Abstract
Coronary artery calcification (CAC) is a well-known marker for coronary artery disease and has important prognostic implications. CAC is able to provide clinicians with a reliable source of information related to cardiovascular atherosclerosis, which carries incremental information beyond Framingham risk. However, non-contrast scans of the heart provide additional information beyond the Agatston score. These studies are also able to measure various sources of fat, including intrathoracic (eg, pericardial or epicardial) and hepatic, both of which are thought to be metabolically active and linked to increased incidence of subclinical atherosclerosis as well as increased prevalence of type 2 diabetes. Testing for CAC is also useful in identifying extracoronary sources of calcification. Specifically, aortic valve calcification, mitral annular calcification, and thoracic aortic calcium (TAC) provide additional risk stratification information for cardiovascular events. Finally, scanning for CAC is able to evaluate myocardial scaring due to myocardial infarcts, which may also add incremental prognostic information. To ensure the benefits outweigh the risks of a scanning for CAC for an appropriately selected asymptomatic patient, the full utility of the scan should be realized. This review describes the current state of the art interpretation of non-contrast cardiac CT, which clinically should go well beyond coronary artery Agatston scoring alone.
Keywords: CAC, Fat, Myocardial scarring, Tumors, Radiation
Introduction
Coronary artery calcification (CAC) is a well-known marker for coronary artery disease (CAD). CAC is well correlated with traditional risk factors such as diabetes, hypertension, and hyperlipidemia.1-3 The Agatston score, typically reported as a total score, represents all calcifications in the coronary tree and has been shown to robustly predict coronary heart disease (CHD) and provide information beyond standard risk factors in major ethnic and racial groups4,5 and has been shown to be clinically useful in persons with diabetes.6 Budoff et al,7 in the Multi-Ethnic study of Atherosclerosis (MESA), has shown that even minimal CAC (Agatston scores of 1–10) increased the risk of CHD 3-fold compared with patients with Agatston scores of zero, and a score of >100 confers a 10-fold risk of future cardiovascular events. However, ample data are emerging that more prognostic information is available on the scans, including location/distribution of calcification, valve and thoracic calcification, scar tissue (occult infarction), and both pericardial and hepatic fat. With gated non-contrast scans of the chest (coronary calcium scan), it is possible to identify multiple other abnormalities, including coronary anomalies that may have a high-risk potential for adverse coronary events.8 Given the small but measurable radiation dose required to obtain a coronary calcium scan, obtaining optimal useful information is desirable and recommended. Thus, non-contrast cardiac computed tomography (CT) is a promising technique for imaging beyond the calcification of the coronary tree. This review describes available information for these ancillary measures, available without additional burden or radiation to the patient.
Calcium distribution/location
The primary focus of testing for CAC and associated risk stratification has been the total Agatston score.9 However, other information is available, including distribution (by vessel or location), nature of calcification (spotty), and even number of calcific foci (lesion count). Each of these has been shown to add independent, incremental information to the total Agatston score.9-11 Left main calcification is associated with an increased mortality compared with other anatomic sites in the coronary tree.4 Williams et al10 reported that both left main calcification and the number of calcific lesions were independent predictors of coronary events. Interestingly, a specific Agatston score, if distributed in only 1 coronary vessel (all calcium in 1 artery), was associated with lower risk than if the same total Agatston score was distributed among 3 coronary vessels and/or the left main.
MESA also evaluated the distribution of CAC and showed incremental prognostic potential. The study evaluated the distance of calcifications from the ostia and calcific coverage of the coronaries, evaluating if both diffuse calcification and more proximal calcification were important prognostic factors.11 After adjustments were made for age, race, ethnicity, and sex, CCCS (coronary calcium coverage score) was associated with hypertension, dyslipidemia, and diabetes (P < 0.001 for all diseases). A 2-fold increase in CCCS was associated with a 52% (95% CI, 34%–72%) increase in risk for any CHD event. When Agatston or mass scores were included with CCCS in a Cox model for prediction of CHD events, neither was a significant predictor. In that study, CCCS remained significantly associated with CHD events. The investigators concluded that both spatial distribution and amount of calcified plaque contribute to risk of CHD.11
We further evaluated whether the number of lesions predict events better than the total score.10 A total of 14,759 asymptomatic patients were evaluated and followed for an average of 6.8 years for all-cause mortality. Risk-adjusted annual mortality was 0.19% (95% CI, 0.18%–0.21%) for patients without any calcified lesions. For patients with >20 lesions, annual risk-adjusted mortality exceeded 2% per year. Mortality rates were significantly higher for left main lesions than for other coronary arteries and increased with number of lesions present. Annual mortality rates were 2.1%, 9.2%, and 13.6% for 1–2, 3–5, and 6 lesions, respectively (P< 0.0001). The investigators concluded that patients with frequent lesions in the left main or patients with a higher number of calcified lesions have a significantly higher risk of mortality. The clinical implications are simple, clinicians can also report lesion counts in addition to total Agatston score, noting that larger number of lesions imparts a higher clinical significance.
Finally, the structure of coronary calcification may add incremental value. A study evaluated the calcification structure and found that spotty calcification is more predictive of cardiovascular events than heavy calcification.12 Thus, it is not only the identification of calcium that matters, but the size, extent, and perhaps even shape of the deposits that are important.
Intrathoracic fat
A non-contrast CT can easily assess both pericardial and epicardial fat and other sources of intrathoracic fat without additional radiation or participant burden. Epicardial fat is located between the myocardium and the visceral layer of the pericardium. Pericardial fat is anterior to epicardial fat, located between visceral and parietal layers of the pericardium.13,14 It is well known that adipose tissue (especially epicardial and pericardial fat) is responsible for producing several biochemically active substances that have paracrine effects on the heart. Several studies have reported that epicardial fat acts as a source of biochemically active molecules associated with increased atherosclerosis.15,16 In cross-sectional studies, greater amounts of epicardial or pericardial fat were associated with a greater prevalence of CAD, greater degrees of stenosis in patients undergoing coronary angiography, greater carotid intima-media thickness, and increased amounts of CAC.17,18 Higher amounts of cardiac fat are associated with the presence of the metabolic syndrome (MetS) and its underlying components that include hypertension, dyslipidemia, insulin resistance, and relation to diet.19,20 Associations between pericardial or epicardial fat and indices of coronary vascular disease (CVD) were shown to be independent of diabetes and MetS components.21 Gorter et al17 have shown that epicardial and pericoronary fat (directly surrounding the coronary arteries) alone are not related to the severity of coronary atherosclerosis and extent of CAC but are related when a low body mass index (BMI) is taken into consideration. Alexopoulos et al15 and Bastarrika et al16 have shown that epicardial fat volume was larger in the presence of obstructive CAD and noncalcified plaques. Outcome studies to evaluate pericardial or thoracic fat are under way, but preliminary data from MESA suggested independent prognostic value,22 independent and incremental to testing for CAC and Framingham risk. Along with CAC, these sources of intrathoracic fat evaluated on coronary calcium scans can be used as a tool to stratify risk of a cardiovascular event in a person. Data are currently somewhat divergent, because some studies fail to show incremental value of pericardial fat to predict events when added to assessment for CAC. Most notably, Tamarappoo et al18 recently found a non-significant increase in receiver operating characteristic curve area when added to Agatston score for ischemia assessment. If large cohort studies continue to find an independent and incremental value to fat measures, this could become a routine additional evaluation in the assessment of scans for CAC in addition to calcified plaque. Pericardial fat measures, as a marker of metabolically active tissue, require no additional scanning or patient radiation exposure to obtain, and the measurements can be made in just minutes. There are automated programs within many workstations to quantitate pericardial/epicardial fat, and selective use in persons with risk of MetS or diabetes may be good candidates for this additional measure. Alternatively, when the reader of the coronary calcium scan notes markedly increased fat around the heart, this can be noted in the report.
Hepatic fat
Liver fat has been shown to be associated with nonalcoholic fatty liver disease (NAFLD) and can also be measured on existing cardiac CT scans. Multiple studies have shown that NAFLD is closely associated with visceral obesity, dyslipidemia, and insulin resistance.23-25 It has been shown that liver fat predicts CVD events.25 NAFLD is associated with a 30%–40% increased risk of incident CAD in a diabetic cohort and depends on the degree of NAFLD present.25 In a number of cross-sectional studies, persons with NAFLD have a greater burden of subclinical atherosclerosis as measured by carotid intima-media thickness, carotid plaque burden, or CAC.26,27
Liver fat by CT was also shown to predict the development of type 2 diabetes in 5578 MESA participants without prevalent type 2 diabetes at baseline.28 Fat content in the liver was associated with the risk of type 2 diabetes after adjusting for BMI, waist circumference, pericardial fat, demographics, smoking, alcohol drinking, physical activity, blood pressures, inflammatory cytokines, and antihypertensive and cholesterol-lowering medication (odds ratio per SD increment, 1.35; 95% CI, 1.23–1.47).28 Because the liver is invariably imaged on the lower slices of every study for CAC, routine evaluation of this structure as a measure of metabolic activity may be prudent. Assessing liver fat may further help with the risk stratification by identifying the person with more metabolic activity and higher likelihood of a cardiovascular adverse event. The measurement takes <1 minute to perform, because it only entails a region of interest in the liver, compared with a region of interest in the spleen as a control. The lower the attenuation in the liver (in Hounsfield unit), the more fat is present in the liver tissue. It is commonly expressed as either a liver attenuation number (40 being normal, lower being abnormal) or a ratio of liver to spleen (1 being normal, lower being abnormal).
Extracoronary calcification
Aortic valve calcification (AVC) and mitral annular calcification (MAC) are independent predictors of atherosclerosis and are considered to be independent markers of risk.29,30 Each can be expressed as a volume of calcium in the respective valve. Pressman et al31 showed that MAC is associated with calcification of the aorta and aortic valve. MAC was shown to predict cardiac adverse events, but was not shown to independently predict CVD once the Agatston score is known.
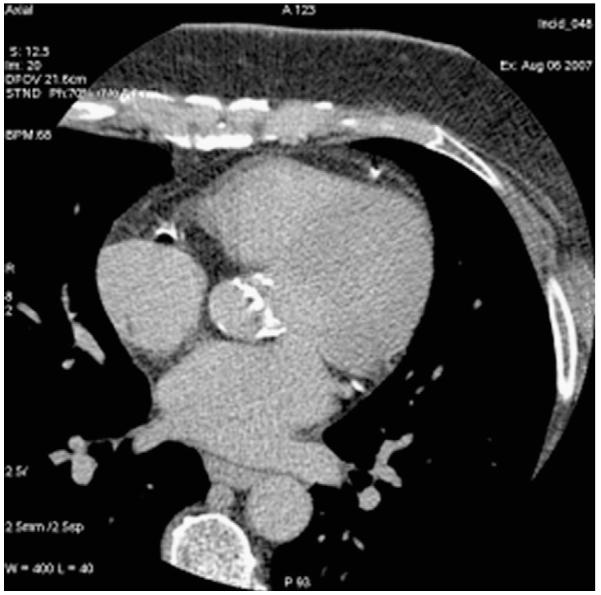
AVC is associated independently with the increasing severity of CAC, independent of age and diabetes.29,32 Messika-Zeitoun et al32 found that AVC (Fig. 1) is common in the aging, strongly associated with atherosclerotic risk factors, and can serve as a marker of subclinical CAD. AVC incrementally adds prognostic and diagnostic information to the scan for CAC. In the MESA cohort, MetS was associated with a significant increase in the incidence of AVC, raising the possibility that MetS may be a potential therapeutic target to prevent AVC development.33-35 Furthermore, in MESA, AVC was shown to be associated with CVD events independent of subclinical CAD severity.35 It was established that an AVC score >500 should lead to echocardiographic evaluation for aortic stenosis.30
Figure 1.
Aortic valve calcium and coronary artery calcification.
TAC is also readily available on the gated CT scans of the heart and is also highly associated with atherosclerotic risk factors and CAC. TAC, another measure available without participant burden on existing coronary calcium scans, has been shown to add incremental prognostic value to Agatston scores in both MESA and other cohorts.36-38 Thus, at least 2 of the 3 extracoronary calcification beds (AVC and TAC) have yielded independent and incremental information, useful for the risk stratification of the individual patient. Mention of TAC and AVC when present, with semiquantitation (mild, moderate, or severe) would allow further risk stratification beyond the Agatston score.
Myocardial scar
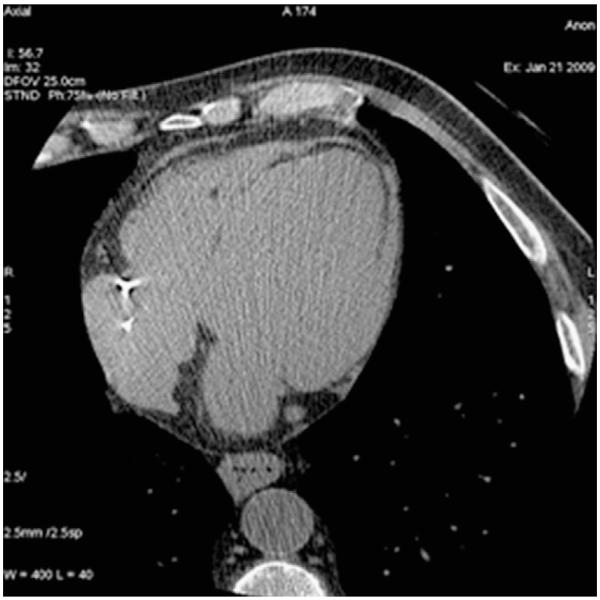
Most recently, the ability to detect occult myocardial infarction (MI; scar) on non-contrast CT scans has been shown (Fig. 2). Population-based studies have found that one-fourth of MIs shown by Q waves on the electrocardiogram are clinically unrecognized.39,40 The true prevalence of unrecognized MI may be even higher, because of the insensitivity of Q waves.40 The 10-year mortality from unrecognized MI was estimated to be 45%–55%, comparable with or higher than for patients with recognized MI.41 Late gadolinium enhancement imaging by contrast-enhanced cardiac magnetic resonance imaging can detect scar due to MI.42,43 Cardiac CT can also evaluate for scar tissue due to an occult MI.44,45 Small pilot studies have documented that the presence of fat and/or calcification in the left ventricular free wall is consistent with MI. MI is characterized by a process of ventricular remodeling, fatty replacement (rarefication), and, in rare cases, calcification. Chronic MI regions of the myocardium appear hypoattenuated on CT during scanning for CAC and are characterized by limited capillary density and a significant amount of adipose tissue, easily appreciated on non-contrast CT. Thus, areas of left ventricular infarction are readily apparent on non-contrast cardiac CT. It has been found that chronic MI can be detected with standard acquisitions of Agatston scoring.44 The attenuation levels of the necrotic region ranged from −10 to −20 HU and were found to be significantly higher than attenuation levels of pericardial fat (which were found to be −100 HU).44 The study found that the additional diagnostic utility of a coronary calcium scan was being able to identify prior unidentified MI.
Figure 2.
Myocardial scar.
Another study evaluated the ability of coronary calcium scans to detect nuclear proven infarctions (fixed defects).44,45 Sixty-two symptomatic patients with fixed perfusion defects on myocardial CT perfusion underwent scanning for CAC and their non-enhanced CT images were studied. The scans for CAC accurately detected irreversible perfusion defect in 59 subjects with sensitivity of 95.2% and a positive predictive value of 100%. This study suggests demonstrates that non-enhanced CT has an excellent agreement with myocardial CT perfusion) The study highlights a novel clinical utility of non-enhanced CT in addition to assessment of overall burden of atherosclerosis measured by Agatston score and provides evidence that it can accurately detect irreversible perfusion defects. Thus, a simple coronary calcium scan can provide crucial information about a previous clinically unrecognized MI.
Bone density assessment on thoracic calcium scans
Osteoporosis is an increasing problem, leading to fractures and disability. Several studies have indicated that multidetector row CT is an effective method to measure trabecular bone mineral density from the thoracic spine on cardiac studies without additional patient imaging or radiation.46-48 Because the thoracic spine is present on the scans, measurement of 3 consecutive thoracic vertebrae provides a measure of bone density for assessment of osteoporosis and baseline measures for serial evaluations. Bone density has been assessed in large cohorts of patients undergoing coronary calcium scans48 to provide normal ranges of thoracic bone density for patients of different ages and sexes. This can be done with or without a calcium phantom under the patient to normalize the data,46,47 allowing easy measurement of patients to assess 2 different diseases of aging simultaneously, osteoporosis and atherosclerosis.
Radiation
There are increased societal concerns about blanket exposure of radiation to an asymptotic population. There is no doubt that high levels of ionizing radiation (ie, atomic bomb exposure) can cause cancers and death; however, the relation between low-dose medical imaging and biologic harm has never been well established.49 For example, interventional cardiologists, airline pilots, and radiologists are exposed to radiation on almost a daily basis without documented increases in cancer rates.49 In addition, each radiation dose a patient receives is optimized and justified to acquire a high-quality image. The US Food and Drug Administration statement recently concluded that managing the risks of CT, fluoroscopy, and nuclear medicine imaging procedures depends on two principles of radiation protection: appropriate justification for ordering and performing each procedure and careful optimization of the radiation dose used during each procedure. These types of imaging examinations should be conducted only when medically justified.
The typical dose of a non-cardiac scan, based on the American Heart Association Scientific Statement on Cardiac CT, noted that prospective scans for CAC are 1.0 mSv per study.50 This dose continues to drop with new protocols and more attention to radiation doses. Guidelines now direct physicians to obtain these studies with the lowest possible radiation exposure and only in appropriate patients.51 A study with the use of various dose-reducing techniques concluded that radiation exposure is lower when adjusted for a patient’s body size and that 50-100 mA in small patients, 150-200 mA in medium patients, and 300 mA in large patients provided a stable estimate of the amount of coronary calcium. Diagnostic studies can be achieved with radiation doses <1 mSv.51
Thus, although the dose of a coronary calcium scan is comparable with mammography and lower than annual background radiation (3-7 mSv per year, depending on altitude), maximizing the yield of this test is clearly worthwhile.50
Conclusions
CT scans of the heart, initially only evaluated for CAC, are now understood to provide ample additional information, readily available on the scan. Almost all of these additional measures, including but not limited to evaluation of thoracic aortic calcification,36-38 aortic valve calcification,33-35 mitral annular calcification,52 pericardial fat,53 liver fat,54 myocardial scar,45 and bone density48 do not require any additional scanning or radiation or any additional participant burden, permitting a number of important phenotypes to be evaluated. Thus, a single scan, able to identify a robust marker of atherosclerosis, can also provide insight into multiple other associated disease states. The only cost for these incremental measures, to the patient or scanning center, would be the time of interpretation. Given the incremental value reported, consideration to adding these measures to routine reporting of coronary calcium scanning is necessary to get at the full prognostic and diagnostic potential of this study (Table 1). Utilization of some or all of these incremental measures would depend on the age and sex of the patient (ie, older postmenopausal women may get more benefit from bone density assessment than hepatic fat measure), whereas patients with certain disease states (ie, MetS or diabetes) may benefit more from pericardial fat measures. The clinician can direct the incremental measurements to the person, maximizing the diagnostic and prognostic yield of the coronary calcium scan.
Table 1.
Clinical pearls
|
AVC, aortic valve calcification; CAC, coronary artery calcification; TAC, thoracic aortic calcium.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
References
- 1.Hadamitzky M, Hein F, Meyer T, Bischoff B, Martinoff S, Schomig A, Hausleiter J. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33:1358–63. doi: 10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hata N, Kunimi T, Kishida H, Miyagawa H, Ikema Y, Hayakawa H. Clinical significance of coronary artery calcification. Int Angiol. 1994;13:281–5. [PubMed] [Google Scholar]
- 3.Shemesh J, Morag-Koren N, Goldbourt U, Grossman E, Tenenbaum A, Fisman EZ, Apter S, Itzchak Y, Motro M. Coronary calcium by spiral computed tomography predicts cardiovascular events in high-risk hypertensive patients. J Hypertens. 2004;22:605–10. doi: 10.1097/00004872-200403000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Rumberger JA. Practical tips and tricks in cardiovascular computed tomography: non-contrast “heartscans”–beyond the calcium score. J Cardiovasc Comput Tomogr. 2009;3:52–6. doi: 10.1016/j.jcct.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr., Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Schenker MP, Dorbala S, Hong EC, Rybicki FJ, Hachamovitch R, Kwong RY, Di Carli MF. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–61. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, Kim YJ, Hur J, Nam JE, Kim TH, Choe KO, Choi BW. Coronary artery anomalies: detection on coronary artery calcium scoring scan. AJR Am J Roentgenol. 2010;194:W382–7. doi: 10.2214/AJR.09.3336. [DOI] [PubMed] [Google Scholar]
- 9.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Williams M, Shaw LJ, Raggi P, Morris D, Vaccarino V, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Nasir K, Budoff M. Prognostic value of number and site of calcified coronary lesions compared with the total score. JACC Cardiovasc Imaging. 2008;1:61–9. doi: 10.1016/j.jcmg.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Brown ER, Kronmal RA, Bluemke DA, Guerci AD, Carr JJ, Goldin J, Detrano R. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology. 2008;247:669–75. doi: 10.1148/radiol.2473071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 14.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–17. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–4. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Bastarrika G, Broncano J, Schoepf UJ, Schwarz F, Lee YS, Abro JA, Costello P, Zwerner PL. Relationship between coronary artery disease and epicardial adipose tissue quantification at cardiac CT: comparison between automatic volumetric measurement and manual bidimensional estimation. Acad Radiol. 2010;17:727–34. doi: 10.1016/j.acra.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–5. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A, Berman DS. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–12. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, Ozdemir R, Aladag M, Yologlu S. Epicardial adipose tissue, hepatic steatosis and obesity. J Endocrinol Invest. 2007;30:459–64. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 20.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, Sherbondy MA, Conjeevaram HS. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–53. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 21.Ditomasso D, Carnethon MR, Wright CM, Allison MA. The associations between visceral fat and calcified atherosclerosis are stronger in women than men. Atherosclerosis. 2010;208:531–6. doi: 10.1016/j.atherosclerosis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care. 2004;27:2498–500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 24.Santos RD, Nasir K, Conceicao RD, Sarwar A, Carvalho JA, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis. 2007;194:517–9. doi: 10.1016/j.atherosclerosis.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–6. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 26.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–6. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Ding J, Hsu FC, Szklo M, Budoff MJ, Carr JJ, Wagenknecht L, Harris TB, Allison M, Bowden D, Criqui MH. Fatty liver and development of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA); Paper presented at: Obesity Society’s 2010 Annual Scientific Meeting; San Diego, CA. October 2010. [Google Scholar]
- 29.Nasir K, Katz R, Al-Mallah M, Takasu J, Shavelle DM, Carr JJ, Kronmal R, Blumenthal RS, O’Brien K, Budoff MJ. Relationship of aortic valve calcification with coronary artery calcium severity: the Multi-Ethnic Study of Atherosclerosis (MESA) J Cardiovasc Comput Tomogr. 2010;4:41–6. doi: 10.1016/j.jcct.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Shavelle DM, Budoff MJ, Buljubasic N, Wu AH, Takasu J, Rosales J, Otto CM, Zhao XQ, O’Brien KD. Usefulness of aortic valve calcium scores by electron beam computed tomography as a marker for aortic stenosis. Am J Cardiol. 2003;92:349–53. doi: 10.1016/s0002-9149(03)00646-5. [DOI] [PubMed] [Google Scholar]
- 31.Pressman GS, Crudu V, Parameswaran-Chandrika A, Romero-Corral A, Purushottam B, Figueredo VM. Can total cardiac calcium predict the coronary calcium score? Int J Cardiol. 2011;146:202–6. doi: 10.1016/j.ijcard.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 32.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–8. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 33.Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O’Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–9. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–9. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 35.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O’Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5:619–25. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic Aortic Calcification and Coronary Heart Disease Events: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos RD, Rumberger JA, Budoff MJ, Shaw LJ, Orakzai SH, Berman D, Raggi P, Blumenthal RS, Nasir K. Thoracic aorta calcification detected by electron beam tomography predicts all-cause mortality. Atherosclerosis. 2010;209:131–5. doi: 10.1016/j.atherosclerosis.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, Budoff MJ. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2008;155:765–71. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kannel WB, Cupples LA, Gagnon DR. Incidence, precursors and prognosis of unrecognized myocardial infarction. Adv Cardiol. 1990;37:202–14. doi: 10.1159/000418828. [DOI] [PubMed] [Google Scholar]
- 40.Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801–11. doi: 10.7326/0003-4819-135-9-200111060-00010. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med. 1984;311:1144–7. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 42.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 43.Chan J, Khafagi F, Young AA, Cowan BR, Thompson C, Marwick TH. Impact of coronary revascularization and transmural extent of scar on regional left ventricular remodelling. Eur Heart J. 2008;29:1608–17. doi: 10.1093/eurheartj/ehn247. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Granillo GA, Rosales MA, Renes P, Diez E, Pereyra J, Gomez E, De Lillo G, Degrossi E, Rodriguez AE, McFadden EP. Chronic myocardial infarction detection and characterization during coronary artery calcium scoring acquisitions. J Cardiovasc Comput Tomogr. 2010;4:99–107. doi: 10.1016/j.jcct.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Gupta M, Kadakia J, Hacioglu Y, Ahmadi N, Patel A, Choi T, Yamada G, Budoff M. Non-contrast cardiac computed tomography can accurately detect chronic myocardial infarction: Validation study. J Nucl Cardiol. 2011;18:96–103. doi: 10.1007/s12350-010-9314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, Child J, Carson S, Mao SS. Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol. 2012;19:179–83. doi: 10.1016/j.acra.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Mueller DK, Kutscherenko A, Bartel H, Vlassenbroek A, Ourednicek P, Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol. 2011;79:375–81. doi: 10.1016/j.ejrad.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J, Carson S, Nee JN, Mao S. Measurement of thoracic bone mineral density with quantitative CT. Radiology. 2010;257:434–40. doi: 10.1148/radiol.10100132. [DOI] [PubMed] [Google Scholar]
- 49.Budoff MJ, Gupta M. Radiation exposure from cardiac imaging procedures: do the risks outweigh the benefits? J Am Coll Cardiol. 2010;56:712–4. doi: 10.1016/j.jacc.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 50.Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–65. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 51.Voros S, Rivera JJ, Berman DS, Blankstein R, Budoff MJ, Cury RC, Desai MY, Dey D, Halliburton SS, Hecht HS, Nasir K, Santos RD, Shapiro MD, Taylor AJ, Valeti US, Young PM, Weissman G. Guideline for minimizing radiation exposure during acquisition of coronary artery calcium scans with the use of multidetector computed tomography: a report by the Society for Atherosclerosis Imaging and Prevention Tomographic Imaging and Prevention Councils in collaboration with the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2011;5:75–83. doi: 10.1016/j.jcct.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–72. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 53.Ahmadi N, Nabavi V, Yang E, Hajsadeghi F, Lakis M, Flores F, Zeb I, Bevinal M, Ebrahimi R, Budoff M. Increased Epicardial, Pericardial, and Subcutaneous Adipose Tissue Is Associated with the Presence and Severity of Coronary Artery Calcium. Acad Radiol. 2010;17:1518–24. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and Antioxidants for the Treatment of Nonalcoholic Fatty Liver Disease: the Saint Francis Heart Study Randomized Clinical Trial. Am J Gastroenterol. 2011;106:71–7. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]