Abstract
Purpose
Patients previously treated with ketoconazole were excluded from phase III trials of abiraterone acetate due to potential overlapping mechanism of action. The purpose of this study was to determine the clinical utility of abiraterone and its impact on circulating androgens following ketoconazole.
Experimental Design
Chemotherapy-naïve patients with progressive metastatic castration-resistant prostate cancer (mCRPC) and prior ketoconazole therapy ≥28 days received abiraterone 1000 mg daily and prednisone 5 mg twice daily. The primary endpoint was the proportion of patients with PSA response, defined as ≥30% PSA decline at 12 weeks. H0=0.30 versus H1=0.50 (α=0.05, power=0.83). Circulating androgens levels were measured using liquid chromatography tandem mass spectrometry.
Results
39 patients were included in the final analysis. Twenty (51%, 95%CI: 36–66%) patients had ≥30% PSA decline; the null hypothesis was rejected. Sixteen (41%) had ≥50% PSA decline. Median PFS was 16 weeks; median rPFS was 36 weeks. Samples for measurement of baseline androgens were available in 37 patients. The PSA response proportion was 59% in 29 patients with DHEA ≥ limit of quantitation (LOQ), compared to 13% in 8 patients with DHEA<LOQ (p=0.042). Median PFS were 6 and 16 weeks in DHEA<LOQ and DHEA≥LOQ patients, respectively (p=0.017); median rPFS were 14 and 36 weeks in DHEA<LOQ and DHEA≥LOQ patients, respectively (p<0.001).
Conclusions
Abiraterone demonstrates modest clinical efficacy in mCRPC patients previously treated with ketoconazole. Patients with DHEA≥LOQ were more likely to demonstrate PSA responses and longer PFS. Analysis of circulating androgens merits further investigation as a biomarker for response to androgen synthesis inhibitor therapy.
Keywords: Ketoconazole, abiraterone acetate, metastatic castration-resistant prostate cancer, androgens, liquid chromatography tandem mass spectrometry
Introduction
Ketoconazole, an imidazole antifungal agent with inhibitory activity of the cytochrome P450 17A1 complex (CYP17), has demonstrated clinical activity in patients with metastatic castration-resistant prostate cancer (mCRPC) in prospective clinical trials (1, 2) and has been used for decades in the treatment of this disease (3). Combined with the understanding that retained androgen receptor (AR) signaling is integral to the progression of prostate cancer to its lethal phenotype, ketoconazole’s clinical utility provided a framework for the development of novel androgen synthesis inhibitors. Abiraterone acetate, an oral CYP17 inhibitor with greater specificity and potency than ketoconazole (4, 5), significantly improved the survival of patients with progressive mCRPC in pivotal phase III trials (6, 7), and is now in broad clinical use. A phase I study of abiraterone in patients who experienced disease progression (or excessive toxicities) on ketoconazole demonstrated PSA responses comparable to those of ketoconazole-naïve patients (8). However, concerns regarding the overlapping mechanism of action (and of resistance) between ketoconazole and abiraterone led to the exclusion of patients previously treated with ketoconazole from the pivotal phase III abiraterone trials (6, 7). Therefore, the clinical efficacy of abiraterone following ketoconazole is not well understood. The purpose of this prospective phase II clinical trial (NCT01199146) was to determine the utility of abiraterone following previous therapy with ketoconazole.
Patients and Methods
Patients
Eligible patients had mCRPC and were treated with ketoconazole for more than 28 days, with evidence of disease progression or grades 3/4 toxicities requiring discontinuation of therapy. Disease progression was defined as a confirmed rise in PSA >2ng/mL above the nadir (or baseline, if no response to ketoconazole), the appearance of new lesions on bone scan, or objective progression defined using RECIST criteria, while on ketoconazole. A minimum washout period of 27 days was required between the final dose of ketoconazole and the first dose of abiraterone acetate. Other key eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; absolute neutrophil count ≥1.5×109/L, hemoglobin ≥9.0g/dL, and platelets ≥100×109/L; serum creatinine and bilirubin ≤1.5x the institutional upper limit of normal (ULN), potassium ≥3.5mmol/L, and AST and ALT ≤2.5x the institutional ULN. Patients previously treated with chemotherapy for mCRPC were excluded from the study.
The study was conducted in compliance with the study protocol and in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by regulatory authorities and institutional review boards (IRBs) of participating institutions. Signed and informed written consent was obtained from all patients prior to study entry.
Study Design and Treatment
This was a single arm, prospective phase II study conducted through the Prostate Cancer Clinical Trials Consortium, at the University of California, San Francisco (UCSF), and the University of Chicago (UoC). Enrolled patients received abiraterone 1000mg by mouth daily and prednisone 5mg by mouth twice daily, in 28-day cycles. Abiraterone was taken on an empty stomach (at least 2 hours after and 1 hour before meals). Dosing was interrupted for grades 3/4 toxicities related to study treatment, and restarted at 25% dose reduction once toxicities resolved to grade 1 or less. Up to 2 dose reductions (to 750 and 500mg by mouth, once daily) were permitted. Patients who required greater than 4 weeks to recover from grade 3/4 toxicities were discontinued from study participation. Patients underwent clinical and laboratory assessment prior to their first dose and on Day 1 of each subsequent cycle; radiographic assessments occurred every 3 cycles.
Treatment continued until disease progression according to Prostate Cancer Working Group 2 (PCWG2) criteria(9). Patients were allowed to continue therapy at the investigators’ discretion if they met the PSA criteria for progression but did not experience unequivocal clinical progression, objective progression per RECIST criteria or ≥2 new bone lesions. Abiraterone was discontinued for unacceptable toxicity, if the patient withdrew consent, or if the patient was withdrawn from the study at the investigators’ discretion.
Study endpoints and assessments
PSA response was defined as ≥30% PSA decline. The primary endpoint was PSA response proportion, defined as the proportion of patients with ≥30% PSA decline at 12 weeks of abiraterone treatment. Secondary endpoints included time to PSA progression (TTPP), safety of abiraterone following prior ketoconazole therapy, and the proportion of patients with ≥50% PSA decline at 12 weeks. Exploratory endpoints included assessment of circulating androgen levels at baseline and their change over time on abiraterone.
Patients were assessed at the beginning of each 28-day cycle for safety and response. Soft tissue and bone imaging were performed every 3 cycles (12 weeks). PSA measurements were taken at baseline and with each cycle of treatment. Androgen levels were measured every 2 cycles (8 weeks) beginning with cycle 1, day 1 (baseline, prior to first dose of abiraterone).
A waterfall plot was constructed to graphically display the PSA response at 12 weeks for each patient (9). PSA progression was defined as ≥25% PSA increase and absolute increase ≥2ng/mL from the nadir, or for patient without PSA decline, ≥25% PSA increase and absolute increase ≥2ng/mL from baseline. Progression-free survival (PFS) was a prespecified assessment. PCWG2 criteria were used to define disease progression, and included PSA progression, unequivocal clinical progression, radiographic progression, initiation of new therapy or death.
Measurement of circulating androgens
Analysis of circulating androgen levels was performed at Roswell Park Cancer Institute. Study samples were analyzed for testosterone (T), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), androstenedione (ASD), and androsterone (AND), using high-pressure liquid chromatography (HPLC) and tandem mass spectrometric detection (LC-MS/MS), a previously validated method (10). Briefly, samples were prepared as follows: a 250μL aliquot of a calibrator, quality controls (QC), plasma blank, or study sample was mixed with 750μL of HPLC-grade water, 100μL internal standard solution (75/225pg/mL d3-T/d3-DHT in 75% methanol), and extracted with 4.0mL methyl-tert-butyl ether (MTBE). Tubes were rotated for 15 minutes and centrifuged at 3,000 rpm and 4°C for 15 minutes to separate liquid phases. The aqueous phase was frozen in a dry-ice acetone bath and the upper MTBE layer poured into a glass conical tube. MTBE was evaporated with nitrogen at 37 °C and the residue reconstituted with 60μL of 60% methanol. The suspension was centrifuged at 3,000 rpm and 4°C for 5 minutes to separate insoluble materials. A 20μL aliquot of the supernatant was injected.
LC-MS/MS was performed using a Shimadzu Prominence UFLC System (Shimadzu Scientific Instruments Inc, Columbia, MD) interfaced with an AB SCIEX QTRAP 5500 mass spectrometer (AB SCIEX, Framingham, MA) in positive ion mode. Voltages for maximum parent/fragment ion pair intensities were optimized using direct infusion and flow injection analysis.
The limits of quantitation (LOQ) for the androgens measured are as follows: T <0.0125ng/mL; DHT <0.025ng/mL; ASD <0.025ng/mL; DHEA <0.250ng/mL; AND <0.250ng/mL.
Serum and plasma specimens were used for analysis of circulating androgen levels. Comparison of androgen concentrations between the two specimen types in a subset of 10 patients in whom serum and plasma were drawn at the same time demonstrated no significant differences.
Statistical Analysis
The study was designed to test the hypothesis that abiraterone retains clinical activity in patients with mCRPC that is refractory to ketoconazole. The null hypothesis of a 30% PSA response proportion (i.e. ≥30% of patients would experience a PSA decline ≥30% at 12 weeks of abiraterone) was compared to an alternative hypothesis of 50% PSA response proportion (i.e. ≥50% of patients would experience a PSA decline ≥30% at 12 weeks), using a Simon’s 2-stage minimax design. Accrual of 39 patients was required assuming directional level of significance 0.05 and power 0.83 using an exact binomial test. Nineteen patients were to be accrued to the first stage, and if 7 achieved a ≥30% PSA decline, then 20 patients were to be accrued to the second stage. If 17 patients entered on the study achieved ≥30% PSA decline, then the null hypothesis would be rejected in favor of the alternative hypothesis.
Frequency distribution and percentage were used to summarize categorical measurements, while mean (with standard deviation) and median (with range) were used to describe symmetric and skewed continuous measurements, respectively. Univariate analysis among variable were assessed using the two-sample t-test, Wilcoxon rank sum test, and the Chi-square test, as appropriate. The distribution of time-to-event variables (such as PFS, TTPP) was estimated using the Kaplan Meier method, and log rank tests were used for comparison.
Results
Demographics
Between August 2010 and December 2012, a total of 42 patients were enrolled at UCSF and UoC. Three patients were replaced during the first stage of accrual due to adverse patient selection: 1 patient with a history of oxygen-dependent pulmonary fibrosis and cardiac disease died of a myocardial infarction prior to week 12 evaluation without evidence of disease progression; 1 patient with rapidly progressing disease at the time of enrollment discontinued therapy after just 9 days and died a few days thereafter; a third patient with symptomatic pleural effusion at the time of enrollment discontinued therapy after 5 weeks with worsening respiratory status and died shortly thereafter. Approvals were obtained from the Data and Safety Monitoring Committees and the IRBs of both institutions prior to study expansion and continued accrual. These 3 patients were excluded from all analyses. Therefore, 39 patients were included in the final analysis (Supplemental Figure 1). Baseline demographics and clinical characteristics are summarized in Table 1. 37 patients discontinued ketoconazole for disease progression; 1 patient discontinued due to QT prolongation and another discontinued due to transaminitis and rash and disease progression. Seven of the 14 patients who had pain due to metastatic disease at baseline prior to starting abiraterone required opiate analgesics. Ninety-two percent had bone metastases; 38% had lymph node metastases and 5% had visceral metastases, which is consistent with the typical metastatic disease burden in this patient population. At the time of final analysis, 2 patients remained on study and on abiraterone.
Table 1.
Demographic information for study cohort, n = 39
| Age, median (range), years | 71 years (59–93) |
| ECOG Performance Status, n (%) | |
| 0 | 28 (72%) |
| 1 | 14 (28%) |
| Metastatic disease burden, n (%) | |
| Bone | 36 (92%) |
| Lymph node | 15 (38%) |
| Visceral | 2 (5%) |
| Pain, n (%) | |
| No | 25 (64%) |
| Yes | 14 (36%) |
| Opiate therapy | 7 (18%) |
| Duration of prior ketoconazole, median (range), weeks | 43 (5–207) |
| Ketoconazole treatment duration >1 year, n (%) | 18 (46%) |
| Baseline PSA, median (range), ng/dL | 48.5 (2.1–1144.0) |
Clinical responses
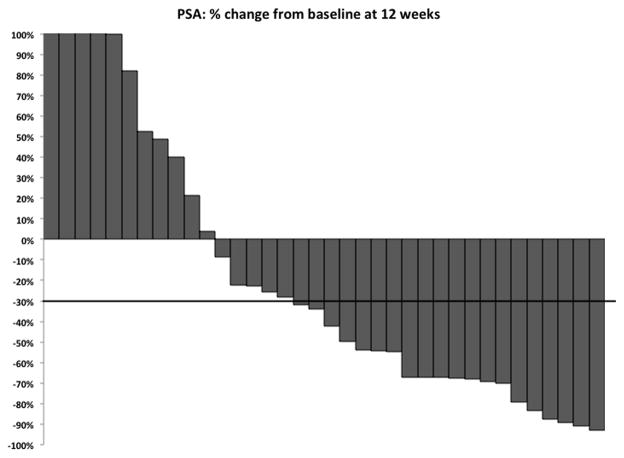
Three patients discontinued study treatment prior to 12 weeks due to disease progression (1 by PSA only and 2 by pain). After 12 weeks of abiraterone treatment, 20 patients (51%, 95% CI 36–66%) had ≥30% PSA decline. Therefore, the null hypothesis of 30% PSA response proportion was rejected. Additionally, 16 patients (41%, 95% CI 26–56%) experienced ≥50% PSA decline after 12 weeks of abiraterone. PSA responses at 12 weeks of abiraterone treatment are summarized on a waterfall plot (Figure 1). Median TTPP was 16 weeks (range, 4–71). Clinical responses to abiraterone, by PSA and radiographic assessment, are summarized in Table 2.
Figure 1.
Waterfall plot representing %change in PSA at 12 weeks of abiraterone therapy. Three out of 39 patients who discontinued therapy prior to 12 weeks are excluded from this waterfall plot.
Table 2.
Summary of clinical responses
| All patients (n=39) | DHEA <LOQ (n=8) | DHEA ≥LOQ (n=29) | p-value | |
|---|---|---|---|---|
| ≥30% PSA decline at 12 weeks, n (%, 95%CI) | 20 (51%, 36–66%) | 1(13%) | 17 (59%) | 0.042 |
| ≥50% PSA decline at 12 weeks, n (%, 95% CI) | 16 (41%, 26–56%) | 1 (13%) | 15 (52%) | 0.103 |
| ≥30% PSA decline at any time, n (%) | 24 (62%) | 2 (25%) | 22 (76%) | 0.013 |
| ≥50% PSA decline at any time, n (%) | 19 (49%) | 1 (13%) | 18 (62%) | 0.018 |
| TTPP, median (range), weeks | 16 (4–71) | 6 (4–32) | 16 (4–71) | 0.017 |
| Bone response at 12 weeks (n=33) | ||||
| Stable disease, n (%) | 31 (94%) | |||
| Soft tissue response at 12 weeks (n=14) | ||||
| Partial response, n (%) | 4 (29%) | |||
| Stable disease, n (%) | 6 (43%) | |||
The relationship between duration of prior ketoconazole therapy and PSA response was explored in a post-hoc analysis, given the long median duration of ketoconazole therapy in enrolled patients. Median duration of prior ketoconazole therapy was significantly shorter in patients who had ≥30% PSA decline (20 vs. 72 weeks, p=0.001; Supplemental Figure 2).
Progression-free survival
Median PFS (Figure 2A) was 16 weeks (range: 4–71 weeks), which was identical to the median time to PSA progression; PSA progression constituted disease progression in all but 2 patients. Both of these patients had clear evidence of clinical disease progression (increasing pain) in spite of neither PSA or radiographic progression.
Figure 2.
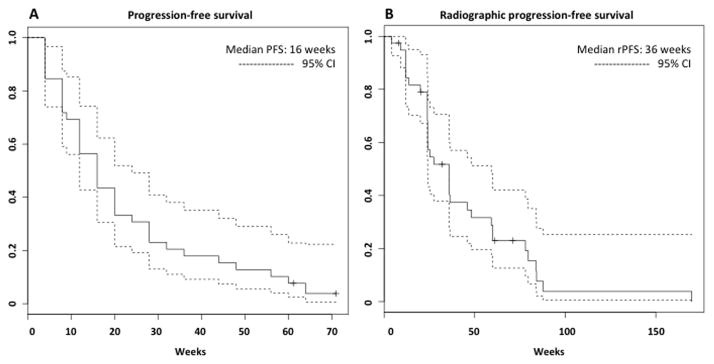
Kaplan-Meier curves representing A) PFS (disease progression was defined per Prostate Cancer Working Group 2 criteria, and included death, radiographic progression, unequivocal clinical progression, and PSA progression), and B) rPFS (radiographic disease progression was defined as death, radiographic progression, or unequivocal clinical progression).
Radiographic progression free survival
Recent studies of abiraterone (COU-AA-302)(7) and enzalutamide (PREVAIL)(11) in chemotherapy-naïve patients utilized a novel definition of radiographic progression-free survival (rPFS), which was defined as death, soft tissue progression per RECIST criteria, bone progression (per modified PCWG2 criteria), or unequivocal clinical progression (defined as worsening symptomatic disease or the need for another therapeutic intervention such as palliative radiation, chemotherapy or surgery). PSA changes were not included in the rPFS definition. rPFS was a co-primary endpoint of both studies.
rPFS, similarly defined, was reported for the present study cohort. (Figure 2B). For the most conservative estimate of rPFS, 3 patients who discontinued study treatment for PSA progression only (all prior to 12 weeks of therapy) were considered to have radiographic progression at the time of PSA progression. Based on this definition, median rPFS was 36 weeks (range: 8–170 weeks).
Safety
The most common adverse events (Table 3) were fatigue (46%), hypophosphatemia (21%), hypertension (18%), transaminitis (13%), and hypokalemia (13%); most of these were grade 1/2 events. Twelve patients (31%) experienced a total of 23 grade 3/4 adverse events, of which one was a grade 4 event (anemia). Hypophosphatemia was the most common grade 3 adverse event, occurring in 3 patients (8%); no other grade 3 adverse event occurred in more than 2 patients. There were no patients who permanently discontinued abiraterone due to adverse events.
Table 3.
Summary of adverse events
| Gd 1 | Gd 2 | Gd 3 | Gd 4 | Total | % | |
|---|---|---|---|---|---|---|
| Fatigue | 13 | 3 | 2 | 0 | 18 | 46 |
| Hypophosphatemia | 1 | 4 | 3 | 0 | 8 | 21 |
| Hypertension | 3 | 2 | 2 | 0 | 7 | 18 |
| Transminitis | 4 | 1 | 0 | 0 | 5 | 13 |
| Hypokalemia | 5 | 0 | 0 | 0 | 5 | 13 |
| Edema | 2 | 1 | 0 | 0 | 3 | 8 |
| Hyperglycemia | 0 | 1 | 2 | 0 | 3 | 8 |
| All grade 3/4 events | 23 events in 12 patients (1 grade 4 event; anemia) | 31 | ||||
Androgen analysis
Baseline androgen levels were available in 37 patients. Median DHEA was 0.614ng/mL; median T was 0.0467ng/mL; median ASD was 0.194ng/mL. DHT was <LOQ in all but 5 patients; AND was <LOQ in all patients.
Median DHEA level was significantly higher in patients who had a ≥30% decline in PSA (0.71ng/mL vs. 0.43ng/mL, p=0.028); Median T and ASD levels were not significantly different between the PSA responders and PSA non-responders (Supplemental Figure 3). Therefore, the relationship between baseline DHEA levels and clinical benefit of abiraterone was explored further. Eight patients had DHEA<LOQ and 29 patients had DHEA≥LOQ. 1 patient (13%) with DHEA<LOQ had a ≥30% PSA decline, compared to 17 patients (59%) with DHEA≥LOQ (p=0.042). The median PFS were 6 and 16 weeks in the DHEA<LOQ and DHEA≥LOQ groups, respectively (p=0.017; Figure 3A). Median rPFS were 14 and 36 weeks in the DHEA<LOQ and DHEA≥LOQ groups, respectively (p<0.001; Figure 3B). All patients had undetectable DHEA levels by cycle 3, day 1 (after 8 weeks of treatment).
Figure 3.
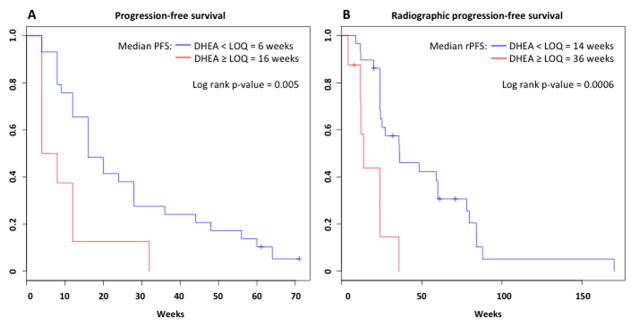
Kaplan-Meier curves representing A) PFS and B) rPFS for patients with DHEA < limit of quantitation (LOQ; in red) and DHEA ≥ LOQ (in blue).
DISCUSSION
Patients previously treated with ketoconazole were excluded from the phase III studies of abiraterone due to concerns regarding the potential overlapping mechanism of action and resistance of these CYP17 inhibitors. In this study, abiraterone demonstrated modest clinical activity in men with progressive mCRPC despite prior CYP17 inhibition therapy with ketoconazole. Over half the patients enrolled on this study had ≥30% PSA declines after 12 weeks of abiraterone treatment; thus the study met its primary endpoint. Moreover, over 40% of patients had ≥50% PSA declines after 12 weeks on abiraterone. Median PFS was 16 weeks, and median rPFS was 36 weeks. Abiraterone was well tolerated in this study population; 31% of patients experienced grade 3/4 adverse events.
PSA decline ≥30% was chosen as the primary endpoint in this study; Petrylak and colleagues (12) and Armstrong and colleagues (13) demonstrated that ≥30% PSA decline satisfied the Prentice criteria (14) as a surrogate for overall survival in first-line chemotherapy studies of mCRPC patients. It is important to note that the association between PSA decline and overall survival in these same studies are modest when reported using PTE, or proportion of treatment effect (15). Furthermore, surrogacy for PSA changes and overall survival has not been demonstrated for AR-targeted therapies. Despite these limitations, the study raises several points that merit further discussion and investigation.
First is whether abiraterone’s greater target inhibitory potency compared to ketoconazole is clinically meaningful. A phase III trial of antiandrogen withdrawal with or without ketoconazole showed significant suppression of serum androgens in patients treated with ketoconazole with subsequent increase in serum androgen levels at the time of disease progression (2), suggesting upregulation of androgen production (perhaps via enhanced CYP17 activity) as a mechanism for ketoconazole resistance; intratumoral CYP17 expression has been shown to be increased in patients previously treated with ketoconazole (16). The ultrasensitive assessment of circulating androgens in this study showed most patients with detectable levels of T, ASD, and DHEA at baseline (after ketoconazole and prior to abiraterone therapy) had undetectable levels after 8 weeks of abiraterone treatment (all 37 patients had undetectable DHEA levels; T remained detectable in 5 patients, and ASD remained detectable in 2 patients). Furthermore, patients with detectable DHEA levels were more likely to respond to abiraterone than those with undetectable levels. Combined, these findings support the conclusions that: 1) upregulated androgen production may be a mechanism of ketoconazole resistance; 2) abiraterone’s greater CYP17 inhibitory potency is indeed clinically meaningful, and can provide clinical benefit in a subset of patients following disease progression.
Second is whether analysis of circulating androgens informs our understanding of therapeutic resistance (as well as response) to androgen synthesis inhibition. Unlike in patients treated with ketoconazole, androgen levels do not rise at the time of disease progression in those treated with abiraterone (8). CYP17 is present in both the endocrine (adrenal) and intracrine (tumor) domains. Whether ketoconazole and abiraterone exert their clinical efficacy in the endocrine versus intracrine domain (or both), and to what degree in each compartment is unknown. While CYP17 expression has been shown to be increased in patients previously treated with ketoconazole(16), whether this is a resistance mechanism that is induced due to suppressed circulating androgens due to adrenal ablation, or in response to intracrine suppression of CYP17 activity, is unknown. Additionally, it is unknown whether upregulation of intratumoral CYP17 expression is the source of the increased androgen concentrations at the time of disease progression on ketoconazole, although adrenal ablation may be incomplete and endocrine androgen synthesis may contribute. How these issues relate to primary and secondary resistance to androgen synthesis inhibition merit further investigation.
The fact that abiraterone can suppress circulating androgens in patients with previous disease progression with ketoconazole demonstrates that abiraterone can suppress CYP17 activity upregulated by ketoconazole. However, as stated above, it remains unclear whether abiraterone exerts its activity via the endocrine versus intracrine compartments (or both). A recent retrospective analysis of serum androgen levels in patients treated with abiraterone after cytotoxic therapy demonstrated that baseline levels (also measured using LC-MS/MS) were prognostic for survival (17). Interestingly, patients with higher androgens had improved survival compared to those with low androgens regardless of treatment arm, and the PSA response proportion was higher in patients with higher androgen levels. This suggests that mCRPC progressing in a low circulating androgen environment has a more aggressive phenotype; it is possible that mCRPC patients who experience disease progression in this setting harbor tumors that may be adapted to CYP17 inhibition.
Baseline DHEA levels were highly associated with response to abiraterone following ketoconazole. Only 1 of 8 patients with undetectable DHEA levels experienced a PSA response, while 17 of 29 patients with detectable DHEA levels treated with abiraterone had a PSA response. Conversely, patients with PSA responses had higher baseline DHEA levels. Patients with detectable DHEA levels had significantly longer PFS and rPFS. These data suggest that DHEA levels may have a potential role as a biomarker for response to CYP17 inhibition therapy. While ultrasensitive assays for androgens are now commercially available, their broad clinical utility as a biomarker requires further investigation and prospective validation prior to incurring additional costs. Currently, T<50ng/dL is the only broadly accepted androgen parameter used to guide the treatment of advanced prostate cancer. Patients with higher “castrate” T levels have been shown to survive longer compared than those with lower “castrate” T levels (17), which questions the adequacy of this parameter. Combined with the results of this study, the utility of circulating androgen levels in the care of prostate cancer patients merits further investigation.
The median duration of prior ketoconazole treatment in this study population was 43 weeks, with nearly half (46%) of enrolled patients having been treated with ketoconazole for more than 1 year. While this could be the result of selection bias to which any small phase II study might be subject, the demographic data are representative of chemotherapy-naïve mCRPC patients (7, 11). In this CYP17 inhibitor-pretreated patient population, the median rPFS on abiraterone was 36 weeks. While this was significantly shorter than the median rPFS reported in the COU-AA-302 phase III study of abiraterone in chemotherapy-naïve mCRPC patients (16.5 months) (7), this result was expected given the prior exposure to CYP17 inhibition, and consistent with ≥50% PSA decline rates (41% in this study, 62% in the COU-AA-302 study). Indeed, the median duration of total CYP17 inhibition therapy (ketoconazole and abiraterone) in the men enrolled on this study was 91 weeks. This compares more favorably to the median rPFS of patients on the COU-AA-302 phase III study (7). While acknowledging the pitfalls of such analysis, this further supports the hypothesis that sequential CYP17 inhibition is a feasible treatment strategy, and is particularly relevant given the broad concerns for rising costs of cancer therapy (18). While abiraterone is clearly the “better” drug (10 fold-greater potency, favorable toxicity profile, and improved survival demonstrated in 2 phase III clinical trials), the current data may support the concept of considering total time on androgen synthesis inhibition therapy and may inform the subsequent study of the sequential use of these therapies.
In summary, abiraterone acetate demonstrates clinical efficacy and safety in patients with mCRPC previously treated with ketoconazole. Patients with detectable DHEA levels were more likely to demonstrate a PSA response to abiraterone and longer PFS. Analysis of circulating androgens merits further investigation as a biomarker for response to androgen synthesis inhibitor therapy in patients with mCRPC.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The CYP17 inhibitor abiraterone acetate has significantly improved the survival of men with mCRPC, in both the post-chemotherapy and chemotherapy-naïve settings. The clinical utility of abiraterone following ketoconazole, however, is unknown. Ketoconazole, an imidazole antifungal agent with less potent and specific CYP17 inhibitory activity, remains a part of the clinical armamentarium for clinicians, particularly in areas of the world where abiraterone has not received regulatory approval. This phase II study demonstrated that abiraterone has modest clinical activity in patients with disease progression on ketoconazole. Patients with higher circulating androgen levels prior to starting abiraterone had significantly higher PSA response proportion and longer PFS. The role of circulating androgens in mCRPC disease biology, and as potential biomarkers for response to androgen synthesis inhibition therapy, merits further investigation.
Acknowledgments
FINANCIAL SUPPORT
JLM was supported by the Roswell Park Cancer Institute and National Cancer Institute(NCI) grants P30-CA16056 and P01-CA77739.
Footnotes
CONFLICTS OF INTEREST
CJR has received honoraria from Janssen.
AM is an employee of Johnson & Johnson, with stock ownership.
The other authors have no conflicts of interest.
- Conception and design: EJS, CJR
-
Development of methodology:
- Clinical trial: VKW, EJS, CJR
- LC/MS/MS: JHW, GF, JM
- Acquisition of data: WK, AM, RS, CJR, JW
- Analysis and interpretation of data: WK, LZ, VKW, CJR, JW, JLM
-
Writing and review of manuscript:
- Primary: WK, CJR
- Review: all authors
- Study supervision: WK, CJR
References
- 1.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. The Journal of urology. 1997;157:1204–7. [PubMed] [Google Scholar]
- 2.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Allen JM, Kerle DJ, Ware H, Doble A, Williams G, Bloom SR. Combined treatment with ketoconazole and luteinising hormone releasing hormone analogue: a novel approach to resistant progressive prostatic cancer. British medical journal (Clinical research ed) 1983;287:1766. doi: 10.1136/bmj.287.6407.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) The Journal of steroid biochemistry and molecular biology. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 5.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. Journal of medicinal chemistry. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilton JH, Titus MA, Efstathiou E, Fetterly GJ, Mohler JL. Androgenic biomarker profiling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. The Prostate. 2014;74:722–31. doi: 10.1002/pros.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beer TM, Armstrong AJ, Sternberg CN, Higano C, Iverson P, Loriot Y, et al. Enzalutamide in men with chemotherapy-naive metastatic prostate cancer (mCRPC): Results of phase III PREVAIL study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(suppl 4):abstr LBA1. [Google Scholar]
- 12.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. Journal of the National Cancer Institute. 2006;98:516–21. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 14.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Statistics in medicine. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Statistics in medicine. 1997;16:1515–27. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan CJ, Molina A, Li J, Kheoh T, Small EJ, Haqq CM, et al. Serum Androgens As Prognostic Biomarkers in Castration-Resistant Prostate Cancer: Results From an Analysis of a Randomized Phase III Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:2791–8. doi: 10.1200/JCO.2012.45.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailankody S, Prasad V. Comparative effectiveness questions in oncology. The New England journal of medicine. 2014;370:1478–81. doi: 10.1056/NEJMp1400104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.