Abstract
Background
Past studies examining the effect of vitamin D on statin myalgia have been variable; however, these studies were done in limited samples not representative of the general population. We aimed to evaluate whether vitamin D status modifies the association between statin use and musculoskeletal pain in a sample representative of the general population.
Methods
We conducted a cross-sectional study using the National Health and Nutrition Examination Survey 2001-2004. Musculoskeletal symptoms and statin use were self-reported. Vitamin D status was assessed using serum 25 hydroxyvitamin D (25[OH]D) , categorized as <15 ng/mL or ≥15 ng/mL . To evaluate if vitamin D status modifies the association between statin use and prevalent musculoskeletal pain, we performed multivariable-adjusted logistic regression models stratified by 25(OH)D status.
Results
Among 5907 participants ≥40 years old, mean serum 25(OH)D was 23.6 ng/mL (95% CI, 22.9-24.3). In stratified multivariable-adjusted logistic regression models, individuals with 25(OH)D <15 ng/mL, using a statin had a significantly higher odds of musculoskeletal pain compared to those not using a statin (adjusted odds ratio [aOR], 1.90; 95% CI, 1.18-3.05). Among those with 25(OH)D ≥15 ng/mL, we found no significant association between statin use and musculoskeletal pain (aOR, 0.91; 95% CI, 0.71-1.16).
CONCLUSION
Among adults ≥ 40 years old with 25(OH)D <15ng/mL, statin users had nearly 2 times greater odds of reporting musculoskeletal pain compared to non-statin users. Our findings support the hypothesis that vitamin D deficiency modifies the risk of musculoskeletal symptoms experienced with statin use.
Keywords: Statins, HMG-CoA reductase inhibitors, Vitamin D, 25-Hydroxyvitamin D, Myalgia, Muscle symptoms, Statin intolerance
1. Introduction
Statin use is associated with a higher risk for musculoskeletal pain [1]. Vitamin D status has been proposed to modify the effect of statin use on musculoskeletal pain, with deficient vitamin D increasing the risk of statin-associated musculoskeletal symptoms [2]. However, prior studies have included statin-users only, and have yielded mixed results [2-6]. Therefore, it remains uncertain whether vitamin D deficiency modifies the effect of statin use by increasing the risk of musculoskeletal pain beyond that associated with statin use or vitamin D deficiency alone, or, alternatively, whether any association exists after adjustment for important confounders. We aimed to examine the effect of vitamin D on the relationship between statin use and musculoskeletal pain in the general population.
2. Methods
For this cross-sectional study we analyzed data from the National Health and Nutrition Examination Survey (NHANES), an ongoing, cross-sectional survey that uses a complex, stratified, multistage, probability-cluster design to select a representative sample of the civilian, non-institutionalized US population. All participants gave written informed consent. Public use data files were obtained from the NHANES website, and our analyses of these de-identified data were approved for exemption by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation.
2.1. Study Population
NHANES participants underwent in-home interviews to obtain information about demographics, medical conditions, prescription medication use, and health and lifestyle risk factors, followed by physical exams and laboratory testing at a mobile examination center. Adults ≥40 years who participated in NHANES between 2001-2004 and had serum 25 hydroxyvitamin D (25[OH]D) measured (n=6233) were considered for inclusion in the study. We excluded 326 participants who were missing data on covariates of interest, leaving 5907 participants for our analyses.
2.2. Measurements
Serum 25(OH)D was analyzed at the CDC in Atlanta, GA using the DiaSorin RIA kit (Stillwater, MN). Measurements of 25(OH)D were adjusted to take into account assay drifts due to reagent and calibration lot-to-lot variability over time [7]. To ascertain statin use, and other medication use, all participants were asked if they had used any prescription medications in the past 30 days. Those who responded “yes” were asked to provide their medication containers to the interviewer to assure accurate recording of the medication. Containers were unavailable for about 15% of the medications used; in such cases, participants verbally reported the details of medications they used to the interviewer.
Pain was self-reported in response to the initial question: “During the past month, have you had a problem with pain that lasted more than 24 hours?” Those who answered “yes” were asked to describe the location(s) of their pain. We defined musculoskeletal pain as report of pain in any of the following areas: lower extremities, upper extremities, buttocks, back, and neck.
2.3. Other covariates of interest
Participants reported their age, sex, race/ethnicity, alcohol use, smoking status, physical activity, and health status. Medical conditions were based on a self-report of a physician or health care provider’s diagnosis. Coronary heart disease was based on having a diagnosis of coronary heart disease, angina, or a previous heart attack. Chronic lung disease was defined as having current asthma, emphysema or chronic bronchitis. Diabetes status was determined by self-reported prior diagnosis, a blood glucose level of > 200 mg/dL, or use of anti-diabetic medication. Body mass index (BMI) was based on measured height and weight. Serum albumin levels were measured using the bichromatic digital endpoint method with Bromcresol purple reagent (Beckman Coulter, Los Angeles, CA). Serum iron concentration was obtained using the timed-endpoint method with Ferrozine iron reagent (Beckman Coulter, Los Angeles, CA).
2.4. Statistical Analyses
All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and Stata 12 (College Station, TX) using survey weights to represent the US population and to account for non-response and over-sampling of select demographic populations. Crude analyses (unadjusted logistic regression) and adjusted analyses (multivariable logistic regression) were performed. We performed pre-planned analyses stratified by 25(OH)D <15 ng/mL versus ≥15 ng/mL, in order to assess if vitamin D deficiency modifies the effect of statin use on musculoskeletal pain. Exploratory analyses were conducted to assess additional thresholds of serum 25(OH)D, specifically: <15 ng/mL, 15-30 ng/mL, and ≥30 ng/mL. Additionally, we have previously shown the effect of statin use on musculoskeletal pain differs significantly among individuals with and without arthritis [1], but did not include vitamin D status in our prior studies; therefore, we explored the effects of vitamin D status on that association. Finally, because statin users may restrict dietary fat, which could decrease absorption of fat-soluble vitamins, we explored if statin users versus nonusers had differences in serum concentrations of vitamin E or carotenoids in post-hoc analyses restricted to participants with 25(OH)D <15 ng/mL.
The following confounders, specified a priori, were used in all multivariable models: age, sex, race (non-Hispanic white, non-Hispanic black, or other), smoking (never, past current), average alcohol consumption in the past year (<1, 1 to 3, or >3 servings per day), physical activity during the past 30 days (vigorous, moderate, sedentary), self-reported health status (excellent/very good, good, or fair/poor), coronary heart disease, stroke, congestive heart failure, diabetes, lung disease, arthritis, osteoporosis, body mass index (BMI), serum albumin (continuous), serum iron (continuous, log-transformed due to its skewed distribution), opioid use in the past 30 days, and prescription nonsteroidal anti-inflammatory drug (NSAID) use in the past 30 days.
We considered other factors as potential confounders but did not include them in our final models because they did not substantively alter the association between statin use, vitamin D levels, and musculoskeletal pain. These factors were aspartate aminotransferase, alanine transaminase, cholesterol, glomerular filtration rate, peripheral vascular disease, and cancer.
3. Results
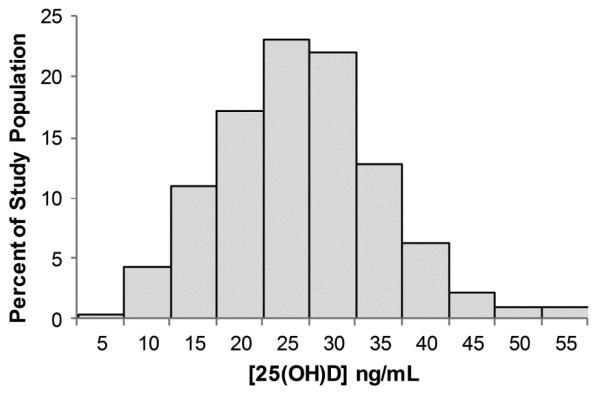
Among 5907 participants ≥40 years, 1057 participants, representing 19.6 million individuals, reported statin use. The distribution of serum 25(OH)D for participants is depicted in Figure 1. The mean serum 25(OH)D for the overall study population (23.6 ng/mL; 95% confidence interval [CI], 22.9-24.3) did not differ from the mean serum 25(OH)D among statin users (23.4 ng/mL; 95% CI, 22.3-24.4). Table 1 shows that participants with 25(OH)D <15 ng/mL, compared to those with higher levels of vitamin D, were less likely to be of non-Hispanic white race/ethnicity and were more likely to be female, current smokers, sedentary; were more likely to report poorer health status and multiple co-morbidities, including coronary heart disease, stroke, congestive heart failure, and diabetes; were more likely to currently use opioids and prescription NSAIDs; and were more likely to have a higher BMI. Characteristics of participants according to statin use or nonuse are shown in Supplementary Table 1. Compared to non-statin users, those who used statins were more likely to be older, male, of non-Hispanic white race/ethnicity, former smokers, and less likely to drink >3 serving of alcohol/day, engage in vigorous physically activity, report very good/excellent health. They were more likely to have a diagnosis of coronary heart disease, stroke, congestive heart failure, diabetes, arthritis, and/or osteoporosis. In addition, they were more likely to currently use NSAIDs and have higher BMI.
Figure 1.
Distribution of serum 25(OH)D (ng/mL) concentration of 2001-2004 NHANES participants ≥40 years (n=5907).
Table 1.
Characteristics of NHANES Participants ≥40 years with Serum Vitamin D Levels <15 ng/mL and Serum Vitamin D Levels ≥15ng/mL (2001-2004)
| Characteristics | Overall n=5907 %* (SE) |
25(OH)D≥15 ng/mL n=4626 %* (SE) |
[25(OH)D]<15 ng/mL n=1281 %* (SE) |
|---|---|---|---|
| Statin use | 16.6 (0.8) | 16.5 (0.8) | 17.2 (1.6) |
| Age, mean, years | 56.3 (0.3) | 56.4 (0.3) | 56.4 (0.5) |
| Sex, female | 52.2 (0.6) | 50.2 (0.7) | 62.8 (1.5) |
| Race/Ethnicity | |||
| Non-Hispanic white | 78.6 (2.0) | 83.5 (1.8) | 52.1 (3.2) |
| Non-Hispanic black | 9.0 (1.1) | 5.2 (0.76) | 29.6 (3.0) |
| Other | 12.4 (1.7) | 11.4 (1.7) | 18.3 (2.2) |
| Smoking status | |||
| Never | 46.5 (1.2) | 47.3 (1.4) | 42.3 (2.1) |
| Former | 33.0 (1.1) | 33.8 (1.1) | 26.9 (1.7) |
| Current | 20.5 (0.8) | 18.7 (0.9) | 30.8 (2.0) |
| Alcohol—average drinks/day | |||
| 0 to < 1/day | 38.1 (2.5) | 36.0 (2.8) | 50.0 (2.3) |
| 1-3/day | 52.0 (2.3) | 54.1 (2.6) | 40.6 (2.2) |
| > 3/day | 9.9 (0.7) | 9.9 (0.7) | 9.4 (1.2) |
| Physical activity | |||
| Sedentary | 38.2 (1.1) | 35.0 (1.3) | 55.4 (1.9) |
| Moderate | 34.6 (0.7) | 35.7 (0.8) | 29.0 (1.9) |
| Vigorous | 27.2 (1.2) | 29.3 (1.2) | 15.7 (1.4) |
| Self-reported health status | |||
| Very good/excellent | 48.3 (1.6) | 51.1 (1.8) | 33.3 (2.0) |
| Good | 31.1 (0.8) | 30.5 (0.8) | 34.7 (1.9) |
| Fair/poor | 20.6 (1.3) | 18.4 (1.4) | 32.1 (1.8) |
| Co-morbidities | |||
| Coronary heart disease | 10.5 (0.7) | 9.9 (0.7) | 13.8 (1.5) |
| Stroke | 3.4 (0.4) | 3.1 (0.4) | 5.2 (1.0) |
| Congestive heart failure | 3.7 (0.4) | 3.3 (0.4) | 5.5 (0.8) |
| Diabetes | 11.4 (0.6) | 10.2 (0.6) | 18.2 (1.6) |
| Lung disease | 11.4 (0.7) | 11.3 (0.7) | 11.9 (1.5) |
| Arthritis | 34.2 (1.1) | 34.9 (1.2) | 35.6 (1.7) |
| Osteoporosis | 8.8 (0.6) | 8.9 (0.6) | 8.0 (1.1) |
| Body mass index, mean, kg/m2 | 28.7 (0.2) | 28.3 (0.1) | 31.3 (0.5) |
| Serum albumin, mean, g/dL | 4.2 (0.01) | 4.2 (0.01) | 4.1 (0.02) |
| Serum iron, mean, μg/dL | 87.1 (0.6) | 88.6 (0.6) | 78.8 (1.0) |
| Prescription analgesic use | |||
| Opioid use | 7.0 (0.6) | 6.6 (0.6) | 9.3 (1.3) |
| Non-steroidal anti-inflammatory | 12.9 (0.7) | 12.3 (0.7) | 16.1 (1.6) |
Percent unless otherwise noted as years or mean.
Prior to stratification by vitamin D status, the prevalence of musculoskeletal pain among statin users was 30.5% (95% CI, 25.9–35.6) and 26.3% (95% CI, 24.4– 28.3) among statin non-users. Figure 2 illustrates the prevalence of musculoskeletal pain according to statin use and vitamin D status categorized as: 25(OH)D <15 ng/mL, 15-30 ng/mL, and ≥30 ng/mL. This figure shows the prevalence of musculoskeletal pain was similar among statin users and nonstatin users for serum 25(OH)D levels of 15-30 ng/mL and ≥30 ng/mL, but was substantially higher among statin users with 25(OH)D <15ng/mL. Few participants who used statins had 25(OH)D ≥30 ng/mL, limiting further analyses in this subcategory. After dichotomizing 25(OH)D by <15ng/mL versus ≥15 ng/m L, we found (1) among participants with 25(OH)D < 15 ng/m L: the prevalence of musculoskeletal pain was 43.8% (95% Cl, 33.5-54.6) among those using a statin, compared to 27.0% (95% Cl, 21.9-31.8) in those who did not use a statin, p=0.005; whereas (2) among participants with 25(0H)D 15 ng/ml: the prevalence of musculoskeletal pain was 28.0% (95% Cl, 23.4-33.2) in those using a statin, compared to 26.3% (95% Cl, 24.4-28.3) in those who did not use a statin, p=0.40.
Figure 2.
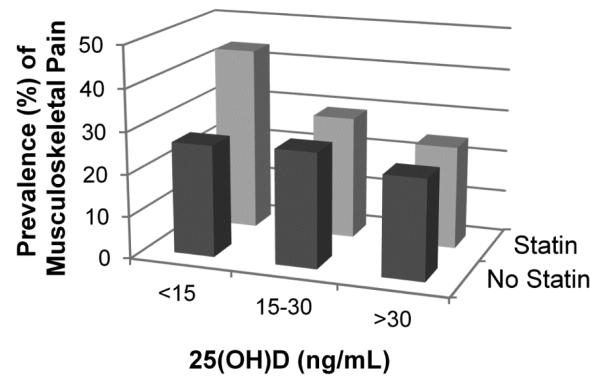
The crude prevalence of musculoskeletal pain according to serum 25(0H)D (ng/mL) concentration and statin use among 2001-2004 NHANES. Among statins users, 43.8% (n=72) who had 25(0H)D<15 ng/mL, 29.2% (n=162) who had 25(0H)D between 15-30 ng/m L, and 24.5% (n=41) who had 25(0H)D 30 ng/m L, reported experiencing musculoskeletal pain in the past month. Among those not using statins, 26.6% (n=255) who had 25(0H)D<15 ng/mL, 27.3% (n=704) who had 25(0H)D between15-30 ng/mL, and 23.7% (n=195) who had 25(0H)D 30 ng/mL, reported experiencing musculoskeletal pain in the past month.
Among the entire sample, crude logistic regression analysis showed a higher risk of musculoskeletal pain in participants using statins compared to those not using a statin (unadjusted OR 1.23; 95% CI, 1.02-1.49). Stratifying the sample by vitamin D status demonstrated that among participants with 25(OH)D <15 ng/mL, statin use, compared to not using a statin, was associated with a higher risk of musculoskeletal pain (unadjusted OR 2.15; 95% CI, 1.36-3.41). Whereas, among participants with 25(OH)D ≥15 ng/mL, there was no association between statin use and musculoskeletal pain (unadjusted OR 1.09; 95% CI, 0.89-1.34).
In multivariable adjusted analysis, 25(OH)D <15ng/mL, compared to 25(OH)D ≥15ng/mL, was not independently associated a higher risk for musculoskeletal pain (aOR 1.04, 95% CI, 0.78-1.39). Including an interaction term between statin and 25(OH)D status showed the association of statins and 25(OH)D <15ng/mL was statistically different from the association of statins and 25(OH)D ≥15ng/mL in multivariable adjusted analysis (p for interaction=0.02). Table 2 shows the results of the multivariable analyses stratified by vitamin D status and illustrates that participants with 25(OH)D levels <15 ng/mL who used statins had significantly higher odds of experiencing musculoskeletal pain compared to those who did not use a statin (aOR 1.90; 95% CI, 1.18-3.05). In contrast, among those with 25(OH)D ≥15 ng/mL, there was no association between statin use and musculoskeletal pain.
Table 2.
Factors associated with musculoskeletal pain, stratified according to serum 25(OH)D
| Variable | 25(OH)D≥15ng/mL (n=4626) |
25(OH)D<15 ng/mL (n=1281) |
||||
|---|---|---|---|---|---|---|
| aOR* | 95% CI | p-value | aOR* | 95% CI | p-value | |
| Statin use | 0.91 | 0.71, 1.16 | 0.43 | 1.90 | 1.18, 3.05 | 0.01 |
| Age | 0.97 | 0.96, 0.98 | <0.001 | 0.99 | 0.98, 1.01 | 0.50 |
| Sex | ||||||
| Male | 1.00 | reference | 1.00 | reference | ||
| Female | 0.95 | 0.78, 1.17 | 0.65 | 1.14 | 0.67, 1.94 | 0.62 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1.00 | reference | 1.00 | reference | ||
| Non-Hispanic Black | 0.63 | 0.45, 0.88 | 0.01 | 0.80 | 0.48, 1.33 | 0.38 |
| Other | 0.72 | 0.50, 1.05 | 0.09 | 0.92 | 0.52, 1.62 | 0.76 |
| Smoking status | ||||||
| Never | 1.00 | reference | 1.00 | reference | ||
| Former | 1.02 | 0.83, 1.25 | 0.86 | 1.24 | 0.77, 2.00 | 0.37 |
| Current | 1.50 | 1.13, 1.97 | 0.01 | 1.60 | 0.97, 2.64 | 0.07 |
| Alcohol—average drinks/day | 0.89† | 0.002† | ||||
| 0-1 servings | 1.00 | reference | 1.00 | reference | ||
| 1-3 servings | 1.02 | 0.75, 1.40 | 0.88 | 1.72 | 1.07, 2.74 | 0.03 |
| >3 servings | 0.92 | 0.59, 1.46 | 0.72 | 2.24 | 1.22, 4.10 | 0.01 |
| Physical activity | 0.11† | 0.05† | ||||
| Sedentary | 1.00 | reference | 1.00 | reference | ||
| Moderate | 0.96 | 0.81, 1.14 | 0.62 | 1.17 | 0.84, 1.63 | 0.33 |
| Vigorous | 0.82 | 0.62, 1.09 | 0.16 | 1.65 | 0.92, 2.96 | 0.09 |
| Self-reported health status | <0.001† | <0.001† | ||||
| Excellent/very good | 1.00 | reference | 1.00 | Reference | ||
| Good | 1.58 | 1.25, 1.99 | <0.001 | 2.30 | 1.32, 3.99 | 0.004 |
| Fair/poor | 2.50 | 1.90, 3.28 | <0.001 | 3.39 | 2.12, 5.44 | <0.001 |
| Medical conditions | ||||||
| Coronary heart disease | 1.18 | 0.82, 1.70 | 0.35 | 0.95 | 0.50, 1.80 | 0.87 |
| History of stroke | 1.06 | 0.66, 1.70 | 0.80 | 1.27 | 0.45, 3.55 | 0.64 |
| Congestive heart failure | 1.00 | 0.50, 2.03 | 0.98 | 1.20 | 0.56, 2.57 | 0.62 |
| Diabetes | 1.13 | 0.85, 1.51 | 0.38 | 0.77 | 0.49, 1.15 | 0.21 |
| Lung disease | 1.33 | 0.97, 1.84 | 0.08 | 0.74 | 0.41, 1.34 | 0.31 |
| Osteoporosis | 1.30 | 0.95, 1.77 | 0.10 | 1.41 | 0.72, 2.75 | 0.31 |
| Arthritis | 2.28 | 1.83, 2.84 | <0.001 | 1.98 | 1.15, 3.41 | 0.05 |
| Body mass index, kg/m2 | 1.02 | 1.00, 1.03 | 0.06 | 1.02 | 1.00, 1.05 | 0.10 |
| Serum albumin, g/dL | 1.14 | 0.80, 1.62 | 0.47 | 2.21 | 1.10, 4.44 | 0.05 |
| Serum iron¥ | 1.10 | 0.83, 1.47 | 0.50 | 0.79 | 0.55, 1.14 | 0.20 |
| Prescription analgesic use | ||||||
| Opioids | 3.07 | 2.06, 4.56 | <0.001 | 8.39 | 4.04, 17.4 | <0.001 |
| Non-steroidal anti-inflammatory | 1.94 | 1.42, 2.64 | <0.001 | 1.38 | 0.70, 2.75 | 0.34 |
Adjusted odds ratio: adjusted for all factors shown in the table.
p for trend.
Serum iron (μg/dL) was log-transformed due to its skewed distribution.
Among those with 25(OH)D levels <15 ng/mL, in addition to statin use, higher risk for musculoskeletal pain was associated with poorer health status, use of opioids, and higher alcohol consumption. Among participants with 25(OH)D ≥15 ng/mL, significant associations with musculoskeletal pain were younger age, poorer health, having arthritis, opioid use, prescription NSAID use, current smoking, and being non-Hispanic white.
3.1. Exploratory analyses among additional 25(OH)D thresholds
Exploratory analyses with vitamin D stratified as 25(OH)D<15 (n=1281), 25(OH)D 15 to <30 ng/mL (n= 3611) and 25(OH)D ≥30 ng/mL (n=1015) revealed adjusted odds ratios of 1.90 (95% CI, 1.18-3.05), 0.96 (95% CI, 0.72-1.26), and 0.68 (95% CI, 0.34-1.34), respectively, for musculoskeletal pain and statin use compared to non-statin use in each vitamin D strata. These analyses were adjusted for all a priori confounders. As noted above, few participants using statins had 25(OH)D ≥30 ng/mL, thus analysis within this category is speculative.
3.2. Exploratory analyses in participants without and with arthritis
Finally, we examined whether the effects of statin and vitamin D were different among persons with or without a diagnoses of arthritis, which is common cause of pain among persons ≥ 40 years. The analyses were adjusted for all other a priori confounders.
3.3. Participants without arthritis
In analyses restricted to those without a diagnosis of arthritis, (1) among those who had 25(OH)D <15 ng/mL (n=818): use of statin, compared to non-statin use, was associated with a substantially higher adjusted odds of musculoskeletal pain of 2.5 (95% CI, 1.10-5.82), while (2) among those who had 25(OH)D ≥15 ng/mL (n=2860): use of statin, compared to non-statin use, was associated with a higher adjusted odds of musculoskeletal pain of 1.41 (95% CI, 1.02-1.93).
3.4. Participants with arthritis
In analysis restricted to participants with a prior diagnosis of arthritis, (1) among those who had 25(OH)D <15 ng/mL (n= 463): use of a statin, compared to non-statin use, we did not find a significant association between statin use with musculoskeletal pain (aOR 1.39; 95% CI, 0.71, 2.72); in contrast (2) among those with 25(OH)D ≥15 ng/mL (n= 1766): statin use, compared to non-statin use, was associated with a lower risk for musculoskeletal pain (aOR 0.61; 95% CI 0.41, 0.92).
3.5. Exploratory subgroup analyses among those with 25(OH)D <15 ng/mL
In post hoc analyses restricted to persons with 25(OH)D <15 ng/mL, we evaluated serum concentrations of vitamin E and carotenoids according to statin use. For vitamin E, we evaluated α-tocopherol (common in supplements and the diet) and gamma-tocopherol (less common in supplements) separately. For carotenoids, we analyzed a combination of six carotenoids: α-carotene, trans-β-carotene, cis β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene. When assessed as continuous variables, no significant associations were found between vitamin E or carotenoids levels according to statin use. Dichotomizing serum concentrations at their medians for bivariable analysis showed statin users, compared to non-statin users, were more likely to have serum levels above the median for α-tocopherol (1326 μg/dL; IQR 150-1785; p=0.02), but not for gamma-tocopherol levels (median 212 μg/dL (IQR 128-316; p=0.89) or carotenoid levels (median 64.9 μg/dL (IQR 45.6-88.7; p=0.80). Adding α-tocopherol to the final adjusted multivariable model for those with 25(OH)D <15 ng/mL showed that α-tocopherol was not independently associated with musculoskeletal pain and it did not materially alter our original findings.
4. Discussion
In this cross-sectional study of 5907 individuals, we found that vitamin D deficiency modified the effect of statin on musculoskeletal pain among adults ≥40 years. Specifically, individuals with 25(OH)D <15ng/mL who were using a statin had a nearly 2 times greater odds of musculoskeletal pain within the past month compared to those with 25(OH)D <15ng/mL who did not use a statin. In contrast, among adults who had serum 25(OH)D ≥15ng/mL, use of statins was not associated with musculoskeletal pain.
Our findings support the concept that vitamin D status modifies the effect of statins on musculoskeletal pain [2,8]. Two recent retrospective cohort studies in statin users came to opposite conclusions regarding the association between vitamin D status and myalgia symptoms in patients using statins, with one study finding an association between vitamin D deficiency and incident statin myalgia [6] and the other finding no association [4]. Although both studies were large, conclusions based on analyses of retrospective chart reviews, such as these, are limited by inclusion of patients using statins who also had blood test results available for vitamin D [6], or vitamin D plus creatine kinase [4].
Because neither 25(OH)D nor creatine kinase tests are universal screening tests performed in all patients on statins, having results of these tests available in a chart as inclusion criteria could lead to a sample that is biased towards selection of participants with more symptoms, increased prevalence of associated co-morbidities (e.g., osteoporosis), and varying providers’ practices. We sought to overcome such potential bias by using data from NHANES, which allowed us to evaluate persons ≥40 years old who had testing performed as part of being enrolled in the study, rather than being based on a clinical decision to perform vitamin D and/or creatine kinase testing. To our knowledge, our study is the first study to assess the effects of statin and vitamin D status on musculoskeletal pain in a nationally representative sample.
Before performing our analyses, we formulated three distinct hypotheses that might explain differences in findings of prior studies examining the association of vitamin D deficiency and musculoskeletal pain in statin users. First, we reasoned that vitamin D deficiency might directly cause musculoskeletal pain in the population that is presumed to be statin myalgia when observed in patients using statins. This hypothesis was not supported by our study. In multivariable adjusted analysis of the entire sample 25(OH)D <15ng/mL compared to 25(OH)D ≥15ng/mL was not independently associated a higher risk for musculoskeletal pain. Although musculoskeletal symptoms may occur with sustained severe vitamin D deficiency, as in the case of osteomalacia, such conditions are likely uncommon and therefore unlikely to substantially impact results of a cross-sectional population-based study.
Second, we hypothesized that vitamin D deficiency might represent a surrogate marker for poorer health in general, which, if not adequately controlled for in prior studies could appear as an association of low vitamin D and statin use with higher musculoskeletal pain. We found some evidence in support of this second hypothesis. However, after including multiple confounders for health, co-morbidities, and lifestyle risks, we observed only modest attenuation of the effect of combined low vitamin D status and statin use on the outcome of musculoskeletal pain. Therefore, if the association observed is predominantly due to poorer health in general, then (1) significant residual confounding exists for poor health that we were unable to account for and (2) low vitamin 25(OH)D serves as a surrogate marker for health in those using statins that is substantially superior to the multiple factors which we included and are commonly used to adjust for health.
Third, assuming no compelling evidence in support of either theory above, we hypothesized there might be a biological interaction between statin therapy and vitamin D deficiency that increases the risk of musculoskeletal symptoms in statin users with vitamin D deficiency. Although the cross-sectional nature of this study does not allow for definitive proof, this scenario is most supported by our findings.
Putative mechanisms to explain why low vitamin D might affect musculoskeletal pain in statin users include direct action on the vitamin D receptor (VDR) and through indirect perturbations in calcium and phosphate balance necessary for maintaining healthy muscle [9]. Severe vitamin D deficiency has been associated with proximal muscle symptoms and atrophy of fast twitch, type II, muscle fibers [10], which are the same muscle fiber type implicated in statin myalgia/myopathy [11]. Therefore, the combination of two states—statin use and vitamin D deficiency—may synergistically lead to adverse effects on type II muscle fibers, resulting in increased risk for injury or musculoskeletal pain with statin use. Of interest is that higher alcohol intake among participants with 25(OH)D <15ng/mL, but not among participants with 25(OH)D ≥15ng/mL, was also significantly associated with increased musculoskeletal pain. Myopathy associated with chronic use of high alcohol intake is also known to be associated with atrophy of type II muscle fibers [12]. Although these findings cannot establish causality, they support recommendations to consider testing vitamin D status in patients with musculoskeletal pain as well as screening for heavy alcohol use when evaluating musculoskeletal symptoms.
Although our exploratory analyses need to be interpreted with caution, assessment of the study population according to presence or absence of a past diagnosis of arthritis showed that the effect of statin on musculoskeletal pain differed by arthritis status. Possible reasons for finding a higher association between statin use and musculoskeletal pain in those without arthritis have previously been discussed [1]. Adding to our prior analyses, however, is that (1) among persons without arthritis, statin use in the setting of low vitamin D status demonstrated an even higher odds for risk of musculoskeletal pain than found in our main models, and (2) among those with arthritis, statin use in the setting of 25(OH)D ≥15ng/mL was associated with significantly lower odds for risk of musculoskeletal pain. Regarding the second finding, other studies have reported similar observations regarding a possible protective effect of statin on reducing the risk or progression of arthritis [13,14]. However, vitamin D supplementation, on its own, was not found to improve symptoms or prevent progression of osteoarthritis [15]. We were not able to identify studies that had investigated the use of statin and vitamin D supplementation together for arthritis.
Limitations of this study should be considered. First, the study cannot address causality or the direction of the association we observed between combined statin use and vitamin D and musculoskeletal pain. Second, portions of our analyses were based on self-report, including health habits and co-morbidities, which are subject to misclassification. Additionally, we lacked sufficient information to assess variations by statin dose and type, and although myalgia is known to occur with all statins, we were not able to evaluate whether higher doses or specific classes of statins modify the strength of the observed associated between combined statin treatment and low vitamin D status and musculoskeletal pain. Additionally, due to the distribution of vitamin D in our sample, we had limited power to evaluate the possible effects of 25(OH)D concentrations at higher levels that have been observed to improve statin myalgia [2,3]. Finally, while our analyses adjusted for a wide array of lifestyle, dietary, and health status covariates, residual confounding may remain.
5. Conclusion
In this cross-sectional nationally-representative study of 5907 individuals, we found that among adults ≥40 years with vitamin D deficiency (25(OH)D<15ng/mL), use of a statin was associated with a nearly 2 times greater odds of reporting musculoskeletal pain compared to non-statin users. However, among individuals with vitamin D levels ≥15 ng/mL, no association between statin use and musculoskeletal pain was observed. Our findings support the hypothesis that vitamin D deficiency modifies the risk of musculoskeletal symptoms experienced with statin use.
Supplementary Material
Highlights.
We examined the effect of vitamin D on statin-associated musculoskeletal pain.
Statin users with low vitamin D were more likely to report musculoskeletal pain.
Statin users with 25OHD ≥15 ng/mL had no increased risk for musculoskeletal pain.
Vitamin D status appears to modify the effect of statins on musculoskeletal pain.
Acknowledgments
Funding Sources
Dr. Buettner was supported by K23 Career Development Award (K23AR055664). Dr. Bertisch was supported by K23 Career Development Award (K23AT005104). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102 and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors’ affiliated organizations and funding sources had no involvement in the study design; collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the article for publication. Dr. Buettner and Beth Israel Deaconess Medical Center have applied for a patent for the combination of statin and vitamin D as a prophylactic treatment in migraine. All other authors have no indicated no finanical conflicts of interests.
References
- 1.Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am. J. Med. 2012;125:176–82. doi: 10.1016/j.amjmed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed W, Khan N, Glueck CJ, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl. Res. 2009;153:11–6. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Glueck CJ, Budhani SB, Masineni SS, et al. Vitamin D deficiency, myositis-myalgia, and reversible statin intolerance. Curr. Med. Res. Opin. 2011;27:1683–90. doi: 10.1185/03007995.2011.598144. [DOI] [PubMed] [Google Scholar]
- 4.Kurnik D, Hochman I, Vesterman-Landes J, et al. Muscle pain and serum creatine kinase are not associated with low serum 25(OH) vitamin D levels in patients receiving statins. Clin. Endocrinol. 2012;77:36–41. doi: 10.1111/j.1365-2265.2011.04321.x. [DOI] [PubMed] [Google Scholar]
- 5.Eisen A, Lev E, Lakobishvilli Z, et al. Low plasma vitamin D levels and muscle-related adverse effects in statin users. Isr. Med. Assoc. J. 2014;16:42–5. [PubMed] [Google Scholar]
- 6.Palamaner Subash Shantha G, Ramos J, Thomas-Hemak L, Pancholy SB. Association of vitamin D and incident statin induced myalgia--a retrospective cohort study. PloS One. 2014;9:e88877. doi: 10.1371/journal.pone.0088877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. (Accessed at http://www.cdc.gov/nchs/data/nhanes/nhanes3/VitaminD_analyticnote.pdf.)
- 8.Lee P, Greenfield JR, Campbell LV. Vitamin D insufficiency--a novel mechanism of statin-induced myalgia? Clin. Endocrinol. 2009;71:154–5. doi: 10.1111/j.1365-2265.2008.03448.x. [DOI] [PubMed] [Google Scholar]
- 9.Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013;92:151–62. doi: 10.1007/s00223-012-9645-y. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa S, Nakamura T, Tanabe H, Imamura T. Osteomalacic myopathy. Endocrinol. Japon. 1979;26:65–72. doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 11.Waclawik AJ, Lindal S, Engel AG. Experimental lovastatin myopathy. J. Neuropathol. Exp. Neurol. 1993;52:542–9. doi: 10.1097/00005072-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Reimers E, Duran-Castellon MC, Lopez-Lirola A, et al. Alcoholic myopathy: vitamin D deficiency is related to muscle fibre atrophy in a murine model. Alcohol. 2010;45:223–30. doi: 10.1093/alcalc/agq010. [DOI] [PubMed] [Google Scholar]
- 13.Clockaerts S, Van Osch GJ, Bastiaansen-Jenniskens YM, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann. Rheum. Dis. 2012;71:642–7. doi: 10.1136/annrheumdis-2011-200092. [DOI] [PubMed] [Google Scholar]
- 14.Kadam UT, Blagojevic M, Belcher J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J. Gen. Intern. Med. 2013;28:943–9. doi: 10.1007/s11606-013-2382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAlindon T, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. J. Am. Med. Assoc. 2013;309:155–62. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.