Abstract
Background
Abnormal P-terminal force in V1 (PTFV1) is associated with an increased risk of heart failure, stroke, atrial fibrillation (AF) and death.
Objective
Our goal was to explore associations of left ventricular (LV) diffuse fibrosis with left atrium (LA) function and ECG measures of LA electrical activity.
Methods
AF-free patients (n=91, mean age 59.5, 61.5% men, 65.9% Caucasian) with structural heart disease (wide spatial QRS-T angle≥105° ± Selvester QRS score≥5 on ECG) but LV ejection fraction >35% underwent clinical evaluation, cardiac magnetic resonance and resting ECG. LA function indices were obtained by multimodality tissue tracking using 2 and 4-chamber long-axis images. T1 mapping and late gadolinium enhancement were used to assess diffuse LV fibrosis and presence of scar. P-prime in V1 amplitude (PPaV1) and duration (PPdV1), averaged P-duration, PR interval and P-axis were automatically measured using 12SL TM algorithm. PTFV1 was calculated as product of PPaV1 by PPdV1.
Results
In linear regression after adjustment for demographic, body mass index, LA volumemax index, presence of scar and LV mass index, each decile increase in LV interstitial fibrosis was associated with 0.76mV*ms increase in negative abnormal PTFV1 [(95%CI −1.42 to −0.09), P=0.025], 15.3ms prolongation in PPdV1 [(95%CI 6.9 to 23.8), P=0.001], and 5.4ms widening in averaged P-duration [(95%CI 0.9 to 10.0), P=0.020]. LV fibrosis did not affect LA function. PPaV1 and PTFV1 were associated with an increase in LA volumes, decrease in LAEF and LA reservoir function.
Conclusion
LV interstitial fibrosis is associated with abnormal PTFV1, prolonged PPdV1 and P-duration, but does not affect LA function.
Keywords: P-terminal force in V1, left atrium function, fibrosis, left ventricle, atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the United States where the prevalence in adults < 65 years of age is about 1% to 2%; it increases up to 9% in elderly1, 2. AF is associated with a two-three-fold risk of cardiovascular mortality and sudden cardiac death3, 4, five-fold risk of stroke5, and a three-fold risk of heart failure (HF)6. Selection of a high-risk subgroup of patients amongst overall low-risk individuals early in the continuum of structural heart disease is needed for successful primary prevention of AF.
Common risk factors of AF (hypertension, coronary heart disease, HF, diabetes) are well acknowledged7, 8. In response to hemodynamic stress or injury, atria undergo structural remodeling with progressive fibrosis9. Cardiac magnetic resonance (CMR) imaging with T1 mapping is a gold standard for quantification of interstitial fibrosis10. However, CMR is not a method of choice for screening of a large population given its complexity, limited accessibility and cost.
ECG carries important information about electrophysiological properties of the heart. P-wave characteristics reflect underlying atrial electrophysiology, as well as structure and function. Prolonged P-wave duration and abnormal P-axis11 are associated with increased risk of AF12, stroke and cardiovascular disease mortality13. Abnormal P-wave terminal force in V1 (PTFV1)14 is associated with increased risk of AF and stroke,15,16 HF and post- MI death17. In a large sample of the adult population in the United States, deep terminal negativity of P-prime in V1 was shown independently associated with increased risk of death due to all-cause, cardiovascular, and ischemic heart disease mortality18. However, while epidemiological data demonstrated a strong association between P-terminal force, deep terminal negativity in lead V1 and mortality, mechanisms behind such association in the absence of left atrial (LA) enlargement remained elusive.
In the Personalized Risk Identification and Management for Arrhythmias and Heart Failure by ECG and CMR (PRIMERI) study19–21, both ECG and CMR were acquired within the same day. We hypothesized that LV interstitial fibrosis is associated with altered atrial electrophysiology and function in PRIMERI study participants.
Methods
Study population
The PRIMERI study is an ongoing prospective observational cohort study19–21 of the Johns Hopkins Hospital patients with ECG signs of structural heart disease. The study protocol was approved by the Johns Hopkins Hospital Institutional Review Board. All study participants signed informed consent upon entering the study. PRIMERI study inclusion criteria were: general hospital population with 12-lead ECG criteria (spatial QRS-T angle22 ≥105° and/or the Selvester QRS score23 ≥5). Exclusion criteria were: age >70 years, LVEF <35%, eGFR<45mL/min, implanted ICD or pacemaker, concomitant non-cardiac diseases with high risk of non-cardiac death within 3 years of follow-up, and other general contraindication to CMR, or claustrophobia. Enrolled patients underwent a comprehensive clinical evaluation, routine 12-lead ECG, and CMR study. PRIMERI participants in sinus rhythm were included in this study, and those with AF on 12-lead ECG (recorded at enrollment) were excluded.
ECG data acquisition and analysis of P-wave indices
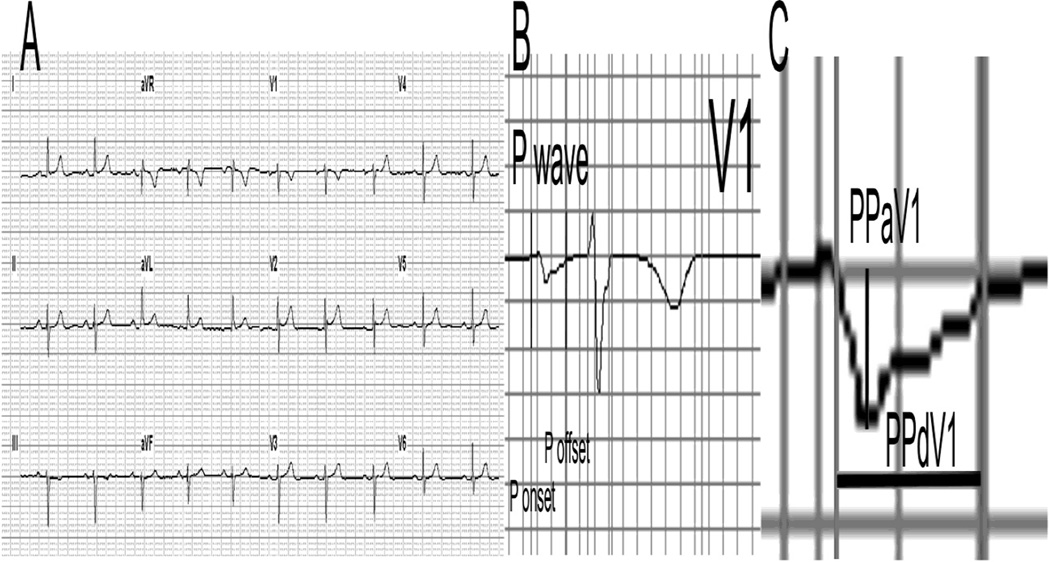
A standard 12-lead ECG was recorded at rest on the same day of the CMR by the Marquette MAC 5000 ECG system with 12SL TM algorithm (GE Medical Systems, Milwaukee, WI) and automatically analyzed by Magellan ECG Research Workstation (Magellan ECG Research Workstation Software, GE Healthcare, Wauwatosa, WI, USA). P-wave duration and PR interval were measured on an averaged median beat (averaged across all recorded beats and all 12 leads). Amplitude (PPaV1) and duration (PPdV1) of a second P-wave deflection (P-prime) in lead V1 were measured (Figure 1) on the median beat (averaged across all recorded beats in V1). PTFV1 was calculated as a product of the duration by the amplitude of the P-prime in lead V1.
Figure 1.
Measurement of P-prime amplitude (PPaV1) and P-prime duration (PPdV1) in V1 on a median beat. A. 12-lead ECG; B. Lead V1 PQRST waveform with marked P onset and offset; C. P-wave in V1 with marked PPaV1 and PPdV1. PPaV1 = −58 µV; PPdV1 = 88 ms.
Cardiac Magnetic Resonance Study Protocol
Enrolled participants underwent CMR using 1.5T whole body MRI scanners (Avanto; Siemens Medical Systems, Erlangen, Germany) on the same day as the ECG recordings.
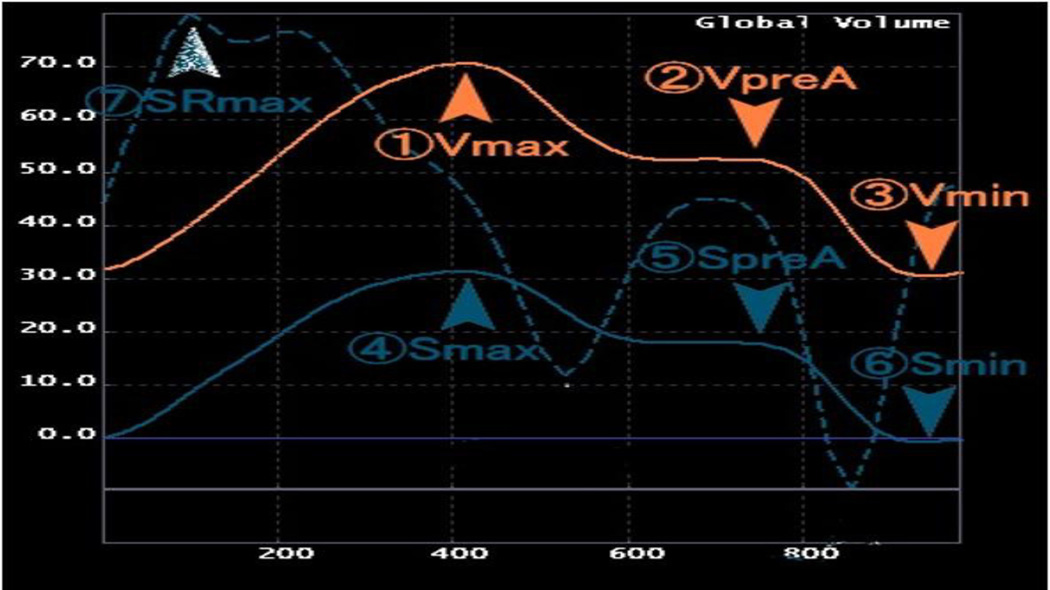
Maximum and minimum LA volume (Vmax and Vmin), LA volume before atrial systole (VpreA), maximum LA strain (Smax), and strain rate (SRmax) (Figure 2) were obtained as relevant LA parameters using a tissue tracking method with semi-automated software (multimodality tissue tracking (MTT) version 5.0, Toshiba, Japan) in untagged long-axis 2-chamber and 4– chamber cine CMR images (Figure 3). Vmax was indexed by body surface area (LAVimax).
Figure 2.
Left atrial parameters: Definitions of parameters from volume (orange), strain (solid blue) and strain rate curves (dotted blue). Vmax=Maximum LA volume, VpreA= LA volume before atrial systole, Vmin=Minimum LAvolume, Smax=Maximum LA strain, SpreA=LA strain before atrial systole, Smin=Minimum LA Strain, SRmax=Maximum LA strain rate.
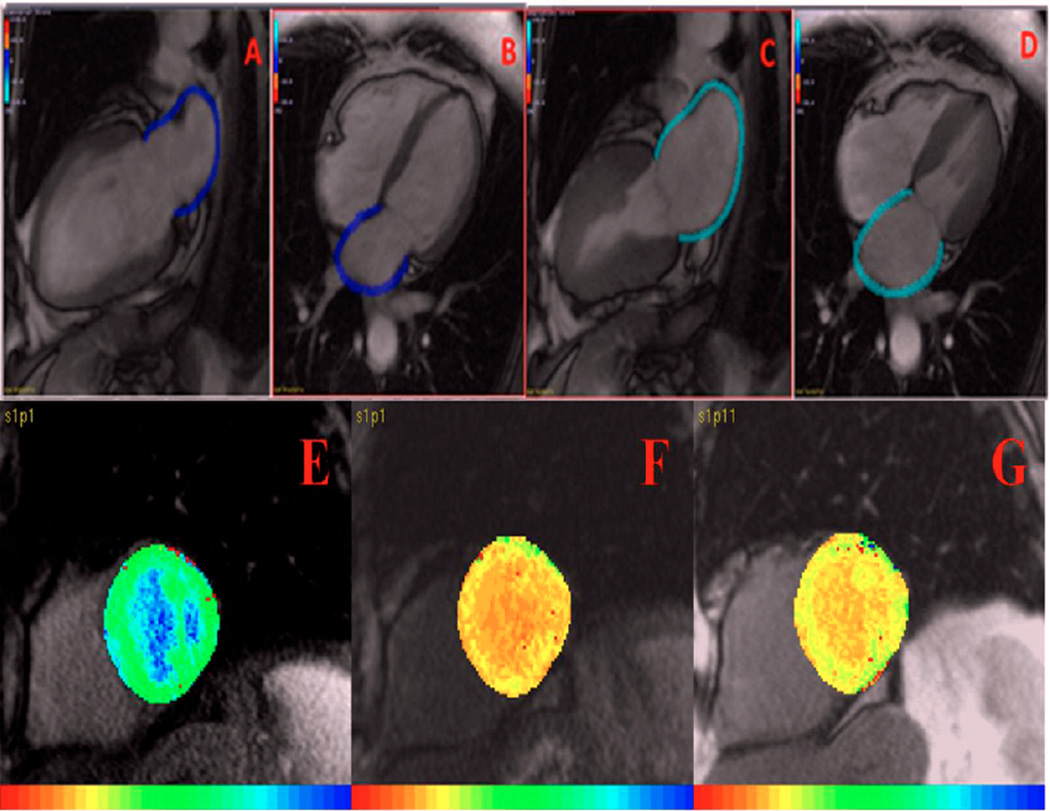
Figure 3.
(A–C) Cardiac Magnetic Resonance multimodality tissue tracking. A: end-diastolic 2 chamber view; B: end-diastolic 4-chamber view; C: end-systolic 2-chamber view; D: end-systolic 4 chamber view. (E–G) Cardiac Magnetic Resonance T1 Mapping. E: Pre-contrast; F: 12 minutes post contrast; G: 25 minutes post contrast showing reduction in T1 time in the presence of diffuse interstitial myocardial fibrosis.
T1 mapping was used to assess diffuse LV fibrosis. One short-axis image in the mid-ventricular level was acquired by modified look-locker inversion recovery24 at three phases; pre-contrast, 12-minute post-contrast, and 25-minute post-contrast using the MASS research software (Medis, Leiden University Medical Center, Leiden, Netherlands) (Figure 3). LV fibrosis index was calculated as following:
Fibrosis index = slope {R1myocardium (pre, post-12min, post-25 min) / R1blood (pre, post-12min, post-25 min25, 26)}. Detailed CMR protocol is provided in Supplemental Methods.
Late gadolinium enhancement CMR (LGE-CMR) was used to detect the presence of regional scar replacement. The images were analyzed using QMass (Medis, The Netherlands). The region of interest for myocardium was manually placed on short-axis slices, and the scar replacement area was then detected as the area with increased intensity manually by the user for each slice.
Statistical Analysis
STATA 13 (StataCorp LP, College Station, TX) was used for statistical analysis. Continuous variables are expressed as mean ± SD after verification of the normal distribution. Categorical data were summarized as frequencies and percentages. The association of LV interstitial fibrosis with P-wave indices (PTFV1, P-duration, P-axis, PR interval) was estimated using general linear models with each of P-wave indices as dependent variables. LV fibrosis index was included in the models as a continuous variable. The difference in P-wave indices associated with increasing LV fibrosis was estimated adjusting for age, race, sex and body mass index (BMI) (Model 1), due to previously reported demographics (age, race, and sex) specificity of P-wave indices27, and their association with BMI28. Model 2 adjusted for all variables from model 1 and included LAVimax. Model 3 adjusted for all variables from model 2 and included LV mass index and the presence of scar on LGE-CMR. Sensitivity analysis was performed after exclusion of participants with LVEF ≤45% (n=3). In addition, statistically significant models were further adjusted by left ventricular ejection fraction (LVEF), and history of myocardial infarction. We examined the possibility of a nonlinear relationship between LV fibrosis and the P-wave indices using restricted cubic splines of LV fibrosis index. All cubic spline analyses were adjusted for demographics, BMI, LAVimax, the presence of LGE-CMR scar and LV mass index.
The associations of P-wave indices (PTFV1, P-duration, P-axis, PR interval) with LA size and function were estimated using general linear models with each of LA function parameters (LA volume index, Smax, SRmax, LAEF) as dependent variables, and each of P-wave indices as predictors. Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for demographics and BMI.
Analysis of associations of LV fibrosis with LA function parameters was performed. Model 1 was adjusted for age, race, sex and BMI. Model 2 included variables from Model 1 and the presence of LGE-CMR scar and LV mass index.
Results
Study population: Comparison of participants with and without atrial fibrillation
The clinical characteristics of participants are reported in Table 1. Less than 20% had a history of MI, less than third patients had NYHA HF class ≥ II. At the same time, risk factors profile was unfavorable in most of the study participants: up to 75% have had hypertension; more than 50% were obese. Systolic blood pressure was not adequately controlled in the study population. LA function and P-wave ECG parameters were normal.
Table 1.
Characteristics of study participants
| n=91 | |
|---|---|
| Age(SD),y | 59.5(9.4) |
| Men,n(%) | 56(61.5) |
| Whites,n(%) | 60(65.9) |
| Body mass index,kg/m2 | 30.5(6.9) |
| Hypertension,n(%) | 65(71.4) |
| Diabetes,n(%) | 24(26.4) |
| Current or former smoker,n(%) | 46(50.6) |
| History of MI,n(%) | 14(15.4) |
| History of PCI/CABG,n(%) | 24(26.4) |
| NYHA class≥II,n(%) | 22(24.2) |
| Valvular Heart Diseases (mild),n(%) | 7(13) |
| Systolic BP(SD),mmHg | 144.3(26.2) |
| Diastolic BP(SD),mmHg | 85.1(16.4) |
| eGFR(SD),ml/min/1.73m2 | 90.5(2.6) |
| Beta-blockers,n(%) | 33(36.3) |
| ACE-I or ARBs,n(%) | 36(39.6) |
| Ca-channel blockers,n(%) | 16(17.6) |
| Statins,n(%) | 51(56.0) |
| LA and LV structure and function parameters | |
| LA Volume index max(SD),ml/m2 | 34.8(11.0) |
| LA Volume index min(SD),ml/m2 | 16.7(7.0) |
| Global LAEF,ml/min | 52.9(9.0) |
| LA conduit function(Passive EF) | 28.0(9.0) |
| LA booster pump function(Active EF) | 34.0(14.5) |
| LA reservoir function | 122.3(56.5) |
| Max LA strain(SD), | 26.4(8.6) |
| Max LA strain rate(SD), | 1.18(0.42) |
| LV ejection fraction(SD),% | 60.64(8.6) |
| LV mass index(SD),g/m2 | 64.4(16.7) |
| LV Fibrosis index | 0.51(0.07) |
| ECG parameters | |
| Heart rate(SD),bpm | 65.0(10.7) |
| QRS duration(SD),ms | 104.7(23.5) |
| P-duration(SD),ms | 111.9(13.7) |
| P-axis(SD),deg | 46.8(33.3) |
| PR interval(SD),ms | 181.3(43.1) |
| PTFV1(SD),mV*ms | −3.3(1.9) |
| P-prime amplitude in V1(SD),µV | −46.3(25.1) |
| P-prime duration in V1,ms | 62.7(26.1) |
Myocardial interstitial fibrosis index and P-wave indices
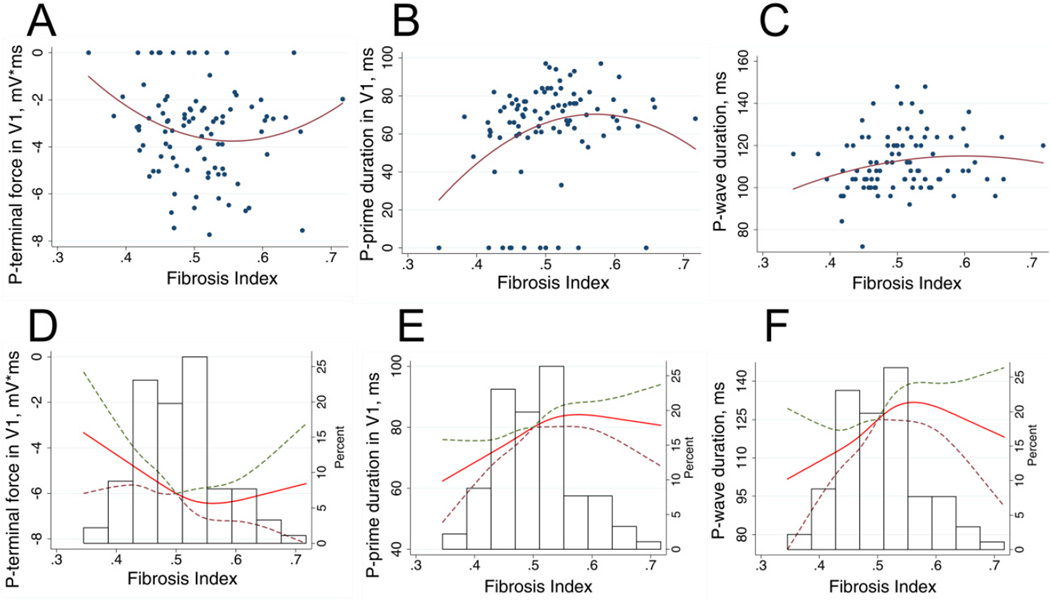
Increasing diffuse LV fibrosis was borderline associated with more abnormal PTFV1 in unadjusted analysis (Table 2). After adjustment for LAVimax, presence of LGE-CMR scar and LV mass index, association between LV fibrosis and PTFV1 strengthened and reached statistical significance: increase in LV fibrosis by each decile of fibrosis index was associated with 0.76 mV*ms increase in negative abnormal PTFV1. LV fibrosis predominantly affected PPdV1, rather than PPaV1. In a fully adjusted model increase in LV fibrosis by each decile of fibrosis index was associated with 15.3 ms prolongation of PPdV1. LV fibrosis impacted averaged P-duration to a lesser degree: increase in the decile of fibrosis index was associated with P-duration prolongation only by 5.4 ms. Association of LV fibrosis with PPdV1, PTFV1, and P-duration was non-linear (Figure 4), with the strongest association in mild and moderate fibrosis. Additional adjustment for LVEF, presence of LGE-CMR scar and history of MI did not attenuate the association between LV fibrosis and PPdV1/PTFV1, or P-duration. Increase in LV fibrosis by each decile of fibrosis index was associated with 0.74 mV*ms increase in negative abnormal PTFV1 [(95%CI from −1.42 to −0.07); P=0.030]. Increase in LV fibrosis by the decile of LV fibrosis index was also associated with 14.7 ms prolongation in PPdV1 [(95%CI 6.2 to 23.2 ms); P=0.001], and 5.2 ms widening in P-duration [(95%CI 0.55 to 9.77 ms); P=0.029]. Neither PR interval nor P-axis was associated with LV fibrosis index. After excluding the participants with LVEF ≤ 45% (n=3), all the previously significant associations became stronger (Supplemental Table 1). LGE-CMR LV scar did not associate with P-wave parameters. LV compliance (assessed by early diastolic global circumferential strain rate) did not associate with P-wave characteristics (data not shown). Adequately adjusted effect of LV interstitial fibrosis on QRS duration was weaker [+2.7 (95%CI −2.4 − 7.9 ms); P=0.298] than on P-wave.
Table 2.
Difference (95%CI) in P-wave indices by LV fibrosis index deciles
| Model | Per 1 decile of LV fibrosis index | P | |
|---|---|---|---|
| PTFV1, mV*ms | 1 | −0.57(−1.22 to +0.08) | 0.086 |
| 2 | −0.62(−1.23 to +0.03) | 0.061 | |
| 3 | −0.76(−1.42 to −0.09) | 0.025 | |
| P-prime amplitude in V1,µV | 1 | −4.86(−13.46 to +3.73) | 0.264 |
| 2 | −5.68(−14.21 to +2.85) | 0.189 | |
| 3 | −7.12(−15.90 to +1.66) | 0.110 | |
| P-prime duration in V1,ms | 1 | +12.34(+3.91 to +20.77) | 0.005 |
| 2 | +14.39(+6.05 to +22.71) | 0.001 | |
| 3 | +15.34(+6.88 to +23.79) | 0.001 | |
| PR interval,ms | 1 | −1.26(−15.29 to +12.78) | 0.859 |
| 2 | −5.39(−19.05 to +8.28) | 0.435 | |
| 3 | −5.79(−19.39 to +7.79) | 0.398 | |
| Average P-duration,ms | 1 | +4.76(+0.30 to +9.22) | 0.0373 |
| 2 | +5.06(+0.65 to +9.46) | 0.0253 | |
| 3 | +5.42(+0.87 to +9.97) | 0.020 | |
| P-axis,deg | 1 | +6.37(−4.87 to +17.62) | 0.263 |
| 2 | −1.00(−8.19 to +6.19) | 0.782 | |
| 3 | +1.39(−6.21 to +8.99) | 0.716 |
General linear models. Model1 is adjusted for age, race, sex, and body mass index. Model 2 includes variables from Model 1 and LA Volumemax index. Model 3 includes variables from Model 2, and scar and LV mass index.
Figure 4.
Unadjusted (A–C) and adjusted (D-F) associations of P-wave indices and fibrosis. Scatterplots of (A) P-terminal force in V1, (B) P-prime duration in V1, (C) average P-wave duration (Y) against fibrosis index (X). Quadratic best fit line is shown. Cubic restricted splines depicting the association of (D) P-terminal force in V1, (E) P-prime duration in V1, (F) average P-wave duration and fibrosis index, adjusted by age, sex, race, body mass index, LA volumemax index and LV mass index. Dashed lines represent 95% confidence intervals surrounding the estimate.
LA function and P-wave indices
Deepening of PPaV1 and more abnormal PTFV1 was associated with an increase in LA volumes, decrease in LAEF, and LA reservoir function (Table 3). Deepening of PPaV1 by 0.1 mV was associated with a decrease in LA strain by 7.5 units. While prolongation of PR interval was associated with LA function impairment, effect size was relatively small. P-duration weakly associated with LA minimum volume index. P-axis did not associate with LA function parameters. LV interstitial fibrosis did not affect LA function parameters (Supplemental Table 2).
Table 3.
Difference (9 5% CI) in LA structure and function indices by ECG P-wave indices participants
| Per 1 mV*ms of PTFV1 |
P | Per 0.1 mV of Pprime V1 amp |
P | Per 1ms of Pprime V1 dur |
P | Per 10 ms of PR interval |
P | Per 10 ms of P-duration |
P | Per 10 degrees of P-axis |
P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA Volume index max,ml/m2 | −1.6(−2.8 to −0.4) | 0.010 | −11.9(−21.2 to −2.57) | 0.013 | +0.07(− 0.03 to +0.16) | 0.155 | +0.9(+0.4 to +1.5) | 0.001 | +1.6(−0.3 to +3.4) | 0.093 | −0.1(− 1.3 to +1.1) | 0.902 |
| LA Volume index min,ml/m2 | −1.2(−1.9 to −0.4) | 0.003 | −8.8(−14.7 to −2.9) | 0.004 | +0.04(− 0.02 to +0.10) | 0.153 | +0.7(+0.3 to +1.0) | <0.0001 | +1.2 (+0.01 to +2.3) | 0.049 | −0.3(− 1.0 to +0.5) | 0.495 |
| Global LAEF,% | +1.1(+0.1 to +2.1) | 0.026 | +8.4(+0.9 to +15.9) | 0.029 | −0.03(− 0.10 to +0.05) | 0.515 | −0.5(−0.9 to − 0.004) | 0.048 | −0.9 (− 2.4 to +0.6) | 0.236 | +0.5(− 0.5 to +1.4) | 0.318 |
| LA reservoir function,% | +7.2(+1.1 to +13.3) | 0.021 | +54.7(+7.8 to +101.5) | 0.023 | −0.28(− 0.75 to +0.20) | 0.253 | −2.6(−5.5 to +0.4) | 0.084 | −5.7(− 15.0 to +3.6) | 0.226 | +2.0(− 4.0 to +8.0) | 0.509 |
| LA conduit function,% | +0.8(−0.2 to +1.8) | 0.107 | +7.5(−0.1 to +15.2) | 0.053 | −0.06(− 0.14 to +0.01) | 0.090 | −0.3(−0.7 to +0.1) | 0.168 | −0.2(− 1.7 to +1.3) | 0.830 | +0.7(− 0.3 to +1.6) | 0.164 |
| LA booster pump function,% | +0.8(−0.9 to +2.4) | 0.378 | +3.5(−9.5 to +16.5) | 0.591 | +0.05(− 0.08 to +0.17) | 0.455 | −0.3(−0.1 to +0.5) | 0.482 | −1.4(− 3.8 to +l.l) | 0.277 | −0.3(− 1.9 to +1.3) | 0.717 |
| LA Strain | +0.9(−0.1 to +1.8) | 0.064 | +7.5(+0.4 to +14.5) | 0.037 | −0.05(− 0.12 to +0.02) | 0.190 | −0.5(−0.9 to −0.1) | 0.027 | −0.8(− 2.2 to +0.6) | 0.259 | −0.6(− 1.5 to +0.3) | 0.167 |
| LA strain Rate | +0.04(− 0.01 to +0.08) | 0.129 | +0.3(−0.04 to +0.7) | 0.083 | −0.003(− 0.006 to +0.001) | 0.144 | −0.03(−0.05 to − 0.01) | 0.007 | −0.04(− 0.1 to +0.02) | 0.163 | −0.00(− 0.05 to +0.04) | 0.922 |
All models are general linear models. All models are adjusted for age, race, sex, and body mass index
Discussion
In this study of patients early in the continuum of structural heart disease, diffuse LV interstitial fibrosis was independently associated with abnormal PTFV1 after adjustment for demographics, BMI, LV mass index, and LV scar detected by LGE-CMR. Specifically, increased LV interstitial fibrosis (and hence potentially LA fibrosis29) resulted in gradual prolongation of PPdV1 and averaged P-duration. LV interstitial fibrosis did not affect LA function. This finding represents the first evidence of the association of LV interstitial fibrosis with interatrial conduction in patients without LA enlargement before impairment of LA function, supports previously reported association of LV fibrosis with LA fibrosis29, and is consistent with previous works30, 31 of the causative role of diffuse fibrosis for cardiac arrhythmias development. PPaV1 demonstrated the strong associations with LA function.
Hospital ECG database screening: the PRIMERI study population
While it is well recognized that primary prevention of cardiac arrhythmias strategy has to be developed, the best screening approach is yet unclear. The PRIMERI study utilized hospital ECG database screening, and enrolled patients with wide QRS-T angle, or high QRS score, who are known to be at risk for cardiac arrhythmias (both AF and ventricular arrhythmias)32, 22, 33, 34.
Effect of LV interstitial fibrosis on atrial electrophysiology and LA function
Fibrosis is categorized into distinct patterns: compact, patchy, interstitial, and diffuse35. Compact/patchy fibrosis is a replacement fibrosis10, quantified by LGE-CMR10. Among patients with AF, atrial fibrosis estimated by LGE-CMR is associated with AF recurrence post-ablation36. LV fibrosis was detected by LGE-CMR in 13% of AF patients, and strongly associated with mortality37. Unfortunately, numerous technical challenges reduce reproducibility of atrial LGE fibrosis quantification38. In this study compact/patchy LV fibrosis did not affect atrial electrophysiology.
Our observation showed that diffuse interstitial LV fibrosis (likely via associated LA fibrosis29) directly affects and impairs interatrial conduction, leading to characteristically abnormal PTFV1. It is known that individuals with PTFV1 in an upper 5th percentile have two-fold increased risk of incident AF15. Interestingly, an association with P-wave measures was observed in the presence of mild-moderate LV fibrosis, but not severe diffuse LV fibrosis which could be explained by the different nature of mild-moderate vs. severe LV fibrosis. Potentially different causes (besides collagen accumulation) could be associated with extreme expansion of extracellular matrix as measured by the high fibrosis index26. Our study is the first study, which quantified diffuse interstitial fibrosis in association with atrial electrophysiology. Further studies of diffuse interstitial fibrosis in patients at risk of cardiac arrhythmias are needed. Interestingly, in this study LV interstitial fibrosis impacted P-wave to a greater degree, as compared to QRS duration. This fact suggests that P-prime in V1 better than QRS duration reflects extend of interstitial fibrosis. Indeed, electrical excitation in atria propagates largely cell-to-cell, whereas QRS duration primarily reflects conduction thru His-Purkinje system, rather than thru LV myocardium, especially in patients early in the continuum of structural heart disease.
Associations between P-wave indices and LA function
LA size is a known predictor of morbidity and mortality. LA volume index predicts survival post-MI39. The present study also showed that PTFV1 and especially PPaV1 can be used as a tool to predict LA size and function. The larger the LA size, the deeper the terminal negativity of P-wave in lead V1, and the larger the PTFV1. Traditionally, LA size and function was characterized by LAVimax. Results of our study are consistent with previously reported studies of P-wave indices association with LA volume40–42. Importantly, in addition, we demonstrated a significant association between negative PPaV1 and LA minimum volume index, global LAEF, and LA reservoir function. We also showed that the weaker the LA strain, the deeper the terminal negativity of P-wave in lead V1. Altogether these observations characterize PPaV1 as a marker of LA dysfunction, which, in turn, can signify the presence of LV diastolic dysfunction.
Out of all P-wave indices PPaV1 showed the strongest association with LA volume, strain, global LAEF and LA reservoir function. Mechanistically, the following sequence of events likely takes place. In consequence of diastolic LV dysfunction in the setting of structural heart disease, LV filling pressure and then LA pressure rise, which leads to increase the LA wall tension. When the LA is overloaded, the vector of P wave rotates to the left and posteriorly in the horizontal plane causing a prominent negative component of the P wave in V1.
Clinical importance and future directions
Our study uncovered underlying mechanisms of electrocardiographic P-wave indices presentation and further advanced our understanding of subclinical structural heart disease. Interstitial fibrosis in LV and possibly in LA is associated with abnormal PTFV1, prolonged PPdV1 and P-duration, which could be used for screening of asymptomatic adults to identify individuals at risk, and serve as an intermediate outcome to track effect of future anti-fibrotic treatment.
At the same time, our study revealed strong association between LA function and PPaV1. We speculate that vasodilators (e.g. ACEI, ARBs), which can unload LV and decrease LA volume43, could possibly acutely affect PTFV1, predominantly PPaV1. Thus, PTFV1, and PPaV1 could be potentially helpful to guide therapy on the day-to-day basis. Future studies are needed to test this hypothesis.
Limitations
Our results must be interpreted in the face of certain methodological limitations. First, because LGE and T1 mapping were not performed in the LA in our study, we were unable to obtain measures of atrial fibrosis. However, association between LV and LA fibrosis was previously shown29. Second, we did not calculate extracellular volume because hematocrit was obtained only in half of our study population. Third, diastolic dysfunction was not the focus of this study. Despite these limitations, our study uncovered mechanisms of abnormal PTFV1 appearance, reflecting interplay between primary and secondary changes in LA electrophysiology. While prolonged PPdV1 reflects interstitial fibrosis in LV and potentially in LA, negative PPaV1 characterizes abnormal LA size and function. Abnormal PTFV1 encompasses both mechanisms.
Supplementary Material
Clinical Perspectives.
Our work for the first time showed that LV diffuse interstitial fibrosis (but not patchy LV scar) is associated with slowing of conduction through LA (measured as duration of P-prime in V1 and averaged P-wave duration) very early in the continuum of structural heart disease, before LA enlargement, impairment of LA function, systolic and diastolic LV function. We also confirmed that impairment of LA function and increase in LA size is associated with deepening of P-prime amplitude in V1, whereas P-terminal force in V1 encompasses both mechanisms.
ECG is an inexpensive and easily available tool, which could be used to identify individuals with interstitial LV fibrosis who have indications for CMR. Duration of P-prime in V1 could serve as an intermediate marker to monitor effect of anti-fibrotic therapy. Amplitude of P-prime in V1 could be used to monitor effect of medications on LA size and function.
Our study is the first cross-sectional observation of an association between LV interstitial fibrosis and duration of P-prime in V1. Therefore, validation of our findings in a prospective cohort study is needed. Then, knowledge about mechanisms behind P-prime in V1 presentation should be disseminated amongst electrophysiologists, cardiologists, primary care physicians and other medical professionals. Routine resting 12-lead ECG is easily available at any medical institution, and healthcare facility. Measurement of P-prime in V1 duration and amplitude could be performed manually with the use of ruler, or automatically by ECG software. Manufacturers of ECG equipment should be encouraged to routinely report P-prime in V1 parameters.
Acknowledgement
This research was supported in part by the National Institute of Health #P20HL101397 (JAL) and #1R01HL118277 (LGT).
Abbreviations
- AF
atrial fibrillation
- LA
left atrium
- LV
left ventricle
- PTFV1
P-terminal force in V1
- PPaV1
P-prime in V1 amplitude
- PPdV1
P-prime in V1 duration
- HF
heart failure
- CMR
cardiac magnetic resonance
- LVEF
left ventricular ejection fraction
- LAEF
left atrium emptying fraction
- LGE
Late gadolinium enhancement
- Vmax
Maximum left atrial volume
- Vmin
minimum left atrial volume
- VpreA
left atrial volume before atrial systole
- Smax
maximum left atrial strain
- SRmax
maximum left atrial strain rate
- LAVimax
left atrial volume indexed by body surface area
- BMI
Body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration Information—URL:http://www.clinicaltrials.gov:NCT01353131
Conflict of Interest Disclosures
None. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
References
- 1.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 2.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–e146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chae CU, Glynn RJ. RIsk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso A, Krijthe BP, Aspelund T, et al. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially and Geographically Diverse Population: the CHARGE-AF Consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J AmCollCardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 10.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J AmCollCardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Shah AJ, Soliman EZ. Effect of electrocardiographic P-wave axis on mortality. AmJCardiol. 2014;113:372–376. doi: 10.1016/j.amjcard.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ. P Wave Duration and Risk of Longitudinal Atrial Fibrillation in Persons GëÑ60 Years Old (from the Framingham Heart Study) The American Journal of Cardiology. 2011;107:917–921. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnani JW, Gorodeski EZ, Johnson VM, Sullivan LM, Hamburg NM, Benjamin EJ, Ellinor PT. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011;8:93–100. doi: 10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MORRIS JJ, Jr., Estes EH, Jr., WHALEN RE, THOMPSON HK, Jr., McINTOSH HD. P-WAVE ANALYSIS IN VALVULAR HEART DISEASE. Circulation. 1964;29:242–252. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 15.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohsaka S, Sciacca RR, Sugioka K, Sacco RL, Homma S, Di Tullio MR. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Tamura A, Torigoe K, Kawano Y, Shinozaki K, Kotoku M, Kadota J. Abnormal P-wave terminal force in lead V1 is associated with cardiac death or hospitalization for heart failure in prior myocardial infarction. Heart and Vessels. 2013;28:690–695. doi: 10.1007/s00380-012-0307-9. [DOI] [PubMed] [Google Scholar]
- 18.Tereshchenko LG, Shah AJ, Li Y, Soliman EZ. Electrocardiographic Deep Terminal Negativity of the P Wave in V1 and Risk of Mortality: The National Health and Nutrition Examination Survey III. J Cardiovasc Electrophysiol. 2014 doi: 10.1111/jce.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy S, Rizzi P, Mewton N, Strauss DG, Liu CY, Volpe GJ, Marchlinski FE, Spooner P, Berger RD, Kellman P, Lima JA, Tereshchenko LG. Number of P-Wave Fragmentations on P-SAECG Correlates with Infiltrated Atrial Fat. AnnNoninvasiveElectrocardiol. 2014;19:114–121. doi: 10.1111/anec.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauss DG, Mewton N, Verrier RL, et al. Screening entire health system ECG databases to identify patients at increased risk of death. CircArrhythmElectrophysiol. 2013;6:1156–1162. doi: 10.1161/CIRCEP.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tereshchenko LG, Rizzi P, Mewton N, Volpe GJ, Murthy S, Strauss DG, Liu CY, Marchlinski FE, Spooner P, Berger RD, Kellman P, Lima JA. Infiltrated atrial fat characterizes underlying atrial fibrillation substrate in patients at risk as defined by the ARIC atrial fibrillation risk score. IntJ Cardiol. 2014;172:196–201. doi: 10.1016/j.ijcard.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. ArchInternMed. 1985;145:1877–1881. [PubMed] [Google Scholar]
- 24.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn ResonMed. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Liu YC, Wu C, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J AmCollCardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong TC, Piehler K, Meier CG, et al. Association Between Extracellular Matrix Expansion Quantified by Cardiovascular Magnetic Resonance and Short-Term Mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soliman EZ, Alonso A, Misialek JR, Jain A, Watson KE, Lloyd-Jones DM, Lima J, Shea S, Burke GL, Heckbert SR. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) JElectrocardiol. 2013;46:702–706. doi: 10.1016/j.jelectrocard.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P Wave Indices, Obesity, and the Metabolic Syndrome: The Atherosclerosis Risk in Communities Study. Obesity. 2012;20:666–672. doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beinart R, Khurram IM, Liu S, Yarmohammadi H, Halperin HR, Bluemke DA, Gai N, van der Geest RJ, Lima JA, Calkins H, Zimmerman SL, Nazarian S. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart Rhythm. 2013;10:1325–1331. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RNW, Kirkels H, Janse MJ, de Bakker JMT. Activation Delay After Premature Stimulation in Chronically Diseased Human Myocardium Relates to the Architecture of Interstitial Fibrosis. Circulation. 2001;104:3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 31.McLenachan JM, Dargie HJ. Ventricular Arrhythmias in Hypertensive Left Ventricular Hypertrophy: Relationship to Coronary Artery Disease, Left Ventricular Dysfunction, and Myocardial Fibrosis. American Journal of Hypertension. 1990;3:735–740. doi: 10.1093/ajh/3.10.735. [DOI] [PubMed] [Google Scholar]
- 32.Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van EL, Swenne CA, Schalij MJ. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. CircArrhythmElectrophysiol. 2009;2:548–554. doi: 10.1161/CIRCEP.109.859108. [DOI] [PubMed] [Google Scholar]
- 33.Gotsman I, Keren A, Hellman Y, Banker J, Lotan C, Zwas DR. Usefulness of electrocardiographic frontal QRS-T angle to predict increased morbidity and mortality in patients with chronic heart failure. Am J Cardiol. 2013;111:1452–1459. doi: 10.1016/j.amjcard.2013.01.294. [DOI] [PubMed] [Google Scholar]
- 34.Pavri BB, Hillis MB, Subacius H, Brumberg GE, Schaechter A, Levine JH, Kadish A. Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117:3181–3186. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 35.de JS, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J CardiovascPharmacol. 2011;57:630–638. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 36.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 37.Neilan TG, Shah RV, Abbasi SA, et al. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J AmCollCardiol. 2013;62:2205–2214. doi: 10.1016/j.jacc.2013.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appelbaum E, Manning WJ. Left atrial fibrosis by late gadolinium enhancement cardiovascular magnetic resonance predicts recurrence of atrial fibrillation after pulmonary vein isolation: do you see what I see? CircArrhythmElectrophysiol. 2014;7:2–4. doi: 10.1161/CIRCEP.114.001354. [DOI] [PubMed] [Google Scholar]
- 39.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume. A powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 40.Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol. 1977;39:967–971. doi: 10.1016/s0002-9149(77)80209-9. [DOI] [PubMed] [Google Scholar]
- 41.Tsao CW, Josephson ME, Hauser TH, O'Halloran TD, Agarwal A, Manning WJ, Yeon SB. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7. doi: 10.1186/1532-429X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truong QA, Charipar EM, Ptaszek LM, Taylor C, Fontes JD, Kriegel M, Irlbeck T, Mahabadi AA, Blankstein R, Hoffmann U. Usefulness of electrocardiographic parameters as compared with computed tomography measures of left atrial volume enlargement: from the ROMICAT trial. Journal of Electrocardiology. 2011;44:257–264. doi: 10.1016/j.jelectrocard.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu T, Ozawa M, Tachibana H, Sato Y, Orii M, Kunugida F, Nakamura M. Combination therapy with amiodarone and enalapril in patients with paroxysmal atrial fibrillation prevents the development of structural atrial remodeling. Int Heart J. 2008;49:435–447. doi: 10.1536/ihj.49.435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.