Abstract
We sought to determine whether Dopamine D2 Receptor (D2R) agonists inhibit lung tumor progression and identify subpopulations of lung cancer patients that benefit most from D2R agonist therapy. We demonstrate D2R agonists abrogate lung tumor progression in syngeneic (LLC1) and human xenograft (A549) orthotopic murine models through inhibition of tumor angiogenesis and reduction of tumor infiltrating myeloid derived suppressor cells. Pathological examination of human lung cancer tissue revealed a positive correlation between endothelial D2R expression and tumor stage. Lung cancer patients with a smoking history exhibited greater levels of D2R in lung endothelium. Our results suggest D2R agonists may represent a promising individualized therapy for lung cancer patients with high levels of endothelial D2R expression and a smoking history.
Keywords: Dopamine, Lung cancer, VEGF, Cabergoline, Angiogenesis, Dopamine D2 receptor agonists
Highlights
D2R agonists inhibit lung tumor progression in murine models of lung cancer.
D2R agonists reduce tumor angiogenesis and myeloid derived suppressor cells.
Endothelial D2R correlates with patients' lung tumor grade and smoking history.
Cabergoline shows promise as a targeted therapy and warrants clinical investigation.
Abbreviations
- D2R
dopamine D2 receptor
- LLC1
Lewis lung carcinoma 1
- NSCLC
non-small cell lung cancer
- VEGF
vascular endothelial growth factor A
- EGFR
epidermal growth factor receptor
- ALK1
activin receptor-like kinase-1
- TKI
tyrosine kinase inhibitors
- HUVEC
human umbilical vein endothelial cells
- DA
dopamine
- MDSCs
myeloid derived suppressor cells
- ROS
reactive oxygen species
1. Introduction
Lung cancer remains the leading cancer related cause of death in the United States and worldwide (Siegel et al., 2013). Non‐small cell lung cancer (NSCLC), the most common subtype (85%) of lung cancer, continues to be associated with a very poor 5‐year survival rate of less than 15% (Cetin et al., 2011). Despite the recent advances in systemic lung cancer treatment due to the introduction new therapies targeting angiogenesis, epidermal growth factor receptor (EGFR), and activin receptor‐like kinase‐1 (ALK1) in selected patient subgroups, the overall mortality of patients with advanced stage disease remains high. The development of new biomarkers and individualized therapies is needed to overcome these challenges and make significant strides towards improving the care of lung cancer patients.
Vascular endothelial growth factor A (VEGF) is a multi‐functional cytokine with important roles in vasculogenesis and angiogenesis (Carmeliet et al., 1996; Ferrara et al., 1996; Leung et al., 1989; Nasevicius et al., 2000; Senger et al., 1983). VEGF was originally discovered as “vascular permeability factor” and described as a tumor‐secreted factor that potently promotes microvascular permeability (Senger et al., 1983). Later, it was discovered separately as VEGF, an endothelial mitogen (Leung et al., 1989) essential for the development of blood vessels (Carmeliet et al., 1996; Ferrara et al., 1996; Nasevicius et al., 2000). VEGF plays a central role in the formation of lung vasculature. Correspondingly, the lungs possess the highest level of VEGF gene expression among normal tissues (Kaner et al., 2000). VEGF and its receptors (VEGFR‐1, VEGFR‐2, and NRP‐1) have been detected in alveolar type II cells, airway epithelial cells, mesenchymal cells, airway and vascular smooth muscle cells, macrophages, and neutrophils (Kazi et al., 2004; Voelkel et al., 2006). In normal lung, VEGF acts in a paracrine fashion by slowly diffusing across the alveolar epithelium to the adjacent vascular endothelium. However, in disease states the expression of VEGF and/or its receptors is affected and often specifically related to the pathophysiology and the particular characteristics of each disease (Lahm et al., 2007). Not surprisingly, NSCLC tumors produce high levels of VEGF (Korpanty et al., 2010), and vascular lung endothelium associated with malignant tissue expresses higher levels of VEGFR‐2 compared to normal lung endothelium (Smith et al., 2010). Increased secretion of VEGF by tumor cells and upregulation of endothelial VEGFR‐2 promotes tumor angiogenesis, which provide lung tumors with the blood supply necessary to grow and metastasize.
Two distinct molecular therapies to target VEGF signaling are currently being investigated clinically. The first involves the blockade of VEGF binding to its extracellular receptors by a VEGF‐specific antibody; the second uses tyrosine kinase inhibitors (TKI) to block intracellular VEGF signaling. Bevacizumab (Avastin®) is a humanized monoclonal antibody that binds all forms of VEGF. In 2004, a phase II clinical trial of bevacizumab in newly diagnosed stage IIIB/IV or recurrent NSCLC patients was conducted. Patients received low or high doses of bevacizumab with standard chemotherapy, and those who received high doses demonstrated improved time to progression (7.4 months) compared to the chemotherapy alone group (Johnson et al., 2004). Although effective in lung adenocarcinoma patients, bevacizumab caused serious pulmonary bleeding in some patients with squamous cell lung tumors and typically is not used in patients with squamous histology or brain metastases (Johnson et al., 2004). Vandetanib (Zactima®) is a small molecule TKI that blocks VEGFR‐1, ‐2, ‐3 and EGFR. Phase II patients with advanced NSCLC who received vandetanib as a single agent experienced an 11.9 week increase in progression‐free survival (Natale, 2008). Because bevacizumab and vandetanib only modestly improve the clinical outcome of NSCLC patients, new therapies are needed for the treatment of lung cancer.
Dopamine (DA) has long been used in the treatment of Parkinson's disease and acute cardiac dysfunction (Lynch et al., 2006). Given that DA is produced by the sympathetic nerves ending in blood vessels, we originally postulated and later revealed that DA and its dopamine D2 receptor (D2R) agonists inhibit VEGF‐mediated angiogenesis and also completely block accumulation of tumor ascites and tumor growth in mice (Basu et al., 2001). Specifically, we demonstrated that DA stimulates endocytosis of VEGFR‐2 via D2R thereby preventing angiogenesis by inhibiting VEGF binding, receptor phosphorylation and subsequent downstream signaling (Basu et al., 2001). These observations define a possible link between DA and vascular biology. Subsequent studies by numerous investigators clearly demonstrate that this strategy can be successfully applied to various diseases including cancer (Basu et al., 2004; Chakroborty et al., 2008; Tilan and Kitlinska, 2010). Correspondingly, we observed significantly more angiogenesis, tumor growth, and VEGFR‐2 phosphorylation in D2R knockout mice (Basu et al., 2004). We documented D2R colocalization with VEGFR‐2 and described the molecular mechanism through which D2R/VEGFR‐2 crosstalk can mediate the dephosphorylation of VEGFR‐2 (Bhattacharya et al., 2008; Sinha et al., 2009). D2R agonists have been shown to increase the efficacy of anti‐cancer drugs in preclinical models of breast and colon cancer (Sarkar et al., 2008). Here we show that D2R agonists inhibit tumor growth in orthotopic murine lung cancer models through inhibition of tumor angiogenesis and reduction of tumor infiltrating myeloid derived suppressor cells. Our findings suggest treatment with D2R agonists may represent a promising therapeutic alternative for NSCLC patients with increased endothelial D2R expression.
2. Materials and methods
2.1. Cell culture
Human NSCLC cell lines A549, H226, H157, H441 and H1650 as well as the murine LLC1 lung cancer cell line were purchased from American Type Culture Collection and maintained according to their instructions. Human umbilical vein endothelial cells (HUVEC; Lonza) were cultured in endothelial basal medium (EBM) supplemented with the EGM‐MV Bullet Kit [5% fetal bovine serum (FBS), 12 mg/mL bovine brain extract, 1 mg/mL hydrocortisone, and 1 mg/mL GA‐1000]. HUVEC of passage three to five were used and cultured in plates coated with bovine collagen type I (BD Biosciences). All cell lines were certified by the indicated cell bank and periodically authenticated by morphologic inspection.
2.2. Antibodies
For immunoblotting and immunohistochemistry, a mouse monoclonal D2R antibody (sc‐5303) from Santa Cruz Biotechnology was used. A rabbit polyclonal CD31 antibody (sc‐1506‐R; Santa Cruz Biotechnology) was used for immunohistochemistry.
2.3. Immunohistochemistry staining
The lungs of tumor bearing mice were intracardially perfused with PBS, harvested, and fixed in neutral buffered 10% formalin at room temperature for 24 h before processing and embedding in paraffin and sectioning. Sections were deparaffinized and then subjected to D2R and CD31 immunochemistry staining. Stable diaminobenzidine (DAB) was used as a chromogen substrate, and the sections were counterstained with a hematoxylin solution. For the D2R immunohistochemistry, the staining was performed using a Bond Autostainer (Leica), and the sections were incubated in Rodent Block M (Biocare RBM961) to eliminate non‐specific mouse IgG staining.
Patient lung cancer tissue microarrays were created using specimens obtained from a variety of NSCLC patients at Mayo Clinic. Tissue microarrays were immunostained using D2R antibody as described above and D2R expression in endothelial cells and myeloid derived cells was assessed by a pulmonary pathologist (ACR).
2.4. Immunofluorescence staining
Tissues sections were deparaffinized, rehydrated, heat‐retrieved with EDTA buffer (PH8.5, Sigma), and then permeabilized with 0.3% Triton in PBS. Immunofluorescence staining was performed with primary antibody against PECAM‐1 (Santa Cruz, sc‐1506‐R) and a rhodamine‐labeled secondary antibody (Licor). After incubation with secondary antibody, the slides were rinsed with PBS and then subjected to TUNEL staining following manufacturer's instructions (Promega, G3250). Briefly, the slides were incubated with equilibration buffer for 5 min and then with the reaction buffer containing rTdT and Nucleotide Mix for 1 h at 37 °C. The reaction was terminated with 2× sodium citrate buffer, rinsed with PBS and counterstained with DAPI. Images were captured using of a Zeiss LSM 780 confocal laser scanning microscope.
2.5. In vivo orthotopic lung cancer model
LLC1 syngeneic model: Six to eight week‐old pathogen‐free female wildtype and D2R knockout C57BL/6 mice were purchased from Jackson Laboratory. The animals were housed in a temperature‐controlled room with alternating 12 h light/dark cycles, allowed 1 week to acclimate to their surroundings, and fed a standard diet. Mice were orthotopically injected with 1 × 105 luciferase‐labeled murine LLC1 cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged four days post‐injection of LLC1 cells. Starting on day 5 post‐injection of LLC1 cells, mice received daily intraperitoneal injections of PBS vehicle (control groups) or 50 mg/kg dopamine, 10 mg/kg quinpirole, or 5 mg/kg cabergoline (treatment groups) for seven days. Mice were xenogen imaged following treatment. The lungs of the mice were perfused with PBS and prepared for histology as indicated.
A549 human xenograft model: Six to eight week‐old pathogen‐free female SCID mice were purchased from the National Cancer Institute and animal husbandry was performed as described above. Mice were orthotopically injected with 2 × 106 luciferase‐labeled human A549 lung cancer cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged eight days post‐injection of A549 cells. Mice received intraperitoneal injections of PBS vehicle (control group, n = 6) or 10 mg/kg quinpirole (treatment group, n = 6) every other day for 17 days. Mice were xenogen imaged following treatment. All animal work was conducted under protocols approved by the Mayo Clinic Institutional Animal Care and Use Committee.
2.6. Flow cytometry
Lungs intracardially perfused with PBS were harvested from euthanized mice and cells were extracted through digestion using Liberase® (Roche), DNase1 (Stemcell Technologies) and mechanical disassociation. Cells were prepared for flow cytometry by incubating for 5 min with Mouse Fc Block (BD Pharmingen) followed by staining in the presence of Fc Block with a lineage cocktail made up of CD45R/B220, CD49b, Ter118, CD8, CD4 and CD3 antibodies conjugated to APC (Biolegend) as well as GR1‐FITC (Biolegend), CD11b‐PerCp (Biolegend) and CD11c‐PE (Biolegend) antibodies for 20 min at 4 °C. The cells were washed three times in FACs Buffer (1% BSA in HBSS (Hank's Balanced Salt Solution, Life Technologies) with 0.035% w/v Sodium Bicarbonate and 0.02% Sodium Azide) and resuspended in 400 μl of 2% paraformaldehyde in PBS, and 100,000 events per tube were analyzed by flow cytometry. The resulting data was analyzed using FlowJo flow cytometry analysis software (Tree Star Inc).
2.7. Statistics
All values are expressed as means ± SEM. Statistical significance was determined using 2‐sided Student's t test and a value of P < 0.05 was considered significant.
3. Results
3.1. Dopamine receptor 2 is upregulated in lung cancer
Given the anti‐tumor and anti‐angiogenesis effects of D2R agonists and the highly vascularized nature of lung tumors, we sought to determine whether D2R agonists may show promise as an effective therapy for NSCLC patients. First, we assessed expression of D2R in lung cancer through immunoblotting and immunohistochemistry. D2R was expressed in all the human lung cancer cell lines we examined (H1650, H157, H226, H441, A549) as well as murine Lewis lung carcinoma 1 (LLC1) cells (Supp. Figure 1A). We also found that D2R is expressed at greater levels in human lung cancer tissue compared to normal human lung (Figure 1A–D). We next sought to identify which cell types express D2R in physiological and pathological lung tissue. To this end, we performed immunohistochemistry‐based colocalization studies in normal and lung tumor tissue using antibodies to D2R and Fli‐1, an endothelial cell marker that is also expressed in some lymphocytes (Pusztaszeri et al., 2006). We observed increased endothelial expression of D2R in lung tumor tissue compared to lung specimens obtained from healthy individuals, as evident by increased colocalization of the D2R and Fli‐1 staining (Figure 1E–F). Based on our observations that D2R is upregulated in human lung tumor endothelium combined with the previously reported tumor angiogenesis inhibitory effects of D2R agonists, we postulated that D2R agonists will significantly reduce angiogenesis and lung tumor growth.
Figure 1.
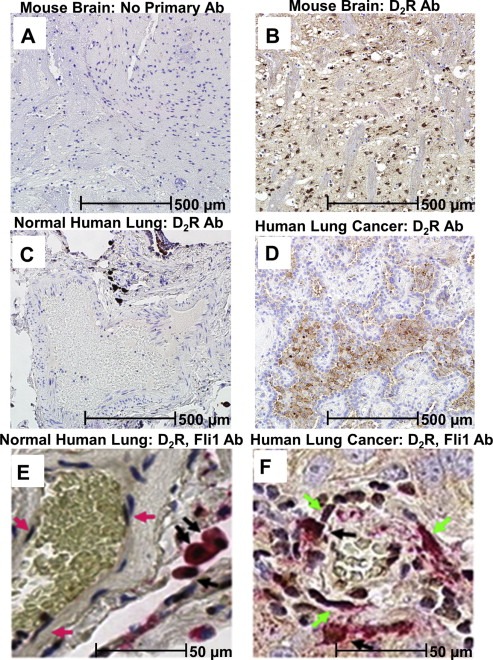
Dopamine D2 Receptor is upregulated in human lung cancer tissue. A–D: Immunohistochemistry was performed using a monoclonal D2R antibody on formalin‐fixed, paraffin‐embedded tissue as follows: A: mouse brain tissue with no primary D2R antibody (negative control), B: mouse brain tissue (positive control), C: human lung tissue from a healthy individual, D: human lung tissue from a lung adenocarcinoma patient. E–F: Immunohistochemistry of formalin‐fixed, paraffin‐embedded human normal lung (E) and lung adenocarcinoma (F) tissue was performed for Fli‐1 (brown staining) and D2R (red staining). Pink arrows indicate Fli+, D2R‐ endothelial cells (E), green arrows indicate Fli+, D2R+ endothelial cells (F) and black arrows indicate D2R+ myeloid cells.
3.2. DA and D2R agonist treatment decreases tumor progression and angiogenesis in an LLC1 murine lung cancer model
To test the hypothesis that D2R agonists reduce angiogenesis and lung cancer growth, we orthotopically injected luciferase‐labeled murine LLC1 cells into the left thorax of C57BL/6 mice, allowed establishment of the lung tumor, and then performed intraperitoneal injections of vehicle or D2R agonists at low, nontoxic doses (DA: 50 mg/kg, corresponding to 5% of the median lethal dose (LD50) (Zaroslinski et al., 1977), quinpirole: 10 mg/kg, cabergoline: 5 mg/kg) (Figure 2A). Mice were luciferase imaged before and after the seven day period of daily D2R agonist treatment. DA and the D2R agonist, quinpirole, each reduced lung tumor growth in wildtype mice but failed to inhibit LLC1 growth in D2R knockout mice (Figure 2B–C), thus supporting the notion that dopamine agonists block tumor growth specifically through D2R. The FDA‐approved D2R agonist cabergoline (Dostinex®) also significantly reduced LLC1 tumor growth (Figure 2D–E). We performed murine dose‐response studies with cabergoline and found LLC1 growth was inhibited by 80–85% with 5 mg/kg cabergoline, 40–45% with 1 mg/kg cabergoline, and 25–30% with 0.5 mg/kg cabergoline (Supp. Figure 2). Taken together, our results suggest DA and D2R agonists act specifically through D2R to inhibit lung tumor growth.
Figure 2.
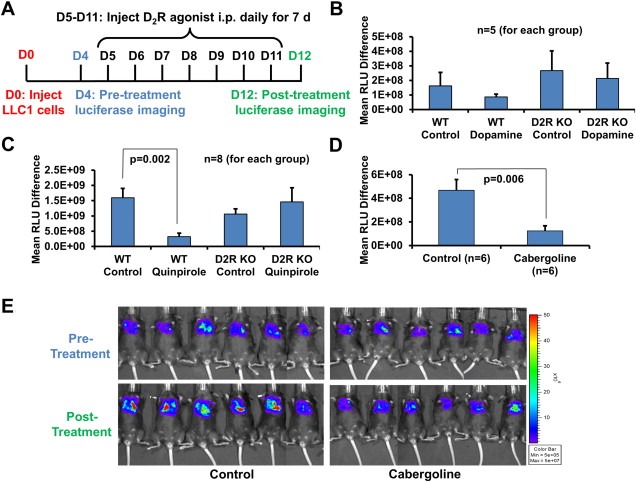
DA and D2R agonist treatment decreases tumor progression in an LLC1 murine lung cancer model. A–E: C57BL/6 wildtype (B–D) and D2R knockout (B–C) mice were orthotopically injected with 1 × 105 luciferase‐labeled murine LLC1 cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged four days post‐injection of LLC1 cells. Mice received daily intraperitoneal injections of PBS vehicle (control groups) or 50 mg/kg dopamine (B), 10 mg/kg quinpirole (C), or 5 mg/kg cabergoline (D) (treatment groups) for seven days. Mice were xenogen imaged following treatment. A: Experimental timeline. B–D: Quantitation of LLC1 tumor growth by determining the difference in relative luciferase units (RLU) before and after treatment. E: Pre‐ and post‐treatment xenogen images of vehicle‐ and cabergoline treated mice.
It has been well established that DA and D2R agonists inhibit tumor angiogenesis by abrogating VEGFR‐2‐mediated signaling in endothelial cells. Specifically, previous studies have shown DA inhibits VEGF‐induced HUVEC proliferation and migration specifically through D2R and ablation of peripheral dopaminergic nerves increased endothelial cell density (Basu et al., 2004). Therefore, we hypothesized that D2R agonist treatment decreases endothelial cells in the lung tumor microenvironment. To that end, we performed CD31 immunohistochemistry with LLC1 tumor tissue harvested from wildtype mice that had been treated with D2R agonist quinpirole or a vehicle control. As expected, we observed a significant decrease in CD31+ endothelial cells in lung tumor tissue from quinpirole‐treated mice compared to tumor tissue from control mice treated with vehicle (Figure 3A–B). Correspondingly, we found that DA or quinpirole treatment does not affect proliferation of a human lung cancer cells (Supp. Figure 1B). We postulated that D2R agonists reduce endothelial cells in the lung tumor microenvironment by stimulating apoptosis. To test this hypothesis, we performed TUNEL immunofluorescence staining in lung tissue derived from LLC1 tumor bearing mice treated with quinpirole or vehicle control. We demonstrated that D2R agonist treatment increases apoptosis in endothelial cells, as evident by the increased prevalence of double positive TUNEL and CD31 cells in the quinpirole‐treated group relative to the control group (Figure 3C–D). Taken together, our findings indicate that DA and D2R agonists decrease VEGF‐induced angiogenesis in lung tumor microenvironment through apoptosis of endothelial cells to reduce LLC1 tumor growth in mice.
Figure 3.
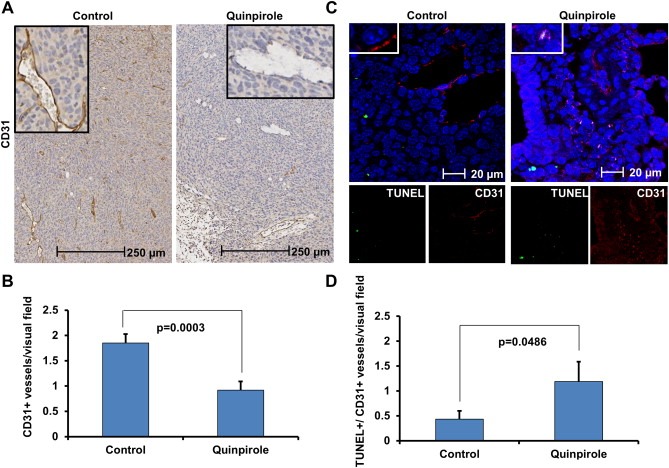
D2R agonist treatment decreases angiogenesis and promotes apoptosis in the endothelium of LLC1 tumor bearing mice. A: Immunohistochemistry was performed using a monoclonal CD31 antibody on formalin‐fixed, paraffin‐embedded tissue harvested from LLC1 tumor bearing mice treated with vehicle (left) or 10 mg/kg quinpirole (right) daily for seven days. B: The amount of CD31‐positive endothelial cells in lung tissue harvested from control and quinpirole‐treated mice (n = 2 for each group) was quantitated by counting the number of CD31‐positive endothelial cells per visual field (10 visual fields were counted for each tissue). C: Co‐immunofluorescence staining for TUNEL (green; bottom left single stain) and CD31 (red; bottom right single stain) was performed on tissue harvested from LLC1 tumor bearing mice treated with vehicle (left) or 10 mg/kg quinpirole (right) daily for seven days. Merged images are shown in the top panel (green: TUNEL, red: CD31, blue: DAPI). D: Colocalization of TUNEL and CD31 staining was quantitated in the immunofluorescent images of lung tissue harvested from control and quinpirole‐treated mice (n = 2 for each group) by counting the number of double positive TUNEL and CD31 endothelial cells per visual field (10 visual fields were counted for each tissue).
3.3. D2R agonist inhibits lung cancer growth in a human xenograft model
We next sought to determine whether the D2R agonist‐mediated inhibition of murine lung cancer growth translates into an effective therapy for human NSCLC. To begin addressing this question, we utilized a human lung xenograft model. Specifically, human A549 adenocarcinoma cells were orthotopically injected into the left thorax of anesthetized SCID mice and allowed to establish themselves for eight days. Mice received intraperitoneal injections of D2R agonist quinpirole or vehicle control every other day for 17 days and were xenogen imaged before and after treatment (Figure 4A). We observed a significant decrease in A549 lung tumor growth in mice treated with D2R agonist compared to controls (Figure 4B–C). These findings support our hypothesis that D2R agonists inhibit human lung tumor growth.
Figure 4.
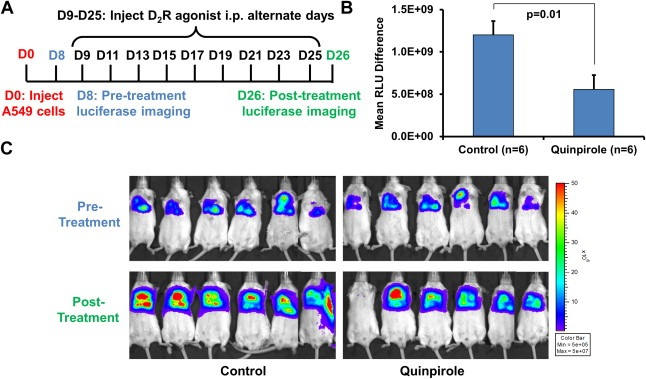
D2R agonist treatment decreases tumor progression in a human lung tumor xenograft model. A–C: SCID mice were orthotopically injected with 2 × 106 luciferase‐labeled human A549 lung cancer cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged eight days post‐injection of A549 cells. Mice received intraperitoneal injections of PBS vehicle (control group, n = 6) or 10 mg/kg quinpirole (treatment group, n = 6) every other day for 17 days. Mice were xenogen imaged following treatment. A: Experimental timeline. B: Quantitation of A549 tumor growth by determining the difference in relative luciferase units (RLU) before and after treatment. C: Pre‐ and post‐treatment xenogen images of vehicle‐ and quinpirole‐treated mice.
3.4. Endothelial expression of D2R is upregulated in lung cancer and correlates with tumor progression
To continue determining whether D2R agonist treatment of NSCLC patients represented an efficacious anti‐cancer therapy, it was necessary to assess the expression levels of D2R in patients with various types and stages of lung cancer. We hypothesized that a subset of NSCLC patients would have high D2R expression, and therefore, envisioned a potential individualized medicine approach in which patients identified to possess high D2R expression would be ideal candidates for D2R agonist therapy. We performed D2R immunohistochemistry on lung cancer tissue microarrays developed using specimens obtained from a variety of NSCLC patients at Mayo Clinic. A pulmonary pathologist (ACR) reviewed the D2R immunohistochemistry staining and scored the percentage of D2R‐positive endothelial cells present in each sample. Not surprisingly, we observed increased endothelial expression of D2R in lung cancer samples compared to normal lung (Figure 5). Furthermore, in both lung adenocarcinoma and squamous cell lung carcinoma, we found a positive correlation between tumor grade and expression of D2R in endothelial cells (Figure 5). As postulated, specific NSCLC patients with high expression of D2R were identified. Taken together, our results suggest lung endothelial D2R expression may increase as lung adenocarcinoma and squamous cell lung carcinoma progress and individual patients with high D2R levels in the lung endothelium that are most likely to benefit from D2R agonist therapy can be identified.
Figure 5.
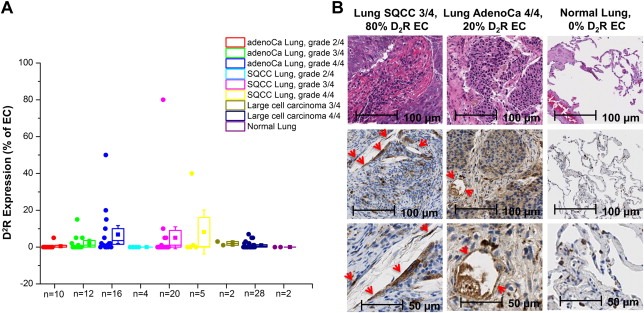
D2R is expressed by endothelial cells in human lung tumor tissue. A: Immunohistochemistry was performed using a monoclonal D2R antibody on a human lung cancer tissue microarray, and each tissue was scored for the percentage of endothelial cells that were D2R‐positive. Each point on the graph represents an individual tissue. B: Representative staining from tissue microarray (top: 10× H&E staining, middle: 10× D2R staining, bottom: 20× D2R staining). Red arrows indicate D2R‐positive endothelial cells.
3.5. D2R agonists reduce lung tumor infiltrating myeloid derived suppressor cells in vivo
Interestingly, pathological analysis of the human lung cancer tissue specimens revealed a considerable amount of D2R‐positive myeloid lineage cells (Figure 1E–F; Figure 6A). Previous reports have linked DA signaling with immune responses in lung cancer patients (Saha et al., 2001). While the presence of pro‐inflammatory immune cells, such as cytotoxic CD8+ T‐lymphocytes, NK‐cells, TH1 polarized T‐helper cells and mature dendritic cells, within the tumor microenvironment have been associated with favorable lung cancer patient outcomes, large numbers of immunosuppressive cells, such as T‐regulatory cells and immature myelo‐monocytic cells (including myeloid derived suppressor cells and tumor‐associated macrophages), correspond to poor patient survival. Based on previous studies and our pathological findings, we hypothesized that tumor infiltrating myeloid derived suppressor cells (MDSCs) express D2R and targeting MDSCs with D2R agonist may reduce their quantity and immunosuppressive function in the lung tumor microenvironment. Therefore, we sought to determine whether the D2R agonist cabergoline decreases MDSCs using the orthotopic LLC1 murine model described earlier. Briefly, following cabergoline treatment of mice bearing LLC1 tumors, lungs were harvested from euthanized mice and cells were extracted through collagenase digestion. Flow cytometry was utilized to assess the number of LIN−, GR1+, CD11b+ MDSCs in cabergoline‐ and vehicle‐treated mice. We found LLC1 tumor burdened mice treated with cabergoline harbored less pulmonary MDSCs than corresponding control mice (Figure 6B–C). These results suggest that D2R agonists may reduce lung tumor growth through inhibition of immunosuppressive MDSCs as well as abrogating tumor angiogenesis. Next, we investigated whether these results translate to humans by determining the effect of D2R agonists on human myeloid cell maturation. CD14+ monocytes isolated from the blood of healthy human donors were treated daily with 10 μM cabergoline and cultured with appropriate cytokines to stimulate the formation of immature and mature myeloid cells. Cabergoline treatment caused a decrease in maturation of both immature and mature myeloid cells, as evident by decreased myeloid cell maturation marker HLA‐DR in cabergoline treated cells compared to controls (Supp. Figure 3).
Figure 6.
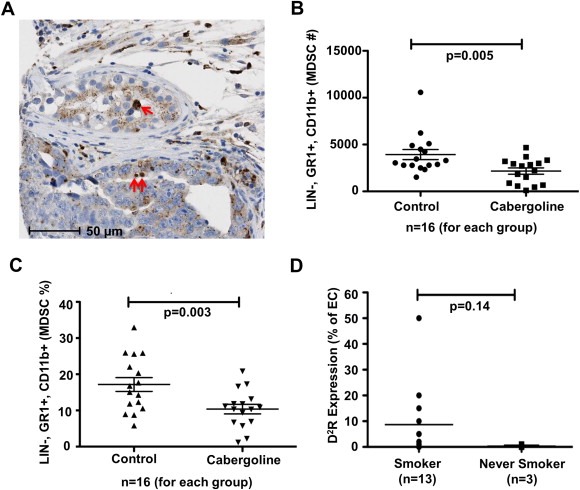
Tumor infiltrating myeloid derived suppressor cells decreased in vivo by D2R agonists and endothelial D2R expression increased in NSCLC patients with a smoking history. A: Representative image of D2R immunohistochemistry of human lung cancer tissue microarray depicting D2R‐positive myeloid/macrophage lineage cells (red arrows). B–C: C57BL/6 wildtype mice were orthotopically injected with 1 × 105 LLC1 cells. Mice were intraperitoneally administered vehicle or cabergoline daily for seven days starting on day five post‐injection of LLC1 cells. After 12 days, the lungs were harvested from euthanized mice and cells were extracted through collagenase digestion. Flow cytometry was used to determine the number (B) and percentage (C) of murine myeloid derived suppressor cells (MDSCs), which were defined as LIN‐, GR1+, and CD11b+. n = 16 mice/treatment group. D: Immunohistochemistry was performed using a monoclonal D2R antibody on a human lung cancer tissue microarray. The graph shows the percentage of endothelial cells stained positive for D2R among lung cancer tissue obtained from smokers (n = 13) compared to those from never smokers (n = 3).
3.6. Increased endothelial D2R expression in lung tumor patients with a smoking history
Smoking is a known risk factor for most cancers, particularly lung carcinomas (Burns, 2003; Hecht, 1999; Tournier and Birembaut, 2011). Interestingly, some initial reports suggest that variant D2R genotypes are associated with a greater likelihood to smoke and a greater smoking intensity and may also be a potential cause for familial aggregation of smoking‐related cancers (Styn et al., 2009; Wu et al., 2000). Hence, the link between the D2R pathway and lung cancer development and progression can be at least partially attributed to smoking. Given the association between D2R, NSCLC, and smoking, we hypothesized that lung cancer patients with a smoking history would exhibit greater endothelial expression of D2R. As expected, we found an increased number of D2R‐positive endothelial cells in lung cancer tissue specimens from smokers compared to samples from NSCLC patients classified as never smokers (Figure 6D).
Further studies beyond the scope of those presented here are necessary to fully understand this association. However, the greater prevalence of endothelial D2R expression in lung cancer patients with a smoking history suggests D2R agonists may represent an intriguing precision therapeutic strategy for treatment of the subset of NSCLC patients with a smoking history and high D2R expression in lung tumor endothelium.
4. Discussion
In this report, we demonstrate that D2R agonists inhibit NSCLC tumor progression using LLC1 syngeneic and A549 human xenograft murine models of lung cancer. Our findings suggest D2R agonists decrease lung cancer tumor growth through two mechanisms: reduction of tumor angiogenesis in lung endothelial cells and abrogation of tumor infiltrating myeloid derived suppressor cells. While additional studies are necessary to elucidate whether these mechanisms are interconnected, we speculate that D2R agonist‐mediated reduction of lung tumor‐associated endothelial cells and angiogenesis may contribute to a tumor microenvironment that is less conducive to tumor infiltration of MDSCs. We previously showed DA and D2R agonists selectively inhibit VEGF‐induced angiogenesis and vascular permeability by negatively regulating VEGFR‐2 phosphorylation (Basu et al., 2001). Specifically, D2R agonists increase the recruitment of Src‐homology‐2‐containing protein tyrosine phosphatase (SHP‐2) to the D2R/VEGFR‐2 complex and promote increased VEGF‐induced phosphorylation of SHP‐2, which stimulates its phosphatase activity (Sinha et al., 2009). Active SHP‐2 then dephosphorylates VEGFR‐2 at Y951, Y996 and Y1059, thus mitigating subsequent VEGF‐dependent signaling events including those that promote angiogenesis (Sinha et al., 2009). Previous studies strongly support the notion that D2R agonist‐mediated inhibition of cancer can be translatable as a therapeutic strategy (Basu et al., 2004; Chakroborty et al., 2008; Tilan and Kitlinska, 2010). It has been shown that disruption of peripheral dopaminergic nerves stimulates tumor growth through induction of VEGF‐dependent angiogenesis (Basu et al., 2004), and DA treatment inhibits tumor‐promoting endothelial progenitor cell mobilization from the bone marrow niche (Chakroborty et al., 2008). Furthermore, D2R agonists increased the efficacy of anti‐cancer drugs in preclinical models of breast and colon cancer (Sarkar et al., 2008). We observed DA, quinpirole and cabergoline each decreased LLC1 lung tumor growth in C57BL/6 mice. Interestingly, the DA‐mediated reduction in LLC1 growth was appreciable but not quite statistically significant (Figure 2B), whereas the quinpirole and cabergoline treatment each resulted in a statistically significant decrease of LLC1 tumor growth (Figure 2C–D). The greater effectiveness of quinpirole and cabergoline likely reflects the fact that they are high affinity agonists for D2R, whereas DA is more promiscuous for other DA receptors.
While the inhibitory effects of D2R agonists on VEGF‐dependent tumor angiogenesis have been well established, we describe D2R agonist‐mediated abrogation of myeloid derived suppressor cell tumor infiltration using a syngeneic murine lung cancer model. The presence of pro‐inflammatory immune cells, such as cytotoxic T‐lymphocytes, NK‐cells, TH1 polarized T‐helper cells and mature dendritic cells, within the tumor microenvironment have been associated with favorable lung cancer patient outcomes. Conversely, large numbers of immunosuppressive cells, such as T‐regulatory cells and immature myelo‐monocytic cells including myeloid derived suppressor cells and tumor‐associated macrophages, correspond to poor patient survival. Most recently the therapeutic success of immunostimulatory checkpoint inhibitors, such as anti‐PD‐1, anti‐PD1‐L1 (B7H1) and anti‐CTLA‐4, for the treatment of various malignancies, including lung cancer, in early phase clinical trials strongly supports a potential role of immunotherapy for the treatment of lung cancer (Lynch et al., 2012; Topalian et al., 2012). Interestingly, circulating DA levels have been shown to be increased in lung cancer patients and corresponding in vitro studies revealed decreased proliferation and cytotoxicity of CD4+ and CD8+ T cells in these patients by a dopamine D1 receptor‐dependent mechanism (Saha et al., 2001). Although beyond the scope of our current study, it would be informative to determine whether administration of D2R agonists to lung cancer patients changes circulating DA levels and/or the effects of endogenous DA on T cell proliferation and cytotoxicity. Regardless, our studies suggest that D2R is expressed on myeloid precursor cells in the lung tumor microenvironment and D2R agonist treatment results in a reduction of tumor infiltrating MDSCs. D2R has been identified as a negative regulator of NADPH oxidase, which promotes angiogenesis and reactive oxygen species (ROS) production (Bhandarkar et al., 2009; Perry et al., 2006; Yang et al., 2012). Therefore, some of the beneficial anti‐angiogenesis effects of D2R agonists may occur through inhibition of NADPH oxidase. Recent studies have demonstrated that one of the major mechanisms of MDSC‐induced immune suppression is mediated by ROS (Corzo et al., 2009). Thus, D2R‐mediated inhibition of ROS may contribute to ablation of the immune suppressive effects of MDSCs.
Smoking is contributing factor in 80–90% of lung cancer deaths, and men and women who smoke are respectively 23 and 13 times more likely to develop lung cancer compared to never smokers (Alberg et al., 2014). Cigarette smoke contains several carcinogens, including polycyclic aromatic hydrocarbons and mutagenic nicotine metabolites such as N‐nitrosonornicotine and 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone (Burns, 2003; Hecht, 1999; Tournier and Birembaut, 2011). Importantly, in most cases, the nicotine and its derived metabolites function through nAChRs that are present not only in the nervous system, but also in numerous peripheral organs such as the lung epithelium and endothelium (Heeschen et al., 2002; Wang et al., 2001). Several reports and detailed reviews have described the implications of nAChRs and their polymorphisms in lung tumor cell proliferation, apoptosis, angiogenesis, and invasion (Benowitz, 2009; Lambrechts et al., 2010; Saccone et al., 2010; Schuller, 2009; Spitz et al., 2008; Tournier and Birembaut, 2011). Interestingly, an association between smoking, D2R signaling, and the development of lung cancer exists as variant D2R genotypes have been linked to an increased likelihood to smoke, greater smoking intensity, and familial aggregation of smoking‐related cancers (Styn et al., 2009; Wu et al., 2000). In addition, the association between the variant D2R TaqIA genotype and smoking cessation suggests that genetic variation in the DA pathway influences smoking cessation (Styn et al., 2009). DA receptors are known to be expressed in alveolar epithelial cells and human lung tumors (Campa et al., 2007). We assessed D2R expression in lung tumor‐associated endothelial cells and found a positive correlation between smoking history and increased levels of endothelial D2R. Our findings are significant in at least three aspects: (1) given the high prevalence of lung cancer patients with a smoking history, D2R agonist treatment results may vary by smoking history; (2) D2R agonist treatment may result in smoking cessation; and more importantly, (3) helping smoking patients quit smoking may improve their treatment outcome and quality of life (Chen et al., 2010, 2012, 2004). While further studies are necessary, our data suggests D2R agonist therapies may be more effective in NSCLC patients with a smoking history compared to never smokers.
Our preclinical studies presented here suggest D2R agonists represent a promising individualized therapeutic strategy for NSCLC patients with high endothelial D2R expression and a smoking history. Cabergoline (Doxtinex™), one of the high affinity D2R agonists used in our murine lung cancer studies, has been FDA approved for the treatment of hyperprolactinemia and advanced Parkinson's disease. A significant advantage of using cabergoline to treat lung cancer is avoidance of direct inhibition of VEGF pathways or VEGF itself, while achieving similar inhibition of tumor angiogenesis. Therefore, we can bypass the adverse effects of anti‐angiogenesis therapy, as well as anti‐angiogenesis resistance of the tumor microenvironment. In view of recent data reporting the challenges of combined chemotherapy and anti‐angiogenesis regimens (Ellis and Al‐Saleh, 2012), it will be of great interest to determine whether D2R agonists have value as therapies for lung cancer, in which angiogenesis has important pathogenic roles. The mechanisms of anti‐VEGF therapies remain under investigation. Jain and colleagues proposed that anti‐angiogenesis drugs “normalize” the tumor vasculature and temporarily improve blood flow within the tumor, thereby enhancing the efficacy of chemotherapy (Jain, 2005). Here we demonstrate that D2R agonists inhibit lung tumor growth by reducing angiogenesis and tumor infiltrating immune cells. Our findings suggest cabergoline as a lung cancer therapeutic strategy and future studies are necessary to determine its efficacy in NSCLC patients in combination with standard chemotherapy regimens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed by the authors.
Supporting information
The following are the supplementary data related to this article:
Supplemental Figure 1Lung cancer cell lines express D2R, but D2R agonists do not affect their proliferation. A: Immunoblotting of whole cell lysates from human umbilical vein endothelial cells (HUVEC) (positive control), human lung cancer cell lines, and murine LLC1 lung cancer cells was performed using a D2R antibody. B: The indicated serum‐starved lung cancer cells were seeded into 96 well plates at a density of 5000 cells/well, treated with vehicle, 10 μM dopamine, or 10 μM quinpirole, incubated for 24 h, and MTT proliferation assays were performed.
Supplemental Figure 2Cabergoline decreases LLC1 tumor progression in a dose‐dependent manner. C57BL/6 mice were orthotopically injected with 1 × 105 luciferase‐labeled murine LLC1 cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged four days post‐injection of LLC1 cells. Mice received daily intraperitoneal injections of 0 mg/kg (PBS vehicle alone), 0.5 mg/kg, 1 mg/kg or 5 mg/kg cabergoline for seven days. Mice were xenogen imaged following treatment.
Supplemental Figure 3D2R agonist treatment decreases maturation of human myeloid cells. CD14+ monocytes were isolated from the whole blood of healthy human donors and cultured in GM‐CSF (2800 U/ml) and IL‐4 (1000 U/ml) to stimulate the formation of immature myeloid cells. Cells were either harvested after 3 days or administered TNF‐alpha (1100 U/ml) and PGE2 (1 μg/ml) and cultured for an additional 2 days to obtain mature myeloid cells. Cells were treated daily with 10 μM cabergoline or vehicle throughout the duration of the experiment. Flow cytometry was performed using the indicated antibodies. Red: Vehicle treated, Green: Cabergoline.
Acknowledgments
The authors thank the Mayo Clinic pathology research core facility for their assistance with this research. This work was partly supported by CA78383, CA150190, and HL70567 (to D. Mukhopadhyay). L.H. Hoeppner is a fellow of NCI‐T32 CA148073 and 13POST14510025 from the American Heart Association. Y. Wang is supported by 14POST20390029 from the American Heart Association.
Supplementary data 1.
1.1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.08.008.
Hoeppner Luke H., Wang Ying, Sharma Anil, Javeed Naureen, Van Keulen Virginia P., Wang Enfeng, Yang Ping, Roden Anja C., Peikert Tobias, Molina Julian R., Mukhopadhyay Debabrata, (2015), Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells, Molecular Oncology, 9, doi: 10.1016/j.molonc.2014.08.008.
References
- Alberg, A.J. , Shopland, D.R. , Cummings, K.M. , 2014. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am. J. Epidemiol. 179, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S. , Nagy, J.A. , Pal, S. , Vasile, E. , Eckelhoefer, I.A. , Bliss, V.S. , Manseau, E.J. , Dasgupta, P.S. , Dvorak, H.F. , Mukhopadhyay, D. , 2001. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569–574. [DOI] [PubMed] [Google Scholar]
- Basu, S. , Sarkar, C. , Chakroborty, D. , Nagy, J. , Mitra, R.B. , Dasgupta, P.S. , Mukhopadhyay, D. , 2004. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 64, 5551–5555. [DOI] [PubMed] [Google Scholar]
- Benowitz, N.L. , 2009. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 49, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandarkar, S.S. , Jaconi, M. , Fried, L.E. , Bonner, M.Y. , Lefkove, B. , Govindarajan, B. , Perry, B.N. , Parhar, R. , Mackelfresh, J. , Sohn, A. , Stouffs, M. , Knaus, U. , Yancopoulos, G. , Reiss, Y. , Benest, A.V. , Augustin, H.G. , Arbiser, J.L. , 2009. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J. Clin. Invest. 119, 2359–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, R. , Sinha, S. , Yang, S.P. , Patra, C. , Dutta, S. , Wang, E. , Mukhopadhyay, D. , 2008. The neurotransmitter dopamine modulates vascular permeability in the endothelium. J. Mol. Signal. 3, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, D.M. , 2003. Tobacco-related diseases. Semin. Oncol. Nurs. 19, 244–249. [DOI] [PubMed] [Google Scholar]
- Campa, D. , Zienolddiny, S. , Lind, H. , Ryberg, D. , Skaug, V. , Canzian, F. , Haugen, A. , 2007. Polymorphisms of dopamine receptor/transporter genes and risk of non-small cell lung cancer. Lung Cancer. 56, 17–23. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. , Ferreira, V. , Breier, G. , Pollefeyt, S. , Kieckens, L. , Gertsenstein, M. , Fahrig, M. , Vandenhoeck, A. , Harpal, K. , Eberhardt, C. , Declercq, C. , Pawling, J. , Moons, L. , Collen, D. , Risau, W. , Nagy, A. , 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380, 435–439. [DOI] [PubMed] [Google Scholar]
- Cetin, K. , Ettinger, D.S. , Hei, Y.J. , O'Malley, C.D. , 2011. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the surveillance, epidemiology and end results program. Clin. Epidemiol. 3, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty, D. , Chowdhury, U.R. , Sarkar, C. , Baral, R. , Dasgupta, P.S. , Basu, S. , 2008. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J. Clin. Invest. 118, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Jiang, R. , Garces, Y.I. , Jatoi, A. , Stoddard, S.M. , Sun, Z. , Marks, R.S. , Liu, Y. , Yang, P. , 2010. Prognostic factors for limited-stage small cell lung cancer: a study of 284 patients. Lung Cancer. 67, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Qi, Y. , Wampfler, J.A. , Jatoi, A. , Garces, Y.I. , Busta, A.J. , Mandrekar, S.J. , Yang, P. , 2012. Effect of cigarette smoking on quality of life in small cell lung cancer patients. Eur. J. Cancer. 48, 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo, C.A. , Cotter, M.J. , Cheng, P. , Cheng, F. , Kusmartsev, S. , Sotomayor, E. , Padhya, T. , McCaffrey, T.V. , McCaffrey, J.C. , Gabrilovich, D.I. , 2009. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, P.M. , Al-Saleh, K. , 2012. Multitargeted anti-angiogenic agents and NSCLC: clinical update and future directions. Crit. Rev. Oncol. Hematol. 84, 47–58. [DOI] [PubMed] [Google Scholar]
- Ferrara, N. , Carver-Moore, K. , Chen, H. , Dowd, M. , Lu, L. , O'Shea, K.S. , Powell-Braxton, L. , Hillan, K.J. , Moore, M.W. , 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380, 439–442. [DOI] [PubMed] [Google Scholar]
- Garces, Y.I. , Yang, P. , Parkinson, J. , Zhao, X. , Wampfler, J.A. , Ebbert, J.O. , Sloan, J.A. , 2004. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 126, 1733–1741. [DOI] [PubMed] [Google Scholar]
- Hecht, S.S. , 1999. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 91, 1194–1210. [DOI] [PubMed] [Google Scholar]
- Heeschen, C. , Weis, M. , Aicher, A. , Dimmeler, S. , Cooke, J.P. , 2002. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Invest. 110, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, R.K. , 2005. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 307, 58–62. [DOI] [PubMed] [Google Scholar]
- Johnson, D.H. , Fehrenbacher, L. , Novotny, W.F. , Herbst, R.S. , Nemunaitis, J.J. , Jablons, D.M. , Langer, C.J. , DeVore, R.F. , Gaudreault, J. , Damico, L.A. , Holmgren, E. , Kabbinavar, F. , 2004. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 22, 2184–2191. [DOI] [PubMed] [Google Scholar]
- Kaner, R.J. , Ladetto, J.V. , Singh, R. , Fukuda, N. , Matthay, M.A. , Crystal, R.G. , 2000. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22, 657–664. [DOI] [PubMed] [Google Scholar]
- Kazi, A.S. , Lotfi, S. , Goncharova, E.A. , Tliba, O. , Amrani, Y. , Krymskaya, V.P. , Lazaar, A.L. , 2004. Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am. J. Physiol. Lung Cell Mol. Physiol. 286, L539–L545. [DOI] [PubMed] [Google Scholar]
- Korpanty, G. , Smyth, E. , Sullivan, L.A. , Brekken, R.A. , Carney, D.N. , 2010. Antiangiogenic therapy in lung cancer: focus on vascular endothelial growth factor pathway. Exp. Biol. Med. (Maywood). 235, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm, T. , Crisostomo, P.R. , Markel, T.A. , Wang, M. , Lillemoe, K.D. , Meldrum, D.R. , 2007. The critical role of vascular endothelial growth factor in pulmonary vascular remodeling after lung injury. Shock. 28, 4–14. [DOI] [PubMed] [Google Scholar]
- Lambrechts, D. , Buysschaert, I. , Zanen, P. , Coolen, J. , Lays, N. , Cuppens, H. , Groen, H.J. , Dewever, W. , van Klaveren, R.J. , Verschakelen, J. , Wijmenga, C. , Postma, D.S. , Decramer, M. , Janssens, W. , 2010. The 15q24/25 susceptibility variant for lung cancer and chronic obstructive pulmonary disease is associated with emphysema. Am. J. Respir. Crit. Care Med. 181, 486–493. [DOI] [PubMed] [Google Scholar]
- Leung, D.W. , Cachianes, G. , Kuang, W.J. , Goeddel, D.V. , Ferrara, N. , 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 246, 1306–1309. [DOI] [PubMed] [Google Scholar]
- Lynch, T.J. , Adjei, A.A. , Bunn, P.A. , Eisen, T.G. , Engelman, J. , Goss, G.D. , Haber, D.A. , Heymach, J.V. , Janne, P.A. , Johnson, B.E. , Johnson, D.H. , Lilenbaum, R.C. , Meyerson, M. , Sandler, A.B. , Sequist, L.V. , Settleman, J. , Wong, K.K. , Hart, C.S. , 2006. Summary statement: novel agents in the treatment of lung cancer: advances in epidermal growth factor receptor-targeted agents. Clin. Cancer Res. 12, 4365s–4371s. [DOI] [PubMed] [Google Scholar]
- Lynch, T.J. , Bondarenko, I. , Luft, A. , Serwatowski, P. , Barlesi, F. , Chacko, R. , Sebastian, M. , Neal, J. , Lu, H. , Cuillerot, J.M. , Reck, M. , 2012. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 30, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Nasevicius, A. , Larson, J. , Ekker, S.C. , 2000. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 17, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale, R.B. , 2008. Dual targeting of the vascular endothelial growth factor receptor and epidermal growth factor receptor pathways with vandetinib (ZD6474) in patients with advanced or metastatic non-small cell lung cancer. J. Thorac. Oncol. 3, S128–S130. [DOI] [PubMed] [Google Scholar]
- Perry, B.N. , Govindarajan, B. , Bhandarkar, S.S. , Knaus, U.G. , Valo, M. , Sturk, C. , Carrillo, C.O. , Sohn, A. , Cerimele, F. , Dumont, D. , Losken, A. , Williams, J. , Brown, L.F. , Tan, X. , Ioffe, E. , Yancopoulos, G.D. , Arbiser, J.L. , 2006. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J. Invest. Dermatol. 126, 2316–2322. [DOI] [PubMed] [Google Scholar]
- Pusztaszeri, M.P. , Seelentag, W. , Bosman, F.T. , 2006. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 54, 385–395. [DOI] [PubMed] [Google Scholar]
- Saccone, N.L. , Culverhouse, R.C. , Schwantes-An, T.H. , Cannon, D.S. , Chen, X. , Cichon, S. , Giegling, I. , Han, S. , Han, Y. , Keskitalo-Vuokko, K. , Kong, X. , Landi, M.T. , Ma, J.Z. , Short, S.E. , Stephens, S.H. , Stevens, V.L. , Sun, L. , Wang, Y. , Wenzlaff, A.S. , Aggen, S.H. , Breslau, N. , Broderick, P. , Chatterjee, N. , Chen, J. , Heath, A.C. , Heliovaara, M. , Hoft, N.R. , Hunter, D.J. , Jensen, M.K. , Martin, N.G. , Montgomery, G.W. , Niu, T. , Payne, T.J. , Peltonen, L. , Pergadia, M.L. , Rice, J.P. , Sherva, R. , Spitz, M.R. , Sun, J. , Wang, J.C. , Weiss, R.B. , Wheeler, W. , Witt, S.H. , Yang, B.Z. , Caporaso, N.E. , Ehringer, M.A. , Eisen, T. , Gapstur, S.M. , Gelernter, J. , Houlston, R. , Kaprio, J. , Kendler, K.S. , Kraft, P. , Leppert, M.F. , Li, M.D. , Madden, P.A. , Nothen, M.M. , Pillai, S. , Rietschel, M. , Rujescu, D. , Schwartz, A. , Amos, C.I. , Bierut, L.J. , 2010. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, B. , Mondal, A.C. , Basu, S. , Dasgupta, P.S. , 2001. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int. Immunopharmacol. 1, 1363–1374. [DOI] [PubMed] [Google Scholar]
- Sarkar, C. , Chakroborty, D. , Chowdhury, U.R. , Dasgupta, P.S. , Basu, S. , 2008. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 14, 2502–2510. [DOI] [PubMed] [Google Scholar]
- Schuller, H.M. , 2009. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors?. Nat. Rev. Cancer. 9, 195–205. [DOI] [PubMed] [Google Scholar]
- Senger, D.R. , Galli, S.J. , Dvorak, A.M. , Perruzzi, C.A. , Harvey, V.S. , Dvorak, H.F. , 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 219, 983–985. [DOI] [PubMed] [Google Scholar]
- Siegel, R. , Naishadham, D. , Jemal, A. , 2013. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30. [DOI] [PubMed] [Google Scholar]
- Sinha, S. , Vohra, P.K. , Bhattacharya, R. , Dutta, S. , Mukhopadhyay, D. , 2009. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J. Cell Sci. 122, 3385–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N.R. , Baker, D. , James, N.H. , Ratcliffe, K. , Jenkins, M. , Ashton, S.E. , Sproat, G. , Swann, R. , Gray, N. , Ryan, A. , Jurgensmeier, J.M. , Womack, C. , 2010. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 16, 3548–3561. [DOI] [PubMed] [Google Scholar]
- Spitz, M.R. , Amos, C.I. , Dong, Q. , Lin, J. , Wu, X. , 2008. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J. Natl. Cancer Inst. 100, 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styn, M.A. , Nukui, T. , Romkes, M. , Perkins, K. , Land, S.R. , Weissfeld, J.L. , 2009. The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilan, J. , Kitlinska, J. , 2010. Sympathetic neurotransmitters and tumor angiogenesis-link between stress and cancer progression. J. Oncol. 2010, 539706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian, S.L. , Hodi, F.S. , Brahmer, J.R. , Gettinger, S.N. , Smith, D.C. , McDermott, D.F. , Powderly, J.D. , Carvajal, R.D. , Sosman, J.A. , Atkins, M.B. , Leming, P.D. , Spigel, D.R. , Antonia, S.J. , Horn, L. , Drake, C.G. , Pardoll, D.M. , Chen, L. , Sharfman, W.H. , Anders, R.A. , Taube, J.M. , McMiller, T.L. , Xu, H. , Korman, A.J. , Jure-Kunkel, M. , Agrawal, S. , McDonald, D. , Kollia, G.D. , Gupta, A. , Wigginton, J.M. , Sznol, M. , 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, J.M. , Birembaut, P. , 2011. Nicotinic acetylcholine receptors and predisposition to lung cancer. Curr. Opin. Oncol. 23, 83–87. [DOI] [PubMed] [Google Scholar]
- Voelkel, N.F. , Vandivier, R.W. , Tuder, R.M. , 2006. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L209–L221. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Pereira, E.F. , Maus, A.D. , Ostlie, N.S. , Navaneetham, D. , Lei, S. , Albuquerque, E.X. , Conti-Fine, B.M. , 2001. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol. Pharmacol. 60, 1201–1209. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Hudmon, K.S. , Detry, M.A. , Chamberlain, R.M. , Spitz, M.R. , 2000. D2 dopamine receptor gene polymorphisms among African-Americans and Mexican-Americans: a lung cancer case-control study. Cancer Epidemiol. Biomarkers Prev. 9, 1021–1026. [PubMed] [Google Scholar]
- Yang, Y. , Zhang, Y. , Cuevas, S. , Villar, V.A. , Escano, C. , D.A, L. , Yu, P. , Grandy, D.K. , Felder, R.A. , Armando, I. , Jose, P.A. , 2012. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic. Biol. Med. 53, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroslinski, J.F. , Possley, L.H. , Schwartz, R.A. , Morris, R.N. , Carone, F.A. , Browne, R.K. , 1977. The pharmacology and subacute toxicology of dopamine. Proc. R. Soc. Med. 70, (Suppl. 2) 2–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplemental Figure 1Lung cancer cell lines express D2R, but D2R agonists do not affect their proliferation. A: Immunoblotting of whole cell lysates from human umbilical vein endothelial cells (HUVEC) (positive control), human lung cancer cell lines, and murine LLC1 lung cancer cells was performed using a D2R antibody. B: The indicated serum‐starved lung cancer cells were seeded into 96 well plates at a density of 5000 cells/well, treated with vehicle, 10 μM dopamine, or 10 μM quinpirole, incubated for 24 h, and MTT proliferation assays were performed.
Supplemental Figure 2Cabergoline decreases LLC1 tumor progression in a dose‐dependent manner. C57BL/6 mice were orthotopically injected with 1 × 105 luciferase‐labeled murine LLC1 cells suspended in 80 μl PBS and Matrigel. After establishment of the lung tumor, mice were xenogen imaged four days post‐injection of LLC1 cells. Mice received daily intraperitoneal injections of 0 mg/kg (PBS vehicle alone), 0.5 mg/kg, 1 mg/kg or 5 mg/kg cabergoline for seven days. Mice were xenogen imaged following treatment.
Supplemental Figure 3D2R agonist treatment decreases maturation of human myeloid cells. CD14+ monocytes were isolated from the whole blood of healthy human donors and cultured in GM‐CSF (2800 U/ml) and IL‐4 (1000 U/ml) to stimulate the formation of immature myeloid cells. Cells were either harvested after 3 days or administered TNF‐alpha (1100 U/ml) and PGE2 (1 μg/ml) and cultured for an additional 2 days to obtain mature myeloid cells. Cells were treated daily with 10 μM cabergoline or vehicle throughout the duration of the experiment. Flow cytometry was performed using the indicated antibodies. Red: Vehicle treated, Green: Cabergoline.


