Agamanolis DP, Cohen ML. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J. Neurol. Sci. 2000;175:33–39. doi: 10.1016/s0022-510x(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 13.Coons SW, Johnson PC, Scheithauer BW, et al. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am. J. Roentgenol. 1998;171:1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]; • One of the initial studies investigating the biological basis for the quantification of cerebral blood volume.
- 15.Barajas RF, Jr, Hodgson JG, Chang JS, et al. Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology. 2010;254(2):564–576. doi: 10.1148/radiol.09090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barajas RF, Jr, Phillips JJ, Parvataneni R, et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro Oncol. 2012;14(7):942–954. doi: 10.1093/neuonc/nos128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagami M, Katayama Y. Angiogenesis of glioma: evaluation of ultrastructural characteristics of microvessels and tubular bodies in endothelial cells and immunohistochemical findings with VEGF and p53 protein. Med. Mol. Morphol. 2005;38:36–42. doi: 10.1007/s00795-004-0273-0. [DOI] [PubMed] [Google Scholar]
- 18.Deane BR, Lantos PL. The vasculature of experimental brain tumours. I. A sequential light and electron microscope study of angiogenesis. J. Neurol. Sci. 1981;49:55–66. doi: 10.1016/0022-510x(81)90188-x. [DOI] [PubMed] [Google Scholar]
- 19.Deane BR, Lantos PL. The vasculature of experimental brain tumours. II. A quantitative assessment of morphological abnormalities. J. Neurol. Sci. 1981;49:67–77. doi: 10.1016/0022-510x(81)90189-1. [DOI] [PubMed] [Google Scholar]
- 20.Cha S, Lupo JM, Chen MH, et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am. J. Neuroradiol. 2007;28(6):1078–1084. doi: 10.3174/ajnr.A0484. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion metrics can differentiate disease etiology of similar appearing contrast-enhancing lesions in patients with glioblastoma and solitary brain metastasis. Tumor-wide percentage of signal intensity recovery values tend to be significantly reduced within metastatic lesions when compared with glioblastoma.
- 21.Jackson A, Kassner A, Annesley-Williams D. Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade. AJNR Am. J. Neuroradiol. 2002;23:7–14. [PMC free article] [PubMed] [Google Scholar]; •• Initial study demonstrating that DSC perfusion MRI is useful in differentiating histologic glioma grade.
- 22.Law M, Oh S, Johnson G, et al. Perfusion magnetic resonance imaging predict patient outcome as an adjunct to histopathology. Neurosurgery. 2006;58:1099–1107. doi: 10.1227/01.NEU.0000215944.81730.18. [DOI] [PubMed] [Google Scholar]
- 23.Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am. J. Neuroradiol. 2006;27:475–487. [PMC free article] [PubMed] [Google Scholar]
- 24.Lev MH, Rosen BR. Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin. N. Am. 1999;9:309–331. [PubMed] [Google Scholar]
- 25.Cha S, Tihan T, Crawford F, et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2005;26(2):266–273. [PMC free article] [PubMed] [Google Scholar]
- 26.Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin. Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- 27.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 28.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann C, von Deimling A. Molecular pathology of oligodendroglial tumors. Recent results. Cancer Res. 2009;171:25–49. doi: 10.1007/978-3-540-31206-2_2. [DOI] [PubMed] [Google Scholar]
- 30.Chawla S1, Krejza J, Vossough A, et al. Differentiation between oligodendroglioma genotypes using dynamic susceptibility contrast perfusion-weighted imaging and proton MR spectroscopy. AJNR Am. J. Neuroradiol. 2013;34(8):1542–1549. doi: 10.3174/ajnr.A3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor GS1, Gocke TA, Chawla S, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J. Neurooncol. 2009;92(3):373–386. doi: 10.1007/s11060-009-9880-x. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006;48:703–713. doi: 10.1007/s00234-006-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Initial study demonstrating that 1p19q genomic mutation in oligogendroglioma can be noninvasively delineated using DSC perfusion MRI.
- 33.Law M, Brodsky JE, Babb J, et al. High cerebral blood volume in human gliomas predicts deletion of chromosome 1p: Preliminary results of molecular studies in gliomas with elevated perfusion. J. Magn. Reson. Imaging. 2007;25:1113–1119. doi: 10.1002/jmri.20920. [DOI] [PubMed] [Google Scholar]
- 34.Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J. Neurosurg. 2007;107:600–609. doi: 10.3171/JNS-07/09/0600. [DOI] [PubMed] [Google Scholar]
- 35.Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25:1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 36.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin. Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 37.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45(6):1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 38.Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 2007;25(16):2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 39.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 40.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc. Natl Acad. Sci. USA. 1998;95(10):5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 42.Li G, Mitra S, Wong AJ. The epidermal growth factor variant III peptide vaccine for treatment of malignant gliomas. Neurosurg. Clin. N. Am. 2010;21(1):87–93. doi: 10.1016/j.nec.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Choi BD, Archer GE, Mitchell DA, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19(4):713–723. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang W, Barth RF, Wu G, et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin. Cancer Res. 2006;12(12):3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 45.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aghi M, Gaviani P, Henson JW, Batchelor TT, Louis DN, Barker FG., 2nd Magnetic resonance imaging characteristics predict epidermal growth factor receptor amplification status in glioblastoma. Clin. Cancer Res. 2005;11:8600–8605. doi: 10.1158/1078-0432.CCR-05-0713. [DOI] [PubMed] [Google Scholar]
- 47.Tykocinski ES, Grant RA, Kapoor GS, et al. Use of magnetic perfusion-weighted imaging to determine epidermal growth factor receptor variant III expression in glioblastoma. Neuro Oncol. 2012;14(5):613–623. doi: 10.1093/neuonc/nos073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A1, Young RJ, Shah AD, et al. Pretreatment dynamic susceptibility contrast MRI perfusion in glioblastoma: prediction of EGFR gene amplification. Clin. Neuroradiol. 2014 doi: 10.1007/s00062-014-0289-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omuro A1, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 50.Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 51.Piepmeier J, Baehring JM. Surgical resection for patients with benign primary brain tumors and low grade gliomas. J. Neurooncol. 2004;69:55. doi: 10.1023/b:neon.0000041871.46785.53. [DOI] [PubMed] [Google Scholar]
- 52.Bampoe J, Bernstein M. The role of surgery in low grade gliomas. J. Neurooncol. 1999;42:259. doi: 10.1023/a:1006103010109. [DOI] [PubMed] [Google Scholar]
- 53.Shaw EG, Tatter SB, Lesser GJ, et al. Current controversies in the radiotherapeutic management of adult low-grade glioma. Semin. Oncol. 2004;31:653. doi: 10.1053/j.seminoncol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 55.Eisenhauer EA1, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Wen PY1, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]; •• Current recommendations by the RANO working group for the diagnosis of recurrent glioma.
- 57.Vöglein J1, Tüttenberg J, Weimer M, et al. Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest. Radiol. 2011;46(6):390–400. doi: 10.1097/RLI.0b013e31820e1511. [DOI] [PubMed] [Google Scholar]
- 58.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575–584. doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 59.Barajas RF, Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253:486–496. doi: 10.1148/radiol.2532090007. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that DSC perfusion metrics may be helpful in differentiating recurrent glioblastoma from treatment effect.
- 60.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am. J. Neuroradiol. 2009;30:552–558. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]