Abstract
BACKGROUND
The 2013 American Urological Association/American Society for Radiation Oncology consensus guidelines recommend offering adjuvant radiotherapy (RT) after radical prostatectomy in patients with high-risk pathologic features for recurrence. In the current study, the authors examined practice patterns of adjuvant RT use in patients with elevated pathologic risk factors over a time period spanning the publication of supporting randomized evidence.
METHODS
Using the National Cancer Data Base, a total of 130,681 patients were identified who underwent surgical resection for prostate cancer between 2004 and 2011 with at least 1 of the following pathologic risk factors for early biochemical failure: pT3a disease or higher, positive surgical margins and/or lymph node-positive disease. Using multivariable logistic regression, the authors examined factors associated with adjuvant RT use including patient, clinical, demographic, and temporal characteristics.
RESULTS
Adjuvant RT was administered to 9.9% of the patients with at least 1 pathologic risk factor. Use of adjuvant RT did not change over the study period (P = .23). On multivariable analysis, we found that patients treated at high-volume surgical facilities were less likely to receive adjuvant RT (15.9% vs 7.8%; odds ratio, 0.58 [95% confidence interval, 0.50–0.65]; P<.0001). Older age, comorbidities, black race, lower income, and lower population density were also associated with lower rates of adjuvant RT.
CONCLUSIONS
Use of adjuvant RT is uncommon and remained unchanged between 2004 and 2011. Patients treated at high-volume surgical facilities are less likely to receive adjuvant RT, irrespective of margin status.
Keywords: prostate cancer, adjuvant, radiation, patterns of care, surgical volume
INTRODUCTION
Three cooperative group randomized clinical trials compared the use of adjuvant radiotherapy (RT) versus observation with optional salvage RT for patients with prostate cancer with high-risk pathologic features.1–5 Adjuvant RT resulted in improved biochemical outcomes in all 3 trials and improved clinical outcomes (metastasis-free survival and freedom from hormonal therapies) in 2 of the 3 trials. Based on the 2013 American Urological Association/ American Society for Radiation Oncology (AUA/ASTRO) guidelines, “physicians should offer adjuvant RT to patients with adverse pathologic findings at prostatectomy including seminal vesicle invasion, positive surgical margins, or extraprostatic extension because of demonstrated reductions in biochemical recurrence, local recurrence and clinical progression.”6
Recently published studies on the patterns of adjuvant RT use in the United States have suggested an overall underuse compared with what might be expected given the randomized data.7–10 However, these studies are limited because they only included men aged >65 years who underwent prostatectomy before 2007 (whereas randomized clinical trial results were published between 2005–2012) and they used data from the Surveillance, Epidemiology, and End Results (SEER) program, which is confined to 17 SEER geographies that only represent 26% of the US population.11
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society and tracks oncology outcomes on 71% of newly diagnosed cancer cases nationwide.12 The NCDB is a registry data set that is complementary to SEER and SEER-Medicare but augments these important resources with more detailed information from a broad set of hospitals that are accredited by the Commission on Cancer.13 Therefore, we conducted an observational study to examine the patterns of adjuvant RT use in men with prostate cancer and at least 1 high-risk pathologic feature between 2004 and 2011 with the hypothesis that adjuvant RT use should have increased over the study period.
MATERIALS AND METHODS
Data Source and Cohort Definition
To test our hypothesis, we used data from the NCDB, which collects information on patients with newly diagnosed cancers at 1508 institutions accredited by the Commission on Cancer of the American College of Surgeons.12 In addition to patient demographics, cancer characteristics, staging, first course of treatment, and survival from time of diagnosis, which are also collected by SEER, the NCDB records variables including insurance status, facility volume, practice setting, RT details (eg, site of treatment and dose), and the use of hormone therapy and immunotherapy.14
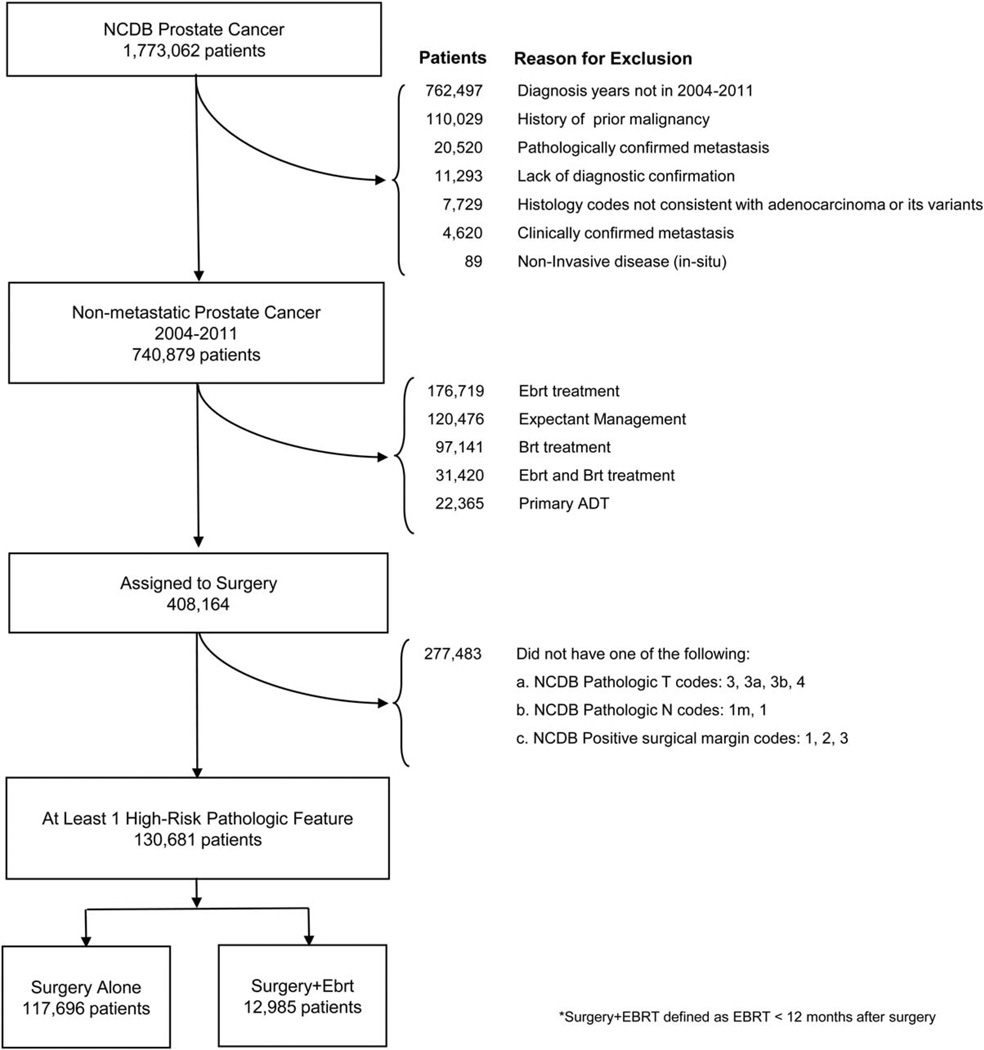
We identified 130,681 patients with prostate cancer reported to the NCDB using International Classification of Diseases for Oncology, 3rd edition codes for site of origin. Figure 1 illustrates how exclusions were applied to these patients to define the study cohort. The study period was limited to patients with diagnostic confirmation between 2004 and 2011. After exclusions, we identified 856,285 patients with nonmetastatic prostate cancer assignable to treatment, and then excluded 448,121 patients who received treatments other than surgery. If patients received multiple definitive treatments, they were assigned to the group consistent with the first definitive treatment. We included 408,164 patients who received surgery.
Figure 1.
Definition of the study cohort is shown. NCDB indicates National Cancer Data Base; EBRT, external beam radiotherapy; BRT, brachytherapy; ADT, androgen deprivation therapy.
From this group, we excluded 277,483 patients without at least 1 high-risk pathologic factor (pathologic T3a or higher disease, pathologic lymph node-positive disease, and/or positive surgical margins). Of these 277,483 excluded patients, 20,247 (7%) had unknown pathologic T classification and 82,453 (29.7%) had unknown pathologic N classification. The final study cohort included 130,681 patients.
Patients in the study cohort were classified as receiving adjuvant RT if RT was administered within 12 months after surgery.15, 16 The NCDB captures all radiation treatment to primary or metastatic sites within the first course of treatment. We chose 12 months as a cutoff for adjuvant RT because 88% of patients received RT within 1 year of surgery in the NCDB data set, with a sharp decrease after 1 year. Other studies have used 6 months as a cutoff for adjuvant RT, but the distribution of patients receiving RT in our data set did not change dramatically between 91 and 180 days after surgery (39%) and 181 and 270 days after surgery (34%). The NCDB does not track postoperative prostate-specific antigen (PSA) values, and therefore this definition of adjuvant RT may include some men with detectable PSA levels. The final cohort included 117,696 patients in the surgery-alone group and 12,985 patients in the group receiving adjuvant RT.
Analytic Variables
The primary outcome was use of adjuvant RT. Use of adjuvant RT was determined by finding the quotient of patients receiving adjuvant RT and the total number of patients in the cohort. We were principally interested in temporal trends of adjuvant RT use from 2004 to 2011.
We were secondarily interested in the association between clinical-level, patient-level, demographic-level, and facility-level variables and adjuvant RT use. Clinical variables included surgical margin status, pathologic T classification, pathologic N classification, and Gleason score. Patient variables included age (< 50 years, 50–64 years, 65–79 years, and >79 years), race (white, black, or other), and Charlson/Deyo comorbidity index. Demographic variables included income, insurance type, and urban/rural residence. Facility variable included facility surgical volume and practice setting.
We defined the surgical volume of each facility as the number of patients treated with definitive surgery for nonmetastatic prostate cancer annually.17 We then categorized facilities into quartiles by annual surgical volume. Facilities in the quartile with the highest surgical volume were grouped as “high” volume, and the remaining facilities were grouped as “low” volume. Prior studies have used high and low categorization of facilities by surgical volume, and although our approach is not identical, the lower limit of annual prostatectomy volume in our high-volume category is consistent with data from earlier studies. 17, 18 Using category classifications assigned to each facility by the Commission on Cancer Accreditation Program, we defined facility practice setting as academic, comprehensive, or community.
Statistical Analysis
We performed univariate and multivariable logistic regression to evaluate temporal trends and use of adjuvant RT over the study period. To evaluate the temporal trend of adjuvant RT use, year of diagnosis was included in the multivariable model as a continuous variable. We evaluated statistical interactions between facility volume and each of the following variables: facility type, surgical margin status, pathologic T classification, pathologic N classification, and Gleason score. We used generalized estimating equations to account for clustering of outcomes within the facility assuming an independent correlation structure with robust variance estimates. We report odds ratios for the use of adjuvant RT with 95% confidence intervals. Statistical analysis was performed using SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC). Associations were considered statistically significant when P<.05; all tests were 2-tailed.
RESULTS
Characteristics of the Study Cohort
As shown in Table 1, the majority of patients were aged <65 years and privately insured with surgical margin-positive (65.5%), lymph node-negative (94.5%), Gleason score 7 (60.1%), and T2 to T3a (76%) disease. More patients in the cohort were treated at high-volume surgical facilities compared with low-volume facilities (73.6% vs 26.4%) (Table 1).
TABLE 1.
Characteristics of the Study Cohort (n = 130,681)
| Characteristic | No. | Percent |
|---|---|---|
| Year of diagnosis | ||
| 2004 | 13,983 | 10.7 |
| 2005 | 13,185 | 10.1 |
| 2006 | 15,171 | 11.6 |
| 2007 | 16,938 | 13.0 |
| 2008 | 17,272 | 13.2 |
| 2009 | 17,905 | 13.7 |
| 2010 | 17,846 | 13.7 |
| 2011 | 18,381 | 14.1 |
| Facility | ||
| Surgical volume | ||
| Low volume | 34,520 | 26.4 |
| High volume | 96,161 | 73.6 |
| Type | ||
| Academic | 52,334 | 40.0 |
| Comprehensive | 69,098 | 52.9 |
| Community | 8305 | 6.4 |
| Unknown | 944 | 0.7 |
| Clinical | ||
| Surgical margin | ||
| Negative | 44,234 | 33.8 |
| Positive | 85,619 | 65.5 |
| Unknown | 828 | 0.6 |
| Pathologic T classification | ||
| T2 | 51,408 | 39.3 |
| T3a | 47,992 | 36.7 |
| T3b | 23,249 | 17.8 |
| T3x | 5914 | 4.5 |
| T4 | 2118 | 1.6 |
| Pathologic N classification | ||
| N0/Nx | 123,503 | 94.5 |
| N1 | 7178 | 5.5 |
| Gleason score | ||
| ≤6 | 24,721 | 18.9 |
| 7 | 78,515 | 60.1 |
| 8–10 | 24,868 | 19.0 |
| Unknown | 2577 | 2.0 |
| Patient | ||
| Age at diagnosis, y | ||
| <50 | 7005 | 5.4 |
| 50–64 | 78,392 | 60.0 |
| 65–79 | 45,029 | 34.5 |
| >79 | 255 | 0.2 |
| Race | ||
| White | 108,081 | 82.7 |
| Black | 15,668 | 12.0 |
| Other | 3644 | 2.8 |
| Unknown | 3288 | 2.5 |
| Charlson/Deyo comorbidity index | ||
| 0 | 108,838 | 83.3 |
| 1 | 19,416 | 14.9 |
| 2 | 2427 | 1.9 |
| Demographic | ||
| Income | ||
| <$30,000 | 13,793 | 10.6 |
| $30–34,999 | 20,481 | 15.7 |
| $35–45,999 | 34,291 | 26.2 |
| ≥$46,000 | 56,251 | 43.0 |
| Unknown | 5865 | 4.5 |
| Insurance | ||
| Private or managed care | 84,367 | 64.6 |
| Medicare or Medicare with supplementary | 40,620 | 31.1 |
| Medicaid | 1686 | 1.3 |
| Not insured | 4008 | 3.1 |
| County size | ||
| Metro areas >250,000 | 86,901 | 66.5 |
| <250,000 | 37,185 | 28.5 |
| Unknown | 6595 | 5.0 |
SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC). Associations were considered statistically significant when P<.05; all tests were 2-tailed.
Overall Use and Temporal Trends
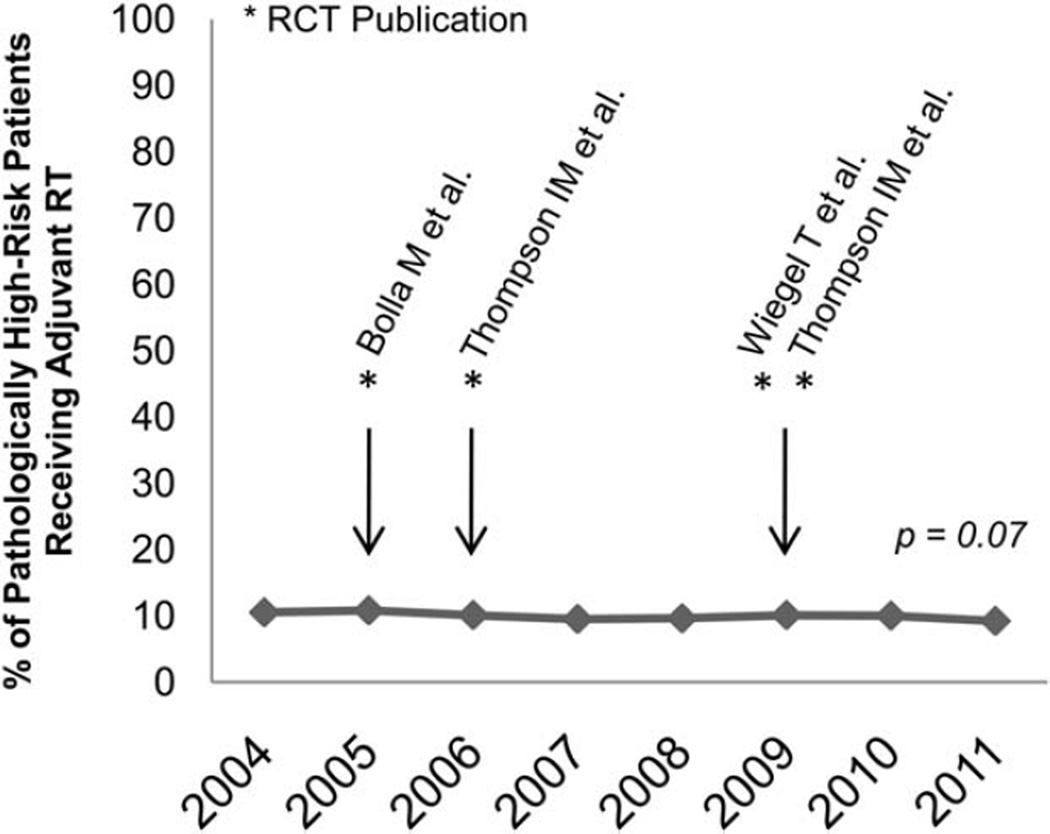
Of the 130,681 patients with nonmetastatic prostate cancer with at least 1 pathologic risk factor after prostatectomy, 12,985 (9.9%) received adjuvant RT. In adjusted analyses, we did not find a statistically significant change in the use of adjuvant RT during the study period (adjusted P = .07) (Fig. 2).
Figure 2.
Temporal trend of adjuvant radiotherapy (RT) is shown in patients with ≥ 1 high-risk pathologic feature (2004–2011). RCT, randomized controlled trial.
Facility Factors Predicting Adjuvant RT Use
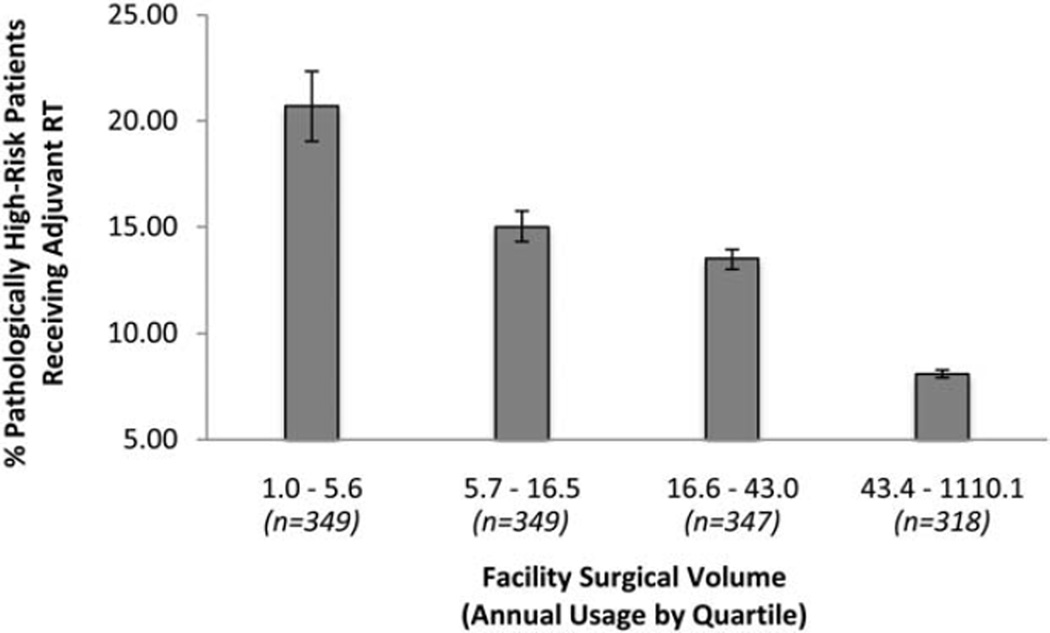
In adjusted analyses accounting for 2-way interactions between facility volume and surgical margin status, pathologic T and N classification, and Gleason score, patients in the cohort treated at high-volume surgical facilities were statistically significantly less likely to receive adjuvant RT compared with patients treated at low-volume facilities (7.8% vs 15.9%; adjusted odds ratio, 0.58 [95% confidence interval, 0.50–0.65]; P<.0001). Adjuvant RT rates declined steadily between facilities in the lowest and highest quartiles of surgical volume (Fig. 3). We identified significant interactions between facility volume and surgical margin status, as well as facility volume and Gleason score.
Figure 3.
Rates of adjuvant radiotherapy (RT) in patients with ≥1 high-risk pathologic feature is shown by facility surgical volume. Error bars represent 95% confidence intervals.
Clinical Factors Predicting Adjuvant RT Use
Patients with positive surgical margins, higher pathologic T classification, lymph node-positive disease, or higher Gleason score were more likely to receive adjuvant RT. We observed low rates of adjuvant RT use (5.2%) in patients with pathologic T2 disease in the cohort (who by definition had either surgical margin-positive or lymph node-positive disease). Patients with Gleason score 6 disease and at least 1 pathologic risk factor also received low rates of adjuvant RT (4.6%). Patients with ≥T3b disease and Gleason score 8 or higher disease received the highest rates of adjuvant RT (21.6% and 20.7%, respectively). Table 2 shows the odds ratios and 95% confidence intervals.
TABLE 2.
Factors Predicting for Adjuvant Radiotherapy
| Characteristic | Adjuvant RT, % |
Unadjusted OR (95% CI) |
P | Adjusted OR (95% CI)a |
P |
|---|---|---|---|---|---|
| Year of diagnosisb | NA | 0.99 (0.98–1.00) | .1723 | 0.99 (0.98–1.00) | .0713 |
| Facility | |||||
| Surgical volume | |||||
| Low volume | 15.9 | Reference | Reference | ||
| High volume | 7.8 | 0.47 (0.42–0.52) | <.0001 | 0.58 (0.50–0.65) | <.0001 |
| Type | |||||
| Academic | 8.1 | Reference | Reference | ||
| Comprehensive | 10.6 | 1.28 (1.12–1.47) | .0003 | 1.14 (0.98–1.32) | .1016 |
| Community | 16.0 | 1.7 (1.40–2.01) | <.0001 | 1.16 (0.95–1.41) | .1382 |
| Unknown | 10.7 | 0.98 (0.64–1.51) | .9304 | 0.65 (0.41–1.03) | .0671 |
| Clinical | |||||
| Surgical margin | |||||
| Negative | 7.6 | Reference | Reference | ||
| Positive | 11.1 | 1.28 (1.23–1.33) | <.0001 | 2.25 (2.14–2.36) | <.0001 |
| Unknown | 18.2 | 2.03 (1.75–2.36) | <.0001 | 1.95 (1.64–2.32) | <.0001 |
| Pathologic T classification | |||||
| T2 | 5.2 | Reference | Reference | ||
| T3a | 8.5 | 1.72 (1.64–1.80) | <.0001 | 2.28 (2.16–2.40) | <.0001 |
| T3b | 21.6 | 3.98 (3.73–4.24) | <.0001 | 4.66 (4.38–4.96) | <.0001 |
| T3x | 12.6 | 2.00 (1.85–2.16) | <.0001 | 2.61 (2.39–2.85) | <.0001 |
| T4 | 23.7 | 4.50 (4.03–5.02) | <.0001 | 4.56 (4.05–5.14) | <.0001 |
| Pathologic N classification | |||||
| N0/Nx | 9.4 | Reference | Reference | ||
| N1 | 19.6 | 2.13 (1.99–2.29) | <.0001 | 1.19 (1.10–1.29) | <.0001 |
| Gleason score | |||||
| ≤6 | 4.6 | Reference | Reference | ||
| 7 | 8.1 | 1.78 (1.70–1.86) | <.0001 | 1.62 (1.53–1.72) | <.0001 |
| 8–10 | 20.7 | 4.27 (4.00–4.57) | <.0001 | 3.17 (2.96–3.40) | <.0001 |
| Unknown | 13.9 | 2.46 (2.20–2.75) | <.0001 | 2.14 (1.89–2.42) | <.0001 |
| Patient | |||||
| Age at diagnosis, y | |||||
| <50 | 11.8 | Reference | Reference | ||
| 50–64 | 10.4 | 0.87 (0.83–0.92) | <.0001 | 0.78 (0.73–0.83) | <.0001 |
| 65–79 | 8.9 | 0.77 (0.73–0.81) | <.0001 | 0.62 (0.57–0.67) | <.0001 |
| >79 | 5.5 | 0.47 (0.33–0.66) | <.0001 | 0.26 (0.16–0.39) | <.0001 |
| Race | |||||
| White | 10.0 | Reference | Reference | ||
| Black | 10.1 | 0.96 (0.92–1.00) | .0795 | 0.92 (0.87–1.03) | .0021 |
| Other | 11.7 | 1.04 (0.96–1.13) | .3632 | 1.01 (0.90–1.13) | .8451 |
| Unknown | 5.1 | 0.74 (0.66–0.83) | <.0001 | 0.75 (0.65–0.87) | <.0001 |
| Charlson/Deyo comorbidity index | |||||
| 0 | 10.1 | Reference | |||
| 1 | 9.1 | 0.88 (0.85–0.92) | <.0001 | 0.85 (0.81–0.89) | <.0001 |
| 2 | 9.0 | 0.86 (0.78–0.95) | .0022 | 0.80 (0.71–0.90) | .0004 |
| Demographic | |||||
| Income | |||||
| <$30,000 | 10.2 | Reference | Reference | ||
| $30–34,999 | 9.7 | 0.98 (0.93–1.03) | .3808 | 0.99 (0.92–1.06) | .7355 |
| $35–45,999 | 10.3 | 1.05 (1.00–1.11) | .033 | 1.07 (1.00–1.14) | .0418 |
| ≥$46,000 | 9.8 | 1.09 (1.04–1.15) | .0009 | 1.10 (1.03–1.17) | 0.006 |
| Unknown | 9.1 | 1.05 (0.98–1.13) | .1752 | 1.09 (0.97–1.22) | .1537 |
| Insurance | |||||
| Private or managed care | 10.2 | Reference | Reference | ||
| Medicare or Medicare with supplementary | 9.1 | 0.89 (0.86–0.91) | <.0001 | 0.95 (0.90–1.01) | .0804 |
| Medicaid | 13.0 | 1.25 (1.11–1.41) | .0002 | 1.32 (1.14–1.52) | .0002 |
| Not insured/unknown | 10.8 | 1.01 (0.93–1.11) | .7344 | 0.93 (0.83–1.04) | .1864 |
| County size | |||||
| Metro areas >250,000 | 9.8 | Reference | Reference | ||
| <250,000 | 10.3 | 0.86 (0.82–0.90) | <.0001 | 0.88 (0.83–0.94) | <.0001 |
| Unknown | 9.1 | 0.92 (0.87–0.98) | .0101 | 0.91 (0.81–1.02) | .097 |
Abbreviations: 95% CI, 95% confidence interval; NA, not available; OR, odds ratio; RT, radiotherapy.
Adjusted ORs account for 2-way interactions between facility volume and each of the following variables: facility type, surgical margin status, pathologic T classification, pathologic N classification, and Gleason score.
Year of diagnosis was included as a continuous variable.
Patient Factors Predicting Adjuvant RT Use
Compared with patients aged <50 years, patients aged 50 years to 64 years, 65 to 79 years, and >79 years received incrementally lower rates of adjuvant RT (11.4%, 10.8%, 8.9%, and 5.5%, respectively; P<.0001). Comorbidity scores of 1 and 2 were also statistically significantly associated with a decreased use of adjuvant RT on multivariate analysis (Table 2).
DISCUSSION
The current study was undertaken to examine trends in the use of adjuvant RT in patients with at least 1 pathologic risk factor between 2004 and 2011. Given the publication of randomized trials demonstrating a benefit for adjuvant RT among patients with pathologic risk factors, we hypothesized an increase in the use of adjuvant RT over the study period.
We observed overall low rates (9.9%) of adjuvant RT in the study cohort, which remained unchanged between 2004 and 2011. This finding is consistent with and expands on the existing literature for patterns of adjuvant RT use, which report only through 2007 using SEER and SEER-Medicare data. One group reported that 10.8% of patients with prostate cancer diagnosed through 2006 with pathologic risk factors received postoperative RT within 6 months of surgery.7 Another study reported 6.1% and 7.4%, respectively, of patients identified from 2004 through 2006 with either positive surgical margins or T3a disease received adjuvant RT.8
Although important, these studies did not include patients treated after publication of the Southwest Oncology Group (SWOG) 8794 study in 2009, which demonstrated a survival benefit from adjuvant RT.2 A vigorous and healthy debate continues about the necessity of adjuvant versus salvage RT; to that end, the 2013 AUA/ ASTRO guidelines recommend that all patients with high-risk pathologic features should be offered adjuvant RT.6 The data from the current study, including patients treated through 2011, highlight a potential discordance between existing evidence and practice patterns; future analysis will determine how 2013 guidelines affect practice patterns.
We also found that patients treated at high-volume surgical facilities were substantially less likely to receive adjuvant RT than patients treated at low-volume surgical facilities (7.8 vs 15.9%). This volume-quality discrepancy is contrary to the literature, which has shown that low-volume facilities, rather than high-volume ones, are less likely to provide guideline-based care.19–21 The discrepancy we found between high-volume and low-volume facilities persisted after adjusting for surgical margin status (a surrogate for surgical quality) and pathologic features. One explanation for these data are that factors inherent to high-volume surgical facilities, apart from surgical quality or disease characteristics, reduce the propensity to refer for adjuvant RT. An alternative explanation is that surgeons at low-volume facilities may be more likely to refer patients who may not derive as much benefit from immediate adjuvant therapy.
We also found that adjuvant RT rates declined significantly in older patients and patients with comorbidities. The decline in adjuvant RT use in older populations may reflect uncertainty regarding the benefits of treatment in patients with a shorter life expectancy, as highlighted by the European Organization for Research and Treatment of Cancer (EORTC) 22911 trial.4 However, we observed a stepwise decline in adjuvant RT use between men aged <50 years and men aged 50 years to 64 years, a group for whom EORTC 22911 reported benefits in both biochemical and clinical recurrence-free survival. Given the safety and efficacy of adjuvant RT, these data highlight the need for a better discussion of adjuvant RT with men aged >50 years.
The current study has limitations. The NCDB does not capture postoperative PSA data. As a result, we were not able to use elevated or rising postoperative PSA as a means to exclude patients receiving salvage RT from the current study cohort. Second, the NCDB gathers data from Commission on Cancer-approved facilities and is not a population-based data set, which limits its generalizability. Facilities approved by the Commission on Cancer are larger and more urban, and provide more dedicated cancer services than non-approved facilities.22 Third, we included patients with lymph node-positive disease, although the indications for adjuvant RT in these patients are controversial. Nonetheless, these results were similar with or without the exclusion of patients with lymph node-positive disease (data not shown). Fourth, although the report of differential rates of adjuvant RT based on facility volume are hypothesis-generating, a causal link cannot be established. This finding may also be impacted by differential reporting of adjuvant RT by facility, although we might expect the bias to favor higher adjuvant RT use in high-volume surgical facilities. Lastly, we cannot ascertain patient preferences or the nature of referrals between urologist and radiation oncologists.23–25
The data from the current study confirm that the use of adjuvant RT in men with at least 1 pathologic risk factor remains uncommon. Although the AUA/ASTRO guidelines recommend that physicians offer adjuvant RT to this population, to our knowledge there is no normative standard for what the overall rate of adjuvant RT should be. However, the rates of adjuvant RT in the current study are low enough to question the extent to which preference-based and participatory decision-making is occurring in routine clinical care among physicians and patients with prostate cancer after prostatectomy.26
Acknowledgments
FUNDING SUPPORT
Supported by National Cancer Institute grant K07-CA163616 and the David and Leslie Clarke Fund.
Footnotes
The data used in the current study were derived from a deidentified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigator.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Sheets NC, Hendrix LH, Allen IM, Chen RC. Trends in the use of postprostatectomy therapies for patients with prostate cancer: a Surveillance, Epidemiology, and End Results Medicare analysis. Cancer. 2013;119:3295–3301. doi: 10.1002/cncr.28222. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber D, Rineer J, Yu JB, et al. Analysis of pathologic extent of disease for clinically localized prostate cancer after radical prostatectomy and subsequent use of adjuvant radiation in a national cohort. Cancer. 2010;116:5757–5766. doi: 10.1002/cncr.25561. [DOI] [PubMed] [Google Scholar]
- 9.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76:1169–1174. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman KE, Nguyen PL, Chen MH, et al. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol. 2011;185:116–120. doi: 10.1016/j.juro.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute Surveillance, Epidemiology, and End Results Program. [Accessed February 7, 2014];About the SEER Registries. seer.cancer.gov/registries/.
- 12.American College of Surgeons Commission on Cancer. [Accessed November 21, 2013];2011 Year in Review. 2011 :1–16. facs.org/cancer/2011-coc-year-in-review.pdf.
- 13.Mettlin CJ, Menck HR, Winchester DP, Murphy GP. A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer. 1997;79:2052–2061. doi: 10.1002/(sici)1097-0142(19970515)79:10<2052::aid-cncr29>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan JR, Kowalczyk KJ, Borza T, et al. Patterns of care and outcomes of radiotherapy for lymph node positivity after radical prostatectomy. BJU Int. 2013;111:1208–1214. doi: 10.1111/bju.12079. [DOI] [PubMed] [Google Scholar]
- 16.Williams SB, Gu X, Lipsitz SR, Nguyen PL, Choueiri TK, Hu JC. Utilization and expense of adjuvant cancer therapies following radical prostatectomy. Cancer. 2011;117:4846–4854. doi: 10.1002/cncr.26012. [DOI] [PubMed] [Google Scholar]
- 17.Begg C, Riedel E, Bach P, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;168(4 pt 1):1642. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 18.Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401–405. doi: 10.1200/JCO.2003.05.169. [DOI] [PubMed] [Google Scholar]
- 19.Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013;63:823–829. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med. 2013;173:559–568. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow R, Chang J, Ziogas A. NCCN Treatment Guidelines for Ovarian Cancer: A Population-Based Validation Study of Structural and Process Quality Measures. Presented at the 2013 Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancer; March 9–March 12, 2013; Los Angeles, CA. Abstract 45. [Google Scholar]
- 22.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of Commission on Cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 23.Sommers BD, Beard CJ, D’Amico AV, Kaplan I, Richie JP, Zeckhauser RJ. Predictors of patient preferences and treatment choices for localized prostate cancer. Cancer. 2008;113:2058–2067. doi: 10.1002/cncr.23807. [DOI] [PubMed] [Google Scholar]
- 24.Sommers BD, Beard CJ, D’Amico AV, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer. 2007;110:2210–2217. doi: 10.1002/cncr.23028. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RM, Couper MP, Zikmund-Fisher BJ, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study) Arch Intern Med. 2009;169:1611–1618. doi: 10.1001/archinternmed.2009.262. [DOI] [PubMed] [Google Scholar]
- 26.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28:4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]